Abstract
This paper evaluates the remediation of soil spiked with lindane using a combined treatment consisting of electrokinetic soil flushing (EKSF) with air stripping to elucidate the main processes occurring in the soil when electric fields of 0.75 V cm−1 and 1.50 V cm−1 are applied. The results demonstrate that lindane is efficiently transported to the anodic and cathodic wells using flushing fluids containing sodium dodecyl sulfate (SDS). Additionally, an important amount is volatilized and stripped with the injected air. In the cathodic well, lindane is rapidly transformed into other species because of the strongly alkaline media. These other species are also found in the portions of soil next to this well, confirming the efficient transport of chlorinated organics with SDS. After 14 days of operation, nearly 50% of the spiked lindane can be removed from the soil. Operation with large electric fields does not improve the performance of the treatment technology and results in lower current intensities and electro-osmotic fluxes and in higher evaporated water, despite the water content in the soil matrix, indicating the coexistence of multiple inputs in these processes.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Lindane (γ-hexachlorocyclohexane, C6H6Cl6) is a very hazardous pollutant banned in many countries around the world and was widely used decades ago as a very efficient synthetic pesticide [1,2,3,4]. Associated with its manufacturing and agricultural application, there are currently many sites around the world with strongly polluted soils, and because of the transport of this species and derivatives from soil to water reservoirs, the problem is becoming increasingly relevant, and efficient technologies for its removal are currently being researched [5,6,7,8,9,10]. The problem of the accumulation of lindane in the environment and their different propagation routes make their natural removal difficult; hence, it necessary to look for alternatives for their elimination directly at the sources of origin. Furthermore, the levels of air contamination are also an important factor, especially in contaminated sites close to cities, acting as a source of secondary pollution. The air/soil partition coefficient in these places facilitates long-range atmospheric transport phenomena, which are regional as well as global [11,12,13,14,15,16,17]. Among the soil remediation processes, transportation of organics with the assistance of electric fields has captured the attention of many scientists in recent years, and it has been demonstrated that these types of technologies can be promising for the removal not only of polar/ionic organics but also for the removal of nonpolar organic compounds such as lindane, for which the use of flushing fluids with a suitable formulation (mainly containing surfactants) is required [18,19,20,21,22,23]. This has opened the possibility of combining electrokinetic soil flushing (EKSF) with many other technologies, with the use of permeable reactive barriers being especially important; that is, traps where pollution mobilized by EK flows is retained or destroyed. These traps can have very different natures, including biological [24, 25], granular activated carbon beds [26, 27], and zero-valent iron beds [28, 29]. Recently, it was pointed out that volatilization of lindane could be of great significance during remediation processes [30] and should be accounted for in the planning of the remediation of polluted sites. In fact, the production of gaseous flows during the application of electrochemical soil remediation technologies has been highlighted as a crucial input to be considered in the design of most electrokinetic remediation technologies [31, 32]. However, electrochemically assisted processes for soil remediation containing lindane are not limited to electrokinetic-based processes but also include other interesting technologies. Thus, other past and recent studies have focused on a combination of electrochemical technologies with conventional soil-washing processes and have demonstrated the suitability of the surfactant sodium dodecyl sulfate for capturing lindane from soil and the compatibility of this compound with electrochemical technology [33,34,35,36,37,38].
This work attempts to obtain more information about the transport of lindane between electrodes and from the soil to the gas phase, which becomes a crucial step in the application of the technology on a large scale. To do this, 14-day tests were performed in bench-scale plants completely sealed in which periodic air was injected to strip volatile pollutants and in which the role of the electric fields was tested. Complete monitoring of the gas, liquid, and soil phases was carried out, and it was completed with a detailed postmortem analysis. A very important point is the full characterization of dehalogenation intermediates produced by the interaction of the basic front produced on the cathode with lindane, which indicates that the electrochemical treatment also promotes an important unexpected advantage: a reduction in the hazardousness of the pollutants contained in the soil.
2 Materials and methods
2.1 Materials
Lindane (Aldrich Chemistry), sodium dodecyl sulfate (SDS) (WWR Chemicals), hexane (Scharlau), and ethyl acetate (Scharlau) were of analytical grade and used as received. Table 1 shows the main characteristic of lindane, SDS, and its main reaction intermediates [1,3-DCB (1,3-dichlorobenzene), 1,2,4-TCB (1,2,4-trichlorobenzene), 1,2,3-TCB (1,2,3-trichlorobenzene), γ-PentaCX (γ-pentachlorocyclohexene), δ-PentaCX (δ-pentachlorocyclohexene), θ-PentaCX (θ-pentachlorocyclohexene), δ-HCH (δ-hexachlorocyclohexane), HeptaCH-1 (heptachlorocyclohexane), and HeptaCH-3 (heptachlorocyclohexane)]. Tap water (conductivity: 476 μS cm−1, pH: 7.56) was used as a solvent to formulate the solubilizing solution used in the wells. To prepare the other solutions, deionized water was used (Millipore Milli-Q system, 18.2 MΩ cm, 25 °C). The silty clay-type soil, extracted from a quarry near Toledo, Spain, contained 4.9% clay, 26.9% sand, and 68.2% silt, in addition to being free from contamination and having a low hydraulic conductivity. The composition of the soil sample was Smectite 28%, Kaolinite 26%, Illite 20%, Feldspar 15%, Quartz 7%, Calcite 4%, and organic content 0%. Considering its granulometric distribution (clay 4.9%, sand 26.9%, and silt 68.2%), the soil can be classified as silty loam. The soil was homogeneously polluted with a solution of lindane until reaching a concentration of 100 mg kg−1 of dry soil. For solvent evaporation, the contaminated soil was placed in a hood for 24 h. The initial soil water content was 20%. A total of 2.4 kg of polluted soil was manually compacted in the cell's central compartment.
2.2 Experimental setup
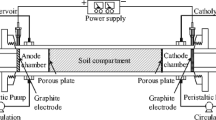
The bench-scale soil remediation plants used in this work consisted of methacrylate boxes divided into three chambers: two small electrolyte wells where graphite electrodes (10 cm × 9 cm × 1 cm) were placed and one larger central chamber [20 cm (length) × 10 cm (width) × 10 cm (height)] where spiked soil was located (Fig. 1). The system was completely sealed, and air was injected periodically (for 10 min every 24 h) on the side close to the anodic chamber and collected at the outlet of the cathodic chamber. The air flows through the soil and electrolyte wells. The outlet of air was connected to a two-bottle hexane trap to capture the volatile species generated during the treatment.
Liquid levels in the two electrolyte wells were kept constant (770 cm3). A solution of 10 g L−1 sodium dodecyl sulfate was used as flushing fluid [35]. The flushing fluid was injected into the anodic wells where a level controller and electro-valves were implemented.
2.3 Experimental procedure
The influence of the electric field was evaluated through two experiments: (I) 0.75 V cm−1 and (II) 1.5 V cm−1. The electrical potential gradient was provided by power sources (MPL-3505 M, Minipa) applied between the electrodes for 14 days. The parameters evaluated in the washing fluid were current intensity, pH, conductivity, temperature, and the concentration of lindane and its intermediates. The hexane contained in the trap was analyzed daily to quantify the volatilized compounds. The distribution of pollution (lindane and intermediates) and other parameters (pH, conductivity, and water content) in the anode–cathode direction and the axial dispersion were obtained by a postmortem analysis in which the soil volume was divided into sixteen portions. Furthermore, after soil treatment, the portions were subdivided and labeled (“Position 1” near the anode and “Position 4” near the cathode).
2.4 Characterization procedures
To determine the contaminants in the liquid phase and the soil after treatment, gas chromatography with an electron capture detector (GC-ECD) (Thermo Fisher Scientific) was used with a TG-5MS capillary column (30 m × 0.25 mm × 0.25 mm) and a 63Ni microelectron capture detector, a split/splitless injector, and ChromCard software. Helium was used as the carrier gas with a flow rate of 1.0 mL min−1. The injector temperature was 210 °C. Under the conditions used, the quantification limit of the GC-ECD was 0.02 mg L−1. All samples were measured by duplicate. The liquid samples collected in the wells were subjected to an L-L extraction with ethyl acetate at a ratio of 1:1 (v/v). This process was carried out in 15 mL separator flasks, vigorously shaken in a vortex mixer (VV3 S040 multitube, VWR International, USA) for 5 min and centrifuged (CENCOM II P-elite, JP Selecta, Barcelona) at 3500 rpm for 10 min. The supernatant was then collected and analyzed. Soil samples were subjected to solid–liquid extraction with a ratio of 1 g of soil: 2 mL of ethyl acetate in 15 mL flasks. The samples were vortexed for 5 min, sonicated for 10 min, and centrifuged at 3500 rpm. The supernatant was then filtered with a 0.22 µm nylon filter and analyzed. The contents of the hexane trap were collected daily and analyzed in the GC-ECD. The moisture of postmortem soil samples was calculated gravimetrically. The pH and conductivity were determined using the standard method EPA 9045C, in which 10 g of soil was mixed with 25 mL of distilled water. The solution was stirred for 10 min, and after sedimentation, the aqueous phase was used for analysis.
3 Results and discussion
Two bench-scale soil remediation plants were operated for 14 days applying electric fields of 0.75 and 1.50 V cm−1, respectively, between the anode and cathode placed on the electrolyte wells in each setup. As seen in Fig. 2a, the resulting current intensity profiles were very different. Initially, the current circulating between the anode and the cathode was higher in the plant undergoing the highest electric field (0.04 A at 1.50 V cm−1), but on day 7, a sudden decrease in the electric current occurred (from 0.04 to 0.01 A) while is remains stable around 0.02 A at 0.75 V cm−1. Thus, the current applied in the process operated at 0.75 V cm−1 was kept almost constant during the complete duration of the tests, reaching a total current charge that overpassed by more than 60% the current passed in the test at a higher electric field. This is a very interesting observation, which confirms that in electrokinetic soil treatment, there is not always a direct relationship between the applied electric field and the resulting charge passed because the electrochemically assisted soil remediation is a very complex process with an extremely large number of inputs to be considered [39].
As observed, in Fig. 2b, the temperatures after 14 days of treatment in both systems were very similar, with average values below the ambient temperature (which may be explained in terms of the evaporative cooling undergone by the evaporation of water) and slightly higher in the case of the plant operated at the highest electric field.
The amounts of water injected in the anode well and injected/extracted in the cathode well are shown in Fig. 3a, where it can be seen that the amount of water injected in the anode is higher than the amount extracted from the cathode, with the difference related to the increase in the water content of the soil during the first days of operation, in which no water was extracted from the cathodic well and, afterward, mainly to the evaporation of water dragged with the air flow. The amount of water evaporated was much higher in the case of the high electric field test, and because of that, only water was extracted from the cathode well on day 7 (with an electro-osmotic flux velocity of 0.23 cm/day). In the low electric field test, the flushing fluid was removed from the well since the very first moment of the treatment, and the electro-osmotic flux velocity was 0.25 cm day−1 [calculated taking into account the volume of water mobilized (cm3), the section (depth × width, cm2), and the time (day)]. However, only very limited water evaporation was observed in this plant. Water balance (taking into account initial and final moisture, volume of water added or extracted from electrolyte wells) was carried out to confirm this observation. As can be observed in Fig. 3b, the final target moisture level of the soil was between 20 and 30%. However, the moisture in the cathodic position was less due to the alkaline front, and consequently favored a decrease the conductivity near the cathode. However, the electro-osmotic fluxes in plant undergoing the highest electric field (1.50 V cm−1), promotes higher axial dispersion.
Changes in the pH and conductivity were as expected, both in the electrolyte wells and in the soil. They are shown in Fig. 4, where the fast acidification of the anodic well and a resulting pH of 4 in the soil portions close to the anode can be seen. Additionally, the rapid basification of the cathodic well raised the pH of the portions of soil in contact with this electrode well up to almost 11.0 units. The production of protons and hydroxyl ions by the oxidation and reduction of water, respectively, could help to explain the changes observed and the increase in the conductivity of the flushing fluids contained in the wells [40, 41]. Additionally, it could also explain the increases in the conductivity of the soil observed in both experiments.
Time course of the a pH and c conductivity profiles in the electrolyte wells for an electric field of 0.75 V cm−1 (○ anode, △ cathode) and 1.5 V cm−1 (● anode, ▲ cathode). b pH and d)conductivity profiles after 14 days remediation teste with an electric field applied of 0.75 V cm−1 (point line, ○) and 1.5 V cm−1 (continuous line, ●)
However, the more important piece of information looked for in this work was related to the changes in lindane pollution. As shown in Fig. 5, lindane reached the anode at significant concentrations in both tests. The total amount collected in this well was 23.4% higher in the treatment carried out applying 1.5 V cm−1 and must be explained in terms of the electrophoretic flux of the micelles formed by the interaction of the SDS contained in the flushing fluid and lindane contained in the portions of soil next to the anode because the electro-osmotic flux drag lindane in the anode–cathode direction and the molecular character (nonpolar) of the molecule prevented its transport by electromigration (both in the opposite direction of that observed experimentally).
However, and opposite to what initially might have been expected, lindane was not observed in the cathode well, where many other chlorinated cyclohexane species were found with a lower chlorination degree. Their occurrence can be explained in terms of the well-known dehalogenation of lindane at alkaline pH values, which has been reported in many recent studies [30, 42,43,44]. Thus, it can be proposed that lindane dragged by the electro-osmotic flux reached the cathodic well, where it was transformed into less halogenated molecules. In addition, periodic air injection in the anode–cathode direction explains the complete exhaustion of this molecule from this well.
This also explains the profiles of lindane obtained in the postmortem characterization of the soil (Fig. 6), which confirms the depletion of lindane in the soil portions next to the cathode wells and the less relevant, but still important, decrease in the proximities of the anode wells, with also an important decrease in the central positions of the soil. Additionally, the profiles of chlorinated derivatives shown in Fig. 5 also indicate their formation in the cathode well and their later diffusion or electro-osmotic transport to the anode, as they were mainly found in positions closer to the cathode wells.
The total amount of intermediates is shown in Fig. 7, where it can be seen that they increased with the electric field. The alkaline pH attained in the cathodic region seems to favor t lindane dechlorination. Thus, lindane is converted to pentachlorocyclohexane, which is unstable and rapidly leads to the formation of trichlorobenzenes and dichlorobenzene. [45, 46]. At the same time, acidic conditions at the anodic region seems to favor chlorination reaction and the formation of heptachlorocyclohexane Anyway, the total amounts measured were low and indicate that stripping by air injection was very successful.
Mass of intermediates eliminated at the end of the treatment. Electric field: □ 0.75 V cm−1 and ■ 1.50 V cm−1. Intermediaries: 1,3-DCB (1,3-dichlorobenzene), 1,2,4-TCB (1,2,4-trichlorobenzene), 1,2,3-TCB (1,2,3-trichlorobenzene), γ-PentaCX (γ-pentachlorocyclohexene), δ-PentaCX (δ-pentachlorocyclohexene), θ-PentaCX (θ-pentachlorocyclohexene), δ-HCH (δ-hexachlorocyclohexane), HeptaCH-1 (heptachlorocyclohexane), and HeptaCH-3 (heptachlorocyclohexane)
Regarding the volatilized species, Fig. 8 shows the mass balance obtained in the plants, where it can be seen that after 14 days of treatment, more than 50.8 and 58.1% of the initial lindane remained in the soil in the 0.75 V cm−1 and 1.50 V cm−1 tests, respectively.
In fact, the gas chromatography data revealed, the amounts of lindane and its derivatives in the electrolyte wells were very low, indicating that electrokinetic transport was slow and the most significant contribution to the removal of this hazardous species was volatilization, which accounted for 46.1 and 39.3% of the total removal of chlorinated organics, respectively, and indicates that volatilization and further drag to the air became more important than EKSF in the removal of this hazardous species. These results highlight the significance of the research on this topic and the wide number of parameters that should be considered to obtain a complete view of the potential performance of the combined EKSF-air stripping remediation technology.
4 Conclusion
Under the application of electric fields, lindane was efficiently transported to the anodic and cathodic wells using flushing fluids containing SDS. Additionally, an important amount was volatilized and stripped with the injected air. Because of the strongly alkaline media, lindane was rapidly transformed into other species in the cathodic well. These other species were also found in the portions of soil next to this well, confirming the efficient transport of chlorinated organics with SDS. After 14 days of operation, nearly 50% of the spiked lindane was removed from the soil. In contrast to what initially was expected, operation with a large electric field did not improve the performance of the treatment technology and resulted in lower current intensities and electro-osmotic fluxes and in a higher ratio of evaporated water.
References
Rodrigo MA, Oturan N, Oturan MA (2014) Electrochemically assisted remediation of pesticides in soils and water: a review. Chem Rev 114(17):8720–8745. https://doi.org/10.1021/cr500077e
Srivastava V, Srivastava T, Kumar MS (2019) Fate of the persistent organic pollutant (POP)hexachlorocyclohexane (HCH) and remediation challenges. Int Biodeterior Biodegrad 140:43–56. https://doi.org/10.1016/j.ibiod.2019.03.004
Waclawek S, Silvestri D, Hrabak P, Padil VVT, Torres-Mendieta R, Waclawek M et al (2019) Chemical oxidation and reduction of hexachlorocyclohexanes: a review. Water Res 162:302–319. https://doi.org/10.1016/j.watres.2019.06.072
Vijgen J, Abhilash PC, Li YF, Lal R, Forter M, Torres J et al (2011) Hexachlorocyclohexane (HCH) as new Stockholm convention POPs—a global perspective on the management of Lindane and its waste isomers. Environ Sci Pollut Res 18(2):152–162. https://doi.org/10.1007/s11356-010-0417-9
Abhilash PC, Singh B, Srivastava P, Schaeffer A, Singh N (2013) Remediation of lindane by Jatropha curcas L: utilization of multipurpose species for rhizoremediation. Biomass Bioenergy 51:189–193. https://doi.org/10.1016/j.biombioe.2013.01.028
Dominguez CM, Parchão J, Rodriguez S, Lorenzo D, Romero A, Santos A (2016) Kinetics of Lindane dechlorination by zerovalent iron microparticles: effect of different salts and stability study. Ind Eng Chem Res 55(50):12776–12785. https://doi.org/10.1021/acs.iecr.6b03434
Merz JP, Gamoke BC, Foley MP, Raghavachari K, Peters DG (2011) Electrochemical reduction of (1R,2r,3S,4R,5r,6S)-hexachlorocyclohexane (Lindane) at carbon cathodes in dimethylformamide. J Electroanal Chem 660(1):121–126. https://doi.org/10.1016/j.jelechem.2011.06.017
Nienow AM, Bezares-Cruz JC, Poyer IC, Hua I, Jafvert CT (2008) Hydrogen peroxide-assisted UV photodegradation of Lindane. Chemosphere 72(11):1700–1705. https://doi.org/10.1016/j.chemosphere.2008.04.080
Peverly AA, Karty JA, Peters DG (2013) Electrochemical reduction of (1R,2r,3S,4R,5r,6S)-hexachlorocyclohexane (Lindane) at silver cathodes in organic and aqueous–organic media. J Electroanal Chem 692:66–71. https://doi.org/10.1016/j.jelechem.2013.01.009
San Román I, Alonso ML, Bartolomé L, Galdames A, Goiti E, Ocejo M et al (2013) Relevance study of bare and coated zero valent iron nanoparticles for lindane degradation from its by-product monitorization. Chemosphere 93(7):1324–1332. https://doi.org/10.1016/j.chemosphere.2013.07.050
Alamdar A, Syed JH, Malik RN, Katsoyiannis A, Liu J, Li J et al (2014) Organochlorine pesticides in surface soils from obsolete pesticide dumping ground in hyderabad city, pakistan: contamination levels and their potential for air-soil exchange. Sci Total Environ 470–471:733–741. https://doi.org/10.1016/j.scitotenv.2013.09.053
Navarro I, de la Torre A, Sanz P, Arjol MA, Fernández J, Martínez MA (2019) Organochlorine pesticides air monitoring near a historical lindane production site in Spain. Sci Total Environ 670:1001–1007. https://doi.org/10.1016/j.scitotenv.2019.03.313
De la Torre A, Navarro I, Sanz P, Arjol MA, Fernández J, Martínez MA (2018) HCH air levels derived from Bailín dumpsite dismantling (Sabiñánigo, Spain). Sci Total Environ 626:1367–1372. https://doi.org/10.1016/j.scitotenv.2018.01.178
Guida Y, Carvalho GO, Capella R, Pozo K, Lino AS, Azeredo A et al (2021) Atmospheric occurrence of organochlorine pesticides and inhalation cancer risk in urban areas at southeast Brazil. Environ Pollut 271:116359. https://doi.org/10.1016/j.envpol.2020.116359
Fang Y, Nie Z, Die Q, Tian Y, Liu F, He J et al (2017) Organochlorine pesticides in soil, air, and vegetation at and around a contaminated site in southwestern China: concentration, transmission, and risk evaluation. Chemosphere 178:340–349. https://doi.org/10.1016/j.chemosphere.2017.02.151
Adu-Kumi S, Kareš R, Literák J, Borůvková J, Yeboah PO, Carboo D et al (2012) Levels and seasonal variations of organochlorine pesticides in urban and rural background air of southern Ghana. Environ Sci Pollut Res Int 19(6):1963–1970. https://doi.org/10.1007/s11356-012-1013-y
Pokhrel B, Gong P, Wang X, Chen M, Wang C, Gao S (2018) Distribution, sources, and air–soil exchange of OCPs, PCBs and PAHs in urban soils of Nepal. Chemosphere 200:532–541. https://doi.org/10.1016/j.chemosphere.2018.01.119
Lacasa E, Cotillas S, Saez C, Lobato J, Cañizares P, Rodrigo MA (2019) Environmental applications of electrochemical technology. What is needed to enable full-scale applications? Curr Opin Electrochem 16:149–156. https://doi.org/10.1016/j.coelec.2019.07.002
Melo Henrique JM, Andrade DC, Barros Neto EL, Silva DR, Santos EV (2019) Solar-powered BDD-electrolysis remediation of soil washing fluid spiked with diesel. J Chem Technol Biotechnol 94(9):2999–3006. https://doi.org/10.1002/jctb.6110
Saichek RE, Reddy KR (2003) Effect of pH control at the anode for the electrokinetic removal of phenanthrene from kaolin soil. Chemosphere 51(4):273–287. https://doi.org/10.1016/S0045-6535(02)00849-4
Hahladakis JN, Latsos A, Gidarakos E (2016) Performance of electroremediation in real contaminated sediments using a big cell, periodic voltage and innovative surfactants. J Hazard Mater 320:376–385. https://doi.org/10.1016/j.jhazmat.2016.08.003
Suanon F, Tang L, Sheng H, Fu Y, Xiang L, Herzberger A et al (2020) TW80 and GLDA-enhanced oxidation under electrokinetic remediation for aged contaminated-soil: does it worth? Chem Eng J 385:123934. https://doi.org/10.1016/j.cej.2019.123934
Suanon F, Tang L, Sheng H, Fu Y, Xiang L, Wang Z et al (2020) Organochlorine pesticides contaminated soil decontamination using TritonX-100-enhanced advanced oxidation under electrokinetic remediation. J Hazard Mater 393:122388. https://doi.org/10.1016/j.jhazmat.2020.122388
Ramírez EM, Camacho JV, Rodrigo MA, Cañizares P (2015) Combination of bioremediation and electrokinetics for the in-situ treatment of diesel polluted soil: a comparison of strategies. Sci Total Environ 533:307–316. https://doi.org/10.1016/j.scitotenv.2015.06.127
Li T, Wang Y, Guo S, Li X, Xu Y, Wang Y et al (2016) Effect of polarity-reversal on electrokinetic enhanced bioremediation of pyrene contaminated soil. Electrochim Acta 187:567–575. https://doi.org/10.1016/j.electacta.2015.11.097
Ruiz C, Mena E, Cañizares P, Villaseñor J, Rodrigo MA (2014) Removal of 2,4,6-trichlorophenol from spiked clay soils by electrokinetic soil flushing assisted with granular activated carbon permeable reactive barrier. Ind Eng Chem Res 53(2):840–846. https://doi.org/10.1021/ie4028022
Sun Y, Gao K, Zhang Y, Zou H (2017) Remediation of persistent organic pollutant-contaminated soil using biosurfactant-enhanced electrokinetics coupled with a zero-valent iron/activated carbon permeable reactive barrier. Environ Sci Pollut Res 24(36):28142–28151. https://doi.org/10.1007/s11356-017-0371-x
Vidal J, Saez C, Cañizares P, Navarro V, Salazar R, Rodrigo MA (2018) ZVI—reactive barriers for the remediation of soils polluted with clopyralid: are they really worth? Chem Eng J 350:100–107. https://doi.org/10.1016/j.cej.2018.05.142
Fu R, Wen D, Chen X, Gu Y, Xu Z, Zhang W (2017) Treatment of decabromodiphenyl ether (BDE209) contaminated soil by solubilizer-enhanced electrokinetics coupled with ZVI-PRB. Environ Sci Pollut Res Int 24(15):13509–13518. https://doi.org/10.1007/s11356-017-8919-3
Vidal J, Carvela M, Saez C, Cañizares P, Navarro V, Salazar R et al (2020) Testing different strategies for the remediation of soils polluted with lindane. Chem Eng J. https://doi.org/10.1016/j.cej.2019.122674
Henrique JMM, Cañizares P, Saez C, Vieira dos Santos E, Rodrigo MA (2021) Relevance of gaseous flows in electrochemically assisted soil thermal sremediation. Curr Opin Electrochem 27:100698. https://doi.org/10.1016/j.coelec.2021.100698
Lopez-Vizcaino R, Risco C, Isidro J, Rodrigo S, Saez C, Canizares P et al (2017) Scale-up of the electrokinetic fence technology for the removal of pesticides. Part II: Does size matter for removal of herbicides? Chemosphere 166:549–555. https://doi.org/10.1016/j.chemosphere.2016.09.114
Beland FA, Farwell SO, Robocker AE, Geer RD (1976) Electrochemical reduction and anaerobic degradation of lindane. J Agric Food Chem 24(4):753–756. https://doi.org/10.1021/jf60206a019
Carboneras MB, Villaseñor J, Fernández FJ, Rodrigo MA, Cañizares P (2020) Selection of anodic material for the combined electrochemical-biological treatment of lindane polluted soil washing effluents. J Hazard Mater 384:121237. https://doi.org/10.1016/j.jhazmat.2019.121237
Muñoz-Morales M, Braojos M, Sáez C, Cañizares P, Rodrigo MA (2017) Remediation of soils polluted with lindane using surfactant-aided soil washing and electrochemical oxidation. J Hazard Mater 339:232–238. https://doi.org/10.1016/j.jhazmat.2017.06.021
Muñoz-Morales M, Sáez C, Cañizares P, Rodrigo MA (2019) Enhanced electrolytic treatment for the removal of clopyralid and lindane. Chemosphere 234:132–138. https://doi.org/10.1016/j.chemosphere.2019.06.059
Estabragh AR, Lahoori M, Ghaziani F, Javadi AA (2018) Electrokinetic remediation of a soil contaminated with anthracene using different surfactants. Environ Eng Sci 36(2):197–206. https://doi.org/10.1089/ees.2018.0089
Boulakradeche MO, Akretche DE, Cameselle C, Hamidi N (2015) Enhanced electrokinetic remediation of hydrophobic organics contaminated soils by the combination of non-ionic and ionic surfactants. Electrochim Acta 174:1057–1066. https://doi.org/10.1016/j.electacta.2015.06.091
Sadeghian F, Jahandari S, Haddad A, Rasekh H, Li J (2021) Effects of variations of voltage and pH value on the shear strength of soil and durability of different electrodes and piles during electrokinetic phenomenon. J Rock Mech Geotech Eng. https://doi.org/10.1016/j.jrmge.2021.07.017
Estabragh AR, Lahoori M, Javadi AA, Abdollahi J (2019) Effect of a surfactant on enhancing efficiency of the electrokinetic method in removing anthracene from a clay soil. J Environ Chem Eng 7(5):103298. https://doi.org/10.1016/j.jece.2019.103298
Saini A, Bekele DN, Chadalavada S, Fang C, Naidu R (2021) Electrokinetic remediation of petroleum hydrocarbon contaminated soil (I). Environ Technol Innov 23:101585. https://doi.org/10.1016/j.eti.2021.101585
Badea S-L, Stegarus D-I, Niculescu V-C, Enache S, Soare A, Ionete R-E et al (2021) Dehalogenation of α-hexachlorocyclohexane by iron sulfide nanoparticles: study of reaction mechanism with stable carbon isotopes and pH variations. Sci Total Environ 801:149672. https://doi.org/10.1016/j.scitotenv.2021.149672
Dominguez CM, Romero A, Fernandez J, Santos A (2018) In situ chemical reduction of chlorinated organic compounds from lindane production wastes by zero valent iron microparticles. J Water Process Eng 26:146–155. https://doi.org/10.1016/j.jwpe.2018.10.011
Liu B, Li G, Mumford KG, Kueper BH, Zhang F (2020) Low permeability zone remediation of trichloroethene via coupling electrokinetic migration with in situ electrochemical hydrodechlorination. Chemosphere 250:126209. https://doi.org/10.1016/j.chemosphere.2020.126209
Wang Z, Peng P, Huang W (2009) Dechlorination of γ-hexachlorocyclohexane by zero-valent metallic iron. J Hazard Mater 166(2):992–997. https://doi.org/10.1016/j.jhazmat.2008.11.106
Peng L, Deng D, Guan M, Fang X, Zhu Q (2015) Remediation HCHs POPs-contaminated soil by activated persulfate technologies: Feasibility, impact of activation methods and mechanistic implications. Sep Purif Technol 150:215–222. https://doi.org/10.1016/j.seppur.2015.07.002
Acknowledgements
This work comprises the research project PDC2021-121105-I00 granted by MCIN/AEI/https://doi.org/10.13039/50110001103310.13039/501100011033/and “Unión Europea NextGenerationEU/PRTR”. Financial support is from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq–306323/2018-4). J. M. M. Henrique gratefully acknowledges Coordenação de Aperfeiçoamento de Pessoal de Nível Superior for Process Number 88887.466691/2019-00 (modalidade: doutorado sanduíche no Exterior-SWE).
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
JMMH: investigation; writing – original draft; methodology; and data curation. JI: investigation; validation; formal analysis; and writing—review and editing. CS: supervision; validation; formal analysis; and writing—review and editing. EVDS, MAR: conceptualization; funding acquisition; supervision; and writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Melo Henrique, J.M., Isidro, J., Saez, C. et al. Removal of lindane using electrokinetic soil flushing coupled with air stripping. J Appl Electrochem 52, 1317–1326 (2022). https://doi.org/10.1007/s10800-022-01715-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-022-01715-9