Abstract
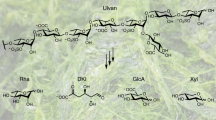
Agars and carrageenans are 1,3-α-1,4-β-galactans from the cell walls of red algae, substituted by zero (agarose), one (κ-), two (ι-), or three (λ-carrageenan) sulfate groups per disaccharidic monomer. Agars, κ-, and ι-carrageenans auto-associate into crystalline fibers and are well known for their gelling properties, used in a variety of laboratory and industrial applications. These sulfated galactans constitute a crucial carbon source for a number of marine bacteria. These microorganisms secrete glycoside hydrolases specific for these polyanionic, insoluble polysaccharides, agarases and carrageenases. This article reviews the microorganisms involved in the degradation of agars and carrageenans, in their environmental and taxonomic diversity. We also present an overview on the biochemistry of the different families of galactanases. The structure–function relationships of the family GH16 β-agarases and κ-caraggeenases and of the family GH82 ι-carrageenases are discussed in more details. In particular, we examine how the active site topologies of these glycoside hydrolases influence their mode of action in heterogeneous phase. Finally, we discuss the next challenges in the basic and applied field of the galactans of red algae and of their related degrading microorganisms.




Similar content being viewed by others
References
Allouch J, Jam M, Helbert W, Barbeyron T, Kloareg B, Henrissat B, Czjzek M (2003) The three-dimensional structures of two beta-agarases. J Biol Chem 278:47171–47180
Allouch J, Helbert W, Henrissat B, Czjzek M (2004) Parallel substrate binding sites in a beta-agarase suggest a novel mode of action on double-helical agarose. Structure 12:623–632
Anderson NS, Campbell JW, Harding MM, Rees DA, Samuel JW (1969) X-ray diffraction studies of polysaccharide sulphates: double helix models for k- and i-carrageenans. J Mol Biol 45:85–99
Antonopoulos A, Favetta P, Helbert W, Lafosse M (2005a) On-line liquid chromatography electrospray ionization mass spectrometry for the characterization of kappa- and iota-carrageenans. Application to the hybrid iota-/nu-carrageenans. Anal Chem 77:4125–4136
Antonopoulos A, Hardouin J, Favetta P, Helbert W, Delmas AF, Lafosse M (2005b) Matrix-assisted laser desorption/ionisation mass spectrometry for the direct analysis of enzymatically digested kappa- iota- and hybrid iota/nu-carrageenans. Rapid Commun Mass Spectrom 19:2217–2226
Aoki T, Araki T, Kitamikado M (1990) Purification and characterization of a novel beta-agarase from Vibrio sp. AP-2. Eur J Biochem 187:461–465
Araki T, Hayakawa M, Lu Z, Karita S, Morishita T (1998) Purification and characterization of agarases from a marine bacterium, Vibrio sp. PO-303. J Mar Biotechnol 6:260–265
Arnott S, Fulmer A, Scott WE, Dea IC, Moorhouse R, Rees DA (1974) The agarose double helix and its function in agarose gel structure. J Mol Biol 90:269–284
Barbeyron T, Henrissat B, Kloareg B (1994) The gene encoding the kappa-carrageenase of Alteromonas carrageenovora is related to beta-1,3-1,4-glucanases. Gene 139:105–109
Barbeyron T, Gerard A, Potin P, Henrissat B, Kloareg B (1998) The kappa-carrageenase of the marine bacterium Cytophaga drobachiensis. Structural and phylogenetic relationships within family-16 glycoside hydrolases. Mol Biol Evol 15:528–537
Barbeyron T, Michel G, Potin P, Henrissat B, Kloareg B (2000) iota-Carrageenases constitute a novel family of glycoside hydrolases, unrelated to that of kappa-carrageenases. J Biol Chem 275:35499–35505
Barbeyron T, L’Haridon S, Corre E, Kloareg B, Potin P (2001) Zobellia galactanovorans gen. nov., sp. nov., a marine species of Flavobacteriaceae isolated from a red alga, and classification of [Cytophaga] uliginosa (Zo Bell and Upham 1944) Reichenbach 1989 as Zobellia uliginosa gen. nov., comb. nov. Int J Syst Evol Microbiol 51:985–997
Bayley ST (1955) X-ray and infrared studies on carrageenin. Biochim Biophys Acta 17:194–205
Belas R (1989) Sequence analysis of the agrA gene encoding beta-agarase from Pseudomonas atlantica. J Bacteriol 171:602–605
Bellion C, Hamer G, Yaphe W (1982) The degradation of Eucheuma spinosum and Eucheuma cottonii carrageenans by ι-carrageenases and k-carrageenases from marine bacteria. Can J Microbiol 28:874–880
Bellion C, Brigand G, Prome J-C, Welti D, Bociek S (1983) Identification et caractérisation des précurseurs biologiques des carraghénanes par spectroscopie de R.M.N.-13C. Carbohydr Res 119:31–48
Bixler H (1996) Recent developments in manufacturing and marketing carrageenan. Hydrobiologia 326/327:35–37
Bongaerts K, Reynaers H, Zanetti F, Paoletti S (1999) Equilibrium and nonequilibrium association processes of kappa-carrageenan in aqueous salt solutions. Macromolecules 32:675–682
Buttner MJ, Fernley IM, Bibb MJ (1987) The agarase gene (dagA) of Streptomyces coelicolor (A3)2: nucleotide sequence and transcriptionnal analysis. Mol Gen Genet 209:101–109
Craigie J (1990) Cell walls. In: Cole K, Sheath R (eds) Biology of the red algae. Cambridge Univ. Press, Cambridge, pp 221–257
Cuppo F, Reynaers H, Paoletti S (2002) Association of kappa-carrageenan induced by Cs+ ions in iodide aqueous solution: a light scattering study. Macromolecules 35:539–547
Davies GJ, Henrissat B (1995) Structures and mechanisms of glycosyl hydrolases. Structure 3:853–859
Day DF, Yaphe W (1975) Enzymatic hydrolysis of agar: purification and characterization of neoagarobiose hydrolase and p-nitrophenyl alpha-galactoside hydrolase. Can J Microbiol 21:1512–1518
De Ruiter G, Rudolph B (1997) Carrageenan biotechnology. Trends Food Sci Technol 8:389–395
Divne C, Stahlberg J, Reinikainen T, Ruohonen L, Pettersson G, Knowles JK, Teeri TT, Jones TA (1994) The three-dimensional crystal structure of the catalytic core of cellobiohydrolase I from Trichoderma reesei. Science 265:524–528
Ekborg NA, Gonzalez JM, Howard MB, Taylor LE, Hutcheson SW, Weiner RM (2005) Saccharophagus degradans gen. nov., sp. nov., a versatile marine degrader of complex polysaccharides. Int J Syst Evol Microbiol 55:1545–1549
Erasmus JH, Cook PA, Coyne VE (1997) The role of bacteria in the digestion of seaweed by the abalone Haliotis midae. Aquaculture 155:377–386
Ford SA, Atkins EDT (1989) New X-ray diffraction results from agarose: extended single helix structures and implications for gelation mechanism. Biopolymers 28:1345–1365
Gauthier G, Gauthier M, Christen R (1995) Phylogenetic analysis of the genera Alteromonas, Shewanella, and Moritella using genes coding for small-subunit rRNA sequences and division of the genus Alteromonas into two genera, Alteromonas (emended) and Pseudoalteromonas gen. nov., and proposal of twelve new species combinations. Int J Syst Bacteriol 45:755–761
Glöckner FO, Kube M, Bauer M, Teeling H, Lombardot T, Ludwig W, Gade D, Beck A, Borzym K, Heitmann K, Rabus R, Schlesner H, Amann R, Reinhardt R (2003) Complete genome sequence of the marine planctomycete Pirellula sp. strain 1. Proc Natl Acad Sci USA 100:8298–8303
Gran HH (1902) Studien über meerebakterien. II. Über die hydrolyse des agars-agars durch ein neues enzym, die gelase. Bergens Museums Arb 2:1–16
Greer CW (1984) A study of carrageenases from marine bacteria. Thèse de Doctorat. McGill University, Montreal, Canada
Greer CW, Yaphe W (1984a) Hybrid (iota–nu–kappa) carrageenan from Eucheuma nudum (Rhodophyta, Solieriaceae), identified using iota- and kappa-carrageenases and 13C-nuclear magnetic resonance spectroscopy. Bot Mar 27:479–484
Greer CW, Yaphe W (1984b) Purification and properties of iota-carrageenase from a marine bacterium. Can J Microbiol 30:1500–1506
Groleau D, Yaphe W (1977) Enzymatic hydrolysis of agar: purification and characterization of beta-neoagarotetraose hydrolase from Pseudomonas atlantica. Can J Microbiol 23:672–679
Guenet JM, Brulet A, Rochas C (1993) Agarose chain conformation in the sol state by neutron scattering. Int J Biol Macromol 15:131–132
Ha JC, Kim GT, Kim SK, Oh TK, Yu JH, Kong IS (1997) Beta-Agarase from Pseudomonas sp. W7: purification of the recombinant enzyme from Escherichia coli and the effects of salt on its activity. Biotechnol Appl Biochem 26:1–6
Hahn M, Olsen O, Politz O, Borriss R, Heinemann U (1995) Crystal structure and site-directed mutagenesis of Bacillus maceransendo-1,3-1,4-beta-glucanase. J Biol Chem 270:3081–3088
Henrissat B, Bairoch A (1996) Updating the sequence-based classification of glycosyl hydrolases. Biochem J 316:695–696
Humm HJ (1946) Marine agar digesting bacteria of the South Atlantic coast. Duke Univ Mar Stn Bull 3:43–75
Jam M, Flament D, Allouch J, Potin P, Thion L, Kloareg B, Czjzek M, Helbert W, Michel G, Barbeyron T (2005) The endo-beta-agarases AgaA and AgaB from the marine bacterium Zobellia galactanivorans: two paralogue enzymes with different molecular organizations and catalytic behaviours. Biochem J 385:703–713
Janaswamy S, Chandrasekaran R (2002) Effect of calcium ions on the organization of iota-carrageenan helices: an X-ray investigation. Carbohydr Res 337:523–535
Johansson P, Brumer H 3rd, Baumann MJ, Kallas AM, Henriksson H, Denman SE, Teeri TT, Jones TA (2004) Crystal structures of a poplar xyloglucan endotransglycosylase reveal details of transglycosylation acceptor binding. Plant Cell 16:874–886
Johnston K, McCandless E (1973) Enzymatic hydrolysis of potassium chloride soluble fraction of carrageenan: properties of “λ-carrageenases” from Pseudomonas carrageenovora. Can J Microbiol 19:779–788
Keitel T, Simon O, Borriss R, Heinemann U (1993) Molecular and active-site structure of a Bacillus 1,3-1,4-beta-glucanase. Proc Natl Acad Sci USA 90:5287–5291
Kimura K, Masuda N, Iwasaki Y, Nakagawa Y, Kobayashi R, Usami S (1999) Purification and characterization of a novel β-agarase from an alkalophylic bacterium, Alteromonas sp. E-1. J Biosci Bioeng 87:436–441
Kleywegt GJ, Zou JY, Divne C, Davies GJ, Sinning I, Stahlberg J, Reinikainen T, Srisodsuk M, Teeri TT, Jones TA (1997) The crystal structure of the catalytic core domain of endoglucanase I from Trichoderma reesei at 3.6 Å resolution, and a comparison with related enzymes. J Mol Biol 272:383–397
Kloareg B, Quatrano R (1988) Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. Oceanogr Mar Biol Annu Rev 26:259–315
Knutsen S, Myslabodski D, Larsen B, Usov A (1994) A modified system of nomenclature for red algal galactans. Bot Mar 37:163–169
Koshland DE (1953) Stereochemistry and the mechanism of enzymatic reactions. Biol Rev Camb Philos Soc 28:416–436
Leon O, Quintana L, Peruzzo G, Slebe JC (1992) Purification and properties of an extracellular agarase from Alteromonas sp. Strain C-1. Appl Environ Microbiol 58:4060–4063
Lewin RA (1969) A classification of flexibacteria. J Gen Microbiol 58:189–206
Mc Hugh DJ (2003) A guide to seaweed industry. In: FAO (ed) FAO fisheries technical paper no 441. FAO, Rome, Italy
McLean MW, Williamson FB (1979a) Glycosulphatase from Pseudomonas carrageenovora. Purification and some properties. Eur J Biochem 101:497–505
McLean MW, Williamson FB (1979b) Kappa-Carrageenase from Pseudomonas carrageenovora. Eur J Biochem 93:553–558
McLean MW, Williamson FB (1981) Neocarratetraose 4-O-monosulphate beta-hydrolase from Pseudomonas carrageenovora. Eur J Biochem 113:447–456
Michel G, Chantalat L, Duee E, Barbeyron T, Henrissat B, Kloareg B, Dideberg O (2001a) The kappa-carrageenase of P. carrageenovora features a tunnel-shaped active site: a novel insight in the evolution of Clan-B glycoside hydrolases. Structure 9:513–525
Michel G, Chantalat L, Fanchon E, Henrissat B, Kloareg B, Dideberg O (2001b) The iota-carrageenase of Alteromonas fortis. A beta-helix fold-containing enzyme for the degradation of a highly polyanionic polysaccharide. J Biol Chem 276:40202–40209
Michel G, Helbert W, Kahn R, Dideberg O, Kloareg B (2003) The structural bases of the processive degradation of iota-carrageenan, a main cell wall polysaccharide of red algae. J Mol Biol 334:421–433
Michel G, Pojasek K, Li Y, Sulea T, Linhardt RJ, Raman R, Prabhakar V, Sasisekharan R, Cygler M (2004) The structure of chondroitin B lyase complexed with glycosaminoglycan oligosaccharides unravels a calcium-dependent catalytic machinery. J Biol Chem 279:32882–32896
Millane RP, Chandrasekaran R, Arnott S, Dea ICM (1988) The molecular structure of kappa-carrageenan and comparison with iota-carrageenan. Carbohydr Res 182:1–17
Mori T (1943) The enzyme catalyzing the decomposition of mucilage of Chondrus ocellatus. III. Purification, unit determination, and distribution of the enzyme. J Agric Chem Soc Jpn 19:740–742
Morrice LM, McLean MW, Long WF, Williamson FB (1983a) Beta-agarases I and II from Pseudomonas atlantica. Substrate specificities. Eur J Biochem 137:149–154
Morrice LM, McLean MW, Williamson FB, Long WF (1983b) beta-Agarases I and II from Pseudomonas atlantica. Purifications and some properties. Eur J Biochem 135:553–558
Morris ER, Rees DA, Robinson G (1980) Cation-specific aggregation of carrageenan helices: domain model of polymer gel structure. J Mol Biol 138:349–362
Ohta Y, Hatada Y, Nogi Y, Li Z, Ito S, Horikoshi K (2004a) Cloning, expression, and characterization of a glycoside hydrolase family 86 beta-agarase from a deep-sea Microbulbifer-like isolate. Appl Microbiol Biotechnol 66:266–275
Ohta Y, Hatada Y, Nogi Y, Miyazaki M, Li Z, Akita M, Hidaka Y, Goda S, Ito S, Horikoshi K (2004b) Enzymatic properties and nucleotide and amino acid sequences of a thermostable beta-agarase from a novel species of deep-sea Microbulbifer. Appl Microbiol Biotechnol 64:505–514
Ohta Y, Nogi Y, Miyazaki M, Li Z, Hatada Y, Ito S, Horikoshi K (2004c) Enzymatic properties and nucleotide and amino acid sequences of a thermostable beta-agarase from the novel marine isolate, JAMB-A94. Biosci Biotechnol Biochem 68:1073–1081
Ohta Y, Hatada Y, Ito S, Horikoshi K (2005a) High-level expression of a neoagarobiose-producing beta-agarase gene from Agarivorans sp. JAMB-A11 in Bacillus subtilis and enzymic properties of the recombinant enzyme. Biotechnol Appl Biochem 41:183–191
Ohta Y, Hatada Y, Miyazaki M, Nogi Y, Ito S, Horikoshi K (2005b) Purification and characterization of a novel alpha-agarase from a Thalassomonas sp. Curr Microbiol 50:212–216
Parsiegla G, Juy M, Reverbel-Leroy C, Tardif C, Belaich JP, Driguez H, Haser R (1998) The crystal structure of the processive endocellulase CelF of Clostridium cellulolyticum in complex with a thiooligosaccharide inhibitor at 2.0 A resolution. EMBO J 17:5551–5562
Petersen TN, Kauppinen S, Larsen S (1997) The crystal structure of rhamnogalacturonase A from Aspergillus aculeatus: a right-handed parallel beta helix. Structure 5:533–544
Potin P (1992) Recherche, production, purification et caractérisation de galactane-hydrolases pour la préparation d’oligosaccharides des parois d’algues rouges. Thèse de Doctorat, Université de Bretagne Occidentale, Brest, Bretagne, France
Potin P, Sanseau A, Le Gall Y, Rochas C, Kloareg B (1991) Purification and characterization of a new kappa-carrageenase from a marine Cytophaga-like bacterium. Eur J Biochem 201:241–247
Potin P, Richard C, Rochas C, Kloareg B (1993) Purification and characterization of the alpha-agarase from Alteromonas agarlyticus (Cataldi) comb. nov., strain GJ1B. Eur J Biochem 214:599–607
Potin P, Richard C, Barbeyron T, Henrissat B, Gey C, Petillot Y, Forest E, Dideberg O, Rochas C, Kloareg B (1995) Processing and hydrolytic mechanism of the cgkA-encoded kappa-carrageenase of Alteromonas carrageenovora. Eur J Biochem 228:971–975
Rees D (1969) Structure, conformation, and mechanism in the formation of polysaccharide gels and networks. Adv Carbohydr Chem Biochem 24:267–332
Rees DA, Morris ER, Thom D, Madden J (1982) In: Aspinall GO (ed) The polysaccharides. Academic, New York, USA, pp 195–290
Rochas C, Rinaudo M (1984) Mechanism of gel formation in k-carrageenan. Biopolymers 23:735–745
Rouvinen J, Bergfors T, Teeri T, Knowles JK, Jones TA (1990) Three-dimensional structure of cellobiohydrolase II from Trichoderma reesei. Science 249:380–386
Sakon J, Irwin D, Wilson DB, Karplus PA (1997) Structure and mechanism of endo/exocellulase E4 from Thermomonospora fusca. Nat Struct Biol 4:810–818
Sarwar G, Matayoshi S, Oda H (1987) Purification of a kappa-carrageenase from marine Cytophaga species. Microbiol Immunol 31:869–877
Schroeder DC, Jaffer MA, Coyne VE (2003) Investigation of the role of a beta(1–4) agarase produced by Pseudoalteromonas gracilis B9 in eliciting disease symptoms in the red alga Gracilaria gracilis. Microbiology 149:2919–2929
Shieh WY, Jean WD (1998) Alterococcus agarolyticus, gen.nov., sp.nov., a halophilic thermophilic bacterium capable of agar degradation. Can J Microbiol 44:637–645
Sinnott ML (1990) Catalytic mechanisms of glycosyl transfer. Chem Rev 90:1171–1202
Skea GL, Mountfort DO, Clements KD (2005) Gut carbohydrases from the New Zealand marine herbivorous fishes Kyphosus sydneyanus (Kyphosidae), Aplodactylus arctidens (Aplodactylidae) and Odax pullus (Labridae). Comp Biochem Physiol B 140:259–269
Smidsrød O, Grasdalen H (1982) Some physical properties of carrageenan in solution and gel state. Carbohydr Polym 2:270–272
Sorbotten A, Horn SJ, Eijsink VG, Varum KM (2005) Degradation of chitosans with chitinase B from Serratia marcescens. Production of chito-oligosaccharides and insight into enzyme processivity. FEBS J 272:538–549
Stanier RY (1941) Studies on marine agar-digesting bacteria. J Bacteriol 42:527–559
Stanier RY (1942) Agar-decomposing strains of the Actinomyces coelicolor species-group. J Bacteriol 1942:555–570
Sugano Y, Matsumoto T, Kodama H, Noma M (1993a) Cloning and sequencing of agaA, a unique agarase 0107 gene from a marine bacterium, Vibrio sp. strain JT0107. Appl Environ Microbiol 59:3750–3756
Sugano Y, Terada I, Arita M, Noma M, Matsumoto T (1993b) Purification and characterization of a new agarase from a marine bacterium, Vibrio sp. strain JT0107. Appl Environ Microbiol 59:1549–1554
Sugano Y, Kodama H, Terada I, Yamazaki Y, Noma M (1994a) Purification and characterization of a novel enzyme, alpha-agarooligosaccharide hydrolase (alpha-NAOS hydrolase), from a marine bacterium, Vibrio sp. strain JT0107. J Bacteriol 176:6812–6818
Sugano Y, Matsumoto T, Noma M (1994b) Sequence analysis of the agaB gene encoding a new beta-agarase from Vibrio sp. strain JT0107. Biochim Biophys Acta 1218:105–108
Suzuki H, Sawai Y, Suzuki T, Kawai K (2003) Purification and characterization of an extracellular beta-agarase from Bacillus sp. MK03. J Biosci Bioeng 95:328–334
Tempel W, Liu ZJ, Horanyi PS, Deng L, Lee D, Newton MG, Rose JP, Ashida H, Li SC, Li YT, Wang BC (2005) Three-dimensional structure of GlcNAcalpha1-4Gal releasing endo-beta-galactosidase from Clostridium perfringens. Proteins 59:141–144
Turvey JR, Christison J (1967) The hydrolysis of algal galactans by enzymes from a Cytophaga species. Biochem J 105:311–321
Usov AI, Miroshnikova LI (1975) Isolation of agarase from Littorina mandshurica by affinity chromatography on Biogel A. Carbohydr Res 43:204–207
Van de Velde F, Peppelman H, Rollema H, Tromp R (2001) On the structure of kappa/iota-hybrid carrageenans. Carbohydr Res 331:271–283
Van de Velde F, Rollema H, Grinberg N, Burova T, Grinberg V, Tromp R (2002) Coil–helix transition of ι-carrageenan as a function of chain regularity. Biopolymers 65:299–312
Van der Meulen HJ, Harder W, Veldkamp H (1974) Isolation and characterization of Cytophaga flevensis sp. Nov., a new agarolytic flexibacterium. Antonie Van Leeuwenhoek 40:329–346
Varrot A, Frandsen TP, von Ossowski I, Boyer V, Cottaz S, Driguez H, Schulein M, Davies GJ (2003) Structural basis for ligand binding and processivity in cellobiohydrolase Cel6A from Humicola insolens. Structure 11:855–864
Vattuone MA, de Flores EA, Sampietro AR (1975) Isolation of neoagarobiose and neoagarotetraose from agarose digested by Pseudomonas elongata. Carbohydr Res 39:164–167
Veldkamp H (1961) A study of two marine agar-degrading, facultatively anaerobic myxobacteria. J Gen Microbiol 26:331–342
Vera J, Alvarez R, Murano E, Slebe JC, Leon O (1998) Identification of a marine agarolytic pseudoalteromonas isolate and characterization of its extracellular agarase. Appl Environ Microbiol 64:4378–4383
Viebke C, Piculell L, Nilsson S (1994) On the mechanism of gelation of helix forming biopolymers. Macromolecules 27:4160–4166
Voget S, Leggewie C, Uesbeck A, Raasch C, Jaeger KE, Streit WR (2003) Prospecting for novel biocatalysts in a soil metagenome. Appl Environ Microbiol 69:6235–6242
Weigl J, Yaphe W (1966) The enzymic hydrolysis of carrageenan by Pseudomonas carrageenovora: purification of a kappa-carrageenase. Can J Microbiol 12:939–947
Yaphe W (1957) The use of agarase from Pseudomonas atlantica in the identification of agar in marine algae (Rhodophyceae). Can J Microbiol 3:893–897
Yaphe W, Baxter B (1955) The enzymatic hydrolysis of carrageenin. Appl Microbiol 3:380–383
Yoder MD, Keen NT, Jurnak F (1993) New domain motif: the structure of pectate lyase C, a secreted plant virulence factor. Science 260:1503–1507
Young KS, Hong KC, Duckworth M, Yaphe W (1971) Enzymic hydrolysis of agar and properties of bacterial agarases. Proc Int Seaweed Symp 7:15–22
Zhong Z, Toukdarian A, Helinski D, Knauf V, Sykes S, Wilkinson JE, O’Bryne C, Shea T, DeLoughery C, Caspi R (2001) Sequence analysis of a 101-kilobase plasmid required for agar degradation by a Microscilla isolate. Appl Environ Microbiol 67:5771–5779
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Michel, G., Nyval-Collen, P., Barbeyron, T. et al. Bioconversion of red seaweed galactans: a focus on bacterial agarases and carrageenases. Appl Microbiol Biotechnol 71, 23–33 (2006). https://doi.org/10.1007/s00253-006-0377-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-006-0377-7