Abstract
Ulva fenestrata is an economically and ecologically important green algal species with a large potential in seaweed aquaculture due to its high productivity, wide environmental tolerance, as well as interesting functional and nutritional properties. Here, we performed a series of manipulative cultivation experiments in order to investigate the effects of irradiance (50, 100, and 160 μmol photons m−2 s−1), temperature (13 and 18 °C), nitrate (< 5, 150, and 500 μM), phosphate (< 1 and 50 μM), and pCO2 (200, 400, and 2500 ppm) on the relative growth rate and biochemical composition (fatty acid, protein, phenolic, ash, and biochar content) in indoor tank cultivation of Swedish U. fenestrata. High irradiance and low temperature were optimal for the growth of this northern hemisphere U. fenestrata strain, but addition of nutrients or changes in pCO2 levels were not necessary to increase growth. Low irradiance resulted in the highest fatty acid, protein, and phenolic content, while low temperature had a negative effect on the fatty acid content but a positive effect on the protein content. Addition of nutrients (especially nitrate) increased the fatty acid, protein, and phenolic content. High nitrate levels decreased the total ash content of the seaweeds. The char content of the seaweeds did not change in response to any of the manipulated factors, and the only significant effect of changes in pCO2 was a negative relationship with phenolic content. We conclude that the optimal cultivation conditions for Swedish U. fenestrata are dependent on the desired biomass traits (biomass yield or biochemical composition).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seaweed aquaculture is a growing industry worldwide, which is worth more than 6 billion US$ per year (Buchholz et al. 2012; Buschmann et al. 2017; FAO 2018). The green seaweed genus Ulva has received a lot of attention due to its high productivity and wide environmental tolerance (Ye et al. 2011; Smetacek and Zingone 2013; Bolton et al. 2016). In general, Ulva spp. have a fast growth rate and are very efficient in absorbing many nitrogen sources such as nitrate, ammonia, and urea (Robertson-Anderson et al. 2008). Therefore, Ulva spp. have great potential for bioremediation of nutrient-polluted water and as extractive species in integrated multi-trophic aquaculture (IMTA) systems (Bolton et al. 2016). Production of Ulva spp. has been successfully integrated with other types of aquaculture such as abalone (Bolton et al. 2009) and fish (Sphigel et al. 2017). Apart from its beneficial function as a nutrient scrubber, the produced biomass also has very interesting functional and nutritional properties and can be used directly as food (Bolton et al. 2016), or turned into value-added products such as functional foods, cosmeceuticals, nutraceuticals, or pharmaceuticals (Hafting et al. 2015). Furthermore, there is a growing interest in Ulva spp. biomass as a feedstock for biofuel production (Coelho et al. 2014).
There is a large variation in the biochemical composition of Ulva spp. (Holdt and Kraan 2011), which is most likely due to genetic differences between species and populations, as well as differences in environmental growth conditions such as temperature, irradiance, salinity, and nutrient composition of the water. The varying responses to environmental factors lead to one of many challenges in seaweed cultivation, i.e., that the optimal cultivation conditions for biomass yield may not correspond to the optimal conditions for the desired biochemical composition. Growth generally increases with increasing irradiance, temperature, nutrient, and pCO2 levels (e.g. Duke et al. 1986; Geertz-Hansen and Sand-Jensen 1992; Riccardi and Solidoro 1996; Taylor et al. 2001; Kalita and Tytlianov 2003; Kalita and Titlyanov 2013; Olischläger et al. 2013; Kumari et al. 2014; Young and Gobler 2016; Gao et al. 2017, 2018a; Tremblay-Gratton et al. 2018; Chen et al. 2019; Mhatre et al. 2019; Sebök et al. 2019), although there are exceptions to this general pattern (e.g. Kerrison et al. 2012; Liu and Zou 2015). The biochemical composition also changes with environmental growth conditions, with increasing temperature, nutrients, and pCO2 levels generally increasing total fatty acid and protein content (e.g. Kumari et al. 2014; Liu and Zou 2015; Gao et al. 2017, 2018a, 2018b; Chen et al. 2019; but see Ak et al. 2015). Increased irradiance, on the other hand, tends to have a positive effect on total fatty acid content, but a negative effect on total protein content (Mhatre et al. 2019; but see Khotimchenko and Yakovleva 2004). The total phenolic content of Ulva spp. increases with increasing nutrient and temperature levels (Cabello-Pasini et al. 2011; Figueroa et al. 2014; McCauley et al. 2018) and decreasing pCO2 levels (Figueroa et al. 2014). From an applied perspective, optimising cultivation conditions to produce high yields of proteins, fatty acids, carbohydrates, and/or bioactive compounds may be more important than optimising growth rate (Hafting et al. 2015).
Most of the mentioned previous studies on cultivation of Ulva spp. investigated the effect of only a few environmental conditions on growth and/or a few selected biochemical constituents (usually proteins, fatty acids, and estimated carbohydrates) simultaneously. The overall aim of the present study was to investigate how changes in several cultivation conditions (irradiance, temperature, nitrate, phosphate, and pCO2) affect the growth and levels of several commonly measured biochemical constituents (total fatty acid, protein, phenolics, ash, and biochar content) of Swedish U. fenestrata in the same study. Effects of environmental factors on the carbohydrate and ulvan content of U. fenestrata are presented in Olsson et al. (2020). To achieve our aim, we conducted three manipulative experiments where the interactive and/or single effects of (1) irradiance and temperature, (2) nitrate and phosphate, and (3) pCO2 at different levels were tested in indoor cultivation tanks with flow-through seawater. Based on the results from the mentioned previous studies, we hypothesised that the (i) growth rate and (ii) fatty acid content of U. fenestrata would be highest at high irradiance, temperature, nutrient, and pCO2 levels; (iii) protein content would be highest at low irradiance, and high temperature, nutrients and pCO2 levels; and (iv) that the total phenolic content would be highest at high irradiance, temperature, and nutrient, and low pCO2 levels. For the ash and biochar content, we did not have specific hypotheses about the direction of change, but still hypothesised that these variables would change with changing cultivation conditions.
Material and methods
Algal material and taxonomic identification
Algal material for the experiments in the present study were taken from a long-term indoor tank culture at the Tjärnö Marine Laboratory (TML, 58° 52′ 36.4″ N 11° 6′ 42.84″ E), where vegetative healthy seaweed thalli were grown in cultivation tanks (90 L) in a greenhouse under a 16:8 h (L:D) light cycle at an irradiance of 140 μmol photons m−2 s−1. The light source was an INDY66 LED 60 W 4000 K 6000 lm. The seaweeds continuously received filtered (5 μm + UV) natural seawater without additional medium or chemicals in a flow-through system (flow = 10–14 L h−1) and water motion was provided by aeration. The salinity and temperature fluctuated depending on the prevailing weather and seasonal conditions.
The taxonomic identification of the Ulva strain used in the present study was based on molecular identification. About 1 cm2 of thallus tissue of four different individuals from the long-term cultivation was frozen and lyophilised, and total DNA was extracted using the DNeasy Plant Mini Kit (Quiagen, Netherlands). Methods for subsequent PCR amplification of the tufA marker gene were described by Steinhagen et al. (2019). Direct sequencing of both strands of the purified amplicons was provided by Eurofins GATC Biotech GmbH (Konstanz, Germany). To determine the taxonomic species affiliations, the obtained sequence of each individual was compared with published sequences of Ulva spp. using the BLAST function in GenBank. To assure the correctness of previous databank searches, representative Ulva spp. sequences were downloaded from GenBank, and phylogenetic controls using the maximum likelihood approach within the software RAxML (v.8; Stamatakis 2014) were performed (data not shown). Resulting sequences from this study were uploaded to GenBank and are publicly available (GenBank accession numbers BankIt2249282 Seq1: MN240309, Seq2: MN240310, Seq3: MN240311).
Experimental set up
The effect of different irradiance, temperature, nutrient, and pCO2 levels on the relative growth rate and chemical composition of Ulva fenestrata were tested in three different manipulative indoor experiments using 15 L aquaria supplied with running filtered (5 μm + UV, 9 L h−1) seawater, which under control conditions had ambient nutrient (nitrate <5 μM, phosphate <1 μM), pCO2 (≈ 400 ppm), and temperature (13–14 °C) levels.
The first experiment was performed in June 2018 in a temperature-controlled room where irradiance and temperature were manipulated in a fully crossed design. The light source was an OSRAM Lumilux Cool daylight L 58 W/865, and irradiance was manipulated by shading the aquaria with layers of semi-opaque plastic netting until the desired intensity (low = 50, medium = 100, and high = 160 μmol photons m−2 s−1) was achieved. Water temperature was manipulated using submersed heaters (EHEIM Aquarium Heaters, 600–1000 L; 230 V, 300 W) and was set to ambient (13 °C) and high (18 °C) temperature. In order to avoid negative effects, irradiance and temperature levels were chosen within the range that is experienced naturally by intertidal macroalgae in Sweden (www.smhi.se). Twelve square centimetre discs were cut from U. fenestrata blades and added to the aquaria at a density of 0.14 g L−1 (n = 6, i.e. a total of 36 aquaria). Seaweeds were cultivated under a 16:8 h (L:D) cycle for 7 days.
In the second experiment, performed in a temperature-controlled room for 5 days in October 2018 (average irradiance 110 μmol photons m−2 s−1, 16:8 h L:D), nitrate and phosphate levels in the water were manipulated in a fully crossed design. Ulva fenestrata blades were added to the aquaria at a density of 0.71 g L−1 (n = 4, i.e. a total of 24 aquaria). Nutrients were added in the morning each day of the experiment by turning off the running water and adding 10 mL of a stock solution prepared from NaNO3 and Na2HPO4, to a final concentration of 150 (medium), and 500 (high) μM nitrate, and 50 (high) μM phosphate. Ambient seawater was used as control. The seaweeds were allowed to assimilate nutrients for 2 h after which the water flow was turned back on.
The third experiment was performed in a greenhouse in October 2018 (natural light cycle 10:14 h L:D, average irradiance between 11:00 am to 01:00 pm = 50 μmol photons m−2 s−1), with constant seawater flow from header tanks (a total of 6 header tanks, n = 2) to 15 L aquaria. Control algae were maintained at ambient pCO2 (target pCO2 = 400 ppm, pH = 8.1), while experimental algae were exposed to either low pCO2 (target pCO2 = 200 ppm, pH = 8.4) or high pCO2 (target pCO2 = 2500 ppm, pH = 7.4). Treatments were regulated using solenoid valves controlled by pH computers (Aqua Medic, Germany, NBS-calibrated), which mixed CO2 (AGA, Sweden) with air that had CO2 removed. pCO2 was monitored using an LI-850 CO2/H2O Gas Analyzer (LI-COR Biosciences, USA). Total alkalinity (AT), salinity, and temperature were 2235 μmol kg−1, 32 PSU, and 13.1 °C, respectively. Total alkalinity was estimated from salinity using long-term salinity:alkalinity relationship data (Falkenberg et al. 2019). Ulva fenestrata blades were added to the aquaria at a density of 0.71 g L−1 (n = 4, i.e. a total of 24 aquaria).
Relative growth rate
The relative growth rate (% day−1) of U. fenestrata was calculated using the formula
where ts and te are the start and end time of the experiments (in days), and Ws and We are the size (in g wet weight) of the algae at the start and the end of the experiments. Wet weight was determined on a lab-scale (Sartorius TE1502S) after removing excess water with a salad spinner. Tissue samples from each aquarium were frozen, lyophilised, homogenised, and stored at < − 20 °C before further analysis of biochemical composition.
Fatty acid content and composition
Fatty acids were analysed by adding 20 μL of internal standard (C23:0, 1000 ppm) to roughly 25 mg of lyophilised and homogenised seaweed, followed by the addition of 1 mL toluene and 1 mL of freshly prepared hydrochloric acid (10% v/v in methanol). The samples were incubated at 70 °C for 120 min and thereafter allowed to cool to room temperature before 1 mL ultra-pure water was added. Thereafter, 1 mL hexane was added; the samples were vortexed for at least 60 s and phase-separation achieved by centrifugation at 100×g for 6 min. The upper phase was injected into a Shimadzu TQ-8030 GC-MS/MS system consisting of a Shimadzu GC-2010 Plus gas chromatograph (GC), Shimadzu TQ-8030 triple quadrupole mass spectrometer, and a Shimadzu AOC-5000 Plus sample handling system (Shimadzu Europe GmbH, Germany). Data was acquired with the Shimadzu GCMSSolutions software version 4.2. One microlitre of each sample was injected with a split ratio of 1, and separation was done on a 30 m × 0.25 mm × 0.25 μm Zebron ZB-WAXplus column (Phenomenex, USA). Injection port temperature was set to 275 °C, and oven temperature programme was as follows: initial temperature 100 °C, ramped to 205 °C at 4 °C min−1 followed by ramping to 230 °C at 1 °C min−1, and held at 230 °C for 5 min. GC was operated in constant linear velocity mode set to 37.2 cm s−1. Septum purge flow was set to 3 mL min−1. Interface and ion source temperatures were 280 and 230 °C, respectively. The autosampler was kept at 8 °C. Helium was used as the carrier gas. Data were collected between 50 and 550 m/z and at single ions at 55, 74, and 87 m/z.
Protein content
Total nitrogen was determined by combustion using a LECO Trumac nitrogen analyser, EDTA was used as standard. Thereafter, the total protein content was calculated using a nitrogen to protein conversion factor of 5 (Angell et al. 2016a).
Total phenolic content
Total phenolic content in U. fenestrata was extracted using 30–50 mg lyophilised and homogenised algal material in 1.5 mL of 70% ethanol for 1.5 h in 20 °C. After extraction, the samples were centrifuged (1 min at 14000 rpm and 20 °C) and the supernatant was collected. Total phenolic content was estimated colorimetrically using the Folin-Ciocalteu phenol reagent (Merck) with gallic acid (Sigma-Aldrich) as a standard. One millilitre supernatant and 0.5 mL Folin-Ciocalteu’s reagent was mixed with 7 mL distilled H2O, after which 1.5 mL Na2CO3 (200 g L−1, Merck) was added. Samples were left for 2 h in 20 °C, after which the absorbance was measured at 765 nm using a spectrophotometer (Lambda XLS+, Perkin Elmer). Total phenolic content was calculated as % of dw.
Biochar and ash content
The biochar and ash contents of U. fenestrata samples were estimated using a Mettler Toledo TGA/DSC. Approximately 4 mg of each lyophilised sample was heated in alumina cups from 40 to 800 °C at a heating rate of 10 °C min−1 under an N2 atmosphere. After being heated to 800 °C, O2 was introduced instead of N2 and the temperature was kept at 800 °C for 15 min. The flow rate was set to 50 mL min−1. The biochar content was calculated as the weight that remained after the samples were heated to 800 °C in N2, and the ash content was calculated as the mass remaining after finalising the heating programme. The data were processed and analysed using STARe software.
Statistical analysis
Data on the relative growth rate and biochemical composition of U. fenestrata from the experiments in the present study were statistically analysed using the analysis of variance (ANOVA). The effects of temperature and irradiance, as well as nitrate and phosphate, were analysed using two separate orthogonal 2-way ANOVAs with fixed 2-level (temperature and phosphate) and 3-level (irradiance and nitrate) factors. The effect of pCO2 was tested using a mixed-model ANOVA with pCO2 as a fixed 3-level factor and header tank as a random 4-level factor nested within pCO2. Significant differences among means were compared using the Tukey’s HSD test. Before statistical analysis, data were tested for homogeneity of variances using Cochran’s test (Underwood 2001). No heterogeneous variances were found, and therefore, data were not transformed.
Results
Taxonomic identification
TufA gene sequences originating from single individuals from the cultivated Ulva strain at TML were all identical with the northern European type of the common “sea lettuce”, formerly known as Ulva lactuca. However, molecular investigations on the Linnaean type material of U. lactuca have revealed that the oldest available name for the European U. lactuca is U. fenestrata (Hughey et al. 2019) and therefore the strain used in this study was identified as U. fenestrata.
Relative growth rate
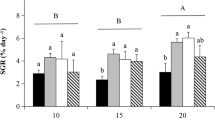
The mean relative growth rate of U. fenestrata varied considerably between the three different experiments, i.e. from 24.64 ± 0.61% day−1 (mean ± SEM) in the temperature/irradiance experiment to 13.40 ± 0.50% day−1 (mean ± SEM) in the nitrate/phosphate experiment and 6.37 ± 0.31% day−1 (mean ± SEM) in the pCO2 experiment (Fig. 1a–c). When data were statistically analysed, we found a significant difference in relative growth rate between algae exposed to different irradiance and temperature levels (Table 1a). The relative growth rate was on average 14.43% higher at medium and high irradiance compared with low irradiance, and 10.81% higher at low compared with high temperature (Tukey’s HSD, p < 0.05, Fig. 1a, b). Furthermore, there was a significant interaction between nitrate and phosphate addition on the relative growth rate of U. fenestrata (Table 1a). However, the relative growth rate was on average 42.65% lower when nitrate was added to the aquaria compared with ambient conditions (Tukey’s HSD, p < 0.05), but this effect was counteracted by adding phosphate (Fig. 1c). We found no statistically significant effect of different pCO2 levels on the relative growth rate of U. fenestrata (Table 1a). The average growth rate of U. fenestrata exposed to high, medium, and low pCO2 treatments was 6.55 ± 0.43, 6.54 ± 0.55, and 6.02 ± 0.68% day−1 (mean ± SEM), respectively.
Mean relative growth rate (% day−1) (a–c), total fatty acid (% dw) (d–f), protein (% dw) (g–i), and phenolic (% dw) (j–l) content in Ulva fenestrata after exposure to different irradiance (n = 12), temperature (n = 18), and nitrate and phosphate (n = 4) levels. Capital letters above bars show significant differences between means based on Tukey’s HSD test (p < 0.05). Error bars show SEM
Fatty acid content
The mean total fatty acid content of U. fenestrata was 1.31 ± 0.03, 1.61 ± 0.05, and 1.83 ± 0.02% dw (mean ± SEM) in the temperature/irradiance (Fig. 1d, e), nitrate/phosphate (Fig. 1f), and pCO2 experiments, respectively. We found statistically significant effects of the factors irradiance and temperature, as well as a significant interaction between nitrate and phosphate addition, on the total fatty acid content in U. fenestrata (Table 1b). The mean fatty acid content decreased significantly (Tukey’s HSD, p < 0.05) by 12.98% in the medium and high compared with low irradiance treatment (Fig. 1d) but increased with 18.70% in the high compared with the low temperature treatment (Fig. 1e). Elevated nitrate levels significantly (Tukey’s HSD, p < 0.05) increased the total fatty acid content with on average 25.13% compared with ambient conditions (Fig. 1f). Elevated phosphate levels alone had no significant (Tukey’s HSD, p > 0.05) effect on the total fatty acid content in U. fenestrata (Fig. 1f). However, in combination with nitrate addition, elevated phosphate levels had additive effects as was shown by the significant (Tukey’s HSD, p < 0.05) 10.51% increase in total fatty acid content between seaweeds exposed to a combination of nitrate and phosphate compared with nitrate only (Fig. 1f). The fatty acid content in algae exposed to different pCO2 treatments did not differ significantly (Table 1b), but there was a statistically significant difference in total fatty acids in algae receiving water from different header tanks (Table 1b). The average total fatty acid content in U. fenestrata exposed to high, medium, and low pCO2 treatments were 1.82 ± 0.03, 1.87 ± 0.03, and 1.80 ± 0.05% day−1 (mean ± SEM) respectively.
When the fatty acid composition was analysed, we found that palmitic acid (C16:0) occurred in highest percentages, followed by alpha-linolenic acid (C18:3n3), hexadecatetraenoic acid (C16:4n3), linoleic acid (C18:2), vaccenic acid (C18:1), and stearidonic acid (C18:4n3) (Fig. 2). The relative proportion of these six most common fatty acids varied between different experiments (Fig. 2). When data were statistically analysed, we found that temperature, irradiance, and pCO2 treatments significantly changed the proportion of some fatty acids in U. fenestrata, but that nutrient addition had no significant effect (Table 2, Fig. 2). The proportion of C18:3n3, C16:4n3, and C18:4n3 significantly (Tukey’s HSD, p < 0.05) increased with decreasing irradiance and temperature, while both C16:0 and C18:1 significantly (Tukey’s HSD, p < 0.05) increased with increasing irradiance, and C18:2 significantly (Tukey’s HSD, p < 0.05) increased with increasing temperature (Fig. 2). Furthermore, C18:2 decreased, and C18:4n3 increased, significantly (Tukey’s HSD, p < 0.05) in response to increased pCO2 (Fig. 2).
Fatty acid composition (% of total fatty acids) in Ulva fenestrata after exposure to different treatment combinations (low I = 50, medium I = 100, and high I = 160 μmol photons m−2 s−1; low T = 13 and high T = 18 °C; ambient N < 5, medium N = 150, and high N = 500 μM; ambient P < 1 and high P = 50 μM; low pCO2 = 200, ambient pCO2 = 400, and high pCO2 = 2500 ppm). Error bars show SEM
Protein content
The mean total protein content of U. fenestrata was 8.09 ± 0.30, 12.36 ± 1.09, and 11.35 ± 0.24% dw (mean ± SEM) in the temperature/irradiance (Fig. 1g, h), nitrate/phosphate (Fig. 1i), and pCO2 experiments, respectively. Similar to relative growth rate and total fatty acid content, there was a statistically significant effect of the factors irradiance and temperature, and a statistically significant interaction between the factors nitrate and phosphate on the total protein content in U. fenestrata (Table 1c). The total protein content decreased significantly (Tukey’s HSD, p < 0.05) with 36.66% between the low and the high irradiance treatments (Fig. 1g), and with 31.57% between the low and the high temperature levels (Fig. 1h). The total protein content increased significantly (Tukey’s HSD, p < 0.05) with 218.38% in the medium and high nitrate treatment compared with the control (Fig. 1i). The addition of phosphate resulted in a significant (Tukey’s HSD, p < 0.05) 14.7% reduction in protein content in the medium nitrate treatment, but no significant change was seen in the ambient and high nitrate treatment (Fig. 1i). There was no statistically significant difference in the total protein content in the different pCO2 treatments (Table 1c), but similar to the results for the fatty acid content, total protein content differed significantly between algae receiving water from different header tanks (Table 1c). The mean total protein content in U. fenestrata exposed to high, medium, and low pCO2 treatments were 10.74 ± 0.41, 11.92 ± 0.44, and 11.39 ± 0.32% day−1 (mean ± SEM), respectively.
Phenolic content
The mean total phenolic content of U. fenestrata was 0.122 ± 0.003, 0.202 ± 0.009, and 0.181 ± 0.004% dw (mean ± SEM) in the temperature/irradiance (Fig. 1j, k), nitrate/phosphate Fig. 1l), and pCO2 experiments, respectively. Temperature had no significant effect on the total phenolic content of U. fenestrata in the present study (Table 1d, Fig. 1k). There was a significant effect of different irradiance treatments (Table 1d), with high irradiance significantly (Tukey’s HSD, p < 0.05) decreasing the total phenolic content with 19.00% compared with the low irradiance treatment (Fig. 1j). Furthermore, both nitrate and phosphate addition significantly increased the total phenolic content of the seaweeds (Table 1d). Elevated nutrient levels significantly (Tukey’s HSD, p < 0.05) increased the total phenolic content with on average 49% (nitrate) and 10% (phosphate) compared with ambient conditions (Fig. 1l). Finally, there was a statistically significant (Table 1d) increase in total phenolic content in response to decreased pCO2 levels. The total phenolic content was 0.169 ± 0.004, 0.181 ± 0.004, and 0.192 ± 0.008% dw (mean ± SEM) in the high, ambient and low pCO2 treatment, respectively.
Biochar and ash content
We found no statistically significant difference in the biochar content of U. fenestrata exposed to any of the different treatments (Table 3a). The mean biochar content was 33.82 ± 0.68, 30.3 ± 0.44, and 33.72 ± 0.59% dw (mean ± SEM) in the temperature/irradiance, nitrate/phosphate, and pCO2 experiments, respectively.
The mean ash content for U. fenestrata was 19.48 ± 0.77, 19.68 ± 0.77, and 20.02 ± 0.41% dw (mean ± SEM) in the temperature/irradiance, nitrate/phosphate, and pCO2 experiments, respectively. The only significant effect of the treatments on the ash content was addition of nitrate (Table 3b), where seaweeds exposed to ambient nitrate conditions had a significantly (Tukey’s HSD, p < 0.05) higher ash content (22.68 ± 0.51% dw, mean ± SEM), compared with seaweeds exposed to medium (19.05 ± 0.88% dw, mean ± SEM), and high (17.33 ± 1.06% dw, mean ± SEM) nitrate concentrations.
Discussion
An initial challenge in the cultivation of Ulva spp. is the confused nomenclature within this genus. Traditional morphologically based taxonomic names do not always correspond to molecular phylogeny (e.g. Hughey et al. 2019) and species identification is difficult without DNA sequencing of appropriate genes. The model species used in the present study was identified as U. fenestrata (previously known as U. lactuca, Hughey et al. 2019) using molecular methods. Proper taxonomic identification of cultivated Ulva species through molecular methods, such as the one used in the present study, is very important to include in future aquaculture studies to disentangle the effect of genetic and/or environmental factors on differences in biomass yield and biochemical composition (c.f. Malta et al. 1999; Biancarosa et al. 2017; Gao et al. 2017). As pointed out by Bolton et al. (2016), the taxonomic identity of Ulva spp. in the aquaculture literature is almost always dubious, and a large number of papers using “Ulva lactuca” as model organisms from northern temperate to tropical areas are probably dealing with different species. Therefore, we mainly chose to focus on previous literature investigating cold-temperate Ulva spp. in the following discussion.
Previous studies have found that the relative growth rates of Ulva spp. in cultivation generally increases with increasing irradiance (Geertz-Hansen and Sand-Jensen 1992; Riccardi and Solidoro 1996; Taylor et al. 2001; Kalita and Tytlianov 2003; Kalita and Titlyanov 2013; Mhatre et al. 2019). Light saturation for the growth of U. lactuca is suggested to occur at 55 μmol photons m−2 s−1 (Sand-Jensen 1988), and the growth of the seaweeds are not inhibited at irradiances up to 225 μmol photons m−2 s−1 (Fortes and Lüning 1980). We found that the relative growth rate of U. fenestrata increased when irradiance levels were increased from 50 to 100 μmol photons m−2 s−1, but we saw no further increase at 160 μmol photons m−2 s−1, confirming previous results. The growth of Ulva spp. also generally increased with increasing temperature in previous studies (Riccardi and Solidoro 1996; Taylor et al. 2001; Gao et al. 2017, 2018b). However, Nejrup et al. (2013) found that the growth rate of Danish populations of U. lactuca was higher at 20 °C compared with 15 and 25 °C. The higher growth rate at low (13 °C) rather than high (18 °C) temperature found in the present study may be a consequence of Swedish U. fenestrata being adapted to cold water conditions. For most algae, tolerance to high temperatures may relate to temperatures experienced in their natural habitat (Gessner 1970).
Because seaweeds are generally nutrient-limited, it has repeatedly been observed that nitrate and phosphate addition result in higher growth in Ulva spp. (Riccardi and Solidoro 1996; Taylor et al. 2001; Kumari et al. 2014; Young and Gobler 2016; Gao et al. 2017, 2018b). Nutrient concentration can also interact with temperature to affect the growth of U. lactuca, which had a higher growth rate under high nitrate and phosphate concentrations at high temperature (10 °C), but no difference was seen at low temperature (5 °C) (Tremblay-Gratton et al. 2018). Surprisingly, nitrate addition decreased the growth rate of U. fenestrata in the present study. The reason for this is unknown, but the nitrate conditions outside TML from April to November (< 5 μM, sharkweb.smhi.se) are usually well below the levels used in our study (150 and 500 μM). An explanation for the decreased growth in response to nitrate addition observed in the present study may be that the unnaturally high nitrate levels used in our experiments adversely affected the seaweeds.
The relatively low growth rate in the pCO2 experiment was probably due to seasonal changes in total irradiance, since these seaweeds were grown in a greenhouse in October without additional light sources. Increased pCO2 levels commonly have positive effect on the growth rate of other Ulva species (e.g. Olischläger et al. 2013; Young and Gobler 2016; Gao et al. 2017, 2018b; Chen et al. 2019; Sebök et al. 2019), although there are examples were changes in pCO2 did not have a significant effect (e.g. Kerrison et al. 2012; Liu and Zou 2015). Differences between seaweed species in response to increased pCO2 may be due to the presence of carbon-concentrating mechanisms (van der Loos et al. 2019). Some Ulva species can use HCO3− as a carbon source and do not have to rely solely on CO2 for photosynthesis and growth (van der Loos et al. 2019). When cultivated in sealed aquaria under saturating light conditions, we found that the U. fenestrata strain used in this study can increase pH of seawater to above 10 (results not shown), indicating that this species is highly effective in using HCO3−. This is probably the reason why we did not find a significant effect of increased pCO2 on growth.
The total fatty acid levels found in the present study (1.31–1.83% dw) are within the upper range of what is reported for Ulva spp. in previous studies (0.3–1.6% dw) (e.g. Holdt and Kraan 2011), while the fatty acid composition is in accordance with what has been found in other Ulva species (e.g. McCauley et al. 2018). In general, previous literature shows that increased irradiance, temperature, nutrient, and pCO2 levels increase the total fatty acid content of Ulva spp. (Figueroa et al. 2014; Kumari et al. 2014; Liu and Zou 2015; Gao et al. 2017, 2018a, 2018b; Chen et al. 2019; Mhatre et al. 2019), with some exceptions (Khotimchenko and Yakovleva 2004; Ak et al. 2015; Liu and Zou 2015; McCauley et al. 2018). We found that the total fatty acid content in U. fenestrata was higher at 50 μmol photons m−2 s−1 than at 100–160 μmol m−2 s−1, mostly due to a decrease in the polyunsaturated fatty acids (PUFA) C18:3n3, C16:4n3, and C18:4n3 at high irradiance levels. These results are in accordance with a previous study where changes in irradiance also affected the composition of fatty acids in U. fenestrata from the Sea of Japan (Khotimchenko and Yakovleva 2004). The highest levels of C16:4n-3 was found in low irradiance, while C16:0 was highest at high irradiance (Khotimchenko and Yakovleva 2004). In the present study, a lower total fatty acid content was found at 13 °C compared with 18 °C, mostly due to a decrease in the C18:2 content. However, the content of PUFA (C18:3n3, C16:4n3, and C18:4n3) significantly increased at 13 °C. This is also in accordance with what is generally known for algae, which accumulate PUFA acids in response to decreased temperature (Khotimchenko 1991).
Lipid metabolism can be regulated by nutrient availability, and nitrogen depletion is hypothesised to increase lipid content, either through a decline in cell division rate (Brennan and Owende 2010) or by diverting fixed carbon from protein to lipid synthesis (Rodolfi et al. 2009). Nitrogen limitation can both enhance (Gordillo et al. 2001) and decrease (Gao et al. 2017, 2018a, 2018b) the lipid content in Ulva rigida. We found a significant increase in the total fatty acid content in response to addition of 150 and 500 μM nitrate, and 50 μM phosphate in the present study, in agreement with the results found by Gao and co-workers (Gao et al. 2017, 2018a, 2018b) but in disagreement with the abovementioned hypothesis. A possible explanation is that the seaweeds become reproductive at the time of harvest and that this may increase the lipid content, as reproductive cells and swarmers contain more lipids (Gao et al. 2017, 2018a). We did not observe any reproductive tissue but our experiment was short (5 days) compared with those in Gao et al. (2017, 2018a) (12 days). It is possible that cells were starting to convert from a vegetative to reproductive state and that we would have observed reproductive tissue if the experiment had continued for another week. No significant changes in the fatty acid composition of U. fenestrata in response to changes in nutrient availability that was found in the present study, indicating that all fatty acids increased in similar amounts. McCauley et al. (2018), on the other hand, found that cultivating Ulva sp. in nutrient replete conditions decreased the percentage of C16:0, while increasing C18:3n3 and C16:4n3 (McCauley et al. 2018). Fatty acid biosynthesis is also dependent of CO2 assimilation (Gordillo et al. 2003). Species that are able to use HCO3− (and are saturated for dissolved organic carbon) may use the elevated CO2 to invest in lipid storage, since their growth rates are not limited by carbon (Van der Loos et al. 2019). Our results do not support this hypothesis, as we did not find any significant changes in total fatty acid content in response to elevated pCO2.
The mean protein content found in the present study (8.09–12.36% dw) was in the lower range compared with previous studies (4–44% dw) (Holdt and Kraan 2011). In general, previous literature show that increased temperature and nutrient levels increased the total protein content of Ulva spp. (e.g. Kumari et al. 2014; Liu and Zou 2015; Gao et al. 2017, 2018a, 2018b). Increased irradiance and pCO2, on the other hand, decreased the protein content of Ulva spp. (Gordillo et al. 2001; Gao et al. 2017, 2018a, 2018b; Chen et al. 2019; Mhatre et al. 2019). We found that the total protein content in U. fenestrata decreased both when irradiance was increased from 50 to 160 μmol photons m−2 s−1 and when temperature was increased from 13 to 18 °C. This is in contrast to metabolic theory that predicts that since the metabolic rates of organisms increase with temperature within a certain range, nitrate assimilation should increase with increasing temperature (Iken 2012). Furthermore, since nitrogen is an essential element in protein metabolism and the amino acid carbon skeleton is derived from CO2 assimilation, high levels of nitrate and CO2 should also increase protein synthesis (Gao et al. 2018a). We found that raising nitrate levels from < 5 to 150 μM had a strong positive effect on the total protein content in U. fenestrata. However, an increase in pCO2 from 200 to 2500 ppm had no significant effect on the total protein content of U. fenestrata, in contrast to theory. The explanation for this result is probably the same as for the lack of effects of increased pCO2 on relative growth rate and fatty acid content, i.e. that the strain used in the present study have an effective CCM mechanism and is not limited by dissolved inorganic carbon.
The total phenolic content in Ulva spp. is of interest from a cultivation perspective since it shows a positive correlation with antibiotic and antioxidant activity (Sirbu et al. 2019), but it is also very interesting for the production of antioxidative seaweed extracts with uses, e.g. in foods and cosmetics (Jacobsen et al. 2019). The total phenolic content was in the range of what has previously been reported for U. lactuca (0.05–0.91% dw) (e.g. Abd El-Baky et al. 2009; Hashem et al. 2019; Sirbu et al. 2019). Nitrate and phosphate addition significantly increased, and pCO2 addition decreased the total phenolic content of the U. fenestrata, which is in accordance with what has been shown in previous studies (Figueroa et al. 2014; McCauley et al. 2018).
Biochar is a material that is produced through pyrolysis, i.e. the combustion of biomass in a low oxygen atmosphere. The biochar content of seaweed biomass has recently received attention because of its potential applications in carbon sequestration and soil amelioration (Lehmann and Joseph 2009). To our knowledge, no previous studies have investigated how different cultivation conditions affect the biochar content in seaweeds. The mean biochar content was 33.82 ± 0.68, 30.3 ± 0.44, and 33.72 ± 0.59% dw (mean ± SEM) in the temperature/irradiance, nitrate/phosphate, and pCO2 experiments, respectively, which is comparable with other species under similar pyrolysis conditions (e.g. Ulva ohnoi 34–35% dw, Roberts et al. 2015). Finally, the ash content was significantly decreased when seaweeds were exposed to nitrate levels above 150 μM. Previous studies have found that increased irradiance, temperature, and nitrate levels can result either in higher (Gao et al. 2017, 2018b; Mhatre et al. 2019) or lower (Ak et al. 2015; Gao et al. 2017) ash content of Ulva spp., whereas increased pCO2 levels did not affect ash content (Gao et al. 2017).
We conclude that the optimal cultivation conditions for Swedish U. fenestrata will depend on the desired biomass trait (yield or biochemical composition). A high protein level is desirable in the light of the growing interest for seaweed for food (e.g. Fleurence 1999; Harrysson et al. 2018) and feed (Angell et al. 2016b; Øverland et al. 2019). Lately, it has been particularly highlighted as a possible contributor to the ongoing protein shift, i.e. the shift from red meat to more sustainable protein sources. Along with high protein content, a high lipid content, especially with lipids from the n-3 family, is beneficial. Hence, the irradiance should be kept low to both increase the total fatty acid content and the relative abundance of C18:3, a precursor to the more long-chained n-3 PUFA EPA and DHA, which are associated with a variety of health benefits (Calder 2010; Maehre et al. 2015). However, given the relatively low lipid content compared with the protein content in U. fenestrata, the lipids rather provide an added extra value to the relatively protein-rich biomass. Thus, from a food perspective, the light intensity should be kept low to enhance both the lipid and protein content, and the temperature should be kept low to favour the protein content. From a growth perspective, on the other hand, light should be kept high to increase yield since maintaining a high total compound yield could be more beneficial economically than increasing the compound concentration.
References
Abd El-Baky HH, El-Baz FK, El-Baroty G (2009) Natural preservative ingredient from marine alga Ulva lactuca L. Int J Food Sci Tech 44:1688–1695
Ak I, Öztaskent C, Özüdogru Y, Göksan T (2015) Effect of sodium acetate and sodium nitrate on biochemical composition of green algae Ulva rigida. Aquacult Int 23:1–11
Angell AR, Mata L, de Nys R, Paul NA (2016a) The protein content of seaweeds: a universal nitrogen-to-protein conversion factor of five. J Appl Phycol 28:511–524
Angell AR, Angell SF, de Nys R, Paul NA (2016b) Seaweed as a protein source for mono-gastric livestock. Trend Food Sci Technol 54:74–84
Biancarosa I, Espe M, Bruckner CG, Heesch S, Liland N, Waagbø R, Torstensen B, Lock EJ (2017) Amino acid composition, protein content, and nitrogen-to-protein converstion factors of 21 seaweed species from Norwegian waters. J Appl Phycol 29:1001–1009
Bolton JJ, Robertson-Andersson DV, Shuuluka D, Kandjengo L (2009) Growing Ulva (Chlorophyta) in integrated systems as a commercial crop for abalone feed in South Africa: a SWOT analysis. J Appl Phycol 21:575–583
Bolton JJ, Cyrus MD, Brand MJ, Joubert M, Macey BM (2016) Why grow Ulva? Its potential role in the future of aquaculture. Perspect Phycol 3:113–120
Brennan L, Owende P (2010) Biofuel from microalgae – a review of technologies for production, processing, and extraction of biofuels and co-products. Renew Sust Energ Rev 14:557–577
Buchholz CM, Krause G, Buck BH (2012) Seaweed and man. In: Wiencke C, Bischof K (eds) Seaweed biology. Springer, Berlin, pp 471–493
Buschmann AH, Camus C, Infante J, Neori A, Israel A, Hernández-González MC, Pereda SV, Gomez-Pinchetti JL, Golberg A, Tadmor-Shalev N, Critchley AT (2017) Seaweed exploitation: overview of the global state of exploitation, farming and emerging research activity. Eur J Phycol 52:391–406
Cabello-Pasini A, Macías-Carranza V, Abdala R, Korbee N, Figueroa FL (2011) Effect of nitrate concentration and UVR on photosynthesis, respiration, nitrate reductase activity, and phenolic compounds in Ulva rigida (Chlorophyta). J Appl Phycol 23:363–369
Calder PC (2010) Omega-3 fatty acids and inflammatory processes. Nutrients 2:355–374
Chen BB, Lin LD, Ma ZL, Zhang TT, Chen WZ, Zou DH (2019) Carbon and nitrogen accumulation and interspecific competition in two algae species, Pyropia haitanensis and Ulva lactuca, under ocean acidification conditions. Aquac Int 27:721–733
Coelho MS, Barbosa FG, de Souza MDAZ (2014) The scientometric research on macroalgal biomass as a source of biofuel feedstock. Algal Res 6:132–138
Duke CS, Lapointe BE, Ramus J (1986) Effects of light on growth, RuBPCase activity and chemical composition of Ulva species (Chlorophyta). J Phycol 22:362–370
Falkenberg LJ, Styan CA, Havenhand JN (2019) Sperm motility of oysters from distinct populations differs in response to ocean acidification and freshening. Sci Rep 9:7970
FAO (2018) The state of world fisheries and aquaculture 2018 – meeting the sustainable development goals. FAO, Rome, 120 pp
Figueroa FL, Barufi JB, Malta EJ, Conde-Álvarez R, Nitschke U, Arenas F, Mata M, Connan S, Abreu MH, Marquardt R, Vaz-Pinto F, Konotchick T, Celis-Plá PSM, Hermoso M, Ordonez G, Ruiz E, Flores P, de los Ríos J, Kirke D, Chow F, CAG N, Robledo D, Pérez-Ruzafa Á, Banares-Espana E, Altamirano M, Jiménez C, Korbee N, Bischof K, Stengel DB (2014) Short-term effects of increasing CO2, nitrate and temperature on three Mediterranean macroalgae: biochemical composition. Aquat Biol 22:177–193
Fleurence J (1999) Seaweed proteins: biochemical, nutritional aspects and potential uses. Trends Food Sci Technol 10:25–28
Fortes MD, Lüning K (1980) Growth rates of North Sea macroalgae in relation to temperature, irradiance and photoperiod. Helgoländer Meeresun 34:15–29
Gao G, Clare AS, Rose C, Caldwell GS (2017) Eutrophication and warming-driven green tides (Ulva rigida) are predicted to increase under future climate change scenarios. Mar Pollut Bull 114:439–447
Gao G, Clare AS, Chatzidimitriou E, Rose C, Caldwell G (2018a) Effects of ocean warming and acidification, combined with nutrient enrichment, on chemical composition and functional properties of Ulva rigida. Food Chem 258:71–78
Gao G, Clare AS, Rose C, Caldwell GS (2018b) Ulva rigida in the future ocean: potential for carbon capture, bioremediation and biomethane production. GCB Bioenergy 10:39–51
Geertz-Hansen O, Sand-Jensen K (1992) Growth rates and photon yield of growth in natural populations of a marine macroalgae Ulva lactuca. Mar Ecol Prod Ser 81:179–183
Gessner F (1970) Temperature: plants. In: Kinne O (ed) Marine ecology, vol 1. Wiley-Interscience, New York, pp 363–406
Gordillo FJL, Jiménez C, Goutx M, Niell X (2001) Effects of CO2 and nitrogen supply on the biochemical composition of Ulva rigida with especial emphasis on lipid class analysis. J Plant Physiol 158:367–373
Gordillo FJL, Figueroa FL, Niell FX (2003) Photon- and carbon-use efficiency in Ulva rigida at different CO2 and N levels. Planta 218:315–322
Hashem HA, Mansour HA, El-Khawas SA, Hassanein RA (2019) The potentiality of marine macro-algae as bio-fertilizer to improve the productivity and salt stress tolerance of canola (Brassica napus L.) plants. Agronomy 9:146
Hafting JT, Craigie JS, Stengel DB, Loureiro RR, Buschmann AH, Yarish C, Edwards MD, Critchley AT (2015) Prospects and challenges for industrial production of seaweed bioactivities. J Phycol 51:821–837
Harrysson H, Hayes M, Eimer F, Carlsson N-G, Toth GB, Undeland I (2018) Production of protein extracts from Swedish red, green, and brown seaweeds, Porphyra umbilicalis Kützing, Ulva lactuca Linnaeus, and Saccharina latissima (Linnaeus) JV Lamouroux using three different methods. J Appl Phycol 30:3565–3580
Holdt SL, Kraan S (2011) Bioactive compounds in seaweed: functional food applications and legislation. J Appl Phycol 23:543–597
Hughey JR, Maggs CA, Mineur F, Jarvis C, Miller KA, Shabaka SH, Gabrielson PW (2019) Genetic analysis of the Linnaean Ulva lactuca (Ulvales, Chlorophyta) holotype and related type specimens reveals name misapplications, unexpected origins, and new synonymies. J Phycol 55:503–508
Iken K (2012) Grazers on benthic seaweeds. In: Wiencke C, Bischof K (eds) Seaweed biology. Springer, Berlin, pp 157–175
Jacobsen C, Sørensen ADM, Holdt SL, Akoh CC, Hermund DB (2019) Source, extraction, characterization, and applications of novel antioxidants from seaweed. Annu Rev Food Sci Technol 10:541–568
Kalita TJ, Tytlianov EA (2003) Effect of temperature and illumination on growth and reproduction of the green alga Ulva fenestrata. Rus J Mar Biol 29:316–322
Kalita TL, Titlyanov EA (2013) Influence of temperature on the infradian growth rhythm in Ulva lactuca (Chlorophyta). Eur J Phycol 48:210–220
Kerrison P, Suggett DJ, Hapburn LJ, Steinke M (2012) Effect of elevated pCO2 on the production of dimethylsulphoniopropionate (DMSP) and dimethylsulphide (DMS) in two species of Ulva (Chlorophyceae). Biogeochem 110:5–16
Khotimchenko SV (1991) Fatty-acid composition of 7 Sargassum species. Phytochemistry 30:2639–2641
Khotimchenko SV, Yakovleva IM (2004) Effect of solar irradiance on lipids of the green alga Ulva fenestrata Postels et Ruprecht. Bot Mar 47:395–401
Kumari P, Kumar M, Reddy CRK, Jha B (2014) Nitrate and phosphate regimes induced lipidomic and biochemical changes in the intertidal macroalga Ulva lactuca (Ulvophyceae, Chlorophyta). Plant Cell Physiol 55:52–63
Lehmann J, Joseph S (2009) Biochar for environmental management: an introduction. Earthscan, Sterling
Liu CX, Zou DH (2015) Responses of elevated CO2 on photosynthesis and nitrogen metabolism in Ulva lactuca (Chlorophyta) at different temperature levels. Mar Biol Res 11:1043–1052
Maehre HK, Jensen I-J, Elvevoll EO, Eilertsen K-E (2015) ω-3 fatty acids and cardiovascular diseases: effects, mechanisms and dietary relevance. Int J Mol Sci 16:22636–22661
Malta E-J, Draisma S, Kamermans P (1999) Free-floating Ulva in the Southwest Netherlands: species or morphotypes? A morphological, molecular and ecological comparison. Eur J Phycol 34:443–454
McCauley JI, Winberg PC, Meyer BJ, Skropeta D (2018) Effects of nutrients and processing on the nutritionally important metabolites of Ulva sp. (Chlorophyta). Algal Res 35:586–594
Mhatre A, Patil S, Agrawal A, Pandit R, Lali AM (2019) Influence of nitrogen source on photochemistry and antenna size of the photosystems in marine green macroalgae, Ulva lactuca. Photosynth Res 139:539–551
Nejrup LB, Staehr PA, Solgaard Thomsen M (2013) Temperature- and light-dependent growth and metabolism of the invasive red algae Gracilaria vermiculophylla – a comparison with two native macroalgae. Eur J Phycol 48:295–308
Olischläger M, Bartsch I, Gutow L, Wiencke C (2013) Effects of ocean acidification on growth and physiology of Ulva lactuca (Chlorophyta) in a rockpool-scenario. Phycol Res 61:180–190
Olsson J, Toth GB, Oerbekke A, Cvijetinovic S, Wahlström N, Harrysson H, Steinhagen S, Kinnby A, White J, Edlund U, Undeland I, Pavia H, Albers E (2020) Cultivation conditions affect the monosaccharide composition in Ulva fenestrata. J Appl Phycol. https://doi.org/10.1007/s10811-020-02138-9
Øverland M, Mydland LT, Skrede A (2019) Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J Sci Food Agric 99:13–24
Riccardi N, Solidoro C (1996) The influence of environmental variables on Ulva rigida C Ag growth and production. Bot Mar 39:27–32
Roberts DA, Paul NA, Dworjanyn SA, Bird MI, de Nys R (2015) Bioochar from commercially cultivated seaweed for soil amelioration. Sci Rep 5:9665
Robertson-Anderson DV, Potgieter M, Bolton JJ, Troell M, Anderson R, Halling C, Probyn T (2008) Integrated seaweed cultivation on an abalone farm in South Africa. J Appl Phycol 20:579–595
Rodolfi L, Chini Zittelli G, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalga for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–112
Sand-Jensen K (1988) Photosynthetic responses of Ulva lactuca at very low light. Mar Ecol Prog Ser 50:195–201
Sebök S, Herppich WB, Hanelt D (2019) Outdoor cultivation of Ulva lactuca in a recently developed ring-shaped photobioreactor: effects of elevated CO2 concentration on growth and photosynthetic performance. Bot Mar 62:179–190
Sirbu R, Stanciu G, Tomescu A, Ionescu AM, Cadar E (2019) Evaluation of antioxidant and antimicrobial activity in relation to total phenolic content of green algae from Black Sea. Rev Chim 70:1197–1203
Sphigel M, Guttman L, Shauli L, Odintsov V, Ben-Ezra D, Harpaz S (2017) Ulva lactuca from an integrated multi-trophic aquaculture (IMTA) biofilter system as a protein supplement in gilthead seabream (Sparus aurata) diet. Aquaculture 481:112–118
Smetacek V, Zingone A (2013) Green and golden seaweed tides on the rise. Nature 504:84–88
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313
Steinhagen S, Karez R, Weinberger F (2019) Cryptic, alien and lost species: molecular diversity of Ulva sensu lato along the German coasts of the north and Baltic seas. Eur J Phycol 54:466–483
Taylor R, Fletcher RL, Raven JA (2001) Preliminary studies on the growth of selected 'Green tide' algae in laboratory culture: effects of irradiance, temperature, salinity and nutrients on growth rate. Bot Mar 44:327–336
Tremblay-Gratton A, Boussin J-C, Tamigneaux É, Vandenberg GW, Le François NR (2018) Bioremediation efficiency of Palmaria palmata and Ulva lactuca for use in a fully recirculated cold-seawater naturalistic exhibit: effect of high NO3 and PO4 concentrations and temperature on growth and nutrient uptake. J Appl Phycol 30:1295–1304
Underwood AJ (2001) Experiments in ecology. Cambridge University Press, Cambridge
van der Loos LM, Schmid M, Leal PP, McGraw CM, Britton D, Revill AT, Virtue P, Nichols PD, Hurd CL (2019) Responses of macroalgae to CO2 enrichment cannot be inferred solely from their inorganic carbon uptake strategy. Ecol Evol 9:125–140
Ye N-H, Zhang X-W, Mao Y-Z, Liang C-W, Xu D, Zou J, Zhuang Z-M, Wang Q-Y (2011) “Green tides” are overwhelming the coastline of our blue planet: taking the world’s largest example. Ecol Res 26:477–485
Young CS, Gobler CJ (2016) Ocean acidification accelerates the growth of two bloom-forming macroalgae. PLoS One 11:e0155152
Acknowledgements
Open access funding provided by University of Gothenburg.
Funding
The authors thank the Swedish Foundation for Strategic Research (SSF), project number RBP14-0045, for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Toth, G.B., Harrysson, H., Wahlström, N. et al. Effects of irradiance, temperature, nutrients, and pCO2 on the growth and biochemical composition of cultivated Ulva fenestrata. J Appl Phycol 32, 3243–3254 (2020). https://doi.org/10.1007/s10811-020-02155-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02155-8