Abstract
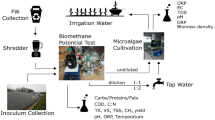
Diversion of food waste from landfill through anaerobic digestion is a sustainable form of energy production (biogas) and the waste effluent (digestate) can be utilised as nutrient supply for microalgae cultivation. However, digestate has very high nutrient concentrations and is highly turbid, making it difficult to utilize as a nutrient source with conventional microalgae cultivation systems. Here we compared the efficiencies of a conventional open raceway pond (ORWP) and an improved inclined thin layer photobioreactor (ITLP) for the utilization and treatment of food waste derived digestate by Chlorella sp. The ITLP improved on volumetric and areal productivities by 17 and 3 times over the ORWP, with values of 0.563 and 31.916 g m −2 day −1 respectively. Areal nutrient removal via microalgae biomass were 2359.759 ± 64.75 and 260.815 ± 7.16 mg m −2 day −1 for nitrogen and phosphorous respectively in the ITLP, which are 2.8 times higher than obtained in the ORWP. The ITLP’s superiority stems from its ability to support a much higher average biomass yield of 6.807 g L −1, which was 7 times higher than in the ORWP. Mean irradiance in-situ was higher in the ITLP, irradiance distribution and utilization by the culture in the ITLP was 44% more efficient than in the ORWP. Our results indicate that the ITLP is a far more productive system than conventional raceway ponds. This demonstrates that integration of ITLP microalgae cultivation using digestate has the potential to make digestate management yield net benefit in food waste biorefinery settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the Food and Agricultural Organization (FAO), one-third (about 1.3 billion tonnes) of the edible parts of food produced for human consumption is wasted globally per year (FAO 2011). The carbon footprint of food waste including land use change contributes 3.6 gigatonnes of CO2 yearly (FAO 2011, 2017). This is approximately 8% of global CO2 emissions (Jain et al. 2018). Food waste is typically disposed via landfilling or incineration, and in the United States of America for example only about 4.1% of food waste is recycled, including composting (EPA 2020). Incineration and landfilling can lead to the release of significant amounts of methane gas and CO2 which has ramifications for global greenhouse gas emissions and also ground water contamination which is a major environmental risk (Kaza et al. 2018; Chuka-ogwude et al. 2020b).
Anaerobic digestion is a mature and widely applied technology used to treat food waste with significantly reduced greenhouse gas emission. The benefits of anaerobic digestion in the biorefinery paradigm include renewable energy generation in the form of bio-methane with accompanying reduction in greenhouse gas emission (Sheets et al. 2015). Anerobic digestion can also be coupled to other bioprocesses, like lactic acid extraction in biorefinery models for further product extraction and value proposition (Bühlmann et al. 2021). However, anaerobic digestion is an incomplete process and produces an effluent (anaerobic digestate) high in inorganic nitrogen in the form of NH4+-N ranging from ~ 700 – 5000 g L−1 (Buhlmann et al. 2019) and phosphate, and pose a hazard if disposed to the environment improperly. Digestate can however, potentially be harnessed in a circular economy as a source of fertilizers (Ren et al. 2020), bio-oil, syngas, biochar, ethanol, electricity, hydroponics, fibre for animal bedding and feed stock for microalgae cultivation (Sheets et al. 2015).
Microalgae have been intensively studied as a sustainable source of various biobased products and they are especially attractive because of their efficiency in carbon sequestration and fixation, and their much higher growth yields and productivities compared to terrestrial plants (Schädler et al. 2019). Microalgal biomass and metabolites have been studied and applied as sources of both high and low value compounds including proteins, carbohydrates, fatty acids, pigments such as carotenoids and phycocyanin, and their applications spread across the feeds and food, pharmaceuticals and energy industries (Mobin et al. 2019). However, microalgae cultivation can be uneconomical if expensive synthetic fertilizers are used as nutrient sources and if appreciable biomass yields are not attained (Fornarelli et al. 2017). Microalgae cultivation can even be unsustainable if natural resources needed for their cultivation such as water are not properly sourced and managed e.g. using waste water instead of fresh water, and water recycling practices (Farooq et al. 2015). In the last few years sustainably derived nutrients from waste water as a nutrient source (e.g. anaerobic digestates) including food waste digestate, have been proposed as a biorefinery model for microalgal cultivation, integrating both wastewater / waste effluent treatment and carbon sequestration (Ayre et al. 2017; Chuka-ogwude et al. 2020b). This is an important advance in the treatment of waste water, especially high strength waste effluents like anaerobic digestates, as regulations around its disposal and end usage, such as land filling and direct application as fertilizers, are becoming more stringent (Rehl and Müller 2011; Torres-Franco et al. 2021).
Food waste derived digestate (henceforth termed Anaerobic digestate of food waste, ADF) as nutrient source for the cultivation of microalgae has its challenges. The most prominent being the very high concentration of nitrogen in the form of NH4+-N (up to 5000 mg L−1) toxic to most microalgae species (Chuka-ogwude et al. 2020c), and very high turbidity (up to 14,000 NTU) from colour and suspended particles. These characteristics significantly increase photolimitation in microalgae cultures and consequently leading to very low productivities (Wang et al. 2010; Raeisossadati et al. 2019). The easiest and most widely applied method used to tackle the problem of toxicity due to high concentrations of NH4+-N in digestates effluents is dilution, but this method is largely uneconomical and unsustainable because of the large amounts of water required for dilution (Cheng et al. 2015). In our previous studies we have isolated, screened and identified microalgae species capable of growing in digestate with very high concentrations of NH4+-N in an effort to minimize the economic and environmental costs associated with dilution practices (Ayre et al. 2017; Chuka-ogwude et al. 2020a). The microalgae species identified in these studies were able to proliferate in digestate with NH4+-N concentrations ranging from 150 – 1500 mg L−1.
Light is the most limiting factor in the cultivation of microalgae species in both open and closed cultivation systems, and the problem of photolimitation when digestates are used is greatly increased due to high turbidity levels (Marcilhac et al. 2014). Even with significant dilution, digestate from piggery waste and food waste are still very turbid with severe light attenuation (Kumar et al. 2019; Chuka-ogwude et al. 2020c) making cultivation using common culture systems like open raceway ponds unattractive due to poor biomass yields. In our previous studies we investigated the use of an inclined thin layer pond (ITLP) (Setlik et al. 1970) as an alternative to open raceway ponds and closed photobioreactor systems for the cultivation of microalgae in turbid piggery effluent digestate (Raeisossadati et al. 2019), and ADF (Chuka-ogwude et al. 2021). This ITLP cultivation system offers a much shorter light path, reduced attenuation, higher turbulence which results in better mixing and potential utilization of the flashing light effect, due to the inclination of the system (Laws et al. 1983; Doucha and Livansky 1995; Chuka-ogwude et al. 2020b). The configuration of the ITLP makes it uniquely suited for microalgae cultivation in turbid medium like digestate. However, because of the large surface area to volume ratio of the ITLP, application of the ITLP for microalgae cultivation using highly turbid digestates have demonstrated suboptimal areal productivities in comparison to regular raceway ponds (Raeisossadati et al. 2019). Our studies have shown that microalgae cultivation in turbid ADF using the ITLP, the light attenuation is reduced leading to increases in biomass yield and that by optimizing the depth of the ITLP, areal productivities are further increased (Chuka-ogwude et al. 2021).
So far there are limited data in the literature on comparative studies between various systems of cultivation for turbid medium such as food waste digestate. The aim of this study is to compare the performance of a NH4+-N tolerant Chlorella sp in an ITLP and in an open raceway pond system using turbid ADF as the growth medium / feedstock. The overarching objective of this study is to determine if the ITLP is a more efficient system for the treatment of ADF and simultaneous production of microalgae biomass. The biomass yields, productivities, nutrient removal from ADF, as well as their photosynthesis and irradiance distribution were used for comparison.
Materials and methods
Inoculum and culture media
This study was performed using Chlorella sp. MUR 268 previously described as robust and selected as suitable for good growth in food waste digestate (Chuka-ogwude et al. 2020a). Prior to use, the strain was maintained in ADF diluted to 300 mg L−1 NH4-N in a 5 L flask with an operational volume of 2 L, under 180 µmol photons m−2 s−1 irradiance and 120 rpm mixing speed. Temperature and humidity were controlled at 210C and 24% respectively. For outdoor cultivation, the culture was scaled up from the flask to a 20 L (0.1 m2) paddle wheel driven raceway pond and then to 200 L in a 1 m2 paddle wheel driven raceway pond. The culture was further scaled up to 400 L in a 2 m2 paddle wheel driven raceway pond from which log phase culture was used to inoculate a 11 m2 ITLP and an 11 m2 open race way pond (ORWP) where the main experiments were performed. The ADF used in this study was obtained from a 50,000 t per annum two-stage mesophilic food waste anaerobic digestion plant (Buhlmann et al. 2019), located in Jandakot, Western Australia. The digestate was used largely untreated and added directly to the cultivation systems until the desired concentration of NH4-N was attained. Large particles such as seeds and fibres were removed by a 1.5 mm pore size sieve briefly installed (for 10 min) in both the ORWP and ITLP after the raw ADF had been added to the ORWP and the sump of the ITLP. The physiochemical properties of the ADF used are described in Table 1.
Experimental setup and operational conditions
The outdoor experiments were performed in a 11 m2 paddle wheel driven raceway pond ORWP and a 11 m2 inclined pond ITLP. The ORWP was made with reinforced plastic with an inner coating of non-adhesive fibreglass. Mixing in the ORWP was performed by a four-blade electric motor driven paddle wheel, operating at a flow rate of approximately 0.2 m s−2. The ITLP design was based on the design of Doucha and Livansky (1995), consisting of a metal frame chassis, an inclined frame, a cultivation surface made of transparent plastic. A detailed design of the ITLP and the ORWP is described in Raeisossadati et al. (2019). Low-stress, open impeller, submersible pumps (Davey water products Pty Ltd, Australia) with a maximum flow rate of 80 L min−1 were used to pump the culture from the sump onto the inclined surface of the ITLP (Chuka-ogwude et al. 2021). The pH in both the ORWP and the ITLP was maintained at 7.0 ± 0.3 by injecting pure CO2 using a TPS miniCHEM pH controller (TPS Pty Ltd, Australia). Temperature of the cultures were monitored using Hobo 64 k Pendant temperature loggers (Onset computer Corp, USA). Evaporation in the two cultivation systems were monitored daily and evaporation loss in the culture was compensated for by replacement with fresh water. Culture in both the ORWP and ITLP were subjected to an acclimation period of 1 – 2 weeks before commencement of the proper experiment and harvest cycles. The cultivation mode was semi-continuous cultivation mode (Hsieh and Wu 2009). Both cultivation systems were grown to the maximum supported biomass concentrations (batch mode for ORWP and fed batch with cell recycling for the ITLP), before transiting to semi-continuous mode of cultivation. Semi-continuous cultivation was performed in both systems by replacement of 30 – 40% of harvested culture volume with fresh ADF and water every 48 h (dilution rate of 0.15 – 0.2 day −1), depending on yield and weather conditions. The operating nutrient concentrations of the systems after the addition of ADF to water through the course of the experiment are described in Table 1. The ORWP was operated at a culture depth of 0.2 m and an operational volume of 2200 L, while the ITLP was operated at a inclined surface culture depth of 0.011 m and an operational volume of 280 L with approximately 40% of the operational volume on the surface of the incline as previously described (Chuka-ogwude et al. 2021). The experiments were performed from November 2020 to March 2021 in outdoor summer conditions of Perth, Western Australia.
Growth rates, productivities, nutrient removal, and water loss
Concentration of the microalgae species used in this study was evaluated as both cell count using a Neubauer hemocytometer and cell dry weight. Cell dry weight was determined as ash free dry weight as described by Moheimani et al. (2013). Specific growth rate (µ) was calculated as the change in the natural logarithm of the microalgae cell numbers per time and productivities were calculated as the change in microalgal biomass concentration per time as described by Moheimani et al. (2013). Nitrogen (NH4-N and NO3-N), phosphates and chemical oxygen demand (COD) concentrations in the cultures were determined using a Hanna HI83099 COD and Multiparameter Laboratory—Photometer (Hanna Instruments, Romania) and accompanying reagents. Mass balance of nitrogen and phosphorus in the systems was estimated by balancing the concentration of these nutrients in the influent medium (at the start of a harvest cycle) against concentration of nutrient in the outlet medium (at end of harvest cycle). Nitrogen and phosphorous assimilated by the microalgal biomass were determined by analysis of digests of dry microalgae biomass via Flow injection analysis of ammonia for nitrogen, and orthophosphate using an automated flow injection analyser (Lachat Instruments, USA). Water loss / evaporation was determined by measuring the amount of fresh water added to the systems to replace evaporated water. Data for ambient temperature, and rainfall were obtained from the Murdoch University weather station.
Light distribution in the cultivation systems
Incident Irradiance spectra in the photosynthetically active radiation (PAR) range was measured using a CXR-SR-50 spectrometer (StellaNet Inc, Florida, USA). The wavelength specific absorbance of the cultures (Absculture(λ) was measured as \(log (\frac{{I}_{0}}{{I}_{L}})\) at each wavelength, where I0 = incident irradiance on the surface of the cultivations systems (ORWP and ITLP), IL = irradiance at distance L, inside the culture. Mean irradiance in the cultures were estimated as described by Holland and Dragavon (2014) and as depicted in Eqs. 1 and 2 (detailed descriptions can also be found in Chuka-ogwude et al. (2021)).
where \({I}_{L}\)= emergent irradiance at the end of the light path L, (µmol photons m−2 s−1), \({I}_{0}\)= incident irradiance, (µmol photons m−2 s−1), \({I}_{mean}\)= mean irradiance inside the culture available to algae (µmol photons m−2 s−1), \(L\)= light path (m), d = culture depth (m), and \(\sigma\)= absorption cross section of culture.
Maximum quantum yields, electron transfer rates, and photosynthesis – irradiance curves
Photosynthesis eas investigated using a pulse amplitude fluorometer (Water-Pam (cuvette version) fluorometer, Heinz Walz,, Germany). Maximum quantum yield in actinic light was calculated as Fv’/Fm ’\(= \frac{{Fm}^{^{\prime}}-F{o}^{^{\prime}}}{F{m}^{^{\prime}}}\) (Genty et al. 1989). Non photosynthetic quenching (NPQ) was determined as NPQ \(= \frac{Fm-F{m}^{^{\prime}}}{F{m}^{^{\prime}}}\) (Cosgrove and Borowitzka 2010), after a 30 min dark adaptation period.
Rapid light curves (RLCs) were generated and plots of relative electron transport rates (rETR) against PAR were made as Photosynthesis – Irradiance (P-I) curves as described in Chuka-ogwude et al., (2021). Functional rETR (FrETR) and functional rETR-ratio of the system were determined as detailed in Chuka-ogwude et al. (2021) and listed in Eqs. 3 and 4, used to quantify the average in-situ rETR across the depth of the culture system, and the proportion of the culture functioning at maximum rETR respectively.
where, \({I}_{i}\)= point irradiance along the trajectory of light as it passes through the culture medium, defined by \({I}_{L}\) in Eq. 2,and \({P}_{max}\) and \({k}_{w}\) are maximum photosynthesis and a scaling constant for the X-axis respectively obtained from fitting rapid light curves.
Statistical analysis
A minimum of five (5) replicates (harvests) were used (n = 5) and the results were expressed as mean ± standard error. Two-tailed independent t-tests were used to evaluate significant differences between the ORWP and the ITLP, and significance was based on p < 0.05. All statistical analysis were performed using IBM SPSS Statistics (version 26) for windows. Curve fittings and modellings were done in python 3.5.
Results
Culture conditions of the systems
The cultures in both systems remained unialgal (Chlorella sp, MUR 268) throughout the experimentation period. Irradiance through the period of cultivation averaged at 1379.72 ± 49.61 µmol photons m−2 s−1, with minimal rainfall. The culture in the ITLP, during the acclimation phase (grey portion of the graphs on Fig. 1), was successfully built up to high densities of up to 7 g L−1 employing fed-batch with cell recycling before transitioning to semi-continuous mode. On the other hand, all attempts to apply the same method of increasing cell densities in the ORWP led to the culture crashing at cell densities around 0.5 g L−1. Culture temperature through the period of cultivation was significantly different (p < 0.05), with the temperature of the ITLP culture being significantly higher at 25.66 ± 0.13 °C in comparison with 22.44 ± 0.13 °C in the ORWP. Maximum temperature was approximately 40.1 °C in the ITLP and 32.5 °C in the ORWP and minimum temperatures were 13.8 °C and 10.9 °C at early hours of the day in the ITLP and ORWP respectively. Evaporation loss of water in the ITLP was significantly higher (p < 0.05) than observed in the ORWP (Table 2).
Time series of weather and culture conditions of the cultivation systems through the period of cultivation. (a) Solar irradiation averaged over 10-min intervals, (b) Rainfall averaged over 10-min intervals (c)pH averaged over 10-min intervals, (d) Daytime temperature readings averaged over 10-min intervals, (e) Biomass density of the Inclined thin later pond (ITLP), (f) Biomass yield (ash free dry weight) of the open raceway pond (ORWP)
Cell growth and productivities
Average biomass yield and cell density maintained through the cultivation period were both significantly higher in the ITLP than in the ORWP (p < 0.001). Volumetric productivities were significantly higher in the ITLP than in the ORWP (p < 0.001), with volumetric productivities of 0.563 ± 0.1 and 0.031 ± 0.01 g L−1 day−1 in the ITLP and the ORWP respectively. Also, areal productivity was significantly higher in the ITLP (p > 0.001), having an areal productivity of 31.916 ± 1.11 g m−2 day−2 in comparison with the ORWP having an areal productivity of 11.46 ± 0.79 g m−2 day−2. However, the results show that there was no significant difference in the maximum specific growth rates, µmax, of the microalgae in the ITLP and the ORWP (t (10) = 15.02, p = 0.452), with growth rates of 0.093 ± 0.02 and 0.073 ± 0.02 day−1 in the ITLP and the ORWP respectively. Figure 2 summarizes the growth rates and productivities of the culture systems.
Average productivities and rates charts for the cultivation systems. (a) areal productivities, (b) volumetric productivities, (c) maximum growth rates. Error bars represent standard error of means. Significant differences are described by the letters at the top of the bars: a is significantly > b. Bars with the same letters are not significantly different
Nutrient removal, utilization, and mass balance
The ITLP was significantly more effective than the ORWP (p < 0.001) regarding the rate of removal of NH4-N, phosphorous, and nitrate, and depleting the levels of COD in the ADF. Regarding percentage efficiency of the nutrient’s removal from the influent ADF, percentage removal was significantly higher (p < 0.001) in the ITLP for ammonia nitrogen and phosphorous at 96.5% and 93.6% respectively, in comparison with 46.6% and 67.9% in the ORWP. Also, percentage efficiency for removal / depletion of nitrate nitrogen, and COD were also higher in the ITLP than in the ORWP (p < 0.05) (Table 3). Mass balance analysis show that over 80% of the total N removed from the influent ADF was utilized by the algal biomass for growth and the rest lost by volatilization, in both the ITLP and the ORWP (Table 4). There was no significant difference in the proportion of the removed ADF nitrogen and phosphorous utilized by the microalgae biomass in both culture systems (p > 0.05). Phosphorous not detected at harvest and not utilized by biomass, could be considered as being precipitated as phosphates. Total nitrogen and phosphorous removed (assimilated) by the biomass were significantly higher in the ITLP than in the ORWP both on a volumetric and areal basis (p < 0.001), with areal removal rates, up to 2359.759 mg m−2 day−1 and 260.815 mg m−2 day−1 for nitrogen and phosphorous respectively in the ITLP (Table 4). Overall treatment capacity in terms of litres of ADF per unit area per day for the systems, determined via dilution rates, was not significantly different at 0.148 ± 0.05 LADF m−2 day−1 for the ITLP and 0.135 ± 0.08 LADF m−2 day−1 for the ORWP. Both culture systems were started with initial ADF-Nitrogen concentrations seen to be most suited to them, especially because of turbidity, as discussed in “Experimental setup and operational conditions”. At the point of harvest, the effluent ammonia nitrogen was below 5 mg L−1 in the ITLP and below 7 mg L−1 in the ORWP. Phosphate concentration was below 2 mg L−1 in both systems.
Light distribution in the culture systems, and photosynthesis
Mean irradiance in the culture systems, as estimated by Eqs. 2 and 3, was seen to be significantly higher (45%) in the ITLP than in the ORWP (p = 0.01). However, there was no significant difference in the optimal irradiance, Ioptimum, for the cells in both systems (p > 0.05). Regarding the photosynthetic responses of the cells in the culture systems, there was no significant difference between the ITLP and the ORWP for both maximum light utilization coefficient (α), and maximum quantum yield in actinic light (Fv’/Fm’) (p > 0.05). Maximum relative electron transfer rate, rETRmax, was slightly higher in the ORWP than in the ITLP (p = 0.046). NPQmax of the cells in both systems were not significantly different. However, functional rETR (FrETR) and functional rETR-ratio (FrETR-ratio) were both significantly higher in the ITLP in comparison to the ORWP (p < 0.05). Table 5 details the distribution of irradiance inside the microalgae cultures in both systems, and the associated photosynthetic responses.
Discussion
Culture conditions of the systems
The first obvious difference between the ITLP and the ORWP was the ORWP’s inability to support microalgae biomass above 0.5 g L−1. This was due to severe photolimitation in the ORWP due to the turbidity of the ADF, as the concentration of nutrients in the ORWP was way below the tolerance threshold of the Chlorella species used in this study. Many studies have demonstrated the negative effect of various digestates via photolimitation due to suspended particles and colour (Wang et al. 2010; Marcilhac et al. 2014). With the food digestate used in this study, the turbidity was very high (Table 1) even after significant dilution, leading to low mean irradiance in culture. As shown in our previous study (Chuka-ogwude et al. 2020c), mean irradiance inside cultures using ADF as feedstock can be very low even at relatively short light paths, and this is further amplified by the higher depth in raceway ponds. The temperature ranges observed in the cultivations systems in this study are within the range for growth of Chlorella species, as the median to upper limits are from 25 – 42 °C (Kessler 1985), and lower limits of 10 – 15 °C (Cho et al. 2007). A consequence of the higher temperature in the ITLP was a higher evaporation rate. However, while evaporation constitutes a significant portion of operational cost in open cultivation systems (Rogers et al. 2014), the working volume of the ITLP is significantly lower (7.8 times) than the ORWP, indicating a significant advantage of the ITLP for less water demand in cultivation and downstream processing operations.
Cell growth and productivities
Since both the ITLP and the ORWP were operated at the same conditions of pH, incident irradiance, and CO2 supply, the comparison of these systems is mainly confined around their abilities to sustain a culture of high density. The results of the maximum growth rates, µmax, of the systems in this study are similar to what was reported in our previous study especially when considering the mean irradiance in the ITLP and ORWP, we demonstrated a linear relationship between growth rate and mean irradiance (Chuka-ogwude et al. 2021). Although µmax, was not statistically different in both systems, the ITLP has a slight edge over the ORWP in terms of growth rates. This is because of higher mean irradiance in the ITLP (discussed in the following sections). The superiority of the ITLP is clearly displayed in the results of both volumetric and areal productivities. Clearly, the ITLP was able to sustain and support growth of high cell density culture of almost 4 × 108 cells mL−1 (Table 2) due to its ability to supply more photons to the algae and higher mixing and turbulence. This contrasts with the ORWP which could not support a high cell density culture. The much lower productivities of the ORWP displayed here highlights its disadvantage especially in utilizing and treating highly turbid substrates like ADF. Higher productivities of 19.24 – 24 g m−2 day−1 under outdoor conditions in various sized ORWPs using abattoir effluent digestate and urban wastewater effluent with the addition of CO2 have been reported while optimizing for CO2 addition, and depth (Morales-Amaral et al. 2015a; Jebali et al. 2018; Shayesteh et al. 2021). Notably, the afore-mentioned examples were done in digestates significantly less turbid that the ADF used in this study. However, productivities obtained for the ORWP in this study are significantly higher than reported for anaerobic digestate of piggery waste effluent of similar turbidity with productivities of 6.2 g m−2 day−1 and 0.024 g L−1 day−1 (Nwoba et al. 2016; Raeisossadati et al. 2019) and similar to that reported by Serejo et al. (2015) at 11.8 g m−2 day−1 using diluted vinasse digestate of lower turbidity. The productivities reported for the ITLP in this study are significantly higher than reported for a lot of the works done on ITLPs in clear synthetic media ranging from 9 – 23 g m−2 day−1 (Doucha et al. 2005; Silva Benavides et al. 2017; Grivalský et al. 2019; Schadler et al. 2020). Also, productivities of the ITLP here are significantly higher than we reported in our first trial of this system on high turbidity anaerobic digestate of piggery effluent, and our depth optimization work using ADF ranging from 2 – 21 g m−2 day−1 (Raeisossadati et al. 2019; Chuka-ogwude et al. 2021). Improvements in the ITLP productivities were achieved by employing fed-batch cultivation of the culture until a high biomass yield was achieved, which the system was able to support, before transiting to semi-continuous culture mode.
Nutrient removal, utilization, and mass balance
The result regarding nutrient removal suggests that the defining factor for performance was biomass yield, and the ability of the ITLP to support a much denser culture makes it a far more superior system for treatment and valorisation of ADF. The volumetric and areal nitrogen removal capacity, via biomass, of 50.16 mg L−1 day−1 and 2359.759 mg m−2 day−1, respectively, reported for the ITLP in this study are among the highest values reported in literature in relation to any wastewater treatment. This is significantly higher than reported in our first trial using ITLP for anaerobic digestate of piggery effluent yielding a total (via biomass and volatilization combined) areal removal of nitrogen of 19 mg L−1 day−1 (Raeisossadati et al. 2019). Phosphate, nitrate, and COD removal are also consequently much higher in this study than in our previous one. The range for nitrogen removal rates reported in literature for outdoor high strength wastewater treatment systems, sub-optimal to optimal conditions, are between 0.5 – 22.7 mg L−1 day−1 (Sevrin-Reyssac 1998; Marcilhac et al. 2014; Shayesteh et al. 2021). Some of the highest removal rates reported are from Morales-Amaral et al., (2015b) with 38 mg L−1 day−1 and 3.9 mg L−1 day−1 for nitrogen and phosphorous respectively. Removal rates for the ITLP reported in this study are similar. Clearly, the ITLP is the superior system in terms of nutrient removal and ADF treatment, and corroborates Morales-Amaral et al., (2015a, b) that biomass concentration is the defining factor required for nutrient removal.
However, volatilization of NH4-N is a significant problem in the use of high strength wastewaters like ADF. Up to 20% of the NH4-N removed in both systems was a result of volatilization, even with pH regulation to keep ammonia nitrogen in NH4+ form. This is relatively good as percentage volatilization can be as high as 60% in uncontrolled systems (Shayesteh et al. 2021). At the pH regulation implemented in this study, ammonia volatilization loss matches that reported by Shayesteh et al., (2021). The percentage volatilization in the ITLP and the ORWP were not different because volatilization is largely dependent on surface area amongst other factors (Montes et al. 2009), and both systems here were operated with the same surface area. Even at very tightly regulated pH control, volatilization can still be up to 14% (Shayesteh et al. 2021). Higher density cultures and pH regulation are ways to limit this as seen here. Also, potential improvements could be realized if a feeding regime is implemented to match microalgal growth rate with nutrient supply, on-demand, using methods such as a combination of exponential feeding and continuous cultivation methods.
Light distribution in the culture systems, and photosynthesis
Mean irradiance in microalgae culture systems is a very important factor to consider in cultivation and design of efficient systems. Also, there is a strong correlation between growth rates and mean irradiance in culture (Chuka-ogwude et al. 2021) as it more accurately describes the availability of light to the cells through the depth of the culture column. The higher mean irradiance in the ITLP in comparison to the ORWP, is reflected in the slightly higher growth rates, µmax, in the ITLP, though this difference in µmax was not significant. However, the difference in mean irradiance did not elicit a matching difference in growth rates, this is not surprising since other parameters relating to the light profile like the optimum irradiance for the cells (Ioptimum) in both systems were the same, indicating that the light profiles in the ITLP and the ORWP were quite similar. This is also further corroborated by the photosynthetic parameters of both culture systems such as rETRmax, α, NPQmax, and Fv’/Fm’. These photosynthetic parameters were not different between the ITLP and the ORWP. These parameters as shown in Table 5 indicate that the cells in both culture systems were not stressed or photoinhibited as indicated by the low NPQmax values, and this is a significant observation especially for the ITLP with a depth of 0.011 m. The high-density culture in the ITLP mitigated against any significant photoinhibition that would otherwise have occurred, considering the high incident irradiance as is the case for lower depth culture in ILTPs (Chuka-ogwude et al. 2021). Given the similarities in the parameters mentioned above, the reason the reason the ITLP was able to sustain a culture of 17 times higher biomass yield in comparison to the ORWP is that light was more efficiently distributed across the depth of the ITLP than in the ORWP. This can be seen in the values of the FrETR which describes the actual functional max rETR of the culture, averaging out the spatial rETRmax across the depth of the culture on the ITLP, and the FrETR-ratio which is an indirect quantification of how much of the culture is in optimal irradiance and hence optimal rETRmax (Chuka-ogwude et al. 2021), Ioptimum and around rETRmax. The values for FrETR indicate that the actual functional electron flux through PSII across the depth of both systems is significantly higher in the ITLP than in the ORWP. It has been established that when grown outdoors microalgae culture of relatively average densities are challenged by supra-optimal irradiance and that approximately 90% of the incident photons could be absorbed in the first 0.01 m of the culture column leaving only a small region of the culture in optimal irradiation conditions (Beardall and Raven 2012). Here the FrETR-ratio values indicate that the portion of the culture functioning in the region of optimal irradiance and rETR is higher in the ITLP than in the ORWP (47% against 33% respectively).
Significance of the study
The above give credence to the ITLPs advantage over the ORWP. Previous studies comparing ITLPs to open raceway ponds for the cultivation of the microalga Scenedesmus sp. in centrate have reported preliminary technoeconomic analysis showing that biomass production cost using the ITLP could be up to 39% less in comparison to open raceway ponds, and 50% less in comparison with tubular photobioreactors (Morales-Amaral et al. 2015a). This is only on the microalgae biomass production side of the food waste biorefinery. Cost analysis of digestate treatment and utilization have shown that using digestate as a fertilizer for crops is less than 10% the cost of digestate management as a waste product, and if the digestate is to be transported over significant distances, including gate fees charged by waste management companies, digestate management becomes a substantial net cost, and at best case scenarios, as a crop fertilizer, it is neither a net cost or benefit (Cannon 2021). Considering the above, if food waste digestate is used for the cultivation of microalgae which has a significantly higher growth rate than traditional crops, integrating microalgae cultivation into food waste digestate management could potentially yield net benefit in food waste biorefinery settings. However, a detailed technoeconomic analysis and a life cycle analysis, especially considering biomass productivity and water savings associated with the ITLP is required to ascertain this.
Conclusions
In this study we have compared an ORWP and an ITLP for the treatment and valorisation of food waste digestate. Utilization of irradiance in the ITLP was 43% more efficient. Biomass yield in the ITLP was 17 times higher. Volumetric and areal productivities were 17 and 3 times higher in the ITLP, nutrient removal capacity was 2.8 times higher in the ITLP. Our results clearly demonstrated that the ITLP is a more efficient system ORWPs for biomass production with ADF. This means that for the same treatment plant, there would be need for third of cultivation area. A massive portion of the cost of microalgal production is the Capex required for building ponds. This would result in significant cost saving in treating anaerobic digestate using microalgae in dry temperate regions like Western Australia. In general, we believe that the use inclined open ponds for microalgal cultivation for treating ADF can make digestate management yield net benefit, not only in food waste biorefinery but also for other similar digestates such as piggery, abattoir, and dairy digestates.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Ayre JM, Moheimani NR, Borowitzka MA (2017) Growth of microalgae on undiluted anaerobic digestate of piggery effluent with high ammonium concentrations. Algal Res 24:218–226
Beardall J, Raven JA (2012) Limits to Phototrophic growth in dense culture: CO2 supply and light. In: Borowitzka MA, Moheimani NR (eds) Algae for Biofuels and Energy. Springer, Cham pp 91–97
Buhlmann CH, Mickan BS, Jenkins SN, Tait S, Kahandawala TKA, Bahri PA (2019) Ammonia stress on a resilient mesophilic anaerobic inoculum : Methane production, microbial community, and putative metabolic pathways. Bioresour Technol 275:70–77
Bühlmann CH, Mickan BS, Tait S, Bahri PA (2021) Developing a food waste biorefinery: Lactic acid extraction using anionic resin and impacts on downstream biogas production. Chem Eng J 431:133243
Cannon SC (2021) A cost analysis and policy review of digestate when deemed a waste product and a fertilizer. MS thesis, Rochester Institute of Technology, NY
Cheng J, Xu J, Huang Y, Li Y, Zhou J, Cen K (2015) Growth optimisation of microalga mutant at high CO2 concentration to purify undiluted anaerobic digestion effluent of swine manure. Bioresour Technol 177:240–246
Cho SH, Ji SC, Hur SB, Bae J, Park IS, Song YC (2007) Optimum temperature and salinity conditions for growth of green algae Chlorella ellipsoidea and Nannochloris oculata. Fish Sci 73:1050–1056
Chuka-ogwude D, Ogbonna J, Borowitzka MA, Moheimani NR (2020a) Screening, acclimation and ammonia tolerance of microalgae grown in food waste digestate. J Appl Phycol 32:3775–3785
Chuka-ogwude D, Ogbonna J, Moheimani NR (2020b) A review on microalgal culture to treat anaerobic digestate food waste effluent. Algal Res 47:101841
Chuka-ogwude D, Ogbonna JC, Moheimani NR (2020c) Adjustments of the photosynthetic unit and compensation mechanisms of tolerance to high ammonia concentration in Chlorella sp . grown in food waste digestate. Algal Res 52:102106.
Chuka-ogwude D, Ogbonna JC, Moheimani NR (2021) Depth optimization of inclined thin layer photobioreactor for efficient microalgae cultivation in high turbidity digestate. Algal Res 60:102509
Cosgrove J, Borowitzka MA (2010) Chlorophyll fluorescence terminology: An introduction. In: Suggett DJ, Prasil O, Borowitzka MA (eds) Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications, Developments in Applied Phycology. Springer, Dordrecht, pp 1–17
Doucha J, Livansky K (1995) Novel outdoor thin-Iayer high density microalgal culture system : Productivity and operational parameters. Arch Hydrobiol Suppl Algol Stud 76:129–147
Doucha J, Straka F, Lívanský K (2005) Utilization of flue gas for cultivation of microalgae (Chlorella sp.) in an outdoor open thin-layer photobioreactor. J Appl Phycol 17:403–412
EPA (2020) National Overview: Facts and Figures on Materials, Wastes and Recycling. United States Environmental Protection Agency. https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/national-overview-facts-and-figures-materials#composting. Accessed 22 Jan 2022
FAO (2011) Global food losses and food waste – Extent, causes and prevention. FAO, Rome
FAO (2017) Save food for a better climate. FAO, Rome
Farooq W, Suh WI, Park MS, Yang JW (2015) Water use and its recycling in microalgae cultivation for biofuel application. Bioresour Technol 184:73–81
Fornarelli R, Bahri PA, Moheimani N (2017) Utilization of microalgae to purify waste streams and production of value added products. Australian Meat Perocessor Corporation, North Sydney https://www.ampc.com.au/getmedia/45586025-7b89-4f92-8e36-36f4679dfcd7/AMPC_UtilizationOfMicroAlgaeToPurifyWasteStreams_FinalReport.pdf?ext=.pdf
Genty B, Briantais J-M, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Grivalský T, Ranglová K, da CâmaraManoel JA, Lakatos GE, Lhotský R, Masojídek J (2019) Development of thin-layer cascades for microalgae cultivation: milestones (review). Folia Microbiol (Praha) 64:603–614
Holland AD, Dragavon JM (2014) Algal reactor design based on comprehensive modeling of light and mixing. In: Bajpai R, Zappi M, Prokop A (eds) Algal Biorefineries: Volume 1: Cultivation of Cells and Products. Springer, Dordrecht pp 25–64
Hsieh CH, Wu WT (2009) Cultivation of microalgae for oil production with a cultivation strategy of urea limitation. Bioresour Technol 100:3921–3926
Jain S, Newman D, Cepeda-Márquez R, Zeller K (2018) Global Food Waste Management: An implamentation guide for cities. Full report. World Biogas Association, Hawthorn, Australia 145 p
Jebali A, Acién FG, Rodriguez Barradas E, Olguín EJ, Sayadi S, Molina Grima E (2018) Pilot-scale outdoor production of Scenedesmus sp. in raceways using flue gases and centrate from anaerobic digestion as the sole culture medium. Bioresour Technol 262:1–8
Kaza S, Yao L, Bhada-Tata P, Van Woerden F (2018) What a waste 2.0, A Global Snapshot of Solid Waste management to 2050. World Bank, Washington DC
Kessler E (1985) Upper limits of temperature for growth in Chlorella (Chlorophyceae). Plant Syst Evol 151:67–71
Kumar PK, Krishna SV, Naidu SS, Verma K, Bhagawan D (2019) Biomass production from microalgae Chlorella grown in sewage, kitchen wastewater using industrial CO2 emissions : Comparative study. Carbon Resour Convers 2:126–133
Laws EA, Terry KL, Wickman J, Chalup MS (1983) A simple algal production system designed to utilize the flashing light effect. Biotechnol Bioeng 25:2319–2335
Marcilhac C, Sialve B, Pourcher AM, Ziebal C, Bernet N, Béline F (2014) Digestate color and light intensity affect nutrient removal and competition phenomena in a microalgal-bacterial ecosystem. Water Res 64:278–287
Mobin SMA, Chowdhury H, Alam F (2019) Commercially important bioproducts from microalgae and their current applications-A review. Energy Procedia 160:752–760
Moheimani NR, Borowitzka MA, Isdepsky A, Fon Sing S (2013) Standard methods for measuring growth of algae and their composition. In: Borowitzka MA, Moheimani NR (eds) Algae for Biofuels and Energy. Springer, Dordrecht, pp 265–283
Montes F, Rotz CA, Chaoui H (2009) Process modeling of ammonia volatilization from ammonium solution and manure surfaces: A review with recommended models. Trans ASABE 52:1707–1719
Morales-Amaral M del M, Gómez-Serrano C, Acién FG, Fernández-Sevilla JM, Molina-Grima E (2015a) Outdoor production of Scenedesmus sp. in thin-layer and raceway reactors using centrate from anaerobic digestion as the sole nutrient source. Algal Res 12:99–108
Morales-Amaral M del M, Gómez-Serrano C, Acién FG, Fernández-Sevilla JM, Molina-Grima E (2015b) Production of microalgae using centrate from anaerobic digestion as the nutrient source. Algal Res 9:297–305.
Nwoba EG, Ayre JM, Moheimani NR, Ubi BE, Ogbonna JC (2016) Growth comparison of microalgae in tubular photobioreactor and open pond for treating anaerobic digestion piggery effluent. Algal Res 17:268–276
Raeisossadati M, Vadiveloo A, Bahri PA, Parlevliet D, Moheimani NR (2019) Treating anaerobically digested piggery effluent (ADPE) using microalgae in thin layer reactor and raceway pond. J Appl Phycol 31:2311–2319
Rehl T, Müller J (2011) Life cycle assessment of biogas digestate processing technologies. Resour Conserv Recycl 56:92–104
Ren AT, Abbott LK, Chen Y, Xiong YC, Mickan BS (2020) Nutrient recovery from anaerobic digestion of food waste: impacts of digestate on plant growth and rhizosphere bacterial community composition and potential function in ryegrass. Biol Fertil Soils 56:973–989
Rogers JN, Rosenberg JN, Guzman BJ, Oh VH, Mimbela LE, Ghassemi A, Betenbaugh MJ, Oyler GA, Donohue MD (2014) A critical analysis of paddlewheel-driven raceway ponds for algal biofuel production at commercial scales. Algal Res 4:76–88
Schädler T, Caballero Cerbon D, de Oliveira L, Garbe D, Brück T, Weuster-Botz D (2019) Production of lipids with Microchloropsis salina in open thin-layer cascade photobioreactors. Bioresour Technol 289:121682
Schadler T, Neumann-Cip A-C, Wieland K, Glockler D, Haisch C, Bruck T, Weuster-Botz D (2020) High density microalgae cultivation in thin layer cascade photobioreactors with water recycling. Appl Sci 10:3883
Serejo ML, Posadas E, Boncz MA, Blanco S, García-Encina P, Muñoz R (2015) Influence of biogas flow rate on biomass composition during the optimization of biogas upgrading in microalgal-bacterial processes. Environ Sci Technol 49:3228–3236
Setlik I, Veladimir S, Malek I (1970) Dual purpose open circulation units for large scale culture of algae in temperate zones. Basic design considerations and scheme of a pilot plant. Algol Stud/ Arch Hydrobiol Supplement 1:111–164
Sevrin-Reyssac J (1998) Biotreatment of swine manure by production of aquatic valuable biomasses. Agric Ecosyst Environ 68:177–186
Shayesteh H, Vadiveloo A, Bahri PA, Moheimani NR (2021) Can CO2 addition improve the tertiary treatment of anaerobically digested abattoir effluent (ADAE) by Scenedesmus sp. (Chlorophyta)? Algal Res 58:102379.
Sheets JP, Yang L, Ge X, Wang Z, Li Y (2015) Beyond land application: Emerging technologies for the treatment and reuse of anaerobically digested agricultural and food waste. Waste Manag 44:94–115
Silva Benavides AM, Ranglová K, Malapascua JR, Masojídek J, Torzillo G (2017) Diurnal changes of photosynthesis and growth of Arthrospira platensis cultured in a thin-layer cascade and an open pond. Algal Res 28:48–56
Torres-Franco A, Passos F, Figueredo C, Mota C, Muñoz R (2021) Current advances in microalgae-based treatment of high-strength wastewaters: challenges and opportunities to enhance wastewater treatment performance. Rev Environ Sci Biotech 20:209–235
Wang L, Li Y, Chen P, Min M, Chen Y, Zhu J, Ruan RR (2010) Anaerobic digested dairy manure as a nutrient supplement for cultivation of oil-rich green microalgae Chlorella sp. Bioresour Technol 101:2623–2628
Acknowledgements
The authors wish to acknowledge the staff of Richgro Anaerobic Digestion Plant, Jandakot Western Australia, for kindly supplying the food waste digestate used in this study.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This research was financially supported by Murdoch University and received no other specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
David Chuka-ogwude: Conceptualization, Investigation, Methodology, Writing—original draft, Writing—review & editing. Bede Michan: Resource, Writing—review & editing. James C Ogbonna: Funding acquisition, Resource, Supervision, Validation, Writing—review & editing. Navid R. Moheimani: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, validation, Writing—review & editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chuka-ogwude, D., Mickan, B.S., Ogbonna, J.C. et al. Developing food waste biorefinery: using optimized inclined thin layer pond to overcome constraints of microalgal biomass production on food waste digestate. J Appl Phycol 34, 2917–2928 (2022). https://doi.org/10.1007/s10811-022-02829-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-022-02829-5