Abstract
Purpose
Glaucoma is the leading cause of irreversible blindness worldwide. It is estimated that over 60 million people around the world have this disease, with only part of them knowing they have it. Timely and early diagnosis is vital to delay/prevent patient blindness. Deep learning (DL) could be a tool for ophthalmologists to give a more informed and objective diagnosis. However, there is a lack of studies that apply DL for glaucoma detection to Latino population. Our contribution is to use transfer learning to retrain MobileNet and Inception V3 models with images of the retinal nerve fiber layer thickness map of Mexican patients, obtained with optical coherence tomography (OCT) from the Instituto de la Visión, a clinic in the northern part of Mexico.
Methods
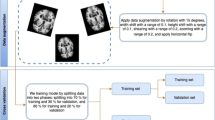
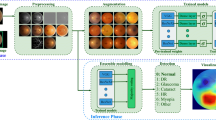
The IBM Foundational Methodology for Data Science was used in this study. The MobileNet and Inception V3 topologies were chosen as the analytical approaches to classify OCT images in two classes, namely glaucomatous and non-glaucomatous. The OCT files were collected from a Zeiss OCT machine at the Instituto de la Visión, and classified by an expert into the two classes under study. These images conform a dataset of 333 files in total. Since this research work is focused on RNFL thickness map images, the OCT files were cropped to obtain only the RNFL thickness map images of the corresponding eye. This action was carried out for images in both classes, glaucomatous and non-glaucomatous. Since some images were damaged (with black spots in which data was missing), these images were cut-out and cut-off. After the preparation process, 50 images per class were used for training. Fifteen images per class, different than the ones used in the training stage, were used for running predictions. In total, 260 images were used in the experiments, 130 per eye. Four models were generated, two trained with MobileNet, one for the left eye and one for the right eye, and another two trained with Inception V3. TensorFlow was used for running transfer learning.
Results
The evaluation results of the MobileNet model for the left eye are, accuracy: 86%, precision: 87%, recall: 87%, and F1 score: 87%. The evaluation results of the MobileNet model for the right eye are, accuracy: 90%, precision: 90%, recall: 90%, and F1 score: 90%. The evaluation results of the Inception V3 model for the left eye are, accuracy: 90%, precision: 90%, recall: 90%, and F1 score: 90%. The evaluation results of the Inception V3 model for the right eye are, accuracy: 90%, precision: 90%, recall: 90%, and F1 score: 90%.
Conclusion
In average, the evaluation results for right eye images were the same for both models. The Inception V3 model showed slight better average results than the MobileNet model in the case of classifying left eye images.







Similar content being viewed by others
Availability of data
The dataset used in this study is available online: shorturl.at/aCGLQ.
Code availability
The code that was used for retraining is available online: https://github.com/tensorflow/hub/blob/master/examples/image_retraining/retrain.py.
References
M. S. Berlin, “Method for treating glaucoma,” English, pat. US9820883B2, Nov. 2017. [Online]. Available: https://patentimages.storage.googleapis.com/c6/78/39/2710a8cad38909/US9820883.pdfx
Glaucoma Research Foundation, January is glaucoma awareness month, Jan. 2020. [Online]. Available: https://www.glaucoma.org/news/glaucoma-awarenessmonth.php.
Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y (2014) Global prevalence of glaucoma and projections of glaucoma burden through 2040. Ophthalmology 121(11):2081–2090. https://doi.org/10.1016/j.ophtha.2014.05.013
M. I. Azcona-Cruz, M. Ríos-Lobo, and S. Amador-Jiménez, “Glaucoma: Aspectos relevantes para la detección oportuna,” Salud y Administración, vol. 2, no. 4, pp. 23–25, Jan. 2015, issn: 2448–6159. [Online]. Available: http://www.unsis.edu.mx/revista/doc/vol2num4/A3_Glaucoma.pdf.
Wiggs JL, Pasquale LR (2017) Genetics of glaucoma. Hum Mol Genet 26(R1):R21–R27. https://doi.org/10.1093/hmg/ddx184.[Online]
K. Boyd, What is Glaucoma? Accessed: 08/28/19, American Academy of Ophthalmology, Aug. 2019. [Online]. Available: https://www.aao.org/eye-health/diseases/what-is-glaucoma
Weinreb RN, Aung T, Medeiros FA (2014) The pathophysiology and treatment of glaucoma. JAMA 311(18):1901. https://doi.org/10.1001/jama.2014.3192
Bussel II, Wollstein G, Schuman JS (2014) “OCT for glaucoma diagnosis, screening and detection of glaucoma progression. Brit J Ophthalmol 98(Suppl II):15–19. https://doi.org/10.1136/bjophthalmol-2013-304326
Ting DS, Peng L, Varadarajan AV, Keane PA, Burlina PM, Chiang MF, Schmetterer L, Pasquale LR, Bressler NM, Webster DR, Abramoff M, Wong TY (2019) Deep learning in ophthalmology: the technical and clinical considerations. Progress Retinal Eye Res. https://doi.org/10.1016/j.preteyeres.2019.04.003
Abbas Q (2017) Glaucoma-Deep: detection of glaucoma eye disease on retinal fundus images using deep learning. Int J Adv Comput Sci Appl 8(6):41–45. https://doi.org/10.14569/ijacsa
X. Chen, Y. Xu, D. W. Kee Wong, T. Y. Wong, and J. Liu, “Glaucoma detection based on deep convolutional neural network,” In: 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 2015, pp. 715–718. doi: https://doi.org/10.1109/EMBC.2015.7318462. [Online]. Available: https://ieeexplore.ieee.org/document/7318462
K. R. Patel, G. Lee, M. Durbin, M. Wall, P. Artes, and J. Flanagan, Deep neural network based glaucoma detection using RNFL thickness map, Poster 1470 - A0154, Apr. 2019. [Online]. Available: https://www.zeiss.com/content/dam/Meditec/ref_master/events/arvo/posters/patel_arvo_glaucoma_detection_using_rnfl_thickness_map_v3.pdf.
Li Z, He Y, Keel S, Meng W, Chang RT, He M (2018) Efficacy of a deep learning system for detecting glaucomatous optic neuropathy based on color fundus photographs. Ophthalmology 125(8):1199–1206
Shibata N, Tanito M, Mitsuhashi K, Fujino Y, Matsuura M, Murata H, Asaoka R (2018) Development of a deep residual learning algorithm to screen for glaucoma from fundus photography. Sci Rep. https://doi.org/10.1038/s41598-018-33013-w
A. G. Howard, Mobilenets: Open-source models for efficient on-device vision, Jun. 2017. [Online]. Available: https://ai.googleblog.com/2017/06/mobilenetsopen-source-models-for.html
A. G. Howard, M. Zhu, B. Chen, D. Kalenichenko, W. Wang, T. Weyand, M. Andreetto, and H. Adam, “MobileNets: Efficient Convolutional Neural Networks for Mobile Vision Applications,” Apr. 17, 2017
C. Szegedy, V. Vanhoucke, S. Ioffe, J. Shlens, and Z. Wojna, “Rethinking the Inception Architecture for Computer Vision,” Dec. 2, 2015. https://arxiv.org/abs/1512.00567v3[cs.CV]
Google Cloud, Advanced guide to Inception V3 on Cloud TPU, English, Google, Jan. [Online]. Available: https://cloud.google.com/tpu/docs/inceptionv3-advanced
American Academy of Ophthalmology, Theories of Glaucomatous Optic Nerve Damage. [Online]. Available: https://www.aao.org/bcscsnippetdetail.aspx?id=f19571e0-b8d7–4679-a2c5-fd30a9b016c1
Ahmad SS (2016) Controversies in the vascular theory of glaucomatous optic nerve degeneration. Taiwan J Ophthalmol 6(4):182–186. https://doi.org/10.1016/j.tjo.2016.05.009
L. A. Remington, “Chapter 4 - retina,” in Clinical Anatomy and Physiology of the Visual System (Third Edition), L. A. Remington, Ed., Third Edition, Saint Louis: Butterworth-Heinemann, 2012, pp. 61–92, ISBN: 978–1–4377–1926–0. https://doi.org/10.1016/B978-1-4377-1926-0.10004-9. [Online]. Available: http://www.sciencedirect.com/science/article/pii/B9781437719260100049.
J. W. Shin, M. Seong, J. W. Lee, E. H. Hong, and K. B. Uhm, “Diagnostic ability of retinal nerve fiber layer thickness deviation map for localized and diffuse retinal nerve fiber layer defects. J Ophthalmol, vol. 2017, pp. 1–9, 2017 https://doi.org/10.1155/2017/8365090. [Online]. Available: https://www.ncbi.nlm.nih.gov/ pmc/articles/PMC5259680/#B1
T. Shaarawy, M. Sherwood, R. Hitchings, and J. Crowston, Glaucoma. Elsevier, 2015. https://doi.org/10.1016/c2011-1-04562-9. [Online]. Available: https:// www.sciencedirect.com/topics/medicine-and-dentistry/retinal-nervefiber-layer.
Burgansky-Eliash Z, Wollstein G, Chu T, Ramsey JD, Glymour C, Noecker RJ, Ishikawa H, Schuman JS (2005) Optical coherence tomography machine learning classifiers for glaucoma detection: a preliminary study. Invest Opthalmol Vis Sci 46(11):4147. https://doi.org/10.1167/iovs.05-0366
Menke MN, Feke GT, Trempe CL (2005) OCT measurements in patients with optic disc edema. Invest Opthalmol Vis Sci 46(10):3807. https://doi.org/10.1167/iovs.05-0352
L. Deng and D. Yu (2014) Deep learning: methods and applications. Foundations and Trends® in Signal Processing 7(3–4):197–387. https://doi.org/10.1561/2000000039
MathWorks, What is Deep Learning? 3 Things You Need To Know, English, MathWorks. [Online]. Available: https://www.mathworks.com/discovery/deep-learning.html
M. Mishra, Convolutional Neural Networks, Explained, English, Oracle, Mar. [Online]. Available: https://www.datascience.com/blog/convolutionalneural-network
G. Zaccone, M. R. Karim, and A. Menshawy, Deep Learning with TensorFlow: Explore neural networks with Python. Packt Publishing, 2017, ISBN: 978-1-78646-978-6
TensorFlow, Why TensorFlow. [Online]. Available: https://www.tensorflow.org/
——, What is transfer learning? [Online]. Available: https://www.tensorflow.org/js/tutorials/transfer/what_is_transfer_learning
I. Goodfellow, Y. Bengio, and A. Courville 2016 Deep learning. MIT Press [Online]. Available: http://www.deeplearningbook.org
Quigley HA (2001) The prevalence of glaucoma in a population-based study of hispanic subjects. Arch Ophthalmol 119(12):1819. https://doi.org/10.1001/archopht.119.12.1819
Leung CK, Lam S, Weinreb RN, Liu S, Ye C, Liu L, He J, Lai GW, Li T, Lam DS (2010) Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography”. Ophthalmology 117(9):1684–1691. https://doi.org/10.1016/j.ophtha.2010.01.026
Murtagh P (2020) Current applications of machine learning in the screening and diagnosis of glaucoma: a systematic review and meta-analysis. Int J Ophthalmol 13(1):149–162. https://doi.org/10.18240/ijo.2020.01.22
L. S. Shigueoka, J. P. C. de Vasconcellos, R. B. Schimiti, A. S. C. Reis, G. O. de Oliveira, E. S. Gomi, J. A. R. Vianna, R. D. dos Reis Lisboa, F. A. Medeiros, and V. P. Costa, “Automated algorithms combining structure and function outperform general ophthalmologists in diagnosing glaucoma,” PLOS ONE, vol. 13, no. 12, B. Mortazavi, Ed., e0207784, 2018. doi: https://doi.org/10.1371/journal.pone.0207784
Christopher M, Belghith A, Weinreb RN, Bowd C, Goldbaum MH, Saunders LJ, Medeiros FA, Zangwill LM (2018) 2018 Retinal nerve fiber layer features identified by unsupervised machine learning on optical coherence tomography scans predict glaucoma progression”. Invest Opthalmol Vis Sci 59(7):2748. https://doi.org/10.1167/iovs.17-23387
S. J. Kim, K. J. Cho, and S. Oh, “Development of machine learning models for diagnosis of glaucoma,” PLOS ONE, vol. 12, no. 5, B. Liu, Ed., e0177726, May 2017. doi: https://doi.org/10.1371/journal.pone.0177726. [Online]. Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5441603/
M. A. Espinoza, G. H. Alferez, and J. Castillo, “Predicción de Glaucoma Mediante Redes Neuronales Convolucionales,” In: Proceedings of the 2018 International Conference on Health Informatics and Medical Systems (HIMS 2018), Las Vegas, NV, USA, 2018
Asaoka R, Murata H, Hirasawa K, Fujino Y, Matsuura M, Miki A, Kanamoto T, Ikeda Y, Mori K, Iwase A, Shoji N, Inoue K, Yamagami J, Araie M (2019) Using deep learning and transfer learning to accurately diagnose early-onset glaucoma from macular optical coherence tomography images. Am J Ophthalmol 198:136–145. https://doi.org/10.1016/j.ajo.2018.10.007
Christopher M, Belghith A, Bowd C, Proudfoot JA, Goldbaum MH, Weinreb RN, Girkin CA, Liebmann JM, Zangwill LM (2018) Performance of deep learning architectures and transfer learning for detecting glaucomatous optic neuropathy in fundus photographs. Sci Rep. https://doi.org/10.1038/s41598-018-35044-9
J. B. Rollins, Metodología Fundamental para la Ciencia de Datos, Jun. 2015. [Online]. Available: https://www.ibm.com/downloads/cas/WKK9DX51
A. Canziani, A. Paszke, and E. Culurciello, “An Analysis of Deep Neural Network Models for Practical Applications,” May 24, 2016. arXiv: http://arxiv.org/abs/1605.07678v4[cs.CV]
Author information
Authors and Affiliations
Contributions
LGO participated in the design of the study, carried out the study, drafted the manuscript and performed the evaluation of the results. GHA participated in the study’s coordination, helped to draft the manuscript, and involved in revising the manuscript. JC helped to coordinate and revise the manuscript. All authors read and approved the final manuscript, Glaucoma detection in Latino population through OCT’s RNFL thickness map using transfer learning.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest or competing interest in this research work.
Ethics approval
All procedures performed in this study were in accordance with the ethical standards of the Instituto de la Visión and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consents were obtained from the subjects after explanation of the nature of the study.
Consent for publication
The patients agreed to collect and publish the data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Olivas, L.G., Alférez, G.H. & Castillo, J. Glaucoma detection in Latino population through OCT’s RNFL thickness map using transfer learning. Int Ophthalmol 41, 3727–3741 (2021). https://doi.org/10.1007/s10792-021-01931-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-021-01931-w