Abstract
This work addresses the evaluation in vitro of different marine (Dunaliella salina REC-0214B and Microchloropsis gaditana REC-0251B) and freshwater (Anabaena sp. BEA-0300B, Arthrospira platensis BEA-0007B, Chlorella vulgaris BEA-0753B, Spirogyra sp. BEA-0666B) microalgae and cyanobacteria as potential dietary ingredients in aquafeeds. For this purpose, total protein content, amino acid composition, and the presence of protease inhibitors were evaluated. In addition, protein bioaccessibility was estimated using a species-specific in vitro assay using Sparus aurata digestive enzymes. Overall, all the microalgae showed high protein content ranging from 25 to 61%, and a balanced essential to non-essential amino acid content (from 0.81 to 0.95). The inhibition assay confirmed the absence of protease inhibitors whatever the microalgae considered. Finally, the in vitro assays showed differences in the degree of protein hydrolysis with values for the coefficient of protein degradation ranging from 49.4% in Spirogyra sp. to 85.5% in D. salina. Similarly, the total amount of free amino acids released from the microalgal biomass (from 12.8 to 20.8 g L-leucine equivalents (100 g protein)−1), as well as their qualitative amino acids profile varied among the different species, although the profile can be considered as well balanced in all cases. In conclusion, the results obtained revealed that, even if significant differences were observed among species in terms of their susceptibility to be hydrolysed by S. aurata digestive enzymes, all the microalgae and cyanobacteria evaluated presented an adequate protein content and a balanced amino acid profile.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The aquaculture industry is continuously trying to reduce the inclusion rates of fishmeal and fish oil in aquafeeds. However, the production of farmed species still depends on these ingredients as usual feedstuffs (Yavad et al. 2020), because they set the basis for balancing the formulation of commercial aquafeeds, especially for feeding crustaceans and carnivorous fish (Tacon and Metian 2008; Younis et al. 2018; Hua et al. 2019).
Increased demand, together with the stagnation of wild fisheries, the over-exploitation of pelagic fishes, and the current environmental concerns associated with extractive fishing, have driven fishmeal prices up by almost three-fold in the past decade. Therefore, finding and testing alternative protein sources, as well as designing sustainable and nutritious aquafeeds including those ingredients, remains a challenge for current industrial aquaculture (Yarnold et al. 2019). In this regard, it is important to point out that any satisfactory alternative feed ingredients must be able to provide a nutritional value comparable to that of regular ingredients but must also be readily available at an affordable cost (Vizcaíno et al. 2014; Guedes et al. 2015; Oliva-Teles et al. 2015).
Over the last decades proteins of plant origin have been introduced in aquafeeds to reduce the dependence on fishmeal, as they provide, roughly, nutrients for adequate fish growth. Indeed, soybean meal is one of the most widespread plant ingredients for aquafeed manufacturing as it is a high-quality protein source with reliable supply at a competitive cost. However, terrestrial plant proteins have some nutritional disadvantages, such as an imbalance in essential amino acids like lysine, methionine, threonine, and tryptophan, also containing anti-nutritional factors (Zheng et al. 2019), which altogether reduce the nutritional quality of aquafeeds (Shah et al. 2018) and jeopardize the bioavailability and digestibility of nutrients (Daniel 2018). Furthermore, recently sustainability concerns are also considered owing to extensive soybean farming areas in developing countries contributing to worldwide deforestation and loss of biodiversity (Pereira et al. 2020). This makes the emergence of other novel protein resources for feeding fish a priority.
Microalgae, including some species of cyanobacteria, have the potential for reducing the dependence on conventional ingredients for aquafeeds, as they are a more reliable and less volatile source of protein (Hemaiswarya et al. 2011; Guedes et al. 2015; Hua et al. 2019). They show the potential to provide protein, lipids, vitamins, carotenoids, among other compounds (Shah et al. 2018). Overall, the protein content of microalgae is in the range of 30—55% of protein (López et al. 2010), although in some genera such as Arthrospira, Scenedesmus and Chlorella it can be even higher (Molino et al. 2018; Shah et al. 2018). In addition, microalgal proteins display well-balanced amino acid profiles, comparable to those of other regular ingredients (Becker 2007; Guedes et al. 2015). Microalgae show high content of aspartate and glutamate (2.9 – 7.1%), whereas cysteine, methionine, tryptophan, and histidine content are in the range of 0.4 to 3.2%, with other amino acids ranging from 3.2 to 13.5% (Wilson 2003).
Despite this, there are some important drawbacks and challenges for extending the use of microalgae in aquafeeds because some species have recalcitrant cell walls that act as a protective barrier that reduces accessibility to intracellular nutrients (Teuling et al. 2019; Vizcaíno et al. 2019). The efficiency of fish enzymes for hydrolysing microalgae cell walls heavily relies on cell wall carbohydrate composition (Vizcaíno et al. 2019). In this regard, little research has been carried out addressing the extent of the hydrolysis of microalgae protein by the fish digestive system (Tibbetts et al. 2017; Vizcaíno et al. 2019).
This work aimed to carry out a comprehensive characterization of the protein nutritional profile and the in vitro protein bioaccessibility of cultured marine (Dunaliella salina REC-0214B, Microchloropsis gaditana REC-0251B) and freshwater (Anabaena BEA-0300B, Arthrospira platensis BEA-0007B, Chlorella vulgaris BEA-0753B, Spirogyra sp. BEA-0666B) microalgae and cyanobacteria to assess their potential as dietary ingredients for gilthead seabream (Sparus aurata) juveniles.
Material and methods
Microalgae
Six species of cyanobacteria or microalgae (Arthrospira platensis BEA-0007B, Dunaliella salina REC-0214B, Microchloropsis gaditana REC-0251B, Spirogyra sp. BEA-0666B, Chlorella vulgaris BEA-0753B and Anabaena sp. BEA-0300B) were provided by the Spanish Bank of Algae of the University of Las Palmas de Gran Canarias (Canary Islands, Spain). Strains were cultivated under laboratory controlled conditions according to the standardised methodology: Erlenmeyer flasks with f/2 medium (Guillard 1975) for seawater strains and BG11 medium (Rippka et al 1979) for freshwater strains, mean light intensity at 240 µmol photons m−2 s−1, photoperiod 12:12 (L:D), temperature 25 ± 2 °C and 1.5% CO2 enriched air continuously supplied during the light period. Artificial light was provided with six white light lamps (Phillips PL-32 W/840/4p) simulating the solar cycle. The biomass was harvested by centrifugation (RINA centrifuge, Spain), frozen at − 20 °C, freeze-dried, and finally milled in 100 g batches using a mortar grinder (RM200 mill, Retsch, Spain) for 20 min at 100 min−1 speed to obtain a fine powder (< 100 μm) that was stored in the dark at − 20 °C until further analysis.
Protein analysis
Chemical analyses of microalgae biomass were carried out as follows: crude protein (N × 6.25) was determined using elemental analysis (C:H:N) using Fisons EA 1108 analyzer (Fisons Instruments, USA). Total amino acid analysis of microalgae biomass was performed using a Biochrom 30 + Series amino acid analyser (Biochrom Ltd, UK) according to the manufacturer’s protocol. Calibration of the instrument was performed with an external amino acid calibration standard (Protein Hydrolysate Standard 2.5 mM, # 80–6002-67 Biochrom, UK)). Briefly, 100 μL of internal standard (2.5 mM norleucine, Sigma-Aldrich) was added to 800 μL sodium loading buffer (pH 2.2) obtaining a final concentration of 250 µM norleucine. This solution was mixed by vortex for 5 s and membrane-filtered (0.2 μm) and then a sample (20 μL) of this mixture was analysed with the amino acid analyser. For amino acid analysis of the microalgae, the freeze-dried biomass was hydrolysed (20 mg in 1 mL HCl 6 M) at 110 ºC for 24 h under an inert atmosphere (N2). Then, 50 μL of the hydrolysate were mixed with 50 μL of 6 M NaOH. The single amino acids were separated by ion exchange chromatography. Amino acids were detected at 570 nm except proline which was measured at 440 nm.
Testing the presence of protease inhibitors
Preparation of fish digestive enzyme extracts
Twenty Gilthead seabream specimens (25 g average body weight) were obtained from a commercial nursery (Predomar SA, Almería, Spain) and fed with a commercial diet (Skretting, crude protein: 47% DM) twice per day (9:00 and 13:00) at a rate of 3% biomass daily for a week. After that, the fish were sacrificed 5 h after feeding by severing their spine according to the requirements of the European Union (Directive 2010/63/UE) and Spanish (Real Decreto 53/2013) legislation, under the protocol number 06/02/2020/011. The abdomen was opened and the whole gut was obtained. Intestines from each five fish were pooled and manually homogenized (1:2, w/v) in distilled water at 4 ºC. Supernatants were obtained after centrifugation (16,000 ×g, 12 min, 4 °C) and stored in aliquots at –20 °C until use. Total alkaline protease activity of the enzymatic extracts was measured spectrophotometrically using 5 g L−1 casein in 50 mM Tris HCl (pH 9.0) as substrate (Alarcón et al. 1998). One unit of total protease activity was defined as the amount of enzyme that released 1 μg of tyrosine per min in the reaction mixture, considering an extinction coefficient for tyrosine of 0.008 μg−1 mL−1 cm−1, measured at 280 nm. Samples were analysed in triplicate.
Inhibition assay
Inhibitory extracts (0.1 g mL−1) were prepared from microalgae by homogenizing 100 mg biomass in 1 mL of distilled water, them shaking for 30 min at room temperature, and then for 24 h at 4 °C. The mixtures were centrifuged (20 min at 13,000 ×g and 4 °C) and supernatants were stored at 4 °C until use in inhibitory assays. The inhibition of S. aurata digestive proteases by microalgae extracts was evaluated according to Alarcón et al. (2001). Briefly, increasing volumes of the microalgae aqueous extract was added to the reaction mixture containing the enzyme extract, to provide different ratios of µg biomass per unit of proteolytic activity (UA) ranging from 0 to 400 µg microalgae UA−1. Enzyme inhibition was expressed as the percentage of inhibition after comparing with a control assay carried out in presence of digestive proteases but without any inhibitory extract (0% inhibition).
in vitro species-specific digestive simulation
in vitro protein hydrolysis assay
The in vitro protein hydrolysis of microalgae was simulated in 10 mL jacketed reaction vessels connected to a circulating water bath at 37 °C under continuous agitation by a magnetic stirrer. Although that temperature is not physiological for sparids, it was selected to increase the activity of the enzymes and reduce the time requested for performing this assay (Hamdan et al. 2009).
An amount of each microalga biomass, providing 80 mg of crude protein, was suspended in 50 mM Tris HCl buffer pH 9.0. After 15 min stirring, the hydrolysis was started by the addition of the S. aurata intestinal extracts providing 200 UA of total alkaline proteolytic activity (Vizcaíno et al. 2019). Blank assays with microalgae biomass but without digestive extract, were carried out. The hydrolysis was maintained for 90 min and samples of the reaction mixture at different sampling points (0, 15, 30, 60 and 90 min) were withdrawn. Each assay was repeated in quadruplicate.
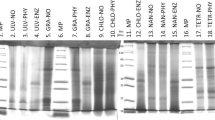
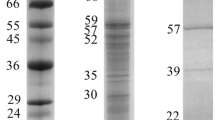
The hydrolysis of microalgae protein was assessed by sequential characterization of the hydrolysis products released, which were separated using sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) according to Laemmli (1970). Prior to electrophoretic separation, samples were diluted (1:1) in sample buffer (0.125 M Tris HCl, pH 6.8; 4% (w/v) SDS; 10% (v/v) β-mercaptoethanol; 20% (v/v) glycerol; 0.04% (w/v) bromophenol blue) and boiled for five minutes to stop the reaction and denature protein fractions. Electrophoresis was performed at a constant voltage of 100 V per gel for 60 min at 4 °C. Gels (12% polyacrylamide and 8 × 10 × 0.075 cm) were stained overnight with 0.1% Coomassie Brillant Blue (BBC R-250) in a methanol-acetic acid solution (50:20:50). For destaining, a methanol-acetic acid–water solution (35:10:55) was used. A protein standard consisting of twelve proteins ranging from 6.5 kDa (aprotinin, bovine lung) to 200 kDa (myosin, porcine heart) was used (wide range molecular weight marker, S-84445, Sigma, USA). The relative molecular mass (Mr, in kDa) of separated proteins fractions were estimated using a linear plot of log Mr of protein standards vs relative mobility (Rf). Changes resulting from protein hydrolysis were measured by densitometric analysis in SDS-PAGE gels (My Image Analysis Software, Thermo Scientific) for the determination of the coefficient of protein degradation (CPD) according to Alarcón et al. (2001).
Quantification of free amino acids released
Total released amino acids from microalgae protein were determined by the o-phthaldialdehyde method (Church et al. 1983) using L-leucine as standard. Before to the assays, the undigested protein was discarded by precipitation with 200 g L−1 trichloroacetic acid (1:1) followed by centrifugation at 12,000 ×g for 15 min. Blank assays were run to estimate free amino acids from enzyme extract and microalgae suspensions, which enabled the determination of the net release of amino acids attributable to the enzymatic hydrolysis. Results were expressed as accumulated values of free amino acids released during the digestive simulation (g of L-leucine equivalents per 100 g protein). In addition, the profile of released amino acids at final time was performed in the supernatants against a blank assay without fish enzymes as detailed before. Assays were performed in triplicate.
Statistical analysis
Results are expressed as mean ± standard deviation (SD). To test data normality and variance homogeneity, the Kolmogorov–Smirnov’s test and Levene’s F-test were used, respectively. Data with parametric distribution were analysed using a one-way analysis of variance (ANOVA) and the significant differences between treatments (p < 0.05) were determined using Tukey’s multiple comparison test. Data with nonparametric distribution were analysed using Kruskal–Wallis test, and significant differences were determined using Box and Whisker Plots graphs. A hierarchical cluster analysis (nearest neighbour method, squared Euclidean) was used to determine a global similarity among the different microalgae evaluated. Clustering is a multivariate technique of grouping together strains that share similar values. This procedure can be used to classify data into groups that are relatively homogeneous within themselves and heterogeneous between each other, based on a defined set of variables. Finally, to assess the feasibility of the protein characterization and in vitro assay to discriminate the protein bioaccessibility of the different microalgae, a Principal Components Analysis (PCA) was carried out. All statistical analyses were performed using the Stagraphics Plus 4.0 (USA) software.
Results
Protein characterization of microalgae
The protein content of microalgae and cyanobacteria is detailed in Table 1. Crude protein ranged from 25 to 61% on dry matter (DM). The highest protein content was found in Anabaena sp. BEA-0300B and D. salina REC-0214B with 60.9% and 52.3%, respectively, whereas Spirogyra sp. BEA-0666B showed the lowest protein content (25.3%).
The amino acid profile is summarised in Table 2. Overall, the microalgae and cyanobacteria showed similar amino acid profiles, and although Anabaena sp. BEA-0300B presented the highest absolute values, however, in relative terms, the proportions of amino acids was similar to that found in the rest of the species. Similarly, Spirogyra sp. BEA-0666B presented the lowest content in amino acids but also showed a similar profile compared to the rest of microalgae and cyanobacteria. The EAA/NEAA ratio ranged from 0.91 to 0.95, which were higher than that of soybean meal (0.7), and within the range of fish meal (0.92). D. salina REC-0214B and Anabaena sp. BEA-0300B showed the highest (0.95 ± 0.02 and 0.95 ± 0.01, respectively) and Spirogyra sp. BEA-0666B showed lowest values (0.81 ± 0.01). Figure 1 graphs the essential amino acid content in the microalgae and cyanobacteria studied. As described, comparable profiles were observed in all of them, with a slightly higher proportion of some amino acids being observed in Anabaena sp. BEA-0300B (i.e., Arg) and D. salina REC-0214B (i.e., Leu and Val).
Presence of protease inhibitors
The inhibitory effect of microalgae and cyanobacteria aqueous extracts on intestinal proteases of gilthead seabream is shown in Fig. 2. A dose–response inhibition curve was obtained by measuring the reduction in the proteolytic activity of a standardized seabream intestinal extract when incubated with different proportions of microalgae extracts. Chlorella vulgaris BEA-0753B showed the highest protease inhibition capacity (23% of inhibition), whereas inhibition with other microalgae and cyanobacteria never reached more than 10%. Each inhibition curve was fitted to a quadratic equation that can be used to predict the expected inhibition for a given amount of each microalga.
In vitro digestive simulation assay using gilthead seabream enzymes
The time-course of protein hydrolysis by the digestive proteases of S. aurata is shown in Fig. 3. Electrophoretic analysis evidenced several protein fractions with different relative molecular mass for each microalga and cyanobacteria biomass. No protein auto-hydrolysis was observed when the in vitro digestive simulation was carried out in the absence of fish enzymes (lanes control at 0 and 90 min). Changes in optical density in the electrophoretic gels were assessed by selecting five protein fractions ranging from 19 to 85 kDa, and from 24.0 to 126.0 kDa in M. gaditana REC-0251B and D. salina REC-0214B, respectively. Four fractions ranging from 11.0 to 59.0 kDa, and from 23.0 to 63.0 kDa in Anabaena sp. BEA-0300B and C. vulgaris BEA-0753B sp., respectively. Six fractions ranging from 10.0 to 63.0 kDa in A. platensis BEA-0007B and three fractions ranging from 24.0 to 62.0 kDa in Spirogyra sp. BEA-0666B. In all the microalgae and cyanobacteria, a gradual hydrolysis of most of the protein fractions above mentioned was observed through the 90 min of digestive simulation. In the case of D. salina REC-0214B, noticeable hydrolysis of all the proteins was found, especially after 30 min (Fig. 4). Some of the microalgae and cyanobacteria presented two protein fractions between 60 – 65 kDa and 20 – 25 kDa whose hydrolysis by fish enzymes was less marked than that observed in the rest of the proteins. This finding was evidenced in C. vulgaris BEA-0753B, where both proteins remained almost undigested after 60 min.
Changes in the average CPD values during the in vitro digestive simulation are shown in Fig. 5. D. salina REC-0214B showed CPD values higher than 80% revealing high bioaccessibility of their proteins to gilthead seabream digestive proteases. The progression revealed initial quick proteolysis followed by less marked but sustained hydrolysis until the end of the in vitro assay. Protein hydrolysis in Anabaena sp. BEA-0300B, A. platensis BEA-0007B and C. vulgaris BEA-0753B yielded similar patterns, reaching CPD values over 60%. The lowest CPD value was obtained for Spirogyra sp. BEA-0666B.
The amount of free amino acids released (ARR) during the enzymatic hydrolysis of protein is shown in Fig. 6. Overall, the accumulation of amino acids in the reaction vessel was progressive in all the microalgae and cyanobacteria evaluated. At the end of the in vitro assay, total free amino acids released ranged from 12.8 to 20.8 g L-leucine equivalents (100 g protein)−1. The highest cumulative values of free amino acids were observed in D. salina REC-0214B (p < 0.05), whereas the lowest value was obtained for Spirogyra sp. BEA-0666B.
The specific amino acids released after 90 min of in vitro proteolysis are shown in Fig. 7. In general, both essential and non-essential amino acids were released owing to the activity of fish digestive enzymes. Specifically, Anabaena sp. BEA-0300B and D. salina REC-0214B showed the highest values of arginine released, whist the lowest values for this amino acid were obtained in Spirogyra sp. BEA-0666B. Arthrospira platensis BEA-0007B and C. vulgaris BEA-0753B yielded relatively high levels of glutamic acid after the hydrolytic process, reaching, in the case of A. platensis BEA-0007B, values up to four times higher than those observed for the rest of microalgae and cyanobacteria. Furthermore, Anabaena sp. BEA-0300B had the highest tyrosine values, while D. salina REC-0214B showed high values of essential amino acids such as valine, leucine or lysine.
When the essential amino acids / non-essential amino acids (EAAR/NEAAR) ratio was calculated (Fig. 8), it was observed that M. gaditana REC-0251B showed the most balanced ratio, with a value close to 1, which represents an equal proportion of essential and non-essential amino acids. Dunaliell salina REC-0214B, Spirogyra sp. BEA-0666B and Anabaena sp. BEA-0300B showed values higher than 1 (1.52, 1.25 and 1.32, respectively), which means that essential amino acids released prevailed in these species, whereas while A. platensis BEA-0007B and C. vulgaris BEA-0753B showed values lower than 1, which represents a higher release of non-essential amino acids.
Figure 9 shows the results of a cluster analysis that provides a global view of the results obtained in this work. This analysis grouped the different microalgae and cyanobacteria species according to the similarity of their characteristics. The dendrogram showed three different groups. Arthrospira platensis BEA-0007B, C. vulgaris BEA-0753B and M. gaditana REC-0251B appear close with less than 5% distance. The second group was shaped by Anabaena sp. BEA-0300B and D. salina REC-0214B (13% distance), whereas Spirogyra sp. BEA-0666B appears separated (28% distance).
Finally, the data on protein characterization and in vitro bioaccessibility were analysed using a Principal Component Analysis (PCA). Principal component (PC) scores were then used in the discrimination analysis to assign each sample to a particular group. A PCA was used here as a simple method to project data to a two-dimensional plane. The PCA revealed that the most influential factors to group the data were the proportion of amino acids released (ARR) and their qualitative profile (EAAR/NEAAR ratio). The PCA results are shown in Fig. 10, which captured 88% of the variance observed in the experiment in the first two PCs. Overall, the groups identified were the same as those from the clustering analysis.
Principal components analysis of the different analytical determinations performed in the different microalgae and cyanobacteria. EAAR: essential amino acids released; NEAAR: non-essential amino acids released; EAA: essential amino acids; NEAA: non- essential amino acids; AAR: amino acids released; CPD: coefficient of protein degradation
Discussion
In the last decade there has been an exponential increase in the number of studies aimed at evaluating microalgae as potential ingredients for aquafeeds (Shah et al. 2018). However, the characterization of algal biomass is still incomplete, especially in terms of protein and amino acid availability, as well as in the lack of knowledge on protein digestibility by marine fish.
The high protein content is one of the major advantages of using microalgae biomass as dietary ingredient for aquafeeds (Shah et al. 2018). In this work, the total protein content of the different microalgae and cyanobacteria species evaluated ranged from 25 to 61%. Anabaena sp. BEA-0300B and D. salina REC-0214B displayed the highest protein content (> 50%, DM), which agree with the values reported previously (Becker 2007; Cheng et al. 2015). However, results obtained for A. platensis BEA-0007B or M. gaditana REC-0251B disagree with those found in the literature. Thus, some authors reported a higher protein content in these microalgae species reaching values within the range of 40–60% DW (Batista et al. 2013; Tibbetts et al. 2015; Teuling et al. 2019). On the other hand, Spirogyra sp. was the alga with the lowest protein content (25.3%, DM) among the species tested, although this value was considerably higher than that reported by Harish et al. (2004). These dissimilar results might well be attributed to different production conditions (Brown et al. 1997; Kumaran et al. 2021). Indeed, it is a well-known phenomenon that the protein content increased when microalgae are grown in culture media rich in nitrogen and at high growth rates (Batista et al. 2013).
Beyond protein content, the amino acid composition, specifically the content in essential amino acids, is a major quality criterion for determining the nutritional value of any alternative protein sources for aquafeeds (Webb and Chu 1983). Roughly, all microalgae and cyanobacteria evaluated in this work showed very similar amino acid profiles, which agrees with Brown et al. (1997) who analysed 40 species of microalgae from six algal classes and pointed out that all species showed similar amino acid composition. As an intrinsic characteristic of microalgae, glutamic acid (2.06 – 5.70 g (100 g biomass)−1) and aspartic acid (1.64 – 5.65 g (100 g biomass)−1) were the predominant NEAA (Tibbetts et al. 2015). Regarding EAA, it is widely acknowledged that lysine and methionine are the most limiting amino acids in ingredients for aquafeeds, not least in land-based crops like grains, pulses and their derivatives (Mai et al. 2006; Tibbetts et al. 2015). In this work, the specific contents measured for these two amino acids were in the range of 1.0 – 2.9 g (100 g biomass)−1 for lysine and of 0.3 – 1.0 g (100 g biomass)−1 for methionine, which are values similar to those reported previously by NRC (2011), ranging around 1.2 – 2.2 g (100 g biomass)−1 for lysine and 0.6 – 1.5 g (100 g biomass)−1 for methionine. Therefore, based on the amino acid requirements of farmed fish (Wilson 2003) all the microalgae and cyanobacteria studied could provide most of the required essential amino acids for ensuring adequate fish growth.
Besides protein content and amino acids profile, some alternative protein sources used in aquafeeds contain antinutritive factors such as protease inhibitors, which can exert negative effects on the digestion and absorption of nutrients, a fact that can reduce the nutritional value of aquafeed (Alarcón et al. 1999; Gatlin et al. 2007). To date, little research has been done assessing the presence of protease inhibitors in microalgae, and their effect on the fish digestive proteases (Diken et al. 2016). The results obtained in this study revealed that a considerably high inclusion level of microalgae would be needed to reach noticeable inhibition values. Thus, according to Martínez-Antequera et al. (2020), a juvenile fish of approximately 20 g body weight, total protease activity released after the intake would be around 1,000 and 1,300 activity units (UA). If fish were fed at 2% rate with a hypothetical feed supplemented with 15% microalgae, the microalgae to activity ratio would be around 60 µg of microalgae UA−1, which would account for less than 3% inhibition in the case on M. gaditana REC-0251B, Anabaena sp. BEA-0300B, D. salina REC-0214B or A. platensis BEA-0007B, and less than 10% inhibition when using C. vulgaris BEA-0753B or Spirogyra sp. BEA-0666B. In the worst-case scenario, it should be also be taken into account that fish have mechanisms to overcome the effects of dietary antinutrients (Haard et al. 1996; Santigosa et al. 2010). Consequently, negligible effects would likely be expected on fish growth if microalgae were included at a low level in practical feeding formulas.
This study also evaluated the digestive capacity of gilthead seabream proteases to hydrolyse microalgae proteins by using a species-specific in vitro protein digestibility assay. This in vitro model has been used previously for estimating other potential feedstuffs for aquafeeds and it has proven useful not only in assessing the suitability of novel dietary ingredients for feeding fish (Alarcón et al. 2002; Vizcaíno et al. 2019), but also in greatly reducing the need to use of experimental animals in the preliminary evaluation of such ingredients.
Protein hydrolysis was monitored by electrophoretic separation, and the results confirmed, overall, that most of the microalgae and cyanobacteria proteins were easily hydrolysed by digestive proteases of S. aurata. Thus, progressive and almost complete in vitro hydrolysis of the main protein fractions (60 – 65 kDa and 20 – 25 kDa) remained undegraded at the end of the in vitro assay. Coefficient of protein degradation (CPD) values ranged from 49.4 to 85.5%, which are similar to those described for other microalgae (Vizcaíno et al. 2019), as well as for other conventional raw materials, such as soybean protein concentrate or fishmeal (Hernández et al. 2015; Sultana et al. 2010). Likewise, the quantification of total free amino acids revealed significant differences among the biomasses evaluated. Thus, fish digestive proteases were able to release only 12.8% of total amino acids in Spirogyra sp. BEA-0666B, but up to 20.8% in D. salina REC-0214B after the 90-min in vitro hydrolysis.
Broadly speaking, the results indicate the high bioavailability of microalgae protein, although the significant differences found in protein hydrolysis among samples suggest the existence of several factors that might have played a role in such variability. One of the main factors that could be related with this fact is the presence of cell walls in some microalgae species characterized by great variability in the structure and composition among species. Thus, species like M. gaditana and C. vulgaris present a rigid cell wall composed mainly of cellulose and hemicellulose together with amounts of other monosaccharides such as mannose, and also characterised by the presence of algaenan, a resistant aliphatic biopolymer composed of ether-linked long alkyl chains of esterified monomers (Bernaerts et al. 2018). To a lesser degree, cell walls of species from genus Spirogyra. and the cyanobacteria Anabaena sp. and A. platensis, are also mainly composed of glucose and mannose, together with minor amounts of galactose and xylose, though it should be noted that in the last one, the polysaccharides represent a small fraction of the cell wall (Bernaerts et al. 2018; Franková and Fry 2021). Therefore, the presence of this cell wall, together with the limitations of the digestive physiology of fish, certainly influence protein digestibility (Kamalam et al. 2017; Bernaerts et al. 2018). Indeed, the microalgae cell wall determines to which extent the intracellular nutrients are accessible to the digestive enzymes. However, this hypothesis is not applicable to all the microalgae species. Species of Dunaliella lack a rigid cellulosic cell wall. The cellular contents are enclosed by a simple cell membrane consisted to a lipid bilayer with integrated and peripheral proteins (D’hondt et al. 2017; Sui and Vlaeminck 2020). This fact could contribute to better bioaccessibility to digestive enzymes, which might explain the higher CDP values obtained in D. salina REC-0214B compared to the other microalgae and cyanobacteria species evaluated.
On the other hand, the structure and spatial conformation of proteins themselves can also determine their susceptibility to being hydrolysed, as is also a difference in amino acid composition (Vizcaíno et al. 2019). The activity of digestive enzymes against proteins with different conformational structures but similar amino acid composition, which might result in different amino acid bioavailability (De la Higuera and Cardenete 1993). Therefore, the analysis of amino acids released during protein hydrolysis by fish enzymes provides useful information about how balanced the biomass of given microalga is, and consequently, the suitability for its inclusion in aquafeeds. The results obtained in this study revealed different profiles of essential and non-essential amino acid released (EAAR and NEAAR, respectively) in each algal biomass studied, despite the fact all of them had similar amino acids profiles in relative terms (Fig. 7). Regarding essential amino acids, it is worth mentioning the high amount of lysine (Lys) released from A. platensis BEA-0007B, D. salina REC-0214B, and M. gaditana REC-0251B, which is one of the most limiting amino acids in regular ingredients used in aquafeeds (Li et al. 2009). The same was found for arginine and branched-chain amino acids (leucine and valine), which play a key role in protein synthesis, immune function, and fish health (Ahmad et al. 2020; Hosseini et al. 2020).
Although most attention is usually paid to EAAR, however, the amount of NEAAR acids released, as well as the EAAR to NEAAR ratio (EAAR/NEAAR), are also important factors when it comes to achieving the highest efficiency in the use of dietary protein, thereby minimizing nitrogen excretion into the environment (Peres and Oliva-Teles 2006). Indeed, if only essential amino acids were considered when formulating inert diets, the requirements of NEAA, or of non-specific N source used to synthesize non-essential amino acids, might not be completely fulfilled (Oliva-Teles et al. 2020).
In this context, M. gaditana REC-0251B, C. vulgaris BEA-0753B and A. platensis BEA-0007B showed a well-balanced profile of released amino acids, yielding values for EAAR/NEAAR ratios close to 1 (0.95, 0.79 and 0.76, respectively), which is the optimal relation for fish nutrition (Oliva-Teles et al. 2020), while D. salina REC-0214B, Anabaena sp. BEA-0300B and Spirogyra sp. BEA-0666B showed ratios even higher than 1. According to Gómez-Requeni et al. (2003), the best growth performance in gilthead seabream is reached when feeding fish on diets that resemble the EAA profile and EAA/NEAA ratio found in muscle tissue.
Finally, the overall view of the whole results obtained in this study revealed that microalgae and cyanobacteria tested might be grouped in three different categories, based on all the data obtained from the different experiments, but mostly influenced by both the proportion of amino acids released and their qualitative profile (Fig. 9). Microchloropsis gaditana REC-0251B, C. vulgaris BEA-0753B and A. platensis BEA-0007B were grouped. In general, these species showed an optimal balance between their crude protein content and the bioaccessibility to the digestive enzymes of gilthead seabream juveniles, this fact suggesting that they could provide an adequate profile of free amino acids for further enterocyte absorption. Close to this group, D. salina REC-0214B and Anabaena sp. BEA-0300B were grouped, whereas Spirogyra sp. BEA-0666B appeared distanced from both groups, likely due to the poor results obtained in the in vitro digestive simulation.
In conclusion, the present study provides useful species-specific information of microalgae and cyanobacteria as potential protein ingredients for aquafeeds. The results obtained revealed that all the microalgae and cyanobacteria evaluated showed an adequate protein content, as well as a balanced amino acid profile, although significant differences were observed in their susceptibility to S. aurata digestive enzymes, and therefore in the bioavailability of their protein fraction for this fish species.
Data availability
The datasets supporting the conclusions of this article are included within the article.
References
Alarcón FJ, Díaz M, Moyano FJ, Abellán E (1998) Characterization and functional properties of digestive proteases in two sparids; gilthead sea bream (Sparus aurata) and common dentex (Dentex dentex). Fish Physiol Biochem 19:257–267
Alarcón FJ, Moyano FJ, Díaz M (1999) Effect of inhibitors present in protein sources on digestive proteases of juvenile sea bream (Sparus aurata). Aquat Living Resour 12:233–238
Alarcón FJ, Moyano FJ, Díaz M (2001) Use of SDS-page in the assessment of protein hydrolysis by fish digestive enzymes. Aquac Int 9:255–267
Alarcón FJ, Martinez TF, Barranco P, Cabello T, Díaz M, Moyano FJ (2002) Digestive proteases during development of larvae of red palm weevil, Rhynchophorus ferrugineus (Olivier, 1790) (Coleptera: Curculionidae). Insect Biochem Mol Biol 32:265–274
Ahmad I, Ahmed I, Fatma S, Peres H (2020) Role of branched-chain amino acids on growth, physiology and metabolism of different fish species: A review. Aquac Nutr 27:1270–1289
Batista AP, Gouveia L, Bandarra NM, Franco JM, Raymundo A (2013) Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res 2:164–173
Becker EW (2007) Micro-algae as source of protein. Biotechnol Adv 25:207–210
Bernaerts TMM, Panazzo A, Verhaegen KAF, Gheysen L, Foubert I, Moldenaers P, Hendrickx ME, Van Loey AM (2018) Impact of different sequences of mechanical and thermal processing on the rheological properties of Porphyridium cruentum and Chlorella vulgaris as functional food ingredients. Food Funct 9:2433–2446
Brown MR, Jeffrey SW, Volkman JK, Dunstan GA (1997) Nutritional properties of microalgae for mariculture. Aquaculture 151:315–331
Cheng J, Xu J, Huang Y, Li Y, Zhou J, Cen K (2015) Growth optimisation of microalga mutant at high CO2 concentration to purify undiluted anaerobic digestion effluent of swine manure. Bioresour Technol 177:240–246
Church FC, Swaisgood HE, Porter DH, Carignani GL (1983) Spectrophotometric assay using o-phthaldehyde for determination of proteolysis in milk proteins. Int J Dairy Sci 66:1219–1227
Daniel N (2018) A review in replacing fish meal in aqua feeds using plant protein sources. Int J Fish Aquatic Stud 6:164–179
De la Higuera M , Cardenete G (1993) La proteína en la nutrición de los peces. In: Castello Orvay F (ed) Acuicultura marina: fundamentos biológicos y tecnología de la producción. Universidad de Barcelona, Barcelona, España, pp. 195–226
D’hondt E, Martin-Juarez J, Bolado S, Kasperoviciene J, Koreiviene J, Sulcius S, Elst K, Bastiaens L (2017) Cell disruption technologies. In: Gonzalez-Fernandez C, Munoz R (eds) Microalgae-based biofuels and bioproducts. Woodhead Publishing, Duxford, pp 133–154
Diken G, Demir O, Naz M (2016) The potential inhibitory effects of microalgae and macroalgae on protease activities of Argyrosomus regius (Pisces, Scanidae) larvae using in vitro assays. J Int Sci Publ 4:473–483
Franková L, Fry SC (2021) Hemicellulose-remodelling transglycanase activities from charophytes: towards the evolution of the land-plant cell wall. Plant J 108:7–28
Gatlin DM III, Barrows FT, Brown P, Dabrowski K, Gaylord TG, Hardy RW, Herman E, Hu G, Krosdahl Å, Nelson R, Overturf K, Rust M, Saeley W, Skonberg D, Souza EJ, Stone D, Wilson R, Wurtele E (2007) Expanding the utilization of sustainable plant products in aquafeeds: a review. Aquac Res 38:551–579
Gómez-Requeni P, Mingarro M, Kirchner S, Calduch-Giner JA, Médale F, Corraze G, Panserat S, Martin SAM, Houlihan DF, Kaushik SJ, Pérez-Sánchez J (2003) Effects of dietary amino acid profile on growth performance, key metabolic enzymes and somatotropic axis responsiveness of gilthead sea bream (Sparus aurata). Aquaculture 220:749–767
Guedes AC, Sousa-Pinto I, Malcata FX (2015) Application of microalgae protein to aquafeed. In: Kim S-K (ed) Handbook of marine microalgae. Academic Press, London, pp 93–125
Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH (eds) Culture of marine invertebrate animals. Plenum Press, New York, pp 29–60
Haard NF, Dimes LE, Arndt RE, Dong FM (1996) Estimation of protein digestibility-IV. Digestive proteinases from the pyloric caeca of Coho salmon (Oncorhynchus kisutch) fed diets containing soybean meal. Comp Biochem Physiol B 115:533–540
Hamdan M, Moyano FJ, Schuhardt D (2009) Optimization of a gastrointestinal model applicable to the evaluation of bioaccessibility in fish feeds. J Sci Food Agric 89:1195–1201
Harish M, Gajaria SC, Radha KS (2004) Growth and development of catla (Catla catla) fed with different levels of diet containing Spirogyra sp. Bioresour Technol 95:73–76
Hemaiswarya S, Raja R, Kumar RR, Ganesan V, Anbazhagan C (2011) Microalgae: a sustainable feed source for aquaculture. World J Microbiol Biotechnol 27:1737–1746
Hernández C, Hardy RW, Márquez-Martínez DG, Domínguez-Jimenez PV, González-Rodríguez B (2015) Evaluation of apparent digestibility coefficients of individual feed ingredients in spotted rose snapper Lutjanus guttatus (Steindachner, 1869). Aquac Res 21:835–842
Hosseini SP, Shamsaie M, Benavreh A, Foroudi F (2020) Partial replacement of fishmeal with corn protein concentrate in diets for rainbow trout (Oncorhynchus mykiss): Effects on growth performance, physiometabolic responses, and fillet quality. Aquac Res 52:249–259
Hua K, Cobcroft JM, Cole A, Condon K, Jerry DR, Mangott A, Praeger C, Vucko MJ, Zeng C, Zenger K, Strugnell JM (2019) The future of aquatic protein: Implications for protein sources in aquaculture diets. One Earth 1:316–329
Kamalam BS, Medale F, Panserat S (2017) Utilisation of dietary carbohydrates in farmed fishes: New insights on influencing factors, biological limitations and future strategies. Aquaculture 467:3–27
Kumaran J, Poulose S, Joseph V, Bright IS (2021) Enhanced biomass production and proximate composition of marine microalga Nannochloropsis oceanica by optimization of medium composition and culture conditions using response surface methodology. Animal Feed Sci Technol 271:114761
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Li P, Mai K, Trushenski J, Wu G (2009) New developments in fish amino acid nutrition: towards functional and environmentally oriented aquafeeds. Amino Acids 37:43–53
López GVG, García MCC, Fernández FGA, Bustos CS, Chisti Y, Sevilla JMF (2010) Protein measurements of microalgal and cyanobacterial biomass. Bioresour Technol 101:7587–7591
Mai K, Zhang L, Ai Q, Duan Q, Zhang C, Li H, Wan J, Liufu Z (2006) Dietary lysine requirement of juvenile Japanese seabass, Lateolabrax Japonicus. Aquaculture 258:535–542
Martínez-Antequera FP, Martos-Sitcha JA, Reyna JM, Moyano FJ (2020) Evaluation of the inclusion of the green seaweed Ulva ohnoi as an ingredient in feeds for gilthead seabream (Sparus aurata) and european seabass (Dicentrarchus labrax). Animals 11:1684
Molino A, Iovine A, Casella P, Mehariya S, Chianese S, Cerbone A, Rimauro J, Musmarra D (2018) Microalgae characterization for consolidated and new application in human food, animal feed and nutraceuticals. Int J Environ 15:2436
NRC (2011) Nutrient requirements of fish and shrimp. The National Academies Press, Washington D.C.
Oliva-Teles A, Enes P, Peres H (2015) Replacing fishmeal and fish oil in industrial aquafeeds for carnivorous fish. In: Davis DA (ed) Feed and feeding practices in aquaculture. Woodhead Publishing, Duxford, pp 203–233
Oliva-Teles A, Couto A, Enes P, Peres H (2020) Dietary protein requirements of fish – a meta-analysis. Rev Aquac 12:1445–1477
Pereira EJDAL, de Santana LC, da Silva LF, de Barros HB (2020) Brazilian policy and agribusiness damage the Amazon rainforest. Land Use Policy 92:104491
Peres H, Oliva-Teles A (2006) The effect of dietary protein replacement by crystalline amino acid on growth and nitrogen utilization of turbot Scophthalmus maximus juveniles. Aquaculture 250:755–764
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Santigosa E, Sáenz MA, Rodiles A, García F, Alarcón FJ (2010) Effect of diets containing a purified soybean trypsin inhibitor on growth performance, digestive proteases and intestinal histology in juvenile sea bream (Sparus aurata L.). Aquac Res 41:187–198
Shah MR, Lutzu GA, Alam A, Sarker P, Chowdhury MK, Parsaeimehr A, Liang Y, Daroch M (2018) Microalgae in aquafeeds for a sustainable aquaculture industry. J Appl Phycol 30:197–213
Sui Y, Vlaeminck SE (2020) Dunaliella microalgae for nutritional protein: an undervalued asset. Trends Biotechnol 38:10–12
Sultana Z, Ahmed MS, Iqball MS, Chisty MAH (2010) Determination of in vitro protein digestibility of different feed ingredients for Nilotica (Oreochromis nilotica). Bangladesh Res Publ J 4:87–94
Tacon AGJ, Metian M (2008) Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trend and future prospects. Aquaculture 285:146–158
Teuling E, Wierenga PA, Agboola JO, Gruppen H, Schrama JW (2019) Cell wall disruption increases bioavailability of Nannochloropsis gaditana nutrients for juvenile Nile tilapia (Oreochromis niloticus). Aquaculture 499:269–282
Tibbetts SM, Milley JE, Lall SP (2015) Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors. J Appl Phycol 27:1109–1119
Tibbetts SM, Yasumaru F, Lemos D (2017) In vitro prediction of digestible protein content of marine microalgae (Nannochloropsis granulate) meals for Pacific white shrimp (Litopenaeus vannamei) and rainbow trout (Oncorhynchus mykiss). Algal Res 21:76–80
Vizcaíno AJ, López G, Sáez MI, Jiménez JA, Barros A, Hidalgo L, Camacho-Rodríguez J, Martínez TF, Cerón-García MC, Alarcón FJ (2014) Effects of the microalga Scenedesmus almeriensis as fishmeal alternative in diets for gilthead sea bream, Sparus aurata, juveniles. Aquaculture 431:34–43
Vizcaíno AJ, Sáez MI, Martínez TF, Acién FG, Alarcón FJ (2019) Differential hydrolysis of proteins of four microalgae by the digestive enzymes of gilthead sea bream and Senegalese sole. Algal Res 37:145–153
Webb KL, Chu FE (1983) Phytoplankton as a food source for bivalve larvae. In: Proceedings of the Second International Conference on Aquaculture Nutrition: Biochemical and Physiological Approaches to Shellfish 2:272–291
Wilson RP (2003) Amino acids and proteins. In: Halver JE, Hardy RW (eds) Fish nutrition. Academic Press, London, pp 143–179
Yarnold J, Karan H, Oey M, Hankamer B (2019) Microalgal aquafeeds as part of a circular bioeconomy. Trends Plant Sci 24:959–970
Yavad AK, Rossi W Jr, Habte-Tsion HM, Kumar V (2020) Impacts of dietary eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) level and ratio on the growth, fatty acids composition and hepatic-antioxidant status of largemouth bass (Micropterus salmoides). Aquaculture 529:735683
Younis EM, Al-Quffaiol AS, Al-Asgah NA, Abdel-Warith AA, Al-Hafedh YS (2018) Effect of dietary fish meal replacement by red algae, Gracilaria arcuate, on growth performance and body composition of Nile tilapia Oreochromis niloticus. Saudi J Biol Sci 25:198–203
Zheng CC, Wu JW, Jin ZH, Ye ZF, Yang S, Sun YQ, Fei H (2019) Exogenous enzymes as functional additives in finfish aquaculture. Aquac Nutr 26:213–224
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research was funded by AquaTech4Feed (grant # PCI2020-112204) granted by MCIN/AEI/10.13039/501100011033 and the EU “NextGenerationEU”/PRTR within the ERA-NET BioBlue COFUND, SABANA project (the European Union’s Horizon 2020 Research and Innovation program, grant # 727874), and MINECO-FEDER (grant # RTI2018-096625-B-C31). Authors thank the Service of Experimental Diets of University of Almería for performing the amino acid analysis (MINECO-FEDER grant # EQC2018-004984-P).
Author information
Authors and Affiliations
Contributions
Alarcón FJ and Martínez TF conceived and designed the experiments. Galafat A, Vizcaíno AJ and Sáez MI performed the in vitro analysis. Acién FG and Gómez-Pinchetti JL produced the microalgae biomass. Galafat A, Vizcaíno AJ, Sáez MI, Martínez TF, Acién FG and Gómez-Pinchetti JL discussed the data. Galafat A, Vizcaíno AJ and Alarcón FJ performed the data analysis and drafted the manuscript. All authors critically revised and approved the manuscript.
Corresponding author
Ethics declarations
Statement of informed consent, human/animal rights
All studies involving fish were conducted following the requirements of the Directive 2010/63/EU, and the Spanish legislation (Real Decreto 53/2013), regarding the ethical rules applicable in research involving laboratory animals.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Galafat, A., Vizcaíno, A.J., Sáez, M.I. et al. Evaluation of the in vitro protein bioaccessibility of several microalgae and cyanobacteria as potential dietary ingredients in gilthead seabream (Sparus aurata) juveniles. J Appl Phycol 34, 2075–2088 (2022). https://doi.org/10.1007/s10811-022-02764-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-022-02764-5