Abstract
The biologically active compounds (fatty acids, pigments, phenolics, and flavonoid content) were studied in supercritical fluid extracts from the biomass of marine (Ulva clathrata, Cladophora glomerata, Polysiphonia fucoides, and their multi-species mixture) and freshwater (C. glomerata) macroalgae. Different extraction techniques were used in order to compare differences in the biologically active compound composition of the macroalgal extracts. The results indicated that the saturated and unsaturated fatty acids ranged from C9:0 to C22:0. The analysis of differences in the composition of unsaturated to saturated fatty acids in extracts showed that palmitic acid (C16:0) and oleic acid (C18:1, n-9) reached the highest value not only in marine monospecies and multi-species biomass but also in the freshwater macroalga C. glomerata. When comparing the similarity between the concentration of fatty acids and the ratio of the concentration of unsaturated fatty acids to saturated in macroalgal extracts, we found small but not statistically significant variations in values between years (up to 10%). This is acceptable for applications as a stable raw material for industrial purposes. Significantly higher values of fatty acids, carotenoids, and chlorophylls were obtained in the case of SC-CO2 extraction. The active ingredients of polyphenols, possessing antioxidant activity ranged from approximately 2–4%. Moreover, flavonoids represented less than 10% of the total content of polyphenolic compounds. The extraction efficiency of polyphenols was higher from a mixture of marine algae for the ultrasound-assisted extraction compared to freshwater. All these findings show that marine and freshwater macroalgae, as a raw material, have the optimal biologically active compounds composition for cosmetics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to literature, fatty acids and pigments are extracted mainly from the biomass of microalgae. Little attention in this respect has been paid to macroalgae. A comparison of the interest in micro- and macroalgae is presented in Table 1. In some cases, instead of the word “macroalgae” the word “seaweed” is used. In the last 15 years, the words “extraction of fatty acids/from macroalgae” appeared in the topic scientific papers 26 times, “extraction of fatty acids/from seaweed” 47 times, whereas “extraction of fatty acids/from microalgae” 435 times (Source: Web of Knowledge, 11 April 2017).
However, the biomass of macroalgae is also a rich source of polyunsaturated fatty acids—PUFAs (both: omega-3 fatty acids: eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) and omega-6 fatty acid: γ-linolenic acid (GLA) and arachidonic acid (AA) (Pereira et al. 2012). For example, the fatty acid profile of green seaweed Cladophora rupestris (L.) Kützing lipidic extract including palmitic, myristic, oleic, α-linolenic, palmitoleic, and linoleic acids (Stabili et al. 2014). Horincar et al. (2014) have reported that the Ulva intestinalis L. extract had a greater content of mono- and polyunsaturated fatty acids of around 46.0%, as compared with 42.0% for Cladophora vagabunda (L.) Hoek and 31.9% for Ceramium virgatum Hooker & Harvey. The most abundant fatty acids were palmitic acid (C16:0), arachidonic acid (C20:4n-6), and oleic acid (C18:1ω-9cis). Chemical characterization of other lipidic extract of Gracilariopsis longissima (Gmelin) Steentoft, Irvine, & Farnham has revealed that palmitic acid methyl ester (16:0) was the predominant saturated fatty acid (42%), while, from among monounsaturated fatty acids, oleic acid methyl ester (18:1) prevailed (8.5%) (Stabili et al. 2012). In this paper, special attention was paid to the biomass of macroalgae collected from the Baltic Sea (Poland, southern Baltic), belonging to the taxa Polysiphonia, Ulva, and Cladophora and the freshwater macroalga, Cladohora, from Lake Oporzynskie (West Poland). This biomass could constitute a valuable source of biologically active compounds (especially fatty acids and pigments: carotenoids and chlorophyll) which could be potentially used by the food, pharmaceutical, and cosmetic industries.
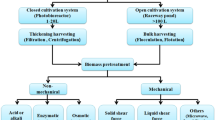
It should be noted that different extraction techniques can be used in order to obtain biologically active compounds from the biomass of algae. The choice of the appropriate method should depend on the nature of the extracted compound (Kadam et al. 2013; Ibañez et al. 2012). Until now, biologically active compounds have been extracted from the biomass of algae mainly by conventional solvent extraction (with the use of organic solvents: i.e., petroleum ether, hexane, cyclohexane, isooctane, toluene, benzene, diethyl ether, dichloromethane, isopropanol, chloroform, acetone, methanol, ethanol) (Stabili et al. 2012, 2014; Horincar et al. 2014). The second method is hydrolysis carried out under alkaline, neutral, or acidic conditions (Booth 1969). However, according to current trends, the use of organic solvent should be minimized. The solution could be the application of supercritical fluid extraction (SFE) with CO2 as a green solvent. It has been shown that SFE is a suitable technology for extraction of nutraceuticals. Bioactive compounds can also be extracted without any loss of volatility and their degradation. SFE offers a high extraction rate and high yield and is an eco-friendly technology with minimal or no use of organic solvents (Kadam et al. 2013). Some examples of the use of SFE for the extraction of lipids from the biomass of macroalgae are summarized in Table 2.
In relation to biological activity, mainly the antioxidant properties, an important group are phenolic compounds and flavonoids. Polymers of phenolic compounds, polyphenols, are a large and diverse group of secondary metabolites produced by plants and fungi, containing in their structure at least one hydroxyl group bonded directly to the aromatic ring. Flavonoids are related to the class of polyphenolic compounds, but their chemical structure consists mainly of two phenyl rings and a heterocyclic ring, which may be substituted at different positions mainly with hydroxyl and methyl groups (Kumar and Pandey 2013).
Among the variety of biological properties of polyphenols, they have a strong radical scavenging ability; therefore, they exhibit noteworthy antioxidant activity. It may occur due to reducing ability, binding of free radicals, chelation of metal ions, stabilization of the free radicals, and inhibition of oxidases. For the above reason, this group of compounds takes part in prevention of many diseases, mostly related to oxidative stress like those caused by harmful solar radiation or attack by pathogens (Lobo et al. 2010).
Colorimetric methods are very useful for identification of classes of compounds, e.g., polyphenols, flavonoids, chlorophylls, or for the evaluation of antioxidant properties of particular extracts or solutions. There are many methods, which allow measurements of the antioxidant activity of single substances and mixtures of compounds. The total quantity of phenolic compounds was determined by using the Folin-Ciocalteu test, using the ferric reducing ability of plasma (FRAP) protocol, 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS) protocol, or 2,2-diphenyl-1-picrylohydrazyl (DPPH) test (Chakraborty et al. 2015). Some of these analyses were conducted in our research, in order to check the radical scavenging ability of the extracts studied and establish the content of biologically active components in macroalgae extracts.
Nonetheless, the results of measurements performed with the use of the above mentioned methods might be compared, when referred to the same, well-defined reference material. The most commonly used reference substance is Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), a synthetic derivative of tocopherol. The antioxidant content is expressed as the amount of Trolox equivalents—TEAC per weight or volume of the sample. Sometimes ascorbic acid, which is considered as powerful antioxidant, is also used for comparison. It is difficult to indicate the best method, which is charged with the least possible error, since any factor may affect the results. The light and oxygen access, pH, and type of solvent are the most common considered ones (Cybul and Nowak 2008).
The aim of this study was to extract by supercritical fluid extraction with CO2 biomass of marine (from the Baltic Sea) and freshwater macroalgae in order to isolate biologically active compounds that have potential applications as natural components of cosmetics and pharmaceuticals. In our previous work, the same extract was examined as a natural plant biostimulant. The utilitarian properties were checked in germination tests with garden cress (Lepidium sativum L.) and wheat (Triticum aestivum L.). The contents of inorganic (macro- and microelements) and organic (plant hormones: auxins and cytokinins; polyphenols) compounds were determined (Michalak et al. 2016).
Materials and methods
Collection of algae and extraction
Collection of individual species (manually) and the mixture of algae (industrial, mechanical collection of biomass) from the Baltic Sea (Ulva clathrata (Roth) Ag., Cladophora glomerata (L.) Kütz., Polysiphonia fucoides (Hudson) Greville) and the production of extract by classical and supercritical fluid extraction, which involved the pretreatment of biomass was described previously (Michalak et al. 2016). Freshwater C. glomerata thalli were collected from the shallow Lake Oporzynskie (N 52° 55′ 70″, E 17° 09′ 60″) situated in the northern part of the Wielkopolska region (western Poland) in the July–August period of 2013 and 2014 when algal biomass was at its annual maximum. Characterization of physical and chemical parameters during the intensive development of C. glomerata in the lake was described earlier (Messyasz et al. 2015a; Pikosz and Messyasz 2016). The algae samples collected from the water were put into plastic container with water coming from the same habitat in the ratio of 3:1 and transported to the laboratory. Next, the thalli were repeatedly rinsed with distilled water to separate any abiotic particles attached to them. The washed fresh algal biomass was weighed immediately and a small portion (5 g) was used for microscopic analysis, using a light microscope (Zeiss Axioskop 2 MOT), at ×200 and ×400 magnification and checking their surface for the presence of microscopic algae. To identify the alga species, the length and width of the cells were measured and algal samples were stained with Lugol’s solution to determine the number of pyrenoids, or with acetocarmine to determine the number of nuclei. Next, the extant material was dried in an oven until water content of biomass was lower than 15% (w/w).
In conventional extraction, macroalgae were extracted with 200 mL of ethanol as a solvent for 3 h.
Supercritical fluid extraction of Baltic algae
In the Fertilizer Research Institute in Puławy, Baltic macroalgal biomass was processed as already described by Michalak et al. (2016). After optimization of the method, the best process parameters were chosen. In this study, seaweed extract obtained in supercritical fluid extraction (in which fine-grained grist was used) was examined. The summary of the SFE of Baltic seaweed extraction is as follows: pressure 500 bar, temperature 40 °C, mass of post extraction remains 9.695 kg, extract mass 179 g, extraction capacity 1.76%, total capacity with total mass loss 4.78%; solvent: CO2 and ethanol.
Ultrasound-assisted extraction (UAE)
Ultrasound-assisted extracts were made out of raw, powdered material of Baltic seaweed and freshwater C. glomerata. For the extract preparation for colorimetric analysis, 10 g of dry weight of material was extracted in the ultrasonic bath (Cole Parmer, 8891, USA) with two portions of methanol as a solvent (2 × 100 mL), over a total time of 1 h. After 30 min, the first part of solvent was removed and new portion was added to continue the extraction for another 30 min. The temperature of the ultrasonic bath did not exceed 35 °C. The extracts were filtrated and the filtrates were combined. The solvent was removed on the rotary evaporator. In order to prepare samples for colorimetric analysis, the methanolic solutions of extracts were made up to a concentration of 10.00 ± 0.06 mg mL−1.
Analytical methods
Determination of fatty acids in extracts from Baltic seaweed
Sigma-Aldrich reagents and standards were used in the fatty acid analysis. Extract − 5 mL was evaporated under reduced pressure (< 1 mmHg) at a temperature of < 40 °C until constant weight. To the dry residue, 0.5 mL of tert-butyl methyl ether (MTBE) was added. Next, 0.25 mL of 0.2 M solution of trimethylsulfonium hydroxide in methanol as a derivatizing agent was added. After 5 min of stirring at room temperature, a solution of 10 μL of methyl undecanoate in MTBE with concentration of methyl undecanoate 42.6 mg mL−1 MTBE was added. The methyl undecanoate as the internal standard was used. Fatty acids as methyl esters were determined using a Varian gas chromatograph GC 450. Operating parameters of the chromatograph are as follows: injector temperature, 250 °C; split, 1:50; carrier gas, He, flow rate 1 mL min−1; column, Varian VF-WAXms, 30 m × 0.53 mm, film thickness 1 μm; temperature program, 50 °C isothermal for 2 min; linear gradient of 10 °C min−1 to 250 °C (20 min), isothermal 250 °C for 23 min; detector, FID detector temperature 250 °C; injection volume of 2 μL of sample. The identification of methyl esters of fatty acids was performed according to retention times of standards. The following acid standards (as methyl esters) were used: butyric acid (C4:0), valeric acid (C5:0), caproic (C6:0), caprylic acid (C8:0), nonanoic (C9:0), capric (C10:0), undecanoic (C11:0), lauric (C12:0), myristic (C14:0), palmitic (C16:0), margarine (C17:0), stearic (C18:0), arachidic (C20:0), behenic (C22:0), myristoleic (C14:1, n-5), palmitoleic (C16:1, n-7), oleic (C18:1, n-9), vaccenic (C18:1, n-7) petroselinic (C18:1, n-12) cis-11-eicosenoic (C20:1, n-9), berucic acid (C22:1, n-9), nervonic (C24:1, n-9), linoleic (C18:2, n-6), α-linolenic (C18:3 n-3), γ-linolenic acid (C18:3, n-6), stearidonic (C18:4, n-3), cis cis-11,14-eicosadienoic (C20:2, n-6), arachidonic (C20:4, n-6); all-cis-5,8,11,14,17-eicosapentaenoic (C20:5, n-3), all-cis-7,10,13,16,19-docosapentaenoic (C22:5, n-3), all-cis-4,7,10,13,16,19-docosahexaenoic acid (C22:6, n-3).
Determination of carotenoids and chlorophyll a and b in extracts
The determination of the total concentration of carotenoids and chlorophyll a was conducted according to the Wellburn method (Wellburn 1994) as described by Macias-Sanchez et al. (2007, 2008) by measuring the absorbance of the different samples using a Cary Spectrophotometer (wavelength from 330 to 800 nm).
Determination of total phenolic compounds (TPC) in extracts
Determination of total polyphenolic compounds in the extracts from Baltic seaweeds and freshwater C. glomerata were obtained using SFE-CO2 extraction. UAE extraction was performed using the method described in (Sim et al. 2010) with slight modifications. For these measurements, Baltic seaweed SFE-CO2 extract obtained in 2014 was used, for which phenolic compounds content was determined (Michalak et al. 2016).
The calibration curve was prepared by dissolving gallic acid in 70% methanol to obtain the stock solution with the concentration of 1 mg mL−1 After that, the subsequent dilutions were made in the range of concentrations from 0.1 to 1 mg mL−1. To the reaction vessel was added 20 μL of gallic acid solution with a particular concentration, 1.58 mL of distilled water, 0.1 mL of Folin-Ciocalteu reagent (Folin-Ciocalteu Phenol Reagent, POCH, Poland) and 0.3 mL of saturated solution of sodium bicarbonate (Na2CO3, POCH, Poland). The final reaction volume was 2 mL and the final concentration of gallic acid ranged between 0.001 and 0.01 mg mL−1. The reaction mixture with real samples were prepared as the samples for the calibration curve (y = 100.28x + 0.0412; R 2 = 0.9975), the 20 μL of 10.00 mg mL−1 extract solution was added instead of the gallic acid solution. The result was expressed as gallic acid equivalent (GAE) using the following equation: C [mg GAE \( {g}_{{\mathrm{extract}}^{-1}} \)] = C i [mg mL−1] * (V 1 [mL]/V 2 [mL]) * (V 3 [mL]/m [g]), where C i [mg mL−1] is the concentration from calibration curve, V 1 is the total volume of reaction vessel, V 2 is the volume of extract/standard added to the reaction, V 3 is the volume in which the extract was dissolved, and m is the mass of the extract dissolved in V 3 in order to prepare real sample of extract. After keeping the reaction vessels in the darkness for 2 h in the room temperature, the samples were measured with UV/VIS spectrometer at 760 nm. Each sample was prepared and measured in triplicate. Data are mean ± SD values.
Determination of total flavonoids (FC) in extracts
The aluminum chloride method was used for the determination of the total flavonoid content of the sample extracts. This method is based on the formation of a complex between the aluminum ion, Al3+, and the carbonyl and hydroxyl groups of flavones and flavonols, which in a results in an yellow color of the solution. Total content of these compounds was determined by the protocol of Baba and Shahid (2015) with some modifications. The volume of the reaction mixture was 2 mL and consisted of 0.8 mL of methanol, 0.2 mL of 10.00 mg mL−1 extract or standard solution, and 0.06 mL of 5% NaNO2. The reaction vessel was placed in the dark for 5 min. After that time, 0.06 mL of 10% AlCl3 was added and again left in the dark for 5 min. In the next step, 0.4 mL of 1 M NaOH and 0.28 mL of methanol were added and the mixture was placed in a dark place for another 15 min. All of the samples were filtered and measured against a blank sample (methanol instead of extract or standard). The calibration curve was prepared using quercetin as a standard in the range of concentrations 0.05–0.25 mg mL−1 (y = 3.204x + 0.0024; R 2 = 0.9975) and the result was expressed as quercetin equivalent (QE) using the following equation: C [mg QE \( {g}_{{\mathrm{extract}}^{-1}} \)] = C i [mg mL−1] * (V 1 [mL]/V 2 [mL]) * (V 3 [mL]/m [g]). Each sample was prepared and measured in triplicate. Data are mean ± SD values.
Determination of antioxidant activity of extracts
One of the most common methods used to evaluate the antioxidant activity is a test with 2,2-diphenyl-1-picrylhydrazyl (DPPH), which was used for preliminary evaluation of the potential of the antioxidant extracts of algae. The solution of free radicals and DPPH has a purple color. During the reduction reaction with the substance with antioxidant properties, component of tested extract, it changes the color to yellow and is spectrophotometrically measured. The reaction mixture was prepared by adding 200 μL of sample (10.00 mg mL−1 extract), 2 mL of methanolic solution of DPPH (0.04 mg mL−1; DPPH, Sigma, Poland) and left for 30 min in the dark. The measurements were conducted at the wavelength of 517 nm.
In addition, the antioxidant content in the analyzed samples is expressed as Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) equivalents (TEAC) per unit volume [mg TEAC (100 mL)−1 of extract], determining the concentration from the standard curve for Trolox. The calibration curve equation was y = −0.0774x + 0.8682 with the regression coefficient 0.9966. The antioxidant properties of ascorbic acid, which is a potent antioxidant were used for the comparison. All of the samples were measured in triplicate. Data are mean ± SD values.
Results
Fatty acid composition in marine and freshwater algae extracts
The differences in fatty acid composition were observed depending on the extraction method and extracted algal biomass (monospecies, multi-species). In our studies, the saturated fatty acids ranged from C9:0 to C22:0. The highest concentration of saturated fatty acids (mainly C16:0) were observed for individual marine species U. clathrata, C. glomerata, and also P. fucoides (Table 3). Analogous results were also obtained for Baltic multi-species biomass extraction, using supercritical CO2. All extracts contained high concentrations of unsaturated fatty acids, especially C16:1 (n-7) and C18:1 (n-3). C16:0 and C18:1 (n-9) fatty acids were detected in the largest percentage in the case of supercritical fluid extraction, and the C18:0, C18:4 (n-3), and C22:6 (n-3) fatty acids were found in the lowest amount in solvent extraction. Traditional extraction of marine multi-species biomass by ethanol as compared to that with using SC-CO2 was characterized by a substantially lower yield of fatty acids.
In order to compare the fatty acid compositions in marine biomass of multi-species macroalgae and single algal species U. clathrata, the samples of the two types of biomass were subjected to the Soxhlet extraction method with the use of two different solvents (acetone, ethanol). Higher values of unsaturated/saturated fatty acid ratio were obtained for the dry matter of the extract from biomass of marine algae (1.75 ± 0.36) and biomass of U. clathrata (1.75 ± 0.35), using ethanol than when using acetone as a solvent (1.38 ± 0.23 and 1.13 ± 0.17, respectively). The fatty acids C16:0, C18:0, C16:1 (n-7), C18:1 (n-9), and C22:1 (n-9) occurred in the highest amounts among other unsaturated fatty acids in the extracts obtained from multi-species marine biomass (Fig. 1). On the other hand, fatty acids, which were obtained in low amounts, belonged to C20:0 and C22:0. Additionally, in the U. clathrata biomass, the percentage weight of fatty acids in dry matter of the extract was often smaller than that in the mixture of marine macroalgae. The extraction efficiency of C18:3 and C18:4 fatty acids was a little better with acetone as a solvent when applying the Soxhlet method (Fig. 2).
The concentration of fatty acid and the ratio of the concentration of unsaturated fatty acids to saturated ones in the extracts obtained by various methods, for the biomass samples of one species of freshwater alga C. glomerata (Table 4, Fig. 3) collected in the same way (the time from harvest to dry biomass approx. 4 h) in 2013 and 2014 differed approximately by 10%. In the freshwater C. glomerata, the fatty acid composition was dominated by the highest amounts of C16:0 (high values also in marine mono- and multi-species biomass), and subsequently C18:1 (n-9) (high values also in marine mono- and multi-species biomass), C18:1 (n-7), C18:2 (n-6), C18:3 (n-3), and C18:3 (n-6) (Fig. 3).
Pigments in extracts from Baltic seaweed
The extraction of carotenoids and chlorophylls using carbon dioxide is an alternative to traditional Soxhlet extraction. The yields of the carotenoid and chlorophyll extractions obtained from the three macroalgae and biomass of multi-species from Baltic seaweeds are shown in Table 5. For Soxhlet extraction by ethanol, the highest concentration of chlorophyll was observed for C. glomerata, while for carotenoids for P. fucoides. The biomass of multi-species seaweeds from Baltic Sea obtained by SC-CO2 extraction contained the highest amounts of carotenoids and chlorophylls extraction relative to the extracts obtained by the Soxhlet method with ethanol.
Total phenolics and flavonoid content; antioxidant properties of marine and freshwater algae extracts
The applied colorimetric method allowed the determination of total polyphenolic compounds and flavonoids in the tested samples (Table 6). The concentrations of these substances ranged from 20.32–41.73 mg GAE g−1 for polyphenols and 1.08–1.72 mg QE g−1 for flavonoids in the samples of extracts. The SFE-CO2 extract, which was analyzed after 1 year showed the same amount of polyphenolic compounds (Michalak et al. 2016). The highest content of polyphenols was extracted from the sample of Baltic algae using UAE and the smallest from the sample of the same biomass, but prepared with SFE-CO2.
All samples showed the antioxidant activity in the range of 59–73%. The highest activity was measured for the sample made out of Baltic algae mixture obtained by the UAE, approx. 73%, and the antioxidant concentration approx. 8 mg TEAC (100 mL)−1.
Discussion
Macroalgae characteristics
In August 2013, in the collected biomass from coastal sites in Sopot, the dominant algae species were filamentous algae from the genera Cladophora sp. and Ulva sp. with a large share of the red alga Polysiphonia. However, the filamentous algae disappeared in September when the number of P. fucoides thalli significantly increased. Irrespective of the collection period, the algae were rough to the touch, due to the presence of numerous epiphytes (diatom, green algae, and cyanobacteria) and macroalgal thalli incrusted on the surface of the cell walls (Michalak et al. 2016).
Filamentous forms of C. glomerata may be either attached to various substrates using rhizoids or loosely floating to form a mat. The color was usually dark green. It is a cosmopolitan species, common in marine and littoral ecosystems (estuaries), saline, and freshwater. It is common in the Polish Baltic Sea: the Gdańsk Gulf (Pliński and Jóźwiak 2004), east coast of the Pomorska Gulf (Rosińska et al. 2013), and Sopot (this study). It occurs particularly in highly morphologically transformed and eutrophic locations.
Ulva intestinalis is the most common Ulva species in Poland (Messyasz and Rybak 2009; Messyasz et al. 2015b) and often occurs with filamentous green algae, mainly C. glomerata. Its thalli are tubular and wrinkled, with numerous proliferations. Young thalli are attached to the substrate, whereas mature thalli float on the water surface. Its thallus is tapered at the scutellum, which attaches to the substrate and further expands and remains cylindrical up to the apical part. It is frequently found in the coastal zone of seas, oceans, and estuaries (Lee 1999; Kirchhoff and Pflugmacher 2002). Ulva intestinalis is a typical euryhaline species (Young et al. 1987). Therefore, its tolerance to salinity is high (Pliński and Hindák 2012). Ulva intestinalis grows best with in a salinity around 24‰, but it is also found in areas with lower values of salinity (Kim and Lee 1996). The alga is widespread, and in some places is growing massively on empty shells, larger stones, and port breakwaters. In the Polish part of the Baltic Sea, U. intestinalis was found in the littoral zone of water bodies in Władysławowo, Świnoujście, Kołobrzeg, Łeba, Mielno, Gdańsk Bay, and Puck Bay (Haroon et al. 1999; Pliński and Jóźwiak 2004; Pliński and Hindák 2012). In our samples, thalli of U. intestinalis were accompanied by U. clathrata, which has an entero-folding type of construction, and can be found in the submerged form and free-floating mats. It is a cosmopolitan euhalinity (salinity values above 28‰) species with a wide distribution in marine and brackish environments throughout the world (Haroon et al. 1999; Bäck et al. 2000; Pliński and Hindák 2012).
Red algae from the genus Polysiphonia occur in the entire Baltic Sea, and also on the Polish coast, (Pliński and Hindák 2012). They form dense mats on a solid surface (e.g., stones), but also loosely floating mats, detached from the substrate. In favorable growth conditions, P. fucoides thalli are red and contain mostly the pigments (phycoerythrin and chlorophyll) that are necessary for photosynthesis. In contrast, in harsh environmental conditions, it produces and accumulates high amounts of xanthophyll and carotenoids, with a concomitant change in coloration of the cells and thalli from red to brown (Van den Hoek et al. 1995; South and Whittick 1996). In this research, the thalli of P. fucoides reached the length of 25 cm and were dark brown in color.
In freshwater Lake Oporzynskie, the presence of filamentous algae was visible in the form of compact and dense mats covering considerable surface area and occurring near the shore. The biomass of C. glomerata was common in conglomeration, which formed large surface mats that tightly covered the surface of the water of this lake.
All of these natural sources for obtaining the biomass of algae have their advantages and limitations. In the climatic conditions of Central Europe, easy acquisition of homogeneous species of algae allows obtaining the best quality material for industrial, agricultural, and cosmetology uses.
Biologically active compounds in marine and freshwater algae extracts
In the supercritical extract of different species of seaweed, the natural bioactive compounds that could be potentially used for the food, pharmaceutical, and cosmetic industries were detected and characterized. In the present study, the extraction of fatty acids from algal biomass was performed by two methods: Soxhlet extraction with ethanol as a solvent and SFE with carbon dioxide as solvent. The algal biomass used for the extraction determines the composition of the extract and the ratio of concentration of unsaturated to saturated fatty acids, irrespective of the technique of extraction. The composition of the extract and concentration of saturated and unsaturated fatty acids depends on the type of algae biomass used for extraction, technique extraction, and solvent used for the extraction. The composition optimal for cosmetic industry had the extract obtained by supercritical CO2 extraction. We also found that the ratio of unsaturated to saturated fatty acids in this extract was about one, which means that the extracts obtained from marine monospecies or multi-species could be used in cosmetic formulations.
Investigation of biomass of freshwater macroalga C. glomerata as a source of fatty acids, amino acids, and other bioactives (Khalid et al. 2012; Messyasz et al. 2015a) has not been as common as that of marine algae (Elenkov et al. 1996; Pereira et al. 2012; Horincar et al. 2014). Harvesting of the freshwater algal biomass studied was made in 2013 and 2014. The above-described results indicate that, regardless of the period of growing, the quantitative composition of fatty acids in the extract from these algae obtained by both the classical Soxhlet apparatus and with the application of supercritical extraction was similar. Variable weather conditions and differences in water properties as a habitat during this period are crucial for the growth of algae in the wild and responsible in about 10% for the composition of the obtained extracts. The SC-CO2 extraction gives better results as compared to methods of extraction using Soxhlet apparatus, both in terms of the number of the extracted fatty acids and in terms of the concentration ratio of saturated to unsaturated fatty acids.
The extract obtained from algae biomass containing unsaturated fatty acids, especially omega-3, omega-6, and omega-9 are valuable material for the production of cosmetic products, as they are natural and environmentally friendly. Unsaturated fatty acids are highly beneficial for the skin, because they are part of its structure. The unsaturated fatty acids keep the skin firm and elastic and at the proper level of moisture and lubrication. The fatty acids slow down the aging process and help the skin cope with the adverse effects of the environment. A very important role in the skin and hair care has a mixture of exogenous unsaturated fatty acids. These acids are used topically and can be incorporated into cell membrane layers of corneocytes (Katsuta et al. 2005), thus preventing water loss and inhibiting the aging of the skin. For this reason, they are often referred to as a factor regulating the skin water and lipids. Creams, conditioners or masks containing a composition of active ingredients derived from algae biomass represents a new line of ecological cosmetics, which not only contribute to the improvement of our skin but also are sourced from renewable, natural biological materials.
Antioxidant activity of marine and freshwater algae extracts
Several studies have been conducted in order to characterize and evaluate Ulva species. For instance two species of U. clathrata and U. prolifera were used from four different locations in Iran were subjected to the FC, TPC, and DPPH tests. Methanolic extracts were prepared and better results were observed for U. clathrata, 4.468 ± 0.379 mg GAE g−1, 45.577 ± 0.949 mg RE g−1 (rutin equivalent) and the radical scavenging activity with a low IC50 (the half-maximal inhibitory concentration) 0.715 ± 0.078 mg mL−1, respectively (Farasat et al. 2013). In another investigation, ethanolic extracts and fractions from solvents with various polarities from green seaweeds such as E. compressa (L.) Ness (syn. Ulva compressa), Cladophera fulvescens, C. moniligera, and U. pertusa were assessed for the same parameters. Samples from U. pertusa contained 9 mg GAE g−1 of phenolic compounds in ethanol, but this amount was approximately three times greater in the fraction from ethyl acetate and 27.4 mg QE g−1 of flavonoids in ethanol and 147 mg QE g−1 in ethyl acetate fraction. From among four tested species, all extracts and fractions from U. pertusa had the lowest content of polyphenols. The ethyl acetate fraction contained the highest amount of flavonoids in comparison to fractions in the same solvent from other species (Cho et al. 2010). Results of our study confirmed how strongly the extracted amounts of compounds depend on the species and type of solvent used for extraction. Interestingly, in the sample of Baltic algae investigated here, a higher content of polyphenols was obtained after UAE when compared to SFE-CO2. Differences between the results could be related to the instability of this group of compounds, age of the sample, the use of various extraction techniques, and the application of different materials. These groups of substances also show antioxidant activity; thus, the samples with high flavonoids and polyphenols content may exhibit strong radical scavenging activity in DPPH test or any other antioxidant protocol.
The levels of secondary metabolites from six species of edible seaweeds from different classes of algae were estimated. Total phenolic content in methanolic extracts of brown algae such as Laminaria digitata, Laminaria saccharina, Himanthalia elongata, red algae Palmaria palmata, Chondrus crispus, and the green macroalga Ulva spirulina ranged from 37.66 to 151.33 mg GAE g−1 of dry extract. The brown seaweed, H. elongata, exhibited the highest content of this group of substances and the lowest was found in the extract of L. digitata. Such a low level of polyphenols in L. digitata might be considered strange, because usually brown seaweeds contain more of these compounds in comparison to red and green seaweeds. However, two other brown seaweeds from this research had significantly higher content of polyphenols. Total flavonoids in the seaweeds ranged from 7.66 to 42.5 mg QE g−1 of extract. Again, the highest value was observed for H. elongata. For the only green seaweed in this study, this value was 19.05 ± 0.73 mg QE g−1 of extract (Cox et al. 2010). This value is more than tenfold greater than the content of flavonoids in the methanolic extract of Cladophora spp. (1.77 ± 0.10 mg QE g−1) from this research.
Ethanolic extracts from C. glomerata collected in Iran contained significantly higher amounts of phenolic compounds and flavonoids, 3077 ± 105 mg GAE g−1 and 595 ± 23 mg QE g−1, respectively, than both extracts we studied (Soltani et al. 2011).
The content of biologically active substances from natural origins depends on the species, site of the material collection, and type and conditions of extraction (temperature, solvent, time). Many authors who worked on the assessment of seaweeds as the potential sources of valuable compounds have used the colorimetric methods, mainly describing the concentration of phenolic compounds and flavonoids in combination with radical scavenging activity tests (Soltani et al. 2011; Tabarsa et al. 2012; Vijayabaskar and Shiyamala 2012; Chakraborty et al. 2013; Mezghani et al. 2013; Gouda et al. 2014; Heffernan et al. 2014; Chakraborty et al. 2015; Farah Diyana et al. 2015; Gall et al. 2015; Machu et al. 2015; Mannino et al. 2015). In some cases, screening tests have been performed for extracts in different solvents, which gives preliminary information about the content of various classes of compounds in seaweed (Jeeva et al. 2012; Domettila et al. 2013; Whankatte and Ambhore 2016). Our investigation has demonstrated the antioxidant activity on a level from 59 to 73% with the highest value (approx. 8 mg TEAC (100 mL)−1) for the sample of Baltic algae mixture. This may be due to the presence of polyphenols in the extract, which increase the antioxidant capacity, but can be also related to the presence of metal ions (Michalak and Chojnacka 2016). Higher activity towards DPPH may be caused by the diversity of species and the preferable polarity of the extrahent-methanol, which increases the content of extracted polyphenols.
On the basis of our results, a strong correlation between the antioxidant activity of the sample and the content of phenolic compounds can be confirmed. However, it should be taken into consideration that the observed free radical scavenging activity may have resulted also from the presence of other compounds in the extract having antioxidant nature. Therefore, the calculated total content of antioxidants, expressed as Trolox equivalents, gives information about all antioxidants in the sample. Tests with DPPH, as other methods used to assess the antioxidant properties, are sensitive to chemical disturbances; thus, the results of measurement of complex matrices in our extracts may be flawed. In addition, a limitation of this method is the fact that DPPH dissolves only in organic solvents, which does not allow determination of hydrophilic antioxidants (Cybul and Nowak 2008). This is another reason why the results obtained by the Folin-Ciocalteu method and DPPH test are independent of each other and why the determined antioxidant capacity can be attributed not only to the phenolic compounds.
In conclusion, a great number of studies concerning the evaluation of seaweed for the content of biologically active compounds have proved that these organisms have a very high potential for their applications in many branches of industry, medicine, and human well-being. We have shown here that the composition of the extract and concentration of saturated and unsaturated fatty acids depends on the type of algae biomass used for extraction, extraction technique, and solvent used for the extraction. Moreover, the optimal composition for cosmetic uses was found in the extract obtained from supercritical CO2 extraction. The content of polyphenols in the extracts, which are the active ingredients showing antioxidant activity, ranged from approximately 2 to 4%. Flavonoids represent less than 10% of the total content of polyphenolic compounds, which is consistent with the literature data concerning the proportions of these compounds in seaweed. Regardless of the method used, the extraction efficiency of polyphenols from freshwater C. glomerata was on a similar level, whereas for the mixture of Baltic algae, the efficiency was higher for the extracts obtained by UAE. This observation may be explained by the fact, that the mixture contains different species of Baltic algae, so the composition of extracted biomass is more diverse. Another explanation is that the use of the polar solvent in UAE is a better extraction medium for polyphenols when compared to CO2.
References
Aresta M, Dibenedetto A, Carone M, Colonna T, Fragale C (2005) Production of biodiesel from macroalgae by supercritical CO2 extraction and thermochemical liquefaction. Environ Chem Lett 3:136–139
Baba SA, Shahid AM (2015) Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J Taibah Univ Med Sci 9(4):449–454
Bäck S, Lehvo A, Blomster J (2000) Mass occurrence of unattached Enteromorpha intestinalis on the Finnish Baltic Sea coast. Ann Bot Fenn 37:155–161
Booth E (1969) The manufacture and properties of liquid seaweed extracts. Proc Int Seaweed Symp 6:655–662
Chakraborty K, Praveen NK, Vijayan KK, Rao GS (2013) Evaluation of phenolic contents and antioxidant activities of brown seaweeds belonging to Turbinaria spp. (Phaeophyta, Sargassaceae) collected from Gulf of Mannar. Asian Pac J Trop Biomed 3:8–16
Chakraborty K, Joseph D, Praveen NK (2015) Antioxidant activities and phenolic contents of three red seaweeds (Division: Rhodophyta) harvested from the Gulf of Mannar of Peninsular India. J Food Sci Technol 52:1924–1935
Chen CY, Chou HN (2002) Screening of red algae filaments as a potential alternative source of eicosapentaenoic acid. Mar Biotechnol 4:189–192
Cheung PCK (1999) Temperature and pressure effects on supercritical carbon dioxide extraction of n-3 fatty acids from red seaweed. Food Chem 65:399–403
Cheung PCK, Leung AYH, Ang JPO (1998) Comparison of supercritical carbon dioxide and soxhlet extraction of lipids from a brown seaweed, Sargassum hemiphyllum (Turn.) C.Ag. J Agric Food Chem 46:4228–4232
Cho M, Kang IJ, Won MH, Lee HS, You S (2010) The antioxidant properties of ethanol extracts and their solvent-partitioned fractions from various green seaweeds. J Med Food 13:1232–1239
Cox S, Abu-Ghannam N, Gupta S (2010) An assessment of the antioxidant and antimicrobial activity of six species of edible Irish seaweeds. Int Food Res J 17:205–220
Cybul M, Nowak R (2008) Review of the methods applied to measuring of antioxidant activity of plant extracts. Herba Pol 54(1):68–78
Domettila C, Joselin J, Jeeva S (2013) Phytochemical analysis on some south Indian seaweeds. J Chem Pharm Res 5:275–278
El Hattab M, Culioli G, Piovetti L, Chitour SE, Valls R (2007) Comparison of various extraction methods for identification and determination of volatile metabolites from the brown alga Dictyopteris membranacea. J Chromatogr A 1143:1–7
Elenkov I, Stefanov K, Dimitrova-Konaklievat S, Popov S (1996) Effect of salinity on lipid composition of Cladophora vagabunda. Phytochemistry:39–44
Esquivel-Hernández DA, Ibarra-Garza IP, Rodríguez-Rodríguez J, Cuéllar-Bermúdez SP, de J Rostro-Alanis M, Alemán-Nava GS, García-Pérez JS, Parra-Saldívar R (2017) Green extraction technologies for high-value metabolites from algae: a review. Biofuels Bioprod Biorefin 11:215–231
Fabrowska J, Ibañez E, Leska B, Herrero M (2016) Supercritical fluid extraction as a tool to valorize underexploited freshwater green algae. Algal Res 19:237–245
Farah Diyana A, Abdullah A, Shahrul Hisham ZA, Chan KM (2015) Antioxidant activity of red algae Kappaphycus alvarezii and Kappaphycus striatum. Int Food Res J 22:1977–1984
Farasat M, Khavari-Nejad RA, Nabavi SMB, Namjooyan F (2013) Antioxidant properties of two edible green seaweeds from northern coasts of the Persian Gulf. Jundishapur J Nat Pharm Prod 8:1
Gall EA, Lelchat F, Hupel M, Jégou C, Stiger-Pouvreau V (2015) Extraction and purification of phlorotannins from brown algae. Methods Mol Biol:131–143
Gao D, Okuda R (2001) Supercritical fluid extraction of halogenated monoterpenes from the red alga Plocamium cartilagineum. J AOAC Int 84:1313–1331
Gouda S, Moharana RR, Das G, Patra JK (2014) Free radical scavenging potential of extracts of Gracilaria verrucosa (L) (Harvey): an economically important seaweed from Chilika lake. India Int J Pharm Pharm Sci 6:707–710
Haroon MA, Szaniawska A, Pliński M (1999) The distribution, species compositions and abundance of Enteromorpha spp. in the Gulf of Gdansk. Oceanol Stud 28:31–39
Heffernan N, Smyth TJ, Fitzgerald RJ, Soler-Vila A, Brunton N (2014) Antioxidant activity and phenolic content of pressurised liquid and solid-liquid extracts from four Irish origin macroalgae. Int J Food Sci Tech 49:1765–1772
Horincar VB, Parfene G, Tyagi AK, Gottardi D, Dinică R, Guerzoni ME, Bahrim G (2014) Extraction and characterization of volatile compounds and fatty acids from red and green macroalgae from the Romanian Black Sea in order to obtain valuable bioadditives and biopreservatives. J Appl Phycol 26:551–559
Ibañez E, Herrero M, Mendiola JA, Castro-Puyana M (2012) Extraction and characterization of bioactive compounds with health benefits from marine resources: macro and micro algae, cyanobacteria, and invertebrates. In: Hayes M (ed) Marine bioactive compounds: sources, characterization and applications. Springer, Berlin, pp 55–98
Jeeva S, Antonisamy JM, Domettila C, Anantham B, Mahesh M (2012) Preliminary phytochemical studies on some selected seaweeds from Gulf of Mannar, India. Asian Pac J Trop Biomed 2(1 Suppl):S30–S33
Kadam SU, Tiwari BK, O’Donnell CP (2013) Application of novel extraction technologies for bioactives from marine algae. J Agric Food Chem 61:4667–4675
Katsuta Y, Iida T, Denda M (2005) Unsaturated fatty acids induce calcium influx into keratinocytes and cause abnormal differentiation of epidermis. J Investig Dermatol 124:108–113
Khalid MN, Shameel M, Ahmad VU (2012) The bioactivity and phycochemistry of two species of Cladophora (Siphonocladophyceae) from Sindh. Proc Pak Acad 49:113–121
Kim KY, Lee IK (1996) The germling growth of Enteromorpha intestinalis (Chlorophyta) in laboratory culture under different combinations of irradiance and salinity and temperature and salinity. Phycologia 35:327–331
Kirchhoff A, Pflugmacher S (2002) Comparison of the detoxication capacity of limnic and marine form of the green algae Enteromorpha compressa. Mar Environ Res 1–5:2–73
Kumar S, Pandey AK (2013) Chemistry and biological activities of flavonoids: an overview. Sci World J:1–49
Lee RE (1999) Phycology. Cambrige University Press, Cambridge, p 614
Lobo V, Patil A, Phatak A, Chandra N (2010) Free radicals, antioxidants and functional foods: impact on human health. Pharmacog Rev 4:118–126
Machu L, Misurcova L, Ambrozova JV, Orsavova J, Mlcek J, Sochor J, Jurikova T (2015) Phenolic content and antioxidant capacity in algal food products. Molecules 20:1118–1133
Macias-Sanchez MD, Mantell C, Rodriguez M, Martinez de la Ossa E, Lubian LM, Montero O (2007) Supercritical fluid extraction of carotenoids and chlorophyll a from Synechococcus sp. J Supercrit Fluids 39:323–329
Macias-Sanchez MD, Mantell Serrano C, Rodriguez Rodriguez M, Martinez de la Ossa E, Lubian LM, Montero O (2008) Extraction of carotenoids and chlorophyll from microalgae with supercritical carbon dioxide and ethanol as cosolvent. J Sep Sci 31:1352–1362
Mannino AM, Vaglica V, Cammarata M, Oddo E (2015) Effects of temperature on total phenolic compounds in Cystoseira amentacea (C.Agardh) Bory (Fucales, Phaeophyceae) from southern Mediterranean Sea. Plant Biosyst:1–9
Messyasz B, Rybak A (2009) The distribution of green algae species from the Ulva genera (syn. Enteromorpha; Chlorophyta) in Polish inland waters. Oceanol Hydrobiol Stud 38:121–138
Messyasz B, Leska B, Fabrowska J, Pikosz M, Roj E, Cieslak A, Schroeder G (2015a) Biomass of freshwater Cladophora as a raw material for agriculture and the cosmetic industry. Open Chem 13:1108–1118
Messyasz B, Pikosz M, Schroeder G, Łęska B, Fabrowska J (2015b) Identification and ecology of macroalgae species existing in Poland. In: Kim SK, Chojnacka K (eds) Marine algae extracts: processes, products, and applications. Wiley – VCH, Weinheim, pp 17–39
Mezghani S, Bourguiba I, Hfaiedh I, Amri M (2013) Antioxidant potential of Ulva rigida extracts: protection of Hela cells against H2O2 cytotoxicity. Biol Bull 225:1–7
Michalak I, Chojnacka K (2016) Innovative technology of algal extracts obtained by supercritical fluid extraction useful in the products for plants, animals and human. In: Chojnacka K, Michalak I (eds) Innovative bio-products for agriculture. Algal extracts in products for humans, animals and plants. Nova Publishers, New York, pp 1–28
Michalak I, Górka B, Wieczorek PP, Rój E, Lipok J, Łęska B, Messyasz B, Wilk R, Schroeder G, Dobrzyńska-Inger A, Chojnacka K (2016) Supercritical fluid extraction of algae enhances levels of biologically active compounds promoting plant growth. Eur J Phycol 51:243–252
Pereira H, Barreira L, Figuieredo F, Custódio L, Vizetto-Duarte C, Polo C, Rešek E, Engelen A, Varela J (2012) Polyunsaturated fatty acids of marine macroalgae: potential for nutritional and pharmaceutical applications. Mar Drugs 10:1920–1935
Pérez-López P, Balboa EM, González-García S, Domínguez H, Feijoo G, Moreira MT (2014) Comparative environmental assessment of valorization strategies of the invasive macroalgae Sargassum muticum. Bioresour Technol 161:137–148
Pikosz M, Messyasz B (2016) Composition and seasonal changes in filamentous algae in floating mats. Oceanol Hydrobiol Stud 44:273–281
Pliński M, Hindák F (2012) Zielenice—Chlorophyta: filamentous green algae. Wydawnictwo Uniwersytetu Gdańskiego, Gdańsk 189 pp
Pliński M, Jóźwiak T (2004) The distribution of water vegetation on the Polish coast oh the Baltic Sea in 1996-2000. Oceanol Hydrobiol Stud 33:29–40
Rosińska B, Chojnacki JC, Klej K, Kowalewska M, Polońska J (2013) The ecological structure macrofauling community of the eastern shore of the Pomeranian Bay (southern Baltic Sea) in 2008 on the anthropogenic substrates. J Ecol Eng 35:60–68
Sim KS, Sri Nurestri AM, Norhanom AW (2010) Phenolic content and antioxidant activity of crude and fractionated extracts of Pereskia bleo (Kunth) DC. (Cactaceae). Afr. J Pharm Pharmacol 4:193–201
Soltani S, Saadatmand S, Khavarinejad R, Nejadsattari T (2011) Antioxidant and antibacterial activities of Cladophora glomerata (L.) Kütz. in Caspian Sea coast, Iran. Afr J Biotechnol 10:7684–7689
South RG, Whittick A (1996) Introduction to phycology. Blackwell, Oxford, p 341
Stabili L, Acquaviva MI, Biandolino F, Cavallo RA, De Pascali SA, Fanizzi FP, Narracci M, Petrocelli A, Cecere E (2012) The lipidic extract of the seaweed Gracilariopsis longissima (Rhodophyta, Gracilariales): a potential resource for biotechnological purposes? New Biotechnol 29:443–450
Stabili L, Acquaviva MI, Biandolino F, Cavallo RA, De Pascali SA, Fanizzi FP, Narracci M, Cecere E, Petrocelli A (2014) Biotechnological potential of the seaweed Cladophora rupestris (Chlorophyta, Cladophorales) lipidic extract. New Biotechnol 31:436–444
Tabarsa M, Rezaei M, Ramezanpour Z, Waaland JR (2012) Chemical compositions of the marine algae Gracilaria salicornia (Rhodophyta) and Ulva lactuca (Chlorophyta) as a potential food source. J Sci Food Agric 92:2500–2506
Van den Hoek C, Mann DG, Jahns HM (1995) Algae. An introduction to phycology. Cambridge University Press, Cambridge, p 623
Vijayabaskar P, Shiyamala V (2012) Antioxidant properties of seaweed polyphenol from Turbinaria ornata (Turner) J. Agardh, 1848. Asian Pac J Trop Biomed 2(1 Suppl):S90–S98
Wellburn AR (1994) The spectral determination of chlorophyll a and chlorophyll b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Whankatte VR, Ambhore JS (2016) Study of phytochemical screening & antioxidant activities of Cladophora glomerata Linn. collected from Raigad Coast of Konkan (M.S.) India. J Sci Nat 7:659–663
Young AJ, Collins JC, Russel G (1987) Solute regulation in the euryhaline marine alga Enteromorpha prolifera (O. F. Müll). J Ag J Exp Bot 38:1298–1308
Acknowledgements
This project is financed in the framework of grant entitled “Innovative technology of seaweed extracts—components of fertilizers, feed and cosmetics” (PBS/1/A1/2/2012) attributed by The National Centre for Research and Development in Poland. We thank Prof. Elliot Shubert for the constructive comments and improving the English text.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Messyasz, B., Michalak, I., Łęska, B. et al. Valuable natural products from marine and freshwater macroalgae obtained from supercritical fluid extracts. J Appl Phycol 30, 591–603 (2018). https://doi.org/10.1007/s10811-017-1257-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1257-5