Abstract
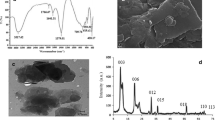
The significance of iron in maintaining physiological homeostasis in the human body is well recognized. However, the presence of unbound iron, specifically the non-transferrin bound iron (NTBI), can potentially induce tissue damage in crucial organs, such as the heart, kidney, and liver. Consequently, there arises a critical need to establish a sensing platform with the capability of detecting exceedingly low concentrations of iron (II). To address this imperative, a layered double-hydroxide (LDH) structure comprising Mg2+ and Al3+ with ethylenediaminetetraacetic acid (EDTA) intercalated between the layers has been successfully synthesized. The proposed methodology involves the utilization of a differential pulse voltammetric detection approach to identify Fe2+ on an EDTA-MgAl LDHs composite-modified glassy carbon electrode. Scanning Electron Microscopy (SEM) analysis of the EDTA-MgAl LDHs revealed the stratified structure of sheets with thicknesses ranging from 95 to 160 nm. Voltammetry studies further demonstrated the effective Fe2+ capturing ability of EDTA and confirmed the existence of multi-layered MgAl LDHs, which contribute to the electrocatalytic oxidation of Fe2+ to Fe3+. The developed sensor exhibited an elevated sensitivity (0.061 µA µM−1) towards Fe2+ and a wide linear detection ranges from 0.1 to 102.1 µM, with an exceptionally low detection limit of 50 nM. Furthermore, the applicability of a two-segmented piecewise linear function to estimate the breakpoint Fe2+ concentration, above and below which the sensor's response behaviour changes, was assessed for the direct determination of Fe2+ in a 0.1 M KCl solution. In conclusion, the developed sensor possesses several advantageous attributes, including a rapid response time, effective Fe2+ capturing ability, electrocatalytic capability, wide linearity, and resistance to interference from other metal ions. These characteristics collectively render it a highly promising and efficient sensing platform for diverse Fe2+ detection applications.
Graphical abstract










Similar content being viewed by others
References
Speich M, Pineau A, Ballereau F (2001) Minerals, trace elements and related biological variables in athletes and during physical activity. Clin Chim Acta 312:1–11. https://doi.org/10.1016/S0009-8981(01)00598-8
Patel M, Ramavataram DVSS (2012) Non transferrin bound iron: nature, manifestations and analytical approaches for estimation. Indian J Clin Biochem 27:322–332. https://doi.org/10.1007/s12291-012-0250-7
Hershko C, Graham G, Bates GW, Rachmilewitz EA (1978) Non-specific serum iron in thalassaemia: an abnormal serum iron fraction of potential toxicity. Br J Haematol 40:255–263. https://doi.org/10.1111/j.1365-2141.1978.tb03662.x
Gutteridge JM, Rowley DA, Halliwell B (1981) Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Detection of “free” iron in biological systems by using bleomycin-dependent degradation of DNA. Biochem J 199:263–265. https://doi.org/10.1042/bj1990263
Matta MK, Beekman CR, Gandhi A et al (2018) Determination of non-transferrin bound iron, transferrin bound iron drug bound iron and total iron in serum in a rats after IV administration of sodium ferric gluconate complex by simple ultrafiltration inductively coupled plasma mass spectrometric dete. Nanomater (Basel, Switzerland). https://doi.org/10.3390/nano8020101
Merli D, Profumo A, Dossi C (2012) An analytical method for Fe(II) and Fe(III) determination in pharmaceutical grade iron sucrose complex and sodium ferric gluconate complex. J Pharm Anal 2:450–453. https://doi.org/10.1016/j.jpha.2012.05.003
Mahmoud WH (2001) Iron ion-selective electrodes for direct potentiometry and potentiotitrimetry in pharmaceuticals. Anal Chim Acta 436:199–206. https://doi.org/10.1016/S0003-2670(01)00892-3
Aghaie M, Giahi M, Aghaie H et al (2009) New Fe(II) Ion-selective electrode based on N-phenylaza-15-crown-5 as neutral carrier in PVC matrix. Desalination 247:346–354. https://doi.org/10.1016/j.desal.2008.10.007
Yazdely M, Taher MA, Tajik S (2013) PVC membrane potentiometric sensor based on (E)-2-acetyl-3-(butyl-amino)-N-phenyl buten-2-thioamide for selective determination of iron(II). Anal Bioanal Electrochem 5:467–480
Kumar S, Mittal S, Kaur N, Kaur R (2017) Improved performance of Schiff based ionophore modified with MWCNT for Fe( ii ) sensing by potentiometry and voltammetry supported with DFT studies. RSC Adv 7:16474–16483
Absalan G, Arabi M, Khalifeh R et al (2014) Coated wire ion selective electrode based on a new crown ether for determination of ${\rm Fe}^{2+}$. IEEE Sens J 14:349–356. https://doi.org/10.1109/JSEN.2013.2282320
Pérez MR, Pavlovic I, Barriga C et al (2006) Uptake of Cu2+, Cd2+ and Pb2+ on Zn–Al layered double hydroxide intercalated with edta. Appl Clay Sci 32:245–251. https://doi.org/10.1016/j.clay.2006.01.008
Zhao D, Sheng G, Hu J et al (2011) The adsorption of Pb(II) on Mg2Al layered double hydroxide. Chem Eng J 171:167–174. https://doi.org/10.1016/j.cej.2011.03.082
Mohamed MEB, Attia NF, Elashery SEA (2021) Greener and facile synthesis of hybrid nanocomposite for ultrasensitive iron (II) detection using carbon sensor. Microporous Mesoporous Mater 313:110832. https://doi.org/10.1016/j.micromeso.2020.110832
Stozhko N, Morosanova E, Kolyadina L, Azarova Z (2004) An electrochemical sol–gel sensor for determining iron by stripping voltammetry. J Anal Chem—J Anal Chem-Engl TR 59:865–870. https://doi.org/10.1023/B:JANC.0000040702.79361.bc
Abounassif MA, Al-Omar MA, Amr A-GE, Mostafa GAE (2011) PVC membrane sensor for potentiometric determination of iron (II) in some pharmaceutical formulations based on a new neutral ionophore. Drug Test Anal 3:373–379. https://doi.org/10.1002/dta.231
Farahani N, Aghaie H (2008) Fe (II) ion-selective membrane electrode based ontetra-phenyl porphyrin in PVC matrix. J Phys Theor Chem 5:17–22
Kamal A, Kumar N, Bhalla V et al (2014) Rhodamine-dimethyliminocinnamyl based electrochemical sensors for selective detection of iron (II). Sensors Actuators B Chem 190:127–133. https://doi.org/10.1016/j.snb.2013.08.079
Aglan RF, Rizk MS, Mohamed GG, El-Wahy AH, Mohamed HA (2014) Preparation and properties of a new carbon paste iron selective electrodes and their applications. Am J Anal Chem 5:140–148. https://doi.org/10.4236/ajac.2014.52017
Frag EY, Abd El-Ghany NA, Fattah MAEL (2018) Physico-chemical properties and characterization of iron (II) electrochemical sensor based on carbon paste electrode modified with novel antimicrobial carboxymethyl chitosan-graft-poly(1-cyanoethanoyl-4-acryloyl-thiosemcarbazide) copolymers. J Electroanal Chem 808:266–277. https://doi.org/10.1016/j.jelechem.2017.12.018
Norocel L, Gutt G (2019) Development and performance testing of an electrochemical sensor for determination of iron ions in wine. Aust J Grape Wine Res 25:161–164. https://doi.org/10.1111/ajgw.12375
Sreekumar A, Durai L, Badhulika S (2023) Facile one-step synthesis of a niobium iron oxide based electrochemical transistor for rapid{,} label-free detection of folic acid in human blood serum samples. New J Chem 47:8845–8853. https://doi.org/10.1039/D3NJ00475A
Acknowledgements
The research was supported by Professor T. R. Rajagopalan research fund, SASTRA Deemed University. The authors are grateful to Department of Science and Technology, New Delhi (SR/FST/ET-I/2018/221(c)) for the financial support. We acknowledge SASTRA Deemed to be University, Thanjavur for extending infrastructure support to carry out the study.
Author information
Authors and Affiliations
Contributions
Shruthee Sankarlinkam—investigation, methodology, conceptualization, data curation, writing original draft, Indhu Suresh—investigation, methodology, conceptualization, writing original draft, G. Hariharan—mathematical analysis, review and editing, Noel Nesakumar—formal analysis, data curation, writing review & editing, Arockia Jayalatha Kulandaisamy—formal analysis, data curation, original draft, John Bosco Balaguru Rayappan—formal analysis, project administration, funding acquisition, and writing review & editing. All authors participated in the discussion, contributing to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sankarlinkam, S., Suresh, I., Hariharan, G. et al. Ethylenediaminetetraacetic acid intercalated MgAl-layered double-hydroxides nanocomposite as an efficient platform in the development of electrochemical sensor for the detection of iron (II). J Appl Electrochem 54, 309–321 (2024). https://doi.org/10.1007/s10800-023-01970-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-023-01970-4