Abstract
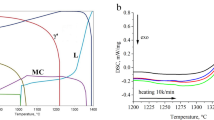
The addition of a ternary element such as boron, silicon, or titanium to the binary alloy Al-40 wt.% Nb produced by rapid solidification after electromagnetic fusion results in a modification of the alloy behavior toward corrosion resistance in a saline medium (3.5 g/l NaCl), as measured by potentiodynamic polarization curves and electrochemical impedance spectroscopy (EIS) on both as-cast and annealed specimens. Optical microscopy, X-ray diffraction (XRD), and scanning electron microscopy (SEM) were used to characterize the binary Al-40 wt.% Nb alloy #4 and ternary Al-40 wt.% Nb-2 wt.% X (X: B alloy #1, Ti alloy #3, Si alloy #2) alloys, as well as EDS quantitative analysis combined with differential thermal calorimetry (DSC). Except for a smaller particle size in alloy #1 when compared to binary alloy #4, the as-cast solidification microstructure reveals no significant shape changes. When compared to other treated alloys, including binary alloys, heat-treated alloy #3 stands out electrochemically, and as-cast binary alloy # 4 also shines out when compared to cast ternary alloys, demonstrating that microstructure is essential.
Graphical Abstract













Similar content being viewed by others
References
Audebert F, Galano M, Saporiti F (2014) The use of Nb in rapid solidified Al alloys and composites. J. Alloys Compounds 615:S621–S626. https://doi.org/10.1016/j.jallcom.2013.12.129
Schwarz H-G, Briem S, Zapp P (2001) Future carbon dioxide emissions in the global material flow of primary aluminium. Energy 26(8):775–795. https://doi.org/10.1016/S0360-5442(01)00032-9
Hirsch J (2011) Aluminium in innovative light-weight car design. Mater Trans 52(5):818–824. https://doi.org/10.2320/matertrans.L-MZ201132
Toros S, Ozturk F, Kacar I (2008) Review of warm forming of aluminum–magnesium alloys. J Mater. Process Technol 207(1–3):1–12. https://doi.org/10.1016/j.jmatprotec.2008.03.057
Rambabu P, Prasad NE, Kutumbarao VV, Wanhill RJH (2017) Aluminium alloys for aerospace applications. In: Prasad NE, Wanhill RJH (eds) Aerospace materials and material technologies. Springer, Singapore, pp 29–52
K. A. Yasakau, M. L. Zheludkevich, M. G. S. Ferreira, Role of intermetallics in corrosion of aluminum alloys. In Smart corrosion protection, in Intermetallic Matrix Composites, Elsevier, 2018, p. 425‑462.
Ujah CO, Popoola API, Popoola OM, Aigbodion VS (2018) Electrical conductivity, mechanical strength and corrosion characteristics of spark plasma sintered Al-Nb nanocomposite. Int J Adv Manuf Technol. https://doi.org/10.1007/s00170-018-3128-x
Shibli SMA, Jabeera B, Manu R (2007) Development of high performance aluminium alloy sacrificial anodes reinforced with metal oxides. Mater Lett 61(14–15):3000–3004. https://doi.org/10.1016/j.matlet.2006.10.062
Pramod SL, Bakshi SR, Murty BS (2015) Aluminum-based cast in situ composites: a review. J Mater Eng Perform 24(6):2185–2207. https://doi.org/10.1007/s11665-015-1424-2
Di Franco F, Santamaria M, Di Quarto F, La Mantia F, de Sá AI, Rangel CM (2013) Dielectric properties of Al-Nb amorphous mixed oxides. ECS J State Science Technol 2(11):205–210. https://doi.org/10.1149/2.012311jss
Ghandvar H, Idris MH, Asma T, Bakar A, Nafari A, Ahmad N (2020) Microstructural characterization, solidification characteristics and tensile properties of Al 15%Mg2Si–x(Gd–Sb) in-situ composite. J Mater Res Technol 9(3):3272–3291
Abboud J, Mazumder J (2020) Developing of nano sized fibrous eutectic silicon in hypereutectic Al–Si alloy by laser remelting. Sci Rep 10:12090. https://doi.org/10.1038/s41598-020-69072-1
Saidman SB, Garcia SG, Bessone JB (1995) Electrochemical behaviour of Al-In alloys in chloride solutions. J Appl Electrochem. https://doi.org/10.1007/BF00262964
D. R. Salinas, Infuence of alloying elements and microstructure on aluminium sacri®cial anode performance: case of Al±Zn, p. 9.
Shayeb HAE (2001) Effect of gallium ions on the electrochemical behaviour of Al, Al±Sn, Al±Zn and Al±Zn±Sn alloys in chloride solutions. Corr Sci 43(3):643–654
Gudić S, Smoljko I, Kliškić M (2010) Electrochemical behaviour of aluminium alloys containing indium and tin in NaCl solution. Mater Chem Phys 121(3):561–566. https://doi.org/10.1016/j.matchemphys.2010.02.040
Mostaan H, Karimzadeh F, Abbasi MH (2012) Thermodynamic analysis of nanocrystalline and amorphous phase formation in Nb–Al system during mechanical alloying. Powder Metallurgy 55(2):142–147. https://doi.org/10.1179/1743290111Y.0000000018
Gauthier V, Bernard F, Gaffet E, Vrel D, Gailhanou M, Larpin JP (2002) Investigations of the formation mechanism of nanostructured NbAl3 via MASHS reaction. Intermetallics 10(4):377–389. https://doi.org/10.1016/S0966-9795(02)00010-9
Almeida A, Petrov P, Nogueira I, Vilar R (2001) Structure and properties of Al–Nb alloys produced by laser surface alloying. Mater Sci Eng A 303(1–2):273–280. https://doi.org/10.1016/S0921-5093(00)01838-4
Mareci D, Popa IM, Ungureanu G, Aelenei D, MirzaRosca JC (2006) Electrochemical response of aluminum in contact with beer. Sci Study Res-Chem Chem Eng Biotechnol Food Ind 7(4):769–778
Rana RS, Purohit R, Das S (2012) Reviews on the influences of alloying elements on the microstructure and mechanical properties of aluminum alloys and aluminum. Alloy Composites 2(6):8
Guzowski MM, Sigworth GK, Sentner DA (1987) The role of boron in the grain. MTA 18(4):603–619. https://doi.org/10.1007/BF02649476
Chen Y, Pan Y, Lu T, Tao S, Wu J (2014) Effects of combinative addition of lanthanum and boron on grain refinement of Al–Si casting alloys. Mater Design 64:423–426. https://doi.org/10.1016/j.matdes.2014.07.068
Yuying W, Xiangfa L, Xiufang B (2007) Effect of boron on the microstructure of near-eutectic Al–Si alloys. Mater Characterization 58(2):205–209. https://doi.org/10.1016/j.matchar.2006.04.009
Sarkar R, Ghosal P, Muraleedharan K, Nandy TK, Ray KK (2011) Effect of boron and carbon addition on microstructure and mechanical properties of Ti-15–3 alloy. Mater Sci Eng A 528(13–14):4819–4829. https://doi.org/10.1016/j.msea.2011.03.014
Hernandez-Rodriguez M, Laverde-Cataño D, Lozano D, Martinez-Cazares G, Bedolla-Gil Y (2019) Influence of boron addition on the microstructure and the corrosion resistance of CoCrMo alloy. Metals 9(3):307. https://doi.org/10.3390/met9030307
Wang M, Hu K, Liu G, Liu X (2020) Synchronous improvement of electrical and mechanical performance of A356 alloy reinforced by boron coupling nano-AlNp. J Alloys Compounds 814:152217. https://doi.org/10.1016/j.jallcom.2019.152217
Cui X, Wu Y, Zhang G, Liu Y, Liu X (2017) Study on the improvement of electrical conductivity and mechanical properties of low alloying electrical aluminum alloys. Composites B Eng 110:381–387. https://doi.org/10.1016/j.compositesb.2016.11.042
Cui X, Wu Y, Liu X, Zhao Q, Zhang G (2015) Effects of grain refinement and boron treatment on electrical conductivity and mechanical properties of AA1070 aluminum. Mater Design 86:397–403. https://doi.org/10.1016/j.matdes.2015.06.149
Zhu J, Kamiya A, Yamada T, Shi W, Naganuma K (2003) Influence of boron addition on microstructure and mechanical properties of dental cast titanium alloys. Mater Sci Eng A 339(1–2):53–62. https://doi.org/10.1016/S0921-5093(02)00102-8
Yan BC, Zhang J, Lou LH (2008) Effect of boron additions on the microstructure and transverse properties of a directionally solidified superalloy. Mater Sci Eng A 474(1–2):39–47. https://doi.org/10.1016/j.msea.2007.05.082
Baetzner C, Beuers J, Hoch M, Korniyenko K (2009) Aluminium—Niobium—Silicon. LandoltBörnstein. https://doi.org/10.1007/978-3-540-88053-0_12
El-Aziz KA, Saber D, Sallam HE-DM (2015) Wear and corrosion behavior of Al–Si matrix composite reinforced with alumina. J Bio TriboCorros 1(1):5. https://doi.org/10.1007/s40735-014-0005-5
Osório WR, Goulart PR, Garcia A (2008) Effect of silicon content on microstructure and electrochemical behavior of hypoeutectic Al–Si alloys. Mater Lett 62(3):365–369. https://doi.org/10.1016/j.matlet.2007.05.051
Bandil K et al (2019) Microstructural, mechanical and corrosion behaviour of Al–Si alloy reinforced with SiC metal matrix composite. J Composite Mater 53(28–30):4215–4223. https://doi.org/10.1177/0021998319856679
Osório WR, Cheung N, Spinelli JE, Goulart PR, Garcia A (2007) The effects of a eutectic modifier on microstructure and surface corrosion behavior of Al-Si hypoeutectic alloys. J Solid State Electrochem 11(10):1421–1427. https://doi.org/10.1007/s10008-007-0300-x
Revilla RI, De Graeve I (2018) Influence of Si content on the microstructure and corrosion behavior of additive manufactured Al-Si alloys. J. Electrochem. Soc. 165(13):C926–C932. https://doi.org/10.1149/2.0101814jes
Kumar V, Mehdi H, Kumar A (2015) Effect of silicon content on the mechanical properties of aluminum. Alloy 02(04):6
Sasikumar Y, Indira K, Rajendran N (2019) Surface modification methods for titanium and its alloys and their corrosion behavior in biological environment: a review. J Bio TriboCorros 5(2):36. https://doi.org/10.1007/s40735-019-0229-5
Sherif E-SM, Ammar HR, Khalil KA (2014) Effects of copper and titanium on the corrosion behavior of newly fabricated nanocrystalline aluminum in natural seawater. Appl Surf Sci 301:142–148. https://doi.org/10.1016/j.apsusc.2014.02.019
Yasakau KA, Zheludkevich ML, Ferreira MGS (2018) Role of intermetallics in corrosion of aluminum alloys. In: Mitra R (ed) Smart corrosion protection. Woodhead Publishing, Intermetallic Matrix Composites, pp 425–462
Knipling KE, Dunand DC, Seidman DN (2006) Criteria for developing castable, creep-resistant aluminum-based alloys—a review. Z Metallkd 97(3):246
Witusiewicz VT, Bondar AA, Hecht U, Rex S, Velikanova TY (2008) The Al-B-Nb-Ti system: III. Thermodynamic re-evaluation of the constituent binary system Al-Ti. J. Alloys Compd. 465:64
Fan QH, Zhao YM, Huang J, Ouyang LS, Kuang Q (2012) Large-scale synthesis of aluminum diboride nanowires by Ni(NO3)2 catalyst. J Cryst Growth 346:75–78
Djurdjević MB, Manasijević S, Odanović Z, Dolić N (2013) Calculation of liquidus temperature for aluminum and magnesium alloys applying method of equivalency. Adv Mater Sci Eng 2013:1–8
Wang S, Xiazhang MF, Wang LJ, Su X (2018) J Mater Process Technol 255:105–109
Prach O, Trudonoshyn O, Puchnin M (2017) Effects of chemical composition on mechanical properties of Al-Mg-Si-Mn based alloys. Mater Eng—Materiálové inžinierstvo 24:11–20
Mondolfo LF (1976) Aluminum alloys: structure and properties. Butterworths, London, pp 312–53
Esquivel J, Murdoch HA, Darling KA, Gupta RK (2018) Excellent corrosion resistance and hardness in Al alloys by extended solid solubility and nanocrystalline structure. Mater Res Lett 6(1):79–83. https://doi.org/10.1080/21663831.2017.1396262
Pride ST, Scully JR, Hudson JL (1994) Metastable pitting of aluminum and criteria for the transition to stable pit growth. J Electrochem Soc 141:3028–3040
Palcut M, Priputen P, Šalgó K, Janovec J (2015) Phase constitution and corrosion resistance of Al-Co alloys. Mater Chem Phys 166:95–104
Debili MY, Sassane N, Boukhris N (2017) Structure and corrosion behavior of Al-Co-Ti alloy system. Anti Corros Methods Mater 64:443–451
Shi YZ, Yang B, Xie X, Brechtl J, Dahmen KA, Liaw PK (2017) Corrosion of AlxCoCrFeNi high-entropy alloys: Al content and potential scan-rate dependent pitting behavior. Corros Sci 119:33
Singh D, Dhayal V, Agarwal DC (2019) Surf Eng Appl Electrochem 55(4):436–442
A. Toloei, V. Stoilov, D. Northwood. Proceedings of the ASME 2013 International Mechanical Engineering Congress & Exposition IMECE 2013 November 13–21, 2013, San Diego, California, USA.
Chen Y, Jepson WP (1999) EIS measurement for corrosion monitoring under multiphase flow conditions. Electrochim Acta 44(24):4453–4464
Prakashaiah, B. G., Corros Sci (2018). https://doi.org/10.1016/j.corsci.2018.03.021.
Arthanari S, Jang JC, Shin KS (2019) J Alloys Compd 783:494–502
Seikh AH, Baig M, Ammar HR, Alam MA (2016) The influence of transition metals addition on the corrosion resistance of nanocrystalline Al alloys produced by mechanical alloying. Metals 6:140
Morquech CPC et al (2012) Int. J. Electrochem. Sci. 7:1125–1133
Liyana NK, Fazal MA, Haseeb ASMA (2018) Polarization and EIS studies to evaluate the effect of aluminum concentration on the corrosion behavior of SAC105 solder alloy. Mater Sci-Poland 35(4):694–701
Acknowledgements
The authors are very grateful to the DGRSDT for financial assistance and to researchers at the ENSMM Annaba for SEM observations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Layachi, F., Debili, M.Y. Structure and corrosion of Al-40 wt.% Nb-2 wt.% X alloys rapidly solidified from the melt. J Appl Electrochem 52, 1259–1273 (2022). https://doi.org/10.1007/s10800-022-01704-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-022-01704-y