Abstract
The future of large-scale kelp aquaculture is standing at a crossroad, with the diverging paths being characterized by two fundamentally different cultivation methods that differ on how well gametophyte reproduction can be controlled. The cultivation method that does not directly control gametophyte reproduction is more widely utilized at the moment, but interest in better controlling gametophyte reproduction is growing steadily. Here, we validate a bioreactor system that overcomes a number of implementation challenges for this controlled reproductive method, expanding the possibility of clonal gametophyte cultivation outside of expensive laboratory settings. The main goals of this system include (i) the maintenance of clean gametophyte clonal cultures in non-sterile environments over prolonged periods of time, (ii) the production of large numbers of juvenile sporophytes, and (iii) effective transportation of gametophytes and sporophytes. The “SeaCoRe system” consists out of three parts that correspond to these three challenges: (1) clone-reactors, (2) a clone-inducer, and (3) a transporter. The validation of the system showed that delayed Saccharina latissima and Alaria esculenta gametophytes can grow reliably for 75 days in the clone-reactors. Initial gametophyte densities of 0.4 mg DW and 0.6 mg DW gametophtyes mL−1 were optimal for S. latissima and A. esculenta, resulting in reproductive successes of 604 and 422 sporophytes mL−1, respectively. Lastly, gametophyte transport was simulated, with high reproductive success still achieved within 19 days in ~ 20 °C environments. The SeaCoRe system helps unlock the full potential of large-scale kelp cultivation using multiannual delayed clonal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kelp aquaculture is on the cusp of becoming a large-scale offshore industry on a global scale (Bak et al., 2020). The main driving force behind this sudden increase in interest is the promise of kelp as a sustainable source for food (van den Burg 2021), feed (Carrier et al. 2017), bio energy (Davies et al. 1990), and as scalable carbon sink for the mitigation of climate change (Chung et al. 2013). Standing on its path toward industrially scaled offshore kelp cultivation lies a choice on how kelp should be cultivated. To this day, there is no clear consensus on which cultivation method is best suited for large-scale kelp aquaculture. Kelp aquaculture is still being practiced using two fundamentally different cultivation methods (Kerrison et al. 2019; Goecke et al. 2020), which are categorized here as the controlled- and uncontrolled reproductive methods (Ebbing et al. 2021). The uncontrolled reproductive method (URM) is characterized by releasing substantial amounts of zoospores in large tanks that are filled with twine rope. This method is considered to be the traditional method and is used in large parts of the world (Shan et al. 2013). The released zoospores first need to attach to the twine rope after which they develop into gametophytes. These newly formed gametophytes then sexually reproduce and form young sporophytes, all the while still being attached to these twine ropes (Su et al. 2017). URM is categorized as “uncontrolled” since farmers cannot precisely steer the reproductive success (sporophytes mL−1), or the relative reproductive success (sporophytes mg−1 gametophyte DW) of these kelp gametophytes. Farmers can therefore not predictively quantify and control the amount of sporophytes on their coils or farm ropes. The second method is called the controlled reproductive method (CRM), as it offers a more direct control on gametophyte reproduction because of the in vitro environment where gametophyte growth and reproduction take place (Zhang et al. 2008). Gametophyte reproduction can be induced in a controlled setting and the subsequent cultures with juvenile sporophytes can then be attached to twine strings using techniques like paintbrush-seeding (Hwang et al 2006; Redmond et al., 2014; Umanzor et al. 2021), or direct seeding (Kerrison et al. 2020; Forbord 2020). However, using CRM as a primary cultivation method is still considered risky, more labor-intensive than URM, and showing a higher detachment from the seed strings when applied (Xu et al. 2009). These characteristics explain for a large part why URM is still common practice in most parts of the world (Goecke et al. 2020).
Interest in using CRM as primary cultivation method is growing in the kelp industry because (i) gametophytes can be kept in cultures for prolonged periods of time (Carney 2011; Barrento et al. 2016; Wade et al. 2020), (ii) gametophytes can be cryopreserved (Visch et al. 2019), (iii) gametophyte reproduction still has room to be optimized (Choi et al. 2005; Ratcliff et al. 2017), and (iv) the crossing of distantly related unialgal male and female gametophyte clone cultures may evoke intraspecific hybrid vigor for future F1 hybrid cultivars (Shan et al. 2016; Zhao et al. 2016). Especially breeding can benefit from using CRM since the breeding possibilities that URM cultivation offers are limited, with long-term degeneration in blade quality and productivity observed in their cultivation practices (Shan et al. 2016). This degeneration is caused by the inbreeding of limited numbers of sorus-bearing individuals that are continuously used in URM for multiple generations (Liu et al. 2014). The resulting reduced genetic diversity and narrowed germplasm bank decreases the variety’s adaptability to changing environmental conditions. This reduced adaptability is made only more relevant under climate change, endangering the future performance of kelp with important economic traits (Bi et al. 2011; Liu et al. 2012). However, breeding using CRM can only be done by also fully understanding its limitations and by mastering the growth and reproduction of larger volumes of delayed gametophyte cultures (Zhang et al. 2008). For now, this is still an understudied subject that has proven difficult to master. This includes taking into account the different growth rates between the sexes (Destombe et al. 2011) or age-related changes in fertility, especially since multiannual delayed gametophytes have shown to survive and able to grow vegetatively for decades (Carney and Edwards. 2006). These potential limitations need more research and ultimately necessitate innovation in a bioreactor system that is specifically designed to maintain and propagate sex-specific multiannual delayed clonal cultures to facilitate CRM based cultivation.
Several different bioreactors have been designed for kelp aquaculture, covering both the production of sporophytes (Sato et al. 2017) as well as gametophytes (Zhang et al. 2019). In the case of gametophytes, several different bioreactor designs were developed that successfully propagated gametophyte biomass (Rorrer et al. 1995; Gao et al. 2005; Chen and Qi 2008). However, these available bioreactor designs did not take into account the fact that gametophytes can delay their reproduction (Carney and Edwards 2006) grow vegetatively for prolonged periods of time (Westermeier et al. 2006), even up to years (Barrento et al. 2016; Ebbing et al. 2021). Delayed gametophytes result into aggregated clumps of gametophyte biomass, making it impossible to evenly expose all gametophyte biomass to comparable doses of light and nutrients, potentially compromising their growth and health. Zhang et al. (2019) published a well-designed bioreactor in order to address this issue, with an integrated four blade impeller that maintains and homogenizes gametophyte cultures from within the same bioreactor. The logical next step is to expand the singular bioreactor that only maintains gametophyte biomass, into a system of bioreactors that is not only designed to successfully maintain, and homogenize clonal cultures, but also induce sexual reproduction, and ultimately even addresses culture transportation. Looking into culture transportation is necessary, as this often overlooked segment in kelp aquaculture becomes increasingly important in an ever more globalized effort to establish large scale kelp aquaculture. Transporting cultures can have a wide variety of possibilities. From the transportation of sporophytes between more centralized kelp propagation companies and farmers, to the transportation of gametophyte cultures to farmers that intend to propagate their own cultures. What is most important in the transport bioreactor is that it is sturdy enough to handle rough transports, and simple enough to successfully use without any prior knowledge of kelp cultivation. Ultimately, this system of bioreactors should form a closed loop system that allows for clean gametophyte culture propagation and transportation, without the need of expensive laboratories.
Here, we report on the design of such a continuous bioreactor system, the “Seaweed Continuous bioReactor” system, or SeaCoRe system (Fig. 1), and report on experiments that validate the effectiveness of its three parts: the clone-reactor, the clone-inducer, and the transporter. The main novelty of the SeaCoRe bioreactor system is that it is specifically designed and tailored for the propagation of multiannual delayed clonal gametophyte cultures. Overall, the SeaCoRe system reached TRL-5, which is defined as the validation of the system in its industrially relevant environment. The validation is accompanied with the blueprints and dimensions of the SeaCoRe system, quantitatively measuring the kelp propagation process, from gametophyte clonal cultures to juvenile sporophyte, as a basis to be used in CRM-based kelp aquaculture.
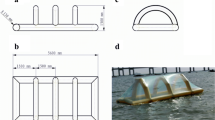
A schematic diagram of the SeaCoRe system, including the three positions in which the clone reactors can be used, a top view of the grinder, the stackable clone-inducer (6 well plates high), the transporter, and valve connector. Specifics on the diameter (d), height (h), and volume (v) of every bioreactor is included under the name. The numbered valves are used for (1) air outflow and medium addition, (2) medium extraction and homogenized culture outflow, (3) unhomogenized culture outflow, and (4) air inflow. The valves can furthermore be opened (green *), functionally opened (yellow *), or closed (red). The stationary (a), refreshment (b), grinding (c) clone reactors all use their valves differently, with the transporter using the valves in a similar fashion as the stationary clone-reactor
Methods
Gametophyte culturing using the SeaCoRe system
Gametophyte culture and maintenance
Ripe sori of Saccharina latissima from Leknesund, Norway (61.212994, 4.896644°E) and Alaria esculenta from Fuhreholmen, Norway (61.043292, 4.868790°E) individuals were collected in the autumn and winter months of 2016. The extraction of zoospores was done in the Netherlands, using a standard extraction protocol (protocol of Ebbing et al. 2020, 2021,similar to earlier methods described by Redmond et al. 2014 and Charrier et al. 2018). Over time, the zoospores developed into wildtype gametophyte cultures, with “wildtype” being characterized by a high genetic diversity and the jointly presence of both sexes in the culture. Male and female gametophytes were isolated from these wildtype cultures to create clean clonal cultures where the sexes are separated and the clonal lines genetically identical. The clonal male and clonal female cultures used here consisted out of a mix of 6 individually bulked up clonal cultures, to increase the genetic diversity of the clonal cultures and deliberately making them more similar to wildtype cultures. We used gametophytes that delayed their sexual reproduction by more than a year, thereby transgressing seasonally induced reproduction, which are categorized here as multiannual delayed (MAD) gametophytes. Both MAD wildtype and clonal cultures derive from the same extraction and were simultaneously used in this experiment to assess potential differences in photosynthetic efficiency and growth rates between the two types of MAD cultures. The medium of all cultures were refreshed once every ~ 60 days with new nutrient rich f/2 medium (Guillard and Ryther 1962), and grew vegetatively for approx. 2.5 years prior to the start of this experiment. The gametophyte cultures were not reused between the three bioreactor validation experiments. All experiments started with pristine stock cultures, so that there was no chance that the results of one bioreactor validation could influence the results of the next.
The SeaCoRe system
The SeaCoRe system consists of 4 bioreactors; two clone-reactors, a clone-inducer, and a transporter, whose culture content is interchangeable in a closed loop system using Luer-lock valve connectors (Fig. 1). The clone-reactors were specifically designed to maintain either male or female MAD gametophyte clonal cultures because separately growing out the sexes is central to the novelty introduced with this design. The clone-inducer was designed for the subsequent remerging of the two sexes, consisting out of stackable interlockable petri-dishes or well plates. Under a gradient of possible gametophyte densities (Fig. 3) in combination with light and temperature conditions (11 °C; 30 µmol photons m−2 s−1 of white light) these cultures initiate gametogenesis and produce young sporophytes. Finally, there is the transporter, a bioreactor that is specifically designed for the transportation of either gametophyte clonal cultures coming from the clone-reactors, or embryonic sporophytes that come from the clone-inducer. All these bioreactors fit in customized incubators (Polar-CE202; Fig. 4) and make the system small enough to easily fit in the back of a pick-up truck. Both incubators were temperature controlled, using external temperature regulators (H tronic TS 1000) in combination with air pumps (Aquaforte V-10) and LED panels (PAN-RGBCCT). The LED panels (LS LED; RGB + CCT 30 × 30 cm 18 W) emitted multiple colors of light (e.g., white, red, green, or blue light), whose spectral distributions (Fig. S1) were analyzed using a modular multispectral radiometer (TriOs Ramses ARC, Germany; Heuermann et al. 1999).
The clone-reactor
The clone-reactor is a vertical tube-shaped bioreactor that includes three fluid valves, a grinder, and an airflow valve. This bioreactor was specifically designed for the prolonged vegetative growth of gametophyte clones without the need for sterile work environments. Gametophyte (vegetative) growth, medium refreshment, and gametophyte homogenization can all be done from within the clone-reactor. Homogenization was achieved using a grinder that functions in a similar way as a French coffee press does, where a sieve/filter separates the coffee grounds from the liquid. The grinder consists of 4 small sieves and a rubber band that circumvents the edge (Fig. 1), making the grinding of gametophyte biomass possible. The sieves are included for easier transfer of seawater past the grinder; otherwise, the grinding becomes too hard and manually straining. The grinder has a second purpose, separating the homogenized culture that is ready for the induction of gametophyte reproduction, from the unhomogenized culture that can remain in the clone reactor for further vegetative growth.
The grinder is an essential part in the design, since its position changes the function of the clone-reactor (Fig. 1 and Fig. S2). The stationary position is the position where gametophytes are allowed to grow vegetatively by placing the grinder outside the culture. The gametophyte culture remains in motion using a filtered airlift system that is connected to valve-4. The refreshment position is used for the refreshment of the cultures by placing the grinder just under valve-2. This way, the medium can be discharged using valve-2 and refilled with new medium using valve-1 without the loss of gametophyte biomass. The grinding position is used to homogenize gametophyte biomass by pushing the grinder to the bottom of the reactor in a pumping motion, so that gametophytes become fragmented by passing past the rubber band of the grinder (Fig. S3). Grinded material can then be discharged using valve 2 without the possibility of discharging unhomogenized material that is kept back by the grinder.
The clone-inducer
The clone-inducer consists out of stackable petri dishes, with a valve on each petri dish (Fig. 1). Male and female gametophyte biomass, coming from the clone-reactors, can be transferred to and merged in the clone-inducer. The clone-inducer is modular in design and can be stacked with as many plates as needed, by placing the large Petri dish-like well plates on top of each other. The clone-inducer described here consists of 6-well plates using well plates with a radius of 22 cm, resulting in a cumulative volume of 7600 mL. This is important to know for future extrapolations using data from the gametophyte reproduction experiment (Fig. 3). In order to make sure that all sides of the bioreactor receive similar quantities of light, a rotating disk can be included in the incubator, rotating the clone-inducer alongside the LED panel that is fitted outside of the incubator (Fig. 4). Temperatures for optimal reproductive success in S. latissima gametophytes that delayed their reproduction (Kinlan 2003) were previously reached using temperatures between 10 and 12 °C, depending on the light intensity that was used (Ebbing et al. 2021). In this case, we decided to use 30 µmol photons m−2 s−1 of white light in combination with 11 °C, which resulted in near optimal reproductive success using S. latissima gametophytes (Lüning et al. 1980).
Transporter
The transporter is similar to the clone-reactor, utilizing two fluid valves, one airflow valve, but without a grinder, all encased in an exoskeleton (Fig. 1). This bioreactor is designed for the transportation of either embryonic sporophytes or gametophyte biomass. This bioreactor has an exoskeleton for sturdiness, protecting the cultures and the valves during transportation in a cooled polystyrene box. The exoskeleton is designed to tightly fit into the polystyrene box, and more importantly prevents any direct contact of the culture with the cooling elements inside the box, preventing unwanted excess cooling (Fig. S4).
Validation of SeaCoRe system
Clone-reactor—gametophyte growth
The vegetative growth and photosynthetic efficiency of both wildtype and clonal cultures were assessed eight times within a timeframe of 75 days, in order to assess the suitability of the clone-reactor design for the maintenance of stock cultures. The cultures were grown vegetatively in the clone-reactors at 11 °C, red light (5 µmol photons m−2 s−1), using f/2 medium (Guillard and Ryther 1962). We used fluorometry measurements (Fast Ocean/Act2 FRRF, Chelsae Technologies Group Ltd) to estimate the Chl-a concentration [Chl-a], a proxy for phytoplankton biomass (Huot et al. 2007). These estimates were then calibrated against freeze dried gametophyte biomasses of both S. latissima and A. esculenta wildtype and clonal cultures (Fig. S5). The maximum PS II photosynthetic efficiency (Fv/Fm), a proxy of cell viability in a dark-adapted state (Suggett et al. 2009), was furthermore measured using the same FRRF, whose maximum PSII photochemical efficiency typically decreases under stressful conditions, such as nutrient starvation or excessive light (Kolber et al. 1988; Suggest et el. 2009). We compared Fv/Fm changes from the starting value, as a sign of increased/decreased stress.
Clone-inducer—reproductive success
In order to see at which initial gametophyte density the reproductive success (sporophytes mL−1) becomes optimal, a gametophyte biomass dilution gradient was used (~ 0.01- and ~ 1.5 mg mL−1), using a 1:1 mixture of male:female gametophyte clones. The used gradient of gametophyte densities was roughly based on the results from a previous study (Ebbing et al. 2020; 2021). The induction of gametophyte reproduction was done using 24-well plates (surface area = 1.9 cm2), whose cylindrical design is identical to the clone-inducer and whose results can be extrapolated to the broader well-shape of the clone-inducer. The reproductive success was determined as the number of successfully formed young sporophytes (≥ 25 µm) per mL on day 21, not including oogonium formation, after initiation of the experiment.
Transporter—transport simulation
Gametophyte culture transportation using the transporter was simulated by monitoring 28 vials containing either S. latissima or A. esculenta gametophyte cultures placed inside the transporter under transport conditions (Fig. S4). The transport conditions consisted of the placement of the bioreactor in a polystyrene box with additional cooling elements placed inside. The transporter was then filled with water so that the temperature between the 28 vials would follow the temperature of a regular culture transport. The temperature within a transporter was monitored during transport in the standard polystyrene box (L:W:H—32:25:36 cm). The photosynthetic efficiency (Fv/Fm) and reproductive success rate (sporophytes mL−1) of the gametophytes was monitored on a regular basis by taking out vials, in order to assess how long these cultures could be transported in between locations without compromising their reproductive success.
Statistical analysis
All statistical analysis was done using SPSS 20.0.0 statistical package (SPSS Inc., USA). All data on the reproductive success (sporophytes mL−1) was normally distributed and analyzed for homogeneity using the Levene’s test of variance. Factors that determined the reproductive success include the initial gametophyte density and transportation days of multiannual delayed gametophyte cultures. In case of unequal variances, a robust test of equality of means for unequal variances was applied (Welch t test). A Games-Howell nonparametric post-hoc comparison was subsequently applied to test for significant differences between the subgroups. If the data was found to be homogeneous, a one-way ANOVA was applied followed by the conservative Scheffe post-hoc test to determine which factor level was responsible for the specific treatment differences. All tests were run with a significance level of 0.05.
Results
Vegetative growth in the clone reactors
Our primary goal was to validate whether the clone reactors could be used for vegetative growth of gametophytes and to maintain stock cultures. The Chl-a concentrations increased in both wildtype cultures (A. esculenta and S. latissima) in a similar fashion, with overall biomass increasing 40% and 35%, respectively (Fig. 2). The highest biomass increase was observed in the S. latissima female clonal culture with a biomass increase of 105% in 75 days. Two cultures showed no increase in their Chl-a concentrations and their growth of 0% are depicted in red. The absence of any vegetative growth corresponded with declining Fv/Fm values in both cultures.
The Chl-a concentration (y-axisa; mg mL−1) of MAD S. latissima and A. esculenta wildtype and clonal cultures followed over a period of 75 days (x-axis). The overall biomass increase at the end of the experiment is depicted as percentages (%) on the right side of the figure. The photosynthetic efficiency of the cultures was followed at the same time (y-axisb; Fv/Fm), using the same cultures. All data are mean ± SD; n = 3
Our secondary goal was to test the grinder and what the influence of the grinder was on the Fv/Fm of gametophyte cultures in the clone reactors. The multipurpose grinder worked as intended during culture refreshments as well as the homogenization of gametophyte biomass (Fig. S2 and Fig. S3). The grinding process also resulted in declining Fv/Fm values and lower Chl-a concentrations in most cultures. We decided to not homogenize the cultures for a prolonged period of time while still following the Fv/Fm and growth (grey background). Not using the grinder resulted in larger standard deviations, and at the end of the first month positive vegetative growth was visible again in all cultures apart from the A. esculenta female clone culture. At the end of the first month, culture grinding resumed, immediately resulting in lower Fv/Fm values in all cultures apart from the S. latissima wildtype. At the end of the experiment, the cultures were refreshed on a weekly basis and grinded 4 times using the grinder of the clone reactors under non-sterile work conditions, during which no contamination was visible.
Gametophyte reproduction
Gametophytes initiated gametogenesis in all cultures with reproductive success in both S. latissima (Welch ANOVA F7,6.4 = 13.3, p ≤ 0.05; Table S1) as well as A. esculenta clonal cultures (Welch ANOVA F7, 6.8 = 4.69, p ≤ 0.05; Table S2) being significantly affected by the initial gametophyte density after 21 days (Fig. 3). In S. latissima cultures, the optimal initial gametophyte density was observed at 0.46 mg gametophyte DW mL−1 with 604 sporophytes mL−1 (± 93 SD), while A. esculenta had no clear optimum with three similarly high initial gametophyte densities (0.3, 0.6, and 1.2 mg gametophyte DW mL−1), with peak reproduction observed at an initial gametophyte density of 0.6 mg gametophyte DW mL−1 with 422 sporophytes mL−1 (± 148 SD).
Culture transport
The transport simulation looked at culture temperature (°C; Fig. 4A), Fv/Fm (Fig. 4B), and reproductive success (sporophytes mL−1; Fig. 4C), taking 31 days to complete (Fig. S7). Transport was successful in the first 19 to 23 days with no observed decline in Fv/Fm and reproductive success rates hovering between ~ 200 and 400 sporophytes mL−1. The three cooling elements, included in the polystyrene boxes for short-term cooled transport, reduced the temperatures in the first 2 to 3 days of transportation (highlighted in blue), with the lowest recorded temperatures being 5.5 °C (grey dashed line) and 4 °C (yellow pointed line) for respectively S. latissima and A. esculenta. Once, on day 20, the outside temperatures increased to more than 30 °C, thereby increasing the inside temperature of the cultures to around 25 °C (highlighted in orange). After this peak in temperature, the reproduction declined significantly after day 27 in both A. esculenta (Welch ANOVA: F13,26 = 552.9, p ≤ 0.05; Table S3) and S. latissima (ANOVA: F13,41 = 9.089, p ≤ 0.05; Table S4). Lastly, strong fluctuations in reproductive success were observed in S. latissima cultures at the start of the experiment, coinciding with strong fluctuations in temperature of the same cultures.
A–C: Transport simulation with the temperature, Fv/Fm, and reproductive success rates of S. latissima and A. esculenta MAD gametophytes followed through time (x-axis; days) with A: the temperature (y-axisa; °C) depicted as line graphs, B: Fv/Fm (y-axisb) depicted as scatter plots, and C: reproductive success (y-axisc; sporophytes mL−1) depicted as line graphs. The reproductive success is depicted as mean ± SD; n = 3. Blue and yellow bands are indicative for the lowest and highest temperatures that were measured
Discussion
SeaCoRe-system validation
The fundamental elements of the system, including the vegetative growth, gametophyte reproduction, and culture transport, were all successfully validated under relevant environments, thereby reaching technology readiness level—5 (Tzinis 2021). The validation of the system can be subdivided into the functionality of the bioreactors and the performance of the gametophytes. The clone reactor performed as envisioned, with gametophyte cultures successfully being kept for 75 days. The cultures were refreshed on a weekly basis, and ground frequently, using the three designed positions, under nonsterile environments (Fig. 1). The gametophytes also performed as envisioned, by slowly growing vegetatively, with the highest overall biomass increase observed in S. latissima female gametophyte cultures (105%). The clonal inducer was emulated successfully and gametophyte reproduction was successful under all initial gametophyte densities. Optimal initial gametophyte densities where reproduction was highest were observed at 0.4 and 0.6 mg gametophyte DW mL−1 for S. latissima and A. esculenta, respectively. The inverse correlation between initial gametophyte density and reproductive success is clearly visible in both species and agrees with previous well documented observations (Reed 1990, 1991; Choi et al. 2005; Ebbing et al. 2020, 2021). The simulated culture transport made clear that although these cultures cannot be transported indefinitely in closed polystyrene boxes, for the first 19 days of transportation it did not have any negative effect on the reproductive success of S. latissima and A. esculenta cultures (Fig. 4). This simulation searched for the limits of gametophyte culture transport, becoming an experiment that looked into the effects of high temperatures on gametophyte reproduction. This was especially informative considering that the overall ambient transport conditions of ~ 20 °C reached the upper limit of what these species normally can handle (Lee and Brinkhuis 1988; Fredersdorf et al. 2009). Regardless of the successful demonstration, there were also aspects of the system that still need closer examination. (1) The S. latissima male clonal culture and A. esculenta female clonal culture reacted strongly to the grinder, whose declining Fv/Fm directly coincided with the usage of the grinder. The grinder clearly functions the way it was meant to function, separating biomass when refreshing, and homogenizing biomass when the gametophyte clumps grow too big (Fig. S2; Fig. S3). All other cultures recuperated well after grinding, raising the question whether the problem lies in the device or in the gametophyte cultures. Follow up experiments should both focus on the device and the health of gametophytes. On the one hand, studies are needed on whether grinders can be fit too tightly and thus become too damaging to gametophyte cultures. On the other hand, emphasis should be placed on the recuperation period after grinding of gametophyte cultures, and whether resting periods without homogenization are needed in order to optimize the vegetative outgrowth. (2) The universal valve type used here (Luer lock) did not work optimally because of the small size of the valve and the bulky nature of gametophyte biomass. The universal interlockability of the valves make them ideal for opensource systems (Nie and Takeuchi 2020); however, if gametophyte biomass cannot easily pass the valve, different interconnectable valves will be needed in following versions.
Key characteristics
The primary objective of the SeaCoRe project was to propagate kelp gametophyte cultures without the need of sterile work environments. An additional aim was using clonal cultures, whose culture characteristics complement our primary objective. Previous gametophyte bioreactor publications looked at single bioreactors, therefore needing wildtype cultures for the successful propagation of kelp sporophytes (Rorrer et al. 1995; Gao et al. 2005; Chen and Qi 2008). Utilizing sex-specific clonal cultures is an essential element in our system, since clean starting cultures are an essential part of this system which cannot be guaranteed by using wildtype gametophyte cultures. Clonal cultures generally start out cleaner from contaminations than their wildtype counterpart because there are reduced risks of microalgal, fungal, and bacterial infections. The main reason why they are cleaner is that clonal cultures start out from isolated gametophytes (Li et al. 1999), instead of mass extractions of zoospores. Moreover, separately growing out the two sexes also opens up unique opportunities like (1) adjustable growing conditions to compensate for the different growth rates between the sexes (Destombe and Oppliger 2011), (2) adjustable male/female ratios for optimal reproduction (Zhang et al. 2008), (3) adjustable genetic diversity through clonal inclusion or exclusion for breeding purposes (Martins et al. 2019), and (4) it paves the way to further optimize the vegetative growth of gametophytes (Zhang et al. 2019), without having to worry for any accidental sexual reproduction. This system is furthermore small enough to easy transport (Fig. 5), but at the same time scalable enough to meet the demands of an ever expanding kelp industry that has already more than doubled between 2000 and 2016 (FAO 2018). Lastly, the entire system, including all bioreactors and incubators, can be built for less than €2000 (Table S2), making it accessible for small-scale seaweed farmers that do not have the resources for the normally expensive lab grade equipment needed for kelp cultivation (Wakamatsu and Miyata 2015).
Future developments
Although the essential parts of the SeaCoRe system were tested successfully, the system still has plenty of room for further improvement. In the end, only actually testing it on large-scale farms will tell us whether the increased complexity of the SeaCoRe system can outperform the output of the more traditional propagation techniques. Nonetheless, studies show that there is still a lot of room for optimization. For example, the vegetative growth rates of the stock cultures, reached in the clone reactors, were intentionally achieved under stock-culture conditions, constituting of minimal light intensities of red light (5 µmol photons m−2 s−1), conventional temperatures (11 °C), and regular nutrient conditions (f/2 medium). Delving deeper in the different light, temperature, and nutrient regimes, in search for optimal growing conditions, are ripe for exploration. Evidence that the vegetative growth rates of these clonal cultures can be further optimized have already been shown by Rorrer et al. (1995) and Chen and Qi (2008). Zhang et al. (2019) even describes Saccharina japonica gametophyte biomass growing up to 12.8 times the initial biomass in just 21 days, using higher light intensities (30 µmol photons m−2 s−1 of white light) and different nutrient conditions than described here. Adjustable airflows can help increase the vegetative growth of kelp gametophytes even more (Gao et al. 2005), although there are also indications that bubble-free bioreactors might be preferable due to the hydrodynamic stress coming from air bubbles (Chen and Qi 2008). Not only vegetative growth can be improved, gametophyte reproduction also has room to be further optimized (Ratcliff et al. 2017), with indications that different male:female ratios of a culture can result in altered reproductive success (Zhang et al. 2008). In order to further optimize, we also need a better understanding of how regular stock culture conditions alters the photosynthetic efficiency of healthy dark adapted gametophytes (Fig. S6), so that our interpretation of what a healthy culture is becomes more accurate. Quantifying the state of a gametophyte culture needs a broad spectrum of trackable parameters, with the Fv/Fm covering only part of it. Perhaps the biggest leap forward we can make is by extending this idea of system compatibility that is exemplified by this compatible system of bioreactors to the entire kelp production process. For example, as of today, it might not be economically sound for an individual kelp farmer to seed for itself, therefore always needing to co-op with more specialized organizations who maintain the gametophytes. A full-scale hatchery or direct seeding machine, that is plug and play compatible with the SeaCoRe system, is therefore something that needs to be incorporated in the future, to make sure a farmer can indeed function independently. The controlled reproductive method, as cultivation method, can be central to this process, since quantitatively being able to assess when, where, and how much gametophyte biomass is needed at your farm is key for the predictability and reliability needed for kelp farms that have the ambition to farm kelp on scales not seen before (Hooft and Slootweg 2021).
Data availability
Data obtained in the experiments herein can be available upon request.
References
Bak UG, Olavur G, Infante J (2020) Technical challenges for offshore cultivation of kelp species : lessons learned and future directions. Bot Mar 63:341–353
Barrento S, Camus C, Sousa-pinto I, Buschmann AH (2016) Germplasm banking of the giant kelp : our biological insurance in a changing environment. Algal Res 13:134–140
Bi Y, Hu Y, Zhou Z (2011) Genetic variation of Laminaria japonica (Phaeophyta) populations in China as revealed by RAPD markers. Acta Oceanol Sin 30:103–112
van den Burg S (2020) Prospects for upgrading by the European kelp sector. J Appl Phycol 33:557–566
Carney LT (2011) A multispecies laboratory assessment of rapid sporophyte recruitment from delayed kelp gametophytes. J Phycol 47:244–251
Carney LT, Edwards MS (2006) Cryptic processes in the sea : a review of delayed development in the microscopic life stages of marine macroalgae. Algae 21:161–168
Carrier TJ, Eddy SD, Redmond S (2017) Solar-dried kelp as potential feed in sea urchin aquaculture. Aquacult Int 25:355–366
Charrier B, Wichard T, Reddy CRK (eds) (2018) Protocols for macroalgae research (1st ed). CRC Press, Boca Raton
Chen S, Qi H (2008) Photolithotrophic cultivation of Laminaria japonica gametophyte cells in a silicone tubular membrane-aerated photobioreactor. Plant Cell Tissue Organ Cult 93:29–38
Choi HG, Young SK, Soon JL, Eun JP, Ki WN (2005) Effects of daylength, irradiance and settlement density on the growth and reproduction of Undaria pinnatifida gametophytes. J Appl Phycol 17:423–430
Chung IK, Oak JH, Lee JA, Shin JA, Kim JG, Park K (2013) Installing kelp forests/seaweed beds for mitigation and adaptation against global warming: Korean Project Overview. Mar Sci 70:1038–1044
Davies RJ (1990) Bio energy: a case study of Kelp Industries, King Island, Tasmania. Solar Wind Technol 7:21–24
Destombe C, Oppliger LV (2011) Male gametophyte fragmentation in Laminaria digitata: a life history strategy to enhance reproductive success. Cah Biol Mar 52:385–394
Ebbing A, Pierik R, Bouma T, Kromkamp JC, Timmermans K (2020) How light and biomass density influence the reproduction of delayed Saccharina latissima gametophytes (Phaeophyceae). J Phycol 56:709–718
Ebbing APJ, Pierik R, Fivash GS, van de Lossdrecht NCJ, Bouma, TJ, Kromkamp JC, Timmermans K (2021) The role of seasonality in reproduction of multiannual delayed gametophytes of Saccharina latissima. J Phycol 57:1580–1589
FAO (2018) The State of World Fisheries and Aquaculture 2018 - Meeting the sustainable development goals. Rome
Forbord S, Steinhovden KB, Solvang T, Handå A, Skjermo J (2020) Effect of seeding methods and hatchery periods on sea cultivation of Saccharina latissima (Phaeophyceae): a Norwegian case study. J Appl Phycol 32:2201–2212
Fredersdorf J, Müller R, Becker S, Wiencke C, Bischof K (2009) Interactive effects of radiation, temperature and salinity on different life history stages of the Arctic kelp Alaria esculenta (Phaeophyceae). Oecologia 160:483–492
Gao J, Zhang Y, Wang H, Qin S (2005) Suspension culture of gametophytes of transgenic kelp in a photobioreactor. Biotech Lett 27:1025–1028
Goecke F, Klemetsdal G, Ergon Å (2020) Cultivar development of kelps for commercial cultivation—past lessons and future prospects. Front Mar Sci 7:110
Guillard RRL, Ryther JH (1962) Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea (Cleve). Can J Microbiol 8:229–239
Heuermann R, Reuter R, Willkomm R (1999) RAMSES: a modular multispectral radiometer for light measurements in the UV and VIS. Environmental Sensing and Applications 3821:279–285
Hooft D, and Slootweg C (2021). Kelp Blue. available on http://kelp.blue/. Accessed 21 May 2021
Huot Y, Babin M, Bruyant F, Grob C, Twardowski TH, Claustre H (2007) Does chlorophyll a provide the best index of phytoplankton biomass for primary productivity studies? Biogeosci Discuss 4:707–745
Hwang EK, Park CS, Baek JM (2006) Artificial seed production and cultivation of the edible brown alga, Sargassum fulvellum (Turner) C. Agardh: developing a new species for seaweed cultivation in Korea. J Appl Phycol 18:251–257
Kerrison PD, Twigg G, Stanley M, De Smet D, Buyle G, Martínez Pina A, Hughes AD (2019) Twine selection is essential for successful hatchery cultivation of Saccharina latissima, seeded with either meiospores or juvenile sporophytes. J Appl Phycol 31:3051–3060
Kerrison, Philip D, Mairi I, Adrian M, Emily M, Peter DE, Michele SS, Adam DH, Maeve SK (2020) Comparing the effectiveness of twine- and binder-seeding in the Laminariales species Alaria esculenta and Saccharina latissima. J Appl Phycol 32:2173–2181
Kolber Z, Zehr J, Falkowski P (1988) Effects of growth irradiance and nitrogen limitation on photosynthetic energy conversion in photosystem II. Plant Physiol 88:923–929
Lee JA, Brinkhuis BH (1988) Seasonal light and temperature interaction effects on development of Laminaria saccharina (Phaeophyta) gametophytes and juvenile sporophytes. J Phycol 24:181–191
Li, D., Zhou, Z., Liu, H., & Wu, C. (1999) A new method of Laminaria japonica strain selection and sporeling raising by the use of gametophyte clones. Hydrobiologia 398:473–476.
Liu F, Sun X, Wang F, Wang W, Liang Z, Lin Z, Dong Z (2014) Breeding, economic traits evaluation, and commercial cultivation of a new Saccharina variety “Huangguan No. 1". Aquac Int 22:1665–1675
Liu F, Yao J, Wang X, Repnikova A, Galanin DA, Duan D (2012) Genetic diversity and structure within and between wild and cultivated Saccharina japonica (Laminariales, Phaeophyta) revealed by SSR markers. Aquaculture 359:139–145
Lüning (1980) Critical levels of light and temperature regulating the gametogenesis of three Laminaria species (Phaeophyceae). J Phycol 16:1–15
Martins N, Pearson GA, Gouveia L, Tavares AI, Serrão EA, Bartsch I (2019) Hybrid vigour for thermal tolerance in hybrids between the allopatric kelps Laminaria digitata and L. pallida (Laminariales, Phaeophyceae) with contrasting thermal affinities. Eur J Phycol 54:548–561
Nie M, Takeuchi S (2020) Luer-lock valve: a pre-fabricated pneumatic valve for 3D printed microfluidic automation. Biomicrofluidics 14:044115
Ratcliff JJ, Soler-Vila A, Hanniffy D, Johnson MP, Edwards MD (2017) Optimisation of kelp (Laminaria digitata) gametophyte growth and gametogenesis: effects of photoperiod and culture media. J Appl Phycol 29:1957-1966
Reddy CRK, Jha B, Fujita Y, Ohno M (2008) Seaweed micropropagation techniques and their potentials: an overview. J Appl Phycol 20:609–617
Redmond S, Kim J, Yarish C, Pietrak M, Bricknell I (2014) Culture of Sargassum in Korea. NOAA Sea Grant, Orono Maine. pp.12
Reed DC (1990) The effects of variable settlement and early competition on patterns of kelp recruitment. Ecology 71:776–787
Reed DC, Neushul M, Ebeling AW (1991) Role of settlement density on gametophyte growth and reproduction in the kelps Pterygophora californica and Macrocystis pyrifera (Phaeophyceae). J Phycol 27:361–366
Rorrer GL, Modrell J, Zhi C, Yoo HD, Nagle DN, Gerwick WH (1995) Bioreactor seaweed cell culture for production of bioactive oxylipins. J Appl Phycol 7:187–198
Sato Y, Yamaguchi M, Hirano T, Fukunishi N, Abe T, Kawano S (2017) Effect of water velocity on Undaria pinnatifida and Saccharina japonica growth in a novel tank system designed for macroalgae cultivation J Appl Phycol 29:1429-1436
Shan TF, Pang SJ, Gao SQ (2013) Novel means for variety breeding and sporeling production in the brown seaweed Undaria pinnatifida (Phaeophyceae): crossing female gametophytes from parthenosporophytes with male gametophyte clones. Phycol Res 61:154–161
Shan TF, Pang SJ, Li J, Gao SQ (2016) Breeding of an elite cultivar Haibao no. 1 of Undaria pinnatifida (Phaeophyceae) through gametophyte clone crossing and consecutive selection. J Appl Phycol 28:2419–2426
Su L, Pang SJ, Shan TF, Li X (2017) Large-scale hatchery of the kelp Saccharina japonica: a case study experience at Lvshun in northern China. J Appl Phycol 29:3003–3013
Suggett DJ, Moore CM, Hickman AE, Geider RJ (2009) Interpretation of fast repetition rate (FRR) fluorescence: signatures of phytoplankton community structure versus physiological state. Mar Ecol Prog Ser 376:1–19
Tzinis, I (2021) NASA. Technology readiness level. available on https://www.nasa.gov/directorates/heo/scan/engineering/technology/technology_readiness_level. Accessed 07 Aug 2021
Umanzor S, Li Y, Bailey D, Augyte S, Huang M, Marty-Rivera M, Jannink J-L, Yarish C, Lindell S (2021) Comparative analysis of morphometric traits of farmed sugar kelp and skinny kelp, Saccharina spp., strains from the Northwest Atlantic. J World Aquacult Soc 52:1059–1068
van den Burg S, Selnes T, Alves L, Giesbers E, Daniel A (2021) Prospects for upgrading by the European kelp sector. J Appl Phycol 33:557–566
Visch W, Rad-Menéndez C, Nylund GM, Pavia H, Ryan MJ, Day J (2019) Underpinning the development of seaweed biotechnology: cryopreservation of brown algae ( Saccharina latissima ) gametophytes. Biopreserv Biobank 17:1–9
Wade R, Augyte S, Harden M, Nuzhdin S, Yarish C, Alberto F (2020) Macroalgal germplasm banking for conservation, food security, and industry. PLoS Biol 18:e3000641
Wakamatsu H, Miyata T (2015) Reforming wakame aquaculture in Japan with relaxed processing standards: From consumer and producer viewpoints. Aquac Econ Manag 19:387–403
Westermeier R, Patin D, Piel MI, Maier I, Mueller DG (2006) A new approach to kelp mariculture in Chile : production of free-floating sporophyte seedlings from gametophyte cultures of Lessonia trabeculata and Macrocystis pyrifera. Aquac Res 37:164–171
Xu B, Zhang QS, Qu SC, Cong YZ, Tang XX (2009) Introduction of a seedling production method using vegetative gametophytes to the commercial farming of Laminaria in China. J Appl Phycol 21:171–178
Zhang QS, Qu SC, Cong YZ, Luo SJ, Tang XX (2008) High throughput culture and gametogenesis induction of Laminaria japonica gametophyte clones. J Appl Phycol 20:205–211
Zhang Y, Zhang Y, Li M, Li L, Tang X, Gao J (2019) Homogenization significantly enhances growth of macroalga Saccharina japonica female gametophytes. Algal Res 43:1–5
Zhao XB, Pang SJ, Liu F, Shan TF, Li J, Gao SQ, Kim HG (2016) Intraspecific crossing of Saccharina japonica using distantly related unialgal gametophytes benefits kelp farming by improving blade quality and productivity at Sanggou Bay, China. J Appl Phycol 28:449–455
Acknowledgements
We would like to thank Hortimare B.V. for the usage of their wildtype and clonal cultures and for the work of Pieter Mulder and Jessica Schiller into the influence of light on Fv/Fm values of kelp gametophytes. We would also like to thank Matthias Schrama at Schrama-metaaltechniek for the construction of the bioreactor prototypes. The authors are also grateful for the help that the technical department of Royal NIOZ gave in the construction of the incubators. We furthermore want to thank Bas Oteman for his help in developing the SeaCoRe mobile app. Lastly, we would like to thank NWO for the Open-mind grant in 2017, making it possible for us to design, construct, and test the open source SeaCoRe system.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ebbing, A.P.J., Fivash, G.S., Pierik, R. et al. The SeaCoRe system for large scale kelp aquaculture: a plug-and-play, compatible, open-source system for the propagation and transport of clonal gametophyte cultures. J Appl Phycol 34, 517–527 (2022). https://doi.org/10.1007/s10811-021-02638-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-021-02638-2