Abstract
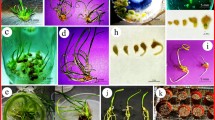
Tissue was cultured and protoplasts isolated from the carrageenophyte Chondracanthus acicularis with the aim of developing micropropagation as an alternative to harvesting raw material from natural beds. Both adventitious shoots and filamentous calluses were induced by tissue culture on medium solidified with 0.4–1 % (w/v) agar. Adventitious shoots were mainly produced from discoid bases while filamentous calluses were mainly induced from basal zones and sub-apical explants. A gradient of the regeneration ability was observed from the top to the bottom of the thallus. The discoid base was the most reactive explant and produced the highest number of adventitious shoots compared to basal zones and sub-apical explants, irrespective of the concentration of agar. Protoplasts were isolated enzymatically from the whole thallus using a combination of cellulase R-10 Onozuka, macerozyme R-10, and crude extract of the gland gut of algivorous molluscs. The highest mean yield of protoplasts (1.2 × 106 protoplasts g−1 fresh weight) was obtained after 16 h of digestion with an enzyme mixture containing 2 % (w/v) cellulase R-10, 1 % (w/v) macerozyme R-10 Onozuka, 4 % (v/v) crude extract of gut gland of Haliotis, 0.8 M mannitol, 50 mM sodium citrate, 0.3 % (w/v) bovine serum albumin. Depending on the conditions, mean protoplast yields ranged from 3.14 × 105 to 1.2 × 106 protoplasts g−1 fresh weight. Different factors (storage duration, mannitol, sodium citrate, crude extract of the gland gut of algivorous molluscs) were tested to improve the yield of protoplasts but none has a significantly effect.









Similar content being viewed by others
References
Asensi A, Ar Gall E, Marie D, Billot C, Dion P, Kloareg B (2001) Clonal propagation of Laminaria digitata (Phaeophyceae) sporophytes through a diploid cell-filament suspension. J Phycol 37:411–417
Avila M, Piel MI, Caceres JH, Alveal K (2011) Cultivation of the red alga Chondracanthus chamissoi: sexual reproduction and seedling production in culture under controlled conditions. J Appl Phycol 23:529–536
Baweja P, Sahoo D, Garcia-Jimenez P, Robaina RR (2009) Seaweed tissue culture as applied to biotechnology: problems, achievements and prospects. Phycol Res 57:45–58
Bixler HJ, Porse H (2011) A decade of change in the seaweed hydrocolloids industry. J Appl Phycol 23:321–335
Bixler HJ, Johndro K, Falshaw R (2001) Kappa-2 carrageenan: structure and performance of commercial extracts. II. Performance in two stimulated dairy applications. Food Hydrocolloids 15:619–630
Bulboa C, Macchiavello JE (2001) The effects of light and temperature on different phases of the life cycle in the carrageenan producing alga Chondracanthus chamissoi (Rhodophyta, Gigartinales). Bot Mar 44:371–374
Bulboa C, Macchiavello J (2006) Cultivation of cystocarpic, tetrasporic and vegetative fronds of Chondracanthus chamissoi (Rhodophyta, Gigartinales) on ropes at two localities in the northern Chile. Invest Mar 34:109–112
Bulboa C, Macchiavello J, Oliveira E, Fonck E (2005) First attempt to cultivate the carrageenan-producing seaweed Chondracanthus chamissoi (C. Agardh) Kützing (Rhodophyta, Gigartinales) in Northern Chile. Aquac Res 36:1069–1074
Bulboa C, Macchiavello J, Véliz K, Oliveira EC (2010) Germination rate and sporeling development of Chondracanthus chamissoi (Rhodophyta, Gigartinales) varies along a latitudinal gradient on the coast of Chile. Aquat Bot 92:137–141
Buschmann AH, Correa JA, Westermeier R, Paredes MA, Aedo D, Potin P, Aroca G, Beltran J, Hernandez-Gonzales MC (2001) Cultivation of Gigartina skottsbergii (Gigartinales, Rhodophyta): Recent advances and challenges for the future. J Appl Phycol 13:255–266
Campo VL, Kawano DF, Braz da Silva D, Carvalho I (2009) Carrageenans: biological properties, chemical modifications and structural analysis—a review. Carbohydr Polym 77:167–180
Chamberlain AHL, Gorham J, Kane DF, Lewey SA (1979) Laboratory growth studies on Sargassum muticum (Yendo) Fensholt. II. Apical dominance. Bot Mar 23:11–19
Correa JA, Beltran J, Buschmann AH, Westermeier R (1999) Healing and regeneration responses in Gigartina skottsbergii (Rhodophyta, Gigartinales): optimization of vegetative propagation for cultivation. J Appl Phycol 11:315–327
Dixon PS, Irvine LM (1977) Seaweeds of the British Isles, vol 1 Rhodophyta. Part 1 Introduction, Nemaliales, Gigartinales. Bristish Museum (Natural History), London
Fonck E, Martinez R, Vasquez J, Bulboa C (2008) Factors that affect the re-attachment of Chondracanthus chamissoi (Rhodophyta, Gigartinales) thalli. J Appl Phycol 20:311–314
Fries L (1984) Induction of plantlets in axenically cultivated rhizoids of Fucus spiralis. Can J Bot 62:1616–1620
Fritsch FE (1945) The structure and reproduction of the algae. I and II. Cambridge University Press, Cambridge
Givernaud T, Cosson J, Givernaud-Mouradi A (1990) Régénération de la Phéophycée Sargassum muticum (Phéophycée, Fucale). Cryptog Algol 11:293–304
Gomez Pinchetti JL, Björk M, Pedersen M, Garcia Reina G (1993) Factors affecting protoplast yield of the carrageenophyte Solieria filiformis (Gigartinales, Rhodophyta). Plant Cell Rep 12:541–545
Gomez IM, Westermeier RC (1991) Frond regrowth from basal disc in Iridaea laminarioides (Rhodophyta, Gigartinales) at Mehuin, southern Chile. Mar Ecol Prog Ser 73:83–91
Gonzalez J, Meneses I (1996) Differences in the early stages of development of gametophytes and tetrasporophytes of Chondracanthus chamissoi (C.Ag.) Kützing from Puerto Aldea, northern Chile. Aquaculture 143:91–107
Gonzalez J, Meneses I, Vasquez J (1997) Field studies in Chondracanthus chamissoi (C. Agardh) Kützing: seasonal and spatial variations in life-cycle phases. Biol Pesqui 26:3–12
Gross W (1990) Preparation of protoplasts from the carrageenophyte Gigartina corymbifera (Kütz.) J. Ag. (Rhodophyta). J Microbiol Methods 12:217–223
Guiry MD, Cunningham EM (1984) Photoperiodic and temperature responses in the reproduction of north-eastern Atlantic Gigartina acicularis (Rhodophyta: Gigartinales). Phycologia 23:357–367
Hayashi L, Yokoya NS, Kikuchi DM, Oliveira EC (2008) Callus induction and micropropagation improved by colchicine and phytoregulators in Kappaphycus alvazerii (Rhodophyta, Solieriaceae). J Appl Phycol 20:653–659
Hernandez-Gonzalez MC, Buschman AH, Cifuentes M, Correa JA, Westermeier R (2007) Vegetative propagation of the carrageenophytic red alga Gigartina skottsbergii Setchell et Gardner: Indoor and fields experiments. Aquaculture 262:120–128
Hernandez-Gonzalez MC, Aroca G, Furci G, Buschmann AH, Filun L, Espinoza R (2010) Population dynamics and culture studies of the edible red alga Callophyllis variegata (Kallymeniaceae). Phycol Res 58:108–115
Huang W, Fujita Y (1997) Callus induction and thallus regeneration in some species of red algae. Phycol Res 45:105–111
Hwang EK, Kim CH, Sohn CH (1994) Callus-like formation and differentiation in Hizikia fusiformis (Harvey) Okamura. Korean J Phycol 9:77–83
Jiao G, Yu G, Zhang J, Ewart HS (2011) Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar Drugs 9:196–223
Jin HJ, Seo GM, Cho YC, Hwang EK, Sohn CH, Hong YK (1997) Gelling agents for tissue culture of the seaweed Hizikia fusiformis. J Appl Phycol 9:489–493
Kevekordes K, Beardall J, Clayton MN (1993) A novel method for extracting protoplasts from large brown algae. J Exp Bot 44:1587–1593
Kloareg B, Quatrano RS (1988) Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. Oceanogr Mar Biol Annu Rev 26:259–315
Kupfermann I, Carew TJ (1974) Behavior patterns of Aplysia californica in its natural environment. Behav Biol 12:317–337
Lafontaine N, Mussio I, Rusig AM (2011) Production and regeneration of protoplasts from Grateloupia turuturu Yamada (Rhodophyta). J Appl Phycol 23:17–24
Macchiavello J, Bulboa C, Edding M (2003) Vegetative propagation and spore-based recruitment in the carrageenophyte Chondracanthus chamissoi (Rhodophyta, Gigartinales) in northern Chile. Phycol Res 51:45–50
Michel G, Nyval-Collen P, Barbeyron T, Czjzek M, Helbert W (2006) Bioconversion of red seaweed galactans: a focus on bacterial agarases and carrageenases. Appl Microbiol Biotechnol 71:23–33
Montanha JA, Bourgougnon N, Boustie J, Amoros M (2009) Antiviral activity of carrageenans from marine red algae. Lat Am J Pharm 28:443–448
Mooney PA, van Staden J (1985) In vitro plantlet formation and multiple shoot induction in Sargassum heterophyllum. S Afr J Bot 51:41–44
Moss B (1965) Apical dominance in Fucus vesiculosus. New Phytol 64:387–392
Mussio I, Rusig AM (2006) Isolation of protoplasts from Fucus serratus and F. vesiculosus (Fucales, Phaeophyceae): factors affecting protoplast yield. J Appl Phycol 18:733–744
Mussio I, Rusig AM (2009) Morphogenetic responses from protoplasts and tissue culture of Laminaria digitata (Linnaeus) J.V. Lamouroux (Laminariales, Phaeophyta): callus and thalloid-like structures regeneration. J Appl Phycol 21:255–264
Pacheco-Ruiz I, Zertruche-Gonzalez JA, Espinoza-Avalos J (2005) The role of the secondary attachment discs in the survival of Chondracanthus squarrulosus (Gigartinales, Rhodophyta). Phycologia 44:629–631
Pereira L, van de Velde F (2011) Portuguese carrageenophytes: carrageenan composition and geographic distribution of eight species (Gigartinales, Rhodophyta). Carbohydr Polym 84:614–623
Pereira L, Critchley AT, Amado AM, Ribeiro-Claro PJA (2009a) A comparative analysis of phycocolloids produced by underutilized versus industrially utilized carrageenophytes (Gigartinales, Rhodophyta). J Appl Phycol 21:599–605
Pereira L, Amado AM, Critchley A, van de Velde F, Ribeiro-Claro PJA (2009b) Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocolloids 23:1903–1909
Perrone C, Felicini GP (1976) Les bourgeons adventifs de Gigartina acicularis (Wulf.) Lamour. (Rhodophyta, Gigartinales) en culture. Phycologia 15:45–50
Polne-Fuller M, Gibor A (1987) Calluses and callus-like growth in seaweeds: induction and culture. Hydrobiologia 151/152:131–138
Polne-Fuller M, Rogerson A, Amano H, Gibor A (1990) Digestion of seaweeds by the marine amoeba Trichosphaerium. Hydrobiologia 204/205:409–413
Rajakrishna Kumar G, Reddy CRK, Jha B (2007) Callus induction and thallus regeneration from callus of phycocolloid yielding seaweeds from the Indian coast. J Appl Phycol 19:15–25
Ram M, Vijayaraghavan MR, Babbar SB (2000) Wound response and regeneration in Coelarthrum opuntia. Aquat Bot 68:345–351
Reddy CRK, Rajakrishna Kumar G, Siddhanta AK, Tewari A, Eswaran K (2003) In vitro somatic embryogenesis and regeneration of somatic embryos from pigmented callus of Kappaphycus alvarezii (Doty) Doty (Rhodophyta, Gigartinales). J Phycol 39:610–616
Reddy CRK, Gupta MK, Mantri VA, Jha B (2008a) Seaweed protoplast: status, biotechnological perspectives and needs. J Appl Phycol 20:619–632
Reddy CRK, Jha B, Fujita Y, Ohno M (2008b) Seaweed micropropagation techniques and their potentials: an overview. J Appl Phycol 20:619–632
Reddy CRK, Gupta V, Jha B (2010) Developments in biotechnology of red algae. In: Chapman DJ (ed) Seckbach J. Red algae in genomic age. Springer, NY, pp 307–341
Rusig AM, Cosson J (2001) Plant regeneration from protoplasts of Enteromorpha intestinalis (Chlorophyta, Ulvophyceae) as seedstock for macroalgal culture. J Appl Phycol 13:103–108
Saez F, Macchiavello J, Fonck J, Bulboa C (2008) The role of the secondary attachment disc in the vegetative propagation of Chondracanthus chamissoi (Gigartinales; Rhodophyta). Aquat Bot 89:63–65
Scherrer B (1984) Biostatistique. Gaëtan Morin, Chicoutimi
Scrosati R (2002) Morphological plasticity and apparent loss of apical dominance following the natural loss of the main apex in Pterocladiella capillaceae (Rhodophyta, Gelidiales) fronds. Phycologia 41:96–98
Sylvester AW, Waaland JR (1983) Cloning the red alga Gigartina exasperata for culture on artificial substrates. Aquaculture 31:305–318
Titlyanov EA, Titlyanova TV, Kadel P, Lüning K (2006a) New methods of obtaining plantlets and tetraspores from fragments and cell aggregates of meristematic and submeristematic tissue of the red algae Palmaria palmata. J Exp Mar Biol Ecol 339:55–64
Titlyanov EA, Titlyanova TV, Kadel P, Lüning K (2006b) Obtaining plantlets from apical meristem of the red alga Gelidium sp. J Appl Phycol 18:167–174
van de Velde F (2008) Structure and function of hybrid carrageenans. Food Hydrocolloids 22:727–734
Yeong HY, Khalid N, Phang SM (2008) Protoplast isolation and regeneration from Gracilaria changii (Gracilariales, Rhodophyta). J Appl Phycol 20:641–651
Yin MY, Hu XY, Tseng CK (2007) Filament formation and differentiation in seven species of red algae. Bot Mar 50:113–118
Yokoya NS, Yoneshigue-Valentin Y (2011) Micropropagation as a tool for sustainable utilization and conservation of populations of Rhodophyta. Rev Bras Farmacogn 21:334–339
Zhang Q (1991) Studies on the isolation, culture and regeneration of protoplasts from Chondrus ocellatus Holm. Ocean Univ Qingdao J 21:52–62
Acknowledgments
Moussa Yagame Bodian was financially supported by the “Ministère de l’Enseignement Supérieur des Universités des Centres Universitaires Régionaux et de la Recherche Scientifique” of Senegal via the foundation FIRST (Fonds d’Impulsion de la Recherche Scientifique et Technique).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bodian, M.Y., Lafontaine, N., Matard, M. et al. Evaluation of the in vitro methods for micropropagation of Chondracanthus acicularis (Roth) Fredericq (Gigartinales, Rhodophyta): tissue culture and production of protoplasts. J Appl Phycol 25, 1835–1845 (2013). https://doi.org/10.1007/s10811-013-0042-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-013-0042-3