Abstract
Although typical electrochemical double-layer capacitors (EDLCs) operate with aqueous or lithium-free organic electrolytes optimized for activated carbon electrodes, there is interest in EDLCs with lithium-ion electrolyte for applications of lithium ion capacitors and hybridized battery-supercapacitor devices. We present an experimental study of symmetric EDLCs with electrolyte 1 M LiPF6 in EC:EMC 50:50 v/v and electrode coatings with 5 wt% SBR or PEDOT:PSS binder at 5 or 10 wt% concentration, where for the PEDOT:PSS containing electrodes pseudocapacitance effects were investigated in the lithium-ion electrolyte. Two different electrode coating fabrication methods were explored, doctor blade coating and spraying. It was found that EDLCs with electrodes with either binder had a stability window of 0–2 V in the lithium-ion electrolyte. EDLCs with electrodes with 10 wt% PEDOT:PSS binder yielded cyclic voltammograms with pseudocapacitance features indicating surface redox pseudocapacitance in the doctor blade coated electrodes, and intercalation and redox phenomena for the sprayed electrodes. The highest energy density in discharge was exhibited by the EDLC with doctor blade-coated electrodes and 10 wt% PEDOT:PSS binder, which combined good capacitive features with surface redox pseudocapacitance. In general, EDLCs with sprayed electrodes reached higher power density than doctor blade coated electrodes.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
There has been increasing interest in supercapacitors with lithium-ion electrolyte targeting applications of lithium-ion capacitors [1] and hybrid battery-supercapacitors [2]. Electrochemical double-layer capacitors (EDLCs) of symmetric or asymmetric type aim for high surface area electrodes, such as activated carbon (AC) fabrics [3, 4], AC coatings [4, 5], graphene [6,7,8] or combinations [9], with conductive additives such as carbon black and multiwall carbon nanotubes (MWCNTs) [10,11,12]. Electrode materials of supercapacitors have been optimized in terms of pore size distribution for aqueous electrolytes, such as phenolic-derived ACs with narrow distribution at small pore sizes [3] or broader or bimodal pore size distributions suitable for organic electrolytes [4, 5, 10, 11] facilitating fast ion transport through pore paths of large pores [13] down to small pores which provide the large surface area and high specific capacitance [14]. Typical organic electrolytes for EDLCs include TEABF4 in acetonitrile or propylene carbonate (PC) with a potential window to 3 V [3,4,5, 9,10,11].
First steps have been made in exploring lithium-ion electrolytes in EDLCs, starting with chemical stability studies. Zhang et al. [15] found that AC electrodes are stable in LiPF6/EC:DMC electrolyte in the range of 1.6–4.4 V against Li/Li+ and in LiPF6/PC electrolyte in the range of 1.3–4.4 V against Li/Li+. In a recent publication by our group [16], computational simulations of ion transport in EDLCs, taking into account the electrode pore size distribution, demonstrated that a peat bog-originated AC coating electrode showed higher specific capacitance than phenolic-originated AC electrodes in electrolyte 1 M LiPF6 in EC:EMC, and, as a result, this best performing AC powder is used for the electrodes of the current work, also in the same electrolyte.
Different binders are used for EDLCs such as PVDF [11], PVA [11], SBR [4], natural polymers [17] and PEDOT:PSS [18], the latter also contributing in terms of pseudocapacitance. Pseudocapacitors function via ion intercalation and redox phenomena [19]. Transition metal oxides, sulfides and carbides [20] and silver nanoparticles [21] exhibit pseudocapacitance and some of them also enhance the conductivity of the composite electrodes, such as silver nanoparticles and iron carbide [21, 22]. Amongst several conducting polymers offering pseudocapacitance, PEDOT:PSS has been the focus of more research studies because of its high conductivity that reduces the equivalent in series resistance (ESR) of the EDLC [23] and also facilitates the redox kinetics for pseudocapacitance. A PEDOT:PSS binder disintegrates in a water stream and, hence, EDLCs with coating electrodes with PEDOT:PSS binder can be recycled according to proposed protocols [24] based on liquid processing [25, 26] and separation methods [27]. PEDOT:PSS pseudocapacitors have typically aqueous electrolytes, such as 1 M H2SO4 [28] or H3PO4/PVA gel electrolyte [29, 30]. It has been reported [31] that their pseudocapacitance is due to the following reversible redox process between PEDOT:PSS and the H+ ion of an aqueous acid electrolyte:
This could be extended to an alkali metal ion, M+[32], either following a surface redox or a bulk redox mechanism [19] the latter taking place if the M+ ion is sufficiently small to be able to diffuse and intercalate through the bulk of PEDOT:PSS. Given that lithium belongs to the first column of the Periodic Table, the Li+ ions in lithium-ion electrolytes offer the best chance of bulk intercalation and redox processes in PEDOT:PSS next to the H+ ions in aqueous electrolytes; in fact, the ion diameter of Li+ is 0.15 nm whereas the hydrogen ion in aqueous electrolytes is in the form of H3O+ with an ion diameter of 0.2 nm [33]. Therefore, PEDOT:PSS has been one of the binders researched in this study, in the hope to explore the pseudocapacitance effect of the binder in the presence of the lithium-ion electrolyte.
Coating via doctor blade is a well-established electrode fabrication technique for EDLCs, able to achieve coatings of large areal density with easily adjustable and well controlled coating thickness [34], and suitable for scale up as it is a continuous process. Spraying is an alternative coating fabrication technique, particularly useful for hybridized battery-supercapacitor architectures [35, 36]. However, spraying can only attain thin coatings, up to 70 µm [37], due to redissolving of binder in consecutive spray passes, which yields low areal density coatings. Aqueous binders such as SBR and PEDOT:PSS are preferred as they provide “green” processing for electrode fabrication via either the doctor blade or the spraying technique. More specifically, PEDOT:PSS has been recently favored over other aqueous binders, as it also offers high conductivity [38]. Apart from differences in electrode thickness and areal density, no other differences between the two electrode fabrication techniques have been reported in terms of the quality and performance of the resulting energy storage devices. Regarding the use of PEDOT:PSS as a binder, it has been found that crystallization of PEDOT:PSS and processing affects its conductivity [39], where, for example, spin coating induces in plane orientation with poor transverse conductivity [40] and drop deposition as in spraying or inkjet printing induces transverse orientation at the drop edges which brings the transverse conductivity to similar level as the in-plane conductivity [41].
In this study, we present experimental investigations of symmetric EDLCs with peat bog-derived AC coating electrodes and lithium-ion electrolyte 1 M LiPF6 in EC:EMC 50:50 v/v, where the pseudocapacitance effect of PEDOT:PSS is explored in the lithium-ion electrolyte, using PEDOT:PSS as a binder at two alternative concentrations of 5 wt% and 10 wt%, and compared to SBR binder at 5 wt%. An additional factor in these investigations is the processing technique for coating fabrication, where two alternative methods are investigated: doctor blade coating and spraying.
2 Experimental part
2.1 Materials
The electrode coatings were based on AC powder, peat bog-derived 4C Norit A charcoal (Sigma Aldrich) with specific surface area BET = 1000 m2 g−1, particle size: 10–15% greater than 74 μm and 70–75% greater than 10 μm. Conductive additive acetylene carbon black (Alfa Aesar) was used at 5 wt% in the electrode coating, with average particle size 42 nm, specific surface area, BET = 75 m2 g−1 and bulk density 170–230 kg m−3. Two alternative binders were used: SBR hydrophilic binder PSBR100 (Targray) comprising a 15% SBR aqueous emulsion; and PEDOT:PSS Clevios PH 1000 (Heraeus) with 1–1.3% PEDOT:PSS. All coatings were deposited on current collector aluminium foil (Goodfellow) with purity > 99.3% and a thickness of 15 μm. In the EDLC cells, the electrolyte was 1 M LiPF6 in EC:EMC 50:50 v/v (Sigma Aldrich). An assembly of two separators was used: a Celgard® 3501 (Celgard), 25 μm thick membrane with an average pore diameter of 0.064 μm, and a Whatman® GF/F borosilicate glass fibre membrane with an average pore size of 0.7 μm (Sigma Aldrich).
2.2 Coating manufacture and characterisation
Slurries for the doctor blade coating method were prepared as follows: 9 g of AC powder for the 5 wt% binder coatings or 8.5 g AC for the 10 wt% binder coatings, and 0.5 g of carbon black were weighed in separate beakers. 40 ml of de-ionised water was added to each of the beakers and they were then stirred and placed in an ultrasonic bath for 20 min to disperse the particles. The contents of the carbon black beaker were poured into the beaker with the AC slurry which was then placed on a magnetic stirrer for about 30 min to ensure thorough mixing. After the sample was mixed, it was treated in a Fisher Scientific Q500 tip sonicator which was operated at 50% amplitude with cycles of 30 s on followed by 59 s off. This was carried out until the probe had spent a total of 3 min on and then the procedure was repeated at 75% amplitude. The beaker was removed and placed on a magnetic stirrer at 50 °C and 100 rpm for 30 min. The binder was then measured out and added to the beaker and the mixture was stirred one final time at 50 °C and 500 rpm for 30 min.
The slurries were either coated using the doctor blade (db) technique with a 400 mm blade gap or spray (s) coated on an aluminium foil substrate of 200 × 200 mm, adhered onto a 2d moving platform [42]. A 176 ViscoMist spray nozzle head Body 5–176. 501 (Lechler) was used at constant distance of 200 mm over the substrate. Spraying was performed at an atomizing air pressure of 138 kPa (20 psi) and fan air pressure of 34.5 kPa (5 psi) and a feed rate of 600 mm s−1, while the substrate was moved by 133 mm at each pass for 3 or 6 passes, depending on the visible quality of the coating. All coatings were dried in an oven at 120 °C for 4 h. Table 1 summarises the fabricated coatings in terms of their composition and fabrication technique.
Scanning electron microscopy (SEM) at 25 kV and energy dispersive X-ray (EDX) analysis were carried out using a JEOL JSM-7100F instrument, equipped with a field emission gun (used at 25 kV in imaging mode) and an UltraDry EDX detector (during elemental analysis).
2.3 EDLC fabrication
Symmetric EDLC coin cells (2032 size of coin case) were fabricated, with all the assembly and fabrication taking place in an argon filled glovebox with moisture and oxygen content below 15 ppm. The EDLC coin cells were symmetric EDLCs, with electrode discs of 15 mm diameter. 200 μl of electrolyte 1 M LiPF6 in EC:EMC 50:50 v/v was used in each cell. An assembly of two separators was used, a Celgard® 3501 membrane and a Whatman® GF/F borosilicate glass fibre filter.
2.4 EDLC tests
EDLCs were tested using a GAMRY 1000 potentiostat in (a) electrochemical impedance spectroscopy (EIS) in the frequency range of 10 MHz-1 MHz; (b) cyclic voltammetry (CV) at 1 mV s−1 to increasingly higher maximum voltage (0.5, 1, 1.5, 2, 2.5 and 3 V) to evaluate electrode and electrolyte materials stability. For the highest voltage in the stability window, CVs were carried out at different voltage scan rates, r, in the range of 1–500 mV s−1, three cycles at each rate; (c) galvanostatic charge–discharge (GCD) at different constant current values in the range of 0.2–50 mA. CVs yielded current, I, versus voltage plots, from which the current axis was translated to specific electrode capacitance, Csp,el according to the relation:
where mel is the mass of one electrode. From the GCD data, Ragone plots were assembled for the discharge phase, comprising the average power and integrated energy during discharge, and translated to power density and energy density, respectively, by dividing with the mass of the two electrodes.
3 Experimental results
In general, the binder was not visible in the SEM images of electrode coatings, especially for the 5 wt% binder concentration. For this reason, the focus was shifted to the 10 wt% binder concentration at which two electrodes were available for examination under SEM: PED10-db and PED10-s. The generated SEM images presented in Fig. 1, still do not show the binder but illustrate the impact which the binder may have on the coating surface topography. The binder enables the accumulation and embedding within its mass of larger carbon black particle domains, covering large areas of the AC on the surface. This part of the study is in agreement with similar observations made by Lei et al. [18] on PED10-db coated AC electrodes of identical composition. The high magnification SEM image of Fig. 1b illustrates an AC particle with clearly visible pore topography, depicting pores of 50–100 nm unblocked by PEDOT:PSS. The sprayed AC electrode PED10-s presented in Fig. 1c, d exhibits thin PEDOT:PSS surface layers, at multiple locations at the upper left-hand side of the captured micrograph in Fig. 1c. Based on SEM, this study illustrates that the binder of the sprayed electrodes is more prevalent over the majority of AC pore surface. In particular, the higher magnification SEM image in Fig. 1d demonstrates that a PEDOT:PSS film has covered macropores of 50–100 nm in the AC particles. In addition, Fig. 1d serves as clear evidence of the well-dispersed and round-shaped, carbon black particles, laced around AC particles, which is in agreement with proposals and reports of past studies that spraying contributes to the good dispersion of nanoparticles [43].
Figure 2 follows with the results of EDX analysis. The EDX line scan in Fig. 2b indicates that both O and S signals are weak and very close to that of the Al background, which is the signal from the current Al foil collector present in the sample. Following the sulfur signal in Fig. 2b, a well distributed presence of PSS is revealed across both the peaks and the valleys of the largely carbonaceous surface of the DB coated electrode. However, the image composition in Fig. 2a shows that the sulfur signal is stronger in valleys together with carbon black particles, indicating that PEDOT:PSS is trapped into the porous carbon black microstructure. Point EDX analysis of the sprayed sample PED10-s, shown in Fig. 2c, indicates a spiked presence of oxygen, also confirmed by the O scan line in Fig. 2d, which might be associated with either PEDOT or aluminium oxide on the current collector. Sulfur is evenly distributed across the entire surface, indicating good spread of the binder. The sulfur concentration in the EDX point analysis indicates more agglomerated PEDOT:PSS, trapped by the carbon black microstructural aggregates, in PED10-db (Fig. 2a) compared to evenly distributed PEDOT:PSS in PED10-s (Fig. 2c). Hence, one may assume that spray coating results in a thin PEDOT:PSS film, homogeneously distributed on the coating surface, which can also be concluded from the SEM image in Fig. 1d.
EDX analysis of electrode coatings: a, b PED10-db; c, d PED10-s, including: a, c in-situ elemental point analysis with b, d corresponding line scans for aluminium (current collector), oxygen and sulfur, the last two used as markers for PEDOT:PSS. All weight percentages have weight % error of not more than ± 0.7 for PED10-db and ± 0.4 for PED10-s
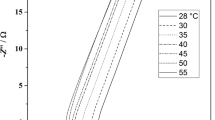
Figure 3 presents the Nyquist plots from the EIS tests in which it can be seen that the sprayed coating with SBR binder, SBR5-s, has no contact resistance and the lowest ESR (equivalent in series resistance) overall, where ESR is considered the sum of the first intercept of the Nyquist curve with the Zre axis and the diameters of all semicircles (or arcs) in the Nyquist plot which represent contact resistances.. The PED10-s sprayed electrode exhibits more than one semicircle in the Nyquist plot, indicating resistances of different layers, which may be attributed to the six passes of spraying (Table 1). Due to different thickness and areal densities of the various coatings, given the different coating fabrication methods and different binder solutions of different composition, it is difficult to compare directly all Nyquist plots in Fig. 3. For this reason, two key performance indicators were extracted from the EIS data and weighted according to the electrode mass: the specific ESR with respect to the mass of both electrodes and electrode specific capacitance, Csp,el, at 10 MHz. The results are presented in Table 2.
It can be seen that the sprayed SBR5-s coating has lower specific resistance than the doctor blade coated SBR5-db, which we believe is due to the better dispersion of the conductive carbon black nanoparticles by the spraying process resulting in an improved conductive network of nanoparticles. Replacing the SBR binder with the same content, 5 wt%, of PEDOT:PSS binder reduces the ESR of both doctor blade and sprayed coatings, due to the higher conductivity of PEDOT:PSS. However, increasing the amount of PEDOT:PSS to 10 wt% increases the ESR value compared to that of the 5 wt% PEDOT:PSS specimens, even for the PED10-db coating, which does not show any multilayer contact resistances: the reason for this is that although PEDOT:PSS is a conductive polymer, it has still a finite conductivity and thicker binder domains would result in an overall decrease of conductivity. The specific electrode capacitance values are similar for all coatings (apart from the high ESR coating PED10-s which inhibits fast ion transport), as the EIS data are close to the OCV and, hence, no pseudocapacitance effects are expected while the charge–discharge rate at 10 MHz allows for timescales up to 100 s, which might be shorter than the time of a slow GCD cycle, as will be seen in the reporting of the GCD experimental results.
Figure 4 presents CV plots for electrodes with 5 wt% SBR binder (Fig. 4a) and 5 wt% PEDOT:PSS (Fig. 4b). In both cases a clear anodic peak rises for voltages higher than 2.7 V that is irreversible in the cathodic part of the curve. The same test for an EDLC with the same electrolyte and the same electrode coating but with 5 wt% PVDF binder produced bow-like CV graphs in the range of 0–3 V [16], where any small increase in the anodic curve above 2 V was reversed in the cathodic curve and an irreversible anodic peak was observed only above 3.3 V [16]. It is clear from Fig. 4 of the present study that the employed SBR and PEDOT:PSS binders seem to result in a more narrow stability window in the range of 0–2 V.
Potential stability window studies via CV tests at 1 mV s−1 to different maximum voltage values for ELDLCs with electrolyte 1 M LiPF6/EC:EMC 50:50 v/v and electrodes manufactured via the doctor blade technique and two alternative binders: SBR and PEDOT:PSS, both at 5 wt% in the coating: a SBR5-db, b PED5-db
Figure 5 presents a comparison of CVs at 1 mV s−1 in the voltage range of 0–2 V for symmetric EDLCs with the different electrodes presented in Table 1. The specific electrode capacitance has been derived from each CV plot as an average during both charge and discharge phases and reported in Table 2. It is clear that the electrodes with 10 wt% PEDOT:PSS have the highest specific capacitance. Figure 5 illustrates that the doctor blade coated PED10-db CV curves exhibits the characteristics of surface redox pseudocapacitance [19] whereas the sprayed PED10-s CV curve clearly indicates intercalation and redox phenomena [19] with an anodic peak at 1.6 V and a cathodic peak at 0.6 V. It is clearly seen that PED10-s has better bulk diffusion characteristics to Li+ ions than PED10-db. As the SEM images and EDX analysis in Figs. 1 and 2 indicated a homogeneous PEDOT:PSS film covering the AC particles for the sprayed PED10-s electrode, Li+ ion transport and intercalation may be easier through this thin film compared to the PEDOT:PSS trapped within the carbon black aggregates in the doctor blade coated PED10-db electrode. Furthermore, it is possible that PED10-s has higher transverse diffusivity than PED10-db, due the fact that drop deposition processes such as spraying induce transverse PEDOT grain orientation [41] that reduces tortuosity in ion transport.
Figure 6 displays the CV plots for the electrodes with 10 wt% PEDOT:PSS at different scan rates. Pseudocapacitance effects are evident only at slow charge–discharge, where for PED10-s no peaks are visible for the CV of 20 mV s−1 or greater scan rates. The reason for this is that at high rates, there is no time for intercalation or redox processes which are slower than the capacitive processes. Fig.SI-1 shows that the SBR5-s electrode seems to have more rectangular voltammograms at high scan rates than the SBR5-db electrode which can be attributed to the low resistance of the former, as also seen in the Nyquist plot in Fig. 3 and Table 2. Similarly, PED5-s has more rectangular voltammograms at high scan rates than PED5-db in Fig. SI-1, which demonstrates that spraying of coating with 5 wt% binder, either of the two binders SBR or PEDOT:PSS, results in lower contact resistance than coating via doctor blade, and this leads to high power density.
Figure 7 presents the results of the GCD plots in the range of 0–2 V. Figure 7a, b display nearly symmetric and linear GCD lines at low currents for the EDLCs with SBR binder in the electrodes. A small curvature is visible in the charge phase at 0.5 and 1 mA for the PED5-s EDLC in Fig. 7d, indicating the pseudocapacitance effect. Such curvature in the charge phase can be seen more clearly for PED10-db in Fig. 7e and more obvious bumps related to intercalation and deeper redox are evident for PED10-s in Fig. 7f, agreeing with the CV results. More specifically, Fig. 7f presents small “plateaus” at 1.4–1.6 V during charge and 0.4–0.7 V during discharge, due to intercalation and bulk redox pseudocapacitance in agreement with the CVs in Fig. 6b. It is evident that PED10-s has lower energy efficiency in slow GCDs than the EDLCs with different electrodes, due to the strong bulk redox processes in PED10-s and the overpotential between the anodic and cathodic redox “plateaus”; this is a common problem in redox-type energy storage devices, such as batteries. PED10-s also demonstrates lower Coulombic efficiency compared to other electrodes which can be further explained by the slow CV in Fig. 6b, where the cathodic peak is smaller than the anodic peak, indicating that the bulk pseudocapacitance redox process is not fully reversible. This might be due to two reasons: (a) the diffusivity of PEDOT:PSS to Li+ ions is higher during the intercalation than the deintercalation stage; (b) the redox reaction is not fully reversible from PEDOT0.PSS−.Li+ to PEDOT+.PSS− in discharge, due to previous distortion of the PEDOT:PSS structure during intercalation.
Table 2 presents the electrode specific capacitance for all types of electrodes in discharge from a slow GCD test at 0.113 mA cm−2. The basic EDLC with SBR5-db electrodes has Cel,sp = 54.4 F g−1 at 0.113 mA cm−2, which becomes Cel,sp = 49.7 F g−1 at 0.56 mA cm−2 that is a little higher than Cel,sp = 46.5 F g−1 at 0.56 mA cm−2 for an EDLC with the same electrolyte and doctor blade coated electrodes of the same active materials but with 5 wt% PVDF binder [16]. The electrodes with 10 wt% PEDOT:PSS binder have the highest electrode specific capacitance during GCD discharge in Table 2, which is attributed to the additional pseudocapacitance effects on top of the capacitive features of the high surface area AC electrodes.
Figure 8 presents the Ragone plots derived from the discharge phase of the GCD curves in the voltage range of 0–2 V: The highest specific energy in discharge amongst all electrodes is exhibited by PED10-db, 7.75 W h kgels−1. In general (with the exception of PED10-s), EDLCs with sprayed electrodes reach higher power density than doctor blade coated electrodes, with PED5-s exhibiting the highest power density, 5 kW kgels−1. The high-ESR resistance for PED10-s displayed in Fig. 3, due to multilayer contact resistances, leads to lower power density than the EDLCs with any of the other electrode types. In our previous study with the same electrolyte and electrode material but with 5 wt% PVDF binder [16], the Ragone plot reached higher energy density due to the fact that that EDLC was stable and tested in a wider potential range of 0–3 V, but still exhibited lower power density than the high values demonstrated in Fig. 8, even within the lower range of 0–2 V in the present study.
Ragone plot derived from the discharge phase of the GCD test data for EDLCs with electrolyte 1 M LiPF6/EC:EMC 50:50 v/v and different electrodes; power and energy density with respect to the mass of both electrodes; solid symbols and solid lines denote doctor blade electrode coatings; open symbols and broken lines denote sprayed electrode coatings
4 Conclusion
The present study explored AC electrode coatings for EDLCs with lithium-ion electrolyte, where two different aqueous binders were investigated, SBR and PEDOT:PSS, at 5 and 10 wt% binder concentration, and two different coating fabrication techniques: doctor blade and spray coating. In particular, the question of pseudocapacitance of PEDOT:PSS against Li+ ions was researched.
The first part of the electrochemical testing focused on establishing the potential window of symmetric EDLCs in electrolyte 1 M LiPF6 in EC:EMC 50:50 v/v. Cyclic voltammetry at 1 mV s−1 showed CVs with clear anodic peak rises for voltages higher than 2.7 V for both types of binder and a stability window of 0–2 V was concluded within which the rest of the electrochemical testing was carried out for all cells.
CVs at 1 mV s−1 in the voltage range of 0–2 V for symmetric EDLCs with the different electrodes displayed evident pseudocapacitance effects for electrodes with 10 wt% PEDOT:PSS binder, manifesting as surface redox pseudocapacitance [19] in the doctor blade coated PED10-db electrodes and as intercalation and redox phenomena [19] for the sprayed PED10-s electrodes. The pseudocapacitance-related peaks or bumps in the CVs decreased with increasing scan rate, with no visible peaks above 20 mV s−1. SEM/EDX was instrumental in showing the morphological characteristics of the coatings fabricated using doctor blade or spraying technique and corroborated the explanation for the difference in pseudcapacitance mechanisms between PED10-s and PED10-db electrodes: The PED10-s microstructure seems to feature an even thin layer of PEDOT:PSS covering the particles, with high transverse diffusivity for Li+ ion intercalation and bulk redox as it has been found in past study [41] that drop deposition techniques result in transverse PEDOT grain orientation. On the other hand, the PED10-db microstructure features PEDOT:PSS trapped in large domains of aggregated carbon black nanoparticles, which would prevent diffusion of Li+ ions in the bulk of the binder; as a result, PED10-db exhibits only surface redox pseudocapacitance in the CV plots.
These effects were replicated in the galvanostatic charge discharge tests in the range of 0–2 V, the data of which were translated to Ragone plots of energy versus power density for discharge. From these Ragone plots it was seen that doctor blade coated electrodes demonstrated the highest energy density compared to sprayed electrodes, with PED10-db coatings (doctor blade coated with 10 wt % PEDOT:PSS) displaying the highest energy density, 7.75 W h kgels−1, which was 5% higher than the energy density of the next high energy performing electrodes, SBR5-db (doctor blade coated with 5 wt% SBR binder). In general, EDLCs with sprayed electrodes reached higher power density than doctor blade coated electrodes, where sprayed electrodes with 5 wt% PEDOT:PSS demonstrated the highest power density, 5 kW kgels−1.
References
Chen K, Xue D (2017) Multiple functional biomass-derived activated carbon materials for aqueous supercapacitors, lithium-ion capacitors and lithium-sulfur batteries. Chin J Chem 35:861–866. https://doi.org/10.1002/cjoc.201600785s
Zuo W, Li R, Zhou C, Li Y, Xia J, Liu J (2017) Battery-supercapacitor hybrid devices: recent progress and future prospects. Adv Sci 4:1600539. https://doi.org/10.1002/advs.201600539
Markoulidis F, Lei C, Lekakou C (2017) Investigations of activated carbon fabric-based supercapacitors with different interlayers via experiments and modelling of electrochemical processes of different timescales. Electrochim Acta 249:122–134. https://doi.org/10.1016/j.electacta.2017.07.182
Fields R, Lei C, Markoulidis F, Lekakou C (2016) The composite supercapacitor. Energy Technol 4:517–525. https://doi.org/10.1002/ente.201500328
Lei C, Amini N, Markoulidis F, Wilson P, Tennison S, Lekakou C (2013) Activated carbon from phenolic resin with controlled mesoporosity for an electric double-layer capacitor (EDLC). J Mater Chem A 1:6037–6042. https://doi.org/10.1039/C3TA01638B
Vermisoglou EC, Giannakopoulou T, Romanos G, Giannouri M, Boukos N, Lei C, Lekakou C, Trapalis C (2015) Effect of hydrothermal reaction time and alkaline conditions on the electrochemical properties of reduced graphene oxide. Appl Surf Sci A 358:100–109. https://doi.org/10.1016/j.apsusc.2015.08.127
Vermisoglou EC, Giannakopoulou T, Romanos GE, Boukos N, Giannouri M, Lei C, Lekakou C, Trapalis C (2015) Non-activated high surface area expanded graphite oxide for supercapacitors. Appl Surf Sci A 358:110–121. https://doi.org/10.1016/j.apsusc.2015.08.123
Vermisoglou EC, Giannakopoulou T, Romanos G, Boukos N, Psycharis V, Lei C, Lekakou C, Petridis D, Trapalis C (2017) Graphene-based materials via benzidine-assisted exfoliation and reduction of graphite oxide and their electrochemical properties. Appl Surf Sci 392:244–255. https://doi.org/10.1016/j.apsusc.2016.08.079
Lei C, Markoulidis F, Wilson P, Lekakou C (2016) Phenolic carbon cloth-based electric double-layer capacitors with conductive interlayers and graphene coating. J Appl Electrochem 46(2):251–258. https://doi.org/10.1007/s10800-015-0909-x
Lei C, Lekakou C (2013) Activated carbon–carbon nanotube nanocomposite coatings for supercapacitor application. Surf Coat Technol 232:326–330. https://doi.org/10.1016/j.surfcoat.2013.05.027
Markoulidis F, Lei C, Lekakou C, Duff D, Khalil S, Martorana B, Cannavaro I (2014) A method to increase the energy density of supercapacitor cells by the addition of multiwall carbon nanotubes into activated carbon electrodes. Carbon 68:58–66. https://doi.org/10.1016/j.carbon.2013.08.040
Lekakou C, Moudam O, Markoulidis F, Andrews T, Watts JF, Reed GT (2011) Carbon-based fibrous EDLC capacitors and supercapacitors. J Nanotechnol 2011:409382. https://doi.org/10.1155/2011/409382
Amico S, Lekakou C (2004) Flow through a two-scale porosity, oriented fibre porous medium. Transp Porous Media 54:35–53. https://doi.org/10.1023/A:1025799404038
Huang J, Sumpter BG, Meunier V (2008) Theoretical model for nanoporous carbon supercapacitors. Angew Chem Int Ed 47:520–524. https://doi.org/10.1002/anie.200703864
Zhang T, Fuchs B, Secchiaroli M, Wohlfahrt-Mehrens M, Dsoke S (2016) Electrochemical behavior and stability of a commercial activated carbon in various organic electrolyte combinations containing Li-salts. Electrochim Acta 218:163–173. https://doi.org/10.1016/j.electacta.2016.09.126
Markoulidis F, Bates J, Lekakou C, Slade R, Laudone GM (2020) Supercapacitors with lithium-ion electrolyte: an experimental study and design of the activated carbon electrodes via modelling and simulations. Carbon 164:422–434. https://doi.org/10.1016/j.carbon.2020.04.017
Ruschhaupt P, Varzi A, Passerini S (2020) Natural polymers as green binders for high-loading supercapacitor electrodes. Chemsuschem 13:763–770. https://doi.org/10.1002/cssc.201902863
Lei C, Wilson P, Lekakou C (2011) Effect of poly(3,4-ethylenedioxythiophene) (PEDOT) in carbon-based composite electrodes for electrochemical supercapacitors. J Power Sources 196:7823–7827. https://doi.org/10.1016/j.jpowsour.2011.03.070
Gogotsi Y, Penner RM (2018) Energy storage in nanomaterials—capacitive, pseudocapacitive, or battery-like? ACS Nano 12:2081–2083. https://doi.org/10.1021/acsnano.8b01914
Subramanian V, Zhu H, Vajtai R, Ajayan PM, Wei B (2005) Hydrothermal synthesis and pseudocapacitance properties of MnO2 nanostructures. Phys Chem B 109(43):20207–20214. https://doi.org/10.1021/jp0543330
Markoulidis F, Todorova N, Grilli R, Lekakou C, Trapalis C (2019) Composite electrodes of activated carbon and multiwall carbon nanotubes decorated with silver nanoparticles for high power energy storage. J Compos Sci 3(4):97. https://doi.org/10.3390/jcs3040097
Vermisoglou EC, Devlin E, Giannakopoulou T, Romanos G, Boukos N, Psycharis V, Lei C, Lekakou C, Petridis D, Trapalis C (2014) Reduced graphene oxide/iron carbide nanocomposites for magnetic and supercapacitor applications. J Alloys Compd 590:102–109. https://doi.org/10.1016/j.jallcom.2013.11.087
Meng Q, Cai K, Chen Y, Chen L (2017) Research progress on conducting polymer based supercapacitor electrode materials. Nano Energy 36:268–285. https://doi.org/10.1016/j.nanoen.2017.04.040
Vermisoglou EC, Giannouri M, Todorova N, Giannakopoulou T, Lekakou C, Trapalis C (2016) Recycling of typical supercapacitor materials. Waste Manag Res 34:337–344. https://doi.org/10.1177/0734242X15625373
Kampouris EM, Papaspyrides CD, Lekakou CN (1988) A model process for the solvent recycling of polystyrene. Polym Eng Sci 28:534–537. https://doi.org/10.1002/pen.760280808
Kampouris EM, Papaspyrides CD, Lekakou CN (1987) A model recovery process for scrap polystyrene foam by means of solvent systems. Conserv Recycl 10:315–319. https://doi.org/10.1016/0361-3658(87)90062-2
Murugesh AK, Uthayanan A, Lekakou C (2010) Electrophoresis and orientation of multiple wall carbon nanotubes in polymer solution. Appl Phys A 100:135–144
Gao X, Zu L, Cai X, Li C, Lian H, Liu Y, Wang X, Cui X (2018) High performance of supercapacitor from PEDOT:PSS electrode and redox iodide ion electrolyte. Nanomaterials 8:335. https://doi.org/10.3390/nano8050335
Ge Y, Jalili R, Wang C, Zheng T, Chao Y, Wallace GG (2017) A robust free-standing MoS2/poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) film for supercapacitor applications. Electrochim Acta 235:348–355. https://doi.org/10.1016/j.electacta.2017.03.069
Khasim S, Pasha A, Badi N, Lakshmi M, Mishra Y-K (2020) High performance flexible supercapacitors based on secondary doped PEDOT–PSS–graphene nanocomposite films for large area solid state devices. RSC Adv 18:10526–10539. https://doi.org/10.1039/D0RA01116A
Li Y, Ren G, Zhang Z, Teng C, Wu Y, Lu X, Zhu Y, Jiangac L (2016) A strong and highly flexible aramid nanofibers/PEDOT:PSS film for all-solid-state supercapacitors with superior cycling stability. J. Mater. Chem. A 4:17324–17332. https://doi.org/10.1039/C6TA06981A
Arena A, Donato N, Saitta G (2007) Electrical characterization of solid-state heterojunctions between PEDOT:PSS and an anionic polyelectrolyte. Microelectron J 38:678–681. https://doi.org/10.1016/j.mejo.2007.05.011
Marcus Y (2015) Ions in solution and their solvation. eBook Wiley, pp. 32–34, 55. https://doi.org/10.1002/9781118892336
Lei C, Fields R, Wilson P, Lekakou C, Amini N, Tennison S, Perry J, Gosso M, Martorana B (2020) Development and evaluation of a composite supercapacitor-based 12 V transient start-stop (TSS) power system for vehicles: modelling, design and fabrication scaling up. Proc Inst Mech Eng A. https://doi.org/10.1177/0957650920930166
Zhu C, Fu Y, Yu Y (2019) Designed nanoarchitectures by electrostatic spray deposition for energy storage. Adv Mater 31:1803408. https://doi.org/10.1002/adma.201803408
Yuan T, Li W-T, Zhang W, He Y-S, Zhang C, Liao X-Z, Ma Z-F (2014) One-pot spray-dried graphene sheets-encapsulated nano-li4ti5o12 microspheres for a hybrid BatCap system. Ind Eng Chem Res 53:10849–10857. https://doi.org/10.1021/ie501287a
Huang C, Zhang J, Young NP, Snaith HJ, Grant PS (2016) Solid-state supercapacitors with rationally designed heterogeneous electrodes fabricated by large area spray processing for wearable energy storage applications. Sci Rep 6:25684. https://doi.org/10.1038/srep25684
Bresser D, Buchholz D, Moretti A, Varzi A, Passerini S (2018) Alternative binders for sustainable electrochemical energy storage—the transition to aqueous electrode processing and bio-derived polymers. Energy Environ Sci 11:3096–3127. https://doi.org/10.1039/C8EE00640G
Wilson P, Lekakou C, Watts JF (2012) A comparative assessment of surface microstructure and electrical conductivity dependence on co-solvent addition in spin coated and inkjet printed poly(3,4-ethylenedioxythiophene):polystyrene sulfonate (PEDOT:PSS). Org Electron 13(3):409–418
Wilson P, Lekakou C, Watts JF (2013) In-plane conduction characterisation and charge transport model of DMSO co-doped, inkjet printed Poly(3,4-ethylenedioxythiophene): polystyrene sulfonate (PEDOT:PSS). Org Electron 14:3277–3285. https://doi.org/10.1016/j.orgel.2013.09.025
Wilson P, Lei C, Lekakou C, Watts JF (2014) Transverse charge transport in inkjet printed poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS). Org Electron 15(9):2043–2051. https://doi.org/10.1016/j.orgel.2014.05.018
Wilson P, Lekakou C, Watts JF (2014) System design and process optimisation for the inkjet printing of PEDOT:PSS. ASME Trans 2(1):1–10. https://doi.org/10.1115/1.4026272
Huang C, Grant PS (2013) One-step spray processing of high power all-solid-state supercapacitors. Sci Rep 3:2393. https://doi.org/10.1038/srep02393
Acknowledgements
We gratefully acknowledge funding of this study by EPSRC under ISCF Wave 1, Project EP/R022852/1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Markoulidis, F., Dawe, A. & Lekakou, C. Electrochemical double-layer capacitors with lithium-ion electrolyte and electrode coatings with PEDOT:PSS binder. J Appl Electrochem 51, 373–385 (2021). https://doi.org/10.1007/s10800-020-01497-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-020-01497-y