Abstract
Purpose
To compare the 27G versus 25G vitrectomy in patients with epiretinal membrane (ERM).
Patients and methods
Sixty pseudophakic eyes of 60 consecutive patients treated by pars plana vitrectomy (PPV) using 27G (30 eyes) or 25G (30 eyes) were prospectively evaluated including eye’s inflammation, surgery time, ERM + ILM removal time and complications. Additionally, 1, 3, 7, 14, 30, 90 and 180 days after PPV, the following were estimated: intraocular pressure (IOP), sclerotomy wound closure time, distance best corrected visual acuity (DBCVA), foveal macular thickness (FMT) and surgically induced astigmatism (SIA).
Results
The eye’s inflammation resolved within 30 days after surgery in both groups. The surgery and ERM + ILM times were longer in the 27G group (p ≤ 0.02). The most common postoperative complication was hypotony in both groups, more common in 25G group (23.3% vs. 10% of eyes). In 27G group, the mean IOP prior to 180 days postoperatively was higher (p < 0.05) and the sclerotomy wound closure time was shorter (p < 0.001). Mean DBCVA values (7, 14, 30 days after surgery) were significantly better in 27G group (p < 0.001). The mean FMT values were similarly and significantly reduced in both groups 1 day postoperatively (p < 0.05) as compared to preoperative values and then stabilized during follow-up. Mean SIA was lower in 27G group 30, 90 and 180 days after surgery (p < 0.001).
Conclusion
The use of 27G PPV in patients with ERM significantly reduced sclerotomy wound closure time and surgically induced astigmatism, better stabilized intraocular pressure and allowed to achieve faster visual acuity improvement, as compared to 25G PPV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transconjunctival microincision vitrectomy with 25G/23G (0.5/0.7 mm in diameter, respectively) instrumentation resulted in a low rate of intraoperative and postoperative complications, such as hypotony and endophthalmitis [1, 2]. Nowadays, progress in vitrectomy devices, stiffness of instruments and light sources have led to the development of 27G (0.4 mm in diameter) transconjunctival sutureless vitrectomy [3]. The use of 27G PPV in the treatment for posterior segment diseases [4,5,6] becomes more and more common, including the surgical treatment of some macular diseases, such as epiretinal membranes. There are no conclusive study results in the literature available that would indicate superiority of 27G vitrectomy over 25G vitrectomy in the treatment for epiretinal membranes [7,8,9,10]. That is why we decided to conduct this prospective study on 60 eyes with idiopathic epiretinal membranes undergoing 27G or 25G vitrectomy and compare postoperative outcomes.
Patients and methods
A prospective, comparative study was conducted, involving pseudophakic 60 eyes of 60 patients with ERM treated with 27G PPV (30 eyes) or 25G PPV (30 eyes) at 2nd Department of Ophthalmology, Pomeranian Medical University in the year 2017. This study adhered to principles of the Declaration of Helsinki and was approved by the local ethics committee. The written informed consent was obtained from all patients participating in the study. For comparison purposes, the surgical methodology, exclusion criteria, parameters analyzed before and after PPV were similar to those described by Mitsui et al. [7]. Patients with prior sclera buckling procedure, PPV, high myopia > than − 8.00 diopters, diabetes or cataract were excluded from the study. All surgeries were performed by one right-handed surgeon (WL) using the Alcon Constellation Vision System (Alcon Laboratories, Fort Worth, TX, USA) through three-port trocar cannula system. The surgical parameters for 25 GPPV were as follows: cutting rate 5000 cuts per minute (cpm), linear aspiration 0–650 mmHg, for 27 GPPV: cutting rate 7500 cpm, linear aspiration 0–650 mmHg. In both types of vitrectomy, IOP was controlled by a system and equal to 20 mmHg. For posterior visualization, surgical microscope, model Hi-R 900 (MÖLLER-WEDEL GmbH & Co. KG, Wedel, Germany), was used. The ERM and ILM were double stained using trypan blue dye.
The eyes with ERM were assigned for 25G or 27G surgery in alternating manner. The conjunctiva was moved from the planned sclerotomy site, and then the trocar was placed approximately 3.5 mm posteriorly to the limbus at 30° angle to the scleral surface in three quadrants: superotemporal, inferotemporal and superonasal. Insertion angle of the trocar was measured by our custom-made protractor (Fig. 1).
At the end of the PPV, the cannulas were removed and gentle massage of the sclerotomy site using a cotton-tipped applicator was performed.
Additionally, 1, 3, 7, 14, 30, 90 and 180 days after PPV, the following outcomes were estimated: inflammation of anterior and posterior segment of the eye (slit lamp, indirect ophthalmoscopy), intraocular pressure (IOP, Goldmann applanation tonometer), presence of sclerotomy gaps (AS-OCT CASIA 2, Tomey, Japan), distance best corrected visual acuity (DBCVA-logMAR), central (foveal) macular thickness (FMT-SD-OCT, Cirrus HD-OCT, Carl Zeiss Meditec, Dublin, CA, USA) and surgically induced astigmatism (SIA). The assessor was masked to the gauge of vitrectomy which the patient had. Hypotony was defined as an IOP < 7 mmHg. The duration of vitrectomy was defined as time of vitreous cutter operation; the surgery time for peeling was the duration of removal of ERM and inner limiting membrane (ILM) using vitreous forceps. The surgically induced astigmatism (SIA) was measured using methodology described by Holladay et al. [11]. Wound closure was defined as the closure of all three sclerotomies in operated eye.
Statistical analysis
All statistical analyses of results obtained from 27G/25G groups were performed by means of Student’s t, Mann–Whitney U or Friedman’s ANOVA tests. p values of < 0.05 were defined as statistically significant.
Results
The characteristics of patients included in the study are shown in Table 1.
There were no significant differences concerning number of eyes, age, axial length, FMT, DBCVA-logMAR and IOP in 27G and 25G PPV groups. All patients from both groups were pseudophakic. The ERM and ILM were removed in all cases. Scleral or conjunctival sutures were not used. Fluid–gas exchange was not performed.
Inflammation of anterior and posterior segment of the eye
The inflammation (flare in the anterior segment—Tyndall effect, and presence of cells in posterior segment determined by means of Volk lens) of the eye resolved in all patients from both groups within 30 days after the surgery.
Surgery time
In 27G PPV group, the mean surgery time was significantly longer in comparison with 25G PPV group (12.2 ± 0.9 vs. 9.3 ± 1.0 min; p = 0.001). The mean time necessary for ERM + ILM removal was slightly but significantly prolonged in 27G group (8.2 ± 0.7 vs. 7.8 ± 0.5 min; p = 0.025) (Table 2).
Scleral wound closure
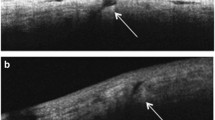
The mean time for wound closure for all sclerotomies was statistically significantly shorter in 27G group as compared to 25G group (14.1 ± 6.2 vs. 30.3 ± 9.5 days) (Figs. 2, 3).
Visual acuity changes
Seven days after surgery, log MAR DBCVA was significantly better as compared to baseline values in 27G group (p < 0.05), but not in 25G group (p > 0.05). In subsequent inspections (14, 30, 90 and 180 days after surgery), log MAR DBCVA was significantly better in both groups (p < 0.05) (Table 3, Fig. 4). There were significant differences in log MAR DBCVA values between the both groups at 7-, 14- and 30-day follow-up visits with better VA for 27G group (p = 0.001) (Table 3, Fig. 4).
Surgically induced astigmatism
In 27 G group, mean of SIA was significantly lower in comparison with mean SIA in 25G group (p < 0.05), Table 4.
Foveal macular thickness changes
In both groups, the mean FMT significantly decreased during follow-up period, as compared to baseline value (p < 0.05) but there were only insignificant differences in FMT between 27G and 25G groups in postoperative period analyzed (Table 5, Fig. 5).
Intraocular pressure changes
In 27G group, at 1, 3 and 7 days after surgery, significant decrease in IOP was observed as compared to preoperative value (p < 0.05), but in 25G group the effect was noted also at 14 and 30 days (p < 0.05). During follow-up between 1 and 90 days after surgery, in 27G group mean IOP values were significantly higher than in 25G group (p < 0.002). No significant differences between groups were observed at 180 day postoperatively (p < 0.372) (Table 6, Fig. 6).
Complications
At the first day after surgery, in 27G group hypotony was observed less commonly (3/30 of eyes 10%) than in 25G group (7/30 of eyes 23.3%). In both groups, retinal detachment in one eye was detected during follow-up period and additionally one macular hole in 27G group. In 27G group, the cause of retinal detachment was subretinal hemorrhage due to sudden increase in systemic blood pressure during the surgery. In 25G group, the cause of retinal detachment was peripheral tear formation 3 month after PPV. In both cases, successful additional PPV was performed. In 27G group, the cause of macular hole formation was remnants of inner limiting membrane. The removal of theses ILM remnants during next PPV resulted in the closure of macular hole and increase in VA. Choroidal detachment and endophthalmitis were not observed in any group.
Discussion
The results of the study presented suggest that in patients with ERM, 27G PPV provides more advantages as compared to 25G PPV, which was confirmed by other authors [8, 10]. The mean time of wound closure for all sclerotomies was significantly shorter (~ 16 days) in 27G PPV group as compared to 25G PPV group. Analysis of logMAR DBCVA showed faster return of visual acuity (difference ~ 1 log MAR DBCVA) between 7 and 30 days after surgery in 27G PPV group. In 27G group, mean SIA was significantly lower (30 days 0.3 D, 90 days 0.2 D, 180 days 0.13 D) as compared to mean SIA in 25G group. During follow-up between 1 and 90 days postoperatively, in 27G group mean IOP values were significantly higher than in 25G group (by ~ 1–4 mm Hg). The most prominent difference was shown at the first day after surgery (mean IOP higher by ~ 4 mmHg in 27G group). At the first day after surgery, in 27G group hypotony was observed less commonly (three eyes) than in 25G group (seven eyes).
One of the disadvantages of 27G PPV is prolonged surgery time of ERM. In our series of patients, the surgery time for 27G PPV group was significantly and approximately 3 min longer than that for 25G PPV. Prolonged surgery time for 27G PPV group was achieved also by Ito et al. (~ 0.5 min) [9], Mitsui et al. (~ 4 min) [7] and Naruse et al. (~ 4 min) [8]. The results obtained indicate that the reduced gauge of PPV instruments (lower infusion and aspiration rates) is responsible for longer time of surgery. Surgical trauma is a known cause of inflammation manifested by protein leakage and cell accumulation within the aqueous humor [12]. Prolonged time of surgery might be a cause for increase in flare in the anterior segment of the eye. The inflammation of the operated eyes resolved in all patients from both groups within 30 days after surgery. So the prolonged time in 27G group, as compared to 25G group, did not affect the duration of inflammation, which was also observed by other authors [7, 8].
It is reasonable to expect that 27G sclerotomies would close faster than 25G sclerotomies, as it was proven in the study. The mean time for all scleral wound closure was significantly shorter in 27G group, suggesting faster wound healing. In our study, the trocar was placed at 30° angle to the scleral surface. For the first time for this purpose, a custom-made protractor was used, permitting precisely performed oblique incisions. Such incisions made at precisely controlled angle contribute significantly to the prevention of wound leakage. The wound edges are pressed together and closed by intraocular pressure [1]. In 27G group, faster sclerotomies healing observed could have resulted in faster recovery of visual acuity, IOP and lower frequency of hypotony. Our results suggest that the size and precise angle of incision play an important role in terms of sclerotomy closure.
Mitsui et al. [7] did not observe significantly shorter scleral wound closure time associated with 27G PPV. It is not surprising, because in that study trocar insertion angle was not precisely determined, so there might be some variations in the angle. In consequence, shorter scleral tunnel could be created with higher possibility of wound leakage and prolonged healing. It is difficult to compare our result with the ones obtained by Naruse et al. [8] and Nakashina et al. [10] because of different angle of trocar placement, use of air or gas exchange, phacovitrectomy performance and more than one operating surgeon involved.
Our study results show that 27G PPV group featured earlier recovery of visual acuity in comparison with 25G PPV group and indicate superiority of surgery with smaller instrument gauge. The data obtained were confirmed by other authors [8].
Surgically induced astigmatism (SIA) may affect recovery of visual acuity after vitrectomy. Changes in the cornea shape after PPV may induce SIA. The SIA was significantly lower after 23G and 25G PPV as compared with 20G vitrectomy [13, 14]. In our study, in 27G PPV group, significantly smaller SIA was detected during the postoperative follow-up, as compared to 25G PPV group. Lower SIA associated with the use of 27G PPV as compared with the 25G system, albeit not significant, was also observed by Mitsui et al. [7]. This suggests that 27G PPV is less invasive than 25G PPV in terms of SIA.
In our study, the FMT was reduced significantly when compared to preoperative values, similarly to other studies [7, 8] and there were no differences between 27G and 25G groups during six-month follow-up period. This relationship suggests that 1-mm-diameter reduction of sclerotomy in 27G PPV, as compared to 25 GPPV, has no influence on the recovery of normal retinal structure in the macular region.
One of the important features which affects the recovery time of visual function is intraocular pressure level after vitrectomy. In our study, in 27G group IOP was significantly higher than in 25G group up to 90 days after the surgery and the most prominent difference was seen at first day postoperatively. This difference strongly suggests that smaller sclerotomy diameter with comparable length of scleral tunnel, as compared to 25G PPV, is responsible for faster sclerotomy closure and recovery of IOP to preoperative values. Similar relationships were noted by others [9]. One of the complications of microincision vitrectomy is hypotony due to evident or subclinical sclerotomy leakage. The oblique incision and gas tamponade are responsible for faster sclerotomy closure [15,16,17]. Also, smaller size of the wound has an impact on the closure of the sclerotomy and plays important role in hypotony prevention [15, 18]. In our study, hypotony (arbitrary defined as IOP less than 7 mmHg) frequency was lower for 27G PPV group than in 25G PPV group and confirmed the above-mentioned data. None of the patients had clinically significant hypotony in the form of hypotony maculopathy or choroidal detachment. In the study presented, we did not use gas tamponade; thus, small (0.4 mm size of sclerotomy) and precise, oblique incision was responsible for the reduction in hypotony incidence. In 27G PPV patients, reduced number or lack of hypotony was also observed by Mitsui [7], Naruse [8] and Ito [9].
In our study, the frequency of postoperative complications in 27/25G PPV groups was low and did not differ between the groups: vitreous hemorrhage and retinal detachment—one eye (1/30 eyes 3.3%) in both groups and additionally one case (1/30 eyes 3.3%) of macular hole formation in 27G PPV group. The results obtained were comparable to those achieved by Naruse et al. [8]. Results from other studies [19, 20] indicate that smaller gauge PPV is associated with low risk of complications. Our study results are consistent with this conclusion.
Postoperative retinal breaks may occur uncommonly (2.6%) [21] after PPV combining epiretinal membrane/inner limiting membrane peeling located centrally or extrafoveally as a full-thickness holes or pseudoholes [22]. It is suggested that weakening of the retinal glial structure as a consequence of Muller cell decapitation, opening of intraretinal cysts, direct mechanical trauma, dye toxicity and contraction of residual ILM may be responsible for hole formation. In our case in 27 GPPV group, residual ILM caused the macular hole formation and was treated successfully by next 27G PPV. There is no reason to associate this complication with small, 0.4-mm-diameter sclerotomies during 27G PPV. However, it cannot be excluded with certainty that this may be related to the tools used during surgery, i.e., 27G forceps.
The results of 6-month follow-up provided in this study indicate that the use of 27G PPV in patients with ERM significantly reduced sclerotomy wound closure time and surgically induced astigmatism, provided better stabilization of intraocular pressure and allowed faster achievement of visual acuity improvement as compared to 25G PPV. The above-mentioned advantages may determine the 27G PPV as a technique of choice in the surgical treatment of ERM.
Further prospective studies with larger number of cases are necessary to verify those findings.
References
Eckhardt C (2005) Transconjunctival sutureless 23G vitrectomy. Retina 25:208–211
Rizzo S, Genovesi-Ebert F, Murri S et al (2006) 25 G, sutureless vitrectomy and standard 20 G paras plana vitrectomy in idiopathic epiretinal membrane surgery: a comparative pilot study. Graefes Arch ClinExpOphthalmol 244:472–479
Oshima Y, Wakabayashi T, Sato T, Ohji M, Tano Y (2010) A 27-gauge instrument system for transconjunctival sutureless microincision vitrectomy surgery. Ophthalmology 117:93–102
Ali Khan M, Shahlaee A, Toussaint B et al (2016) Outcomes of 27 gauge microincision vitrectomy surgery for posterior disease. Am J Ophthalmol 161:36–43
Rizzo S, Barca F, Caporossi T, Mariotti C (2015) Twenty-seven-gauge vitrectomy for various vitreoretinal diseases. Retina 35:1273–1278
Yoneda K, Morikawa K, Oshima Y et al (2017) Surgical outcomes of 27-gauge vitrectomy for a consecutive series of 163 eyes with various vitreous diseases. Retina 37:2130–2137
Mitsui K, Kogo J, Takeda H et al (2016) Comparative study of 27 gauge versus 25 gauge vitrectomy for epiretinal membrane. Eye 30:538–544
Naruse S, Shimada H, Mori R (2017) 27-gauge and 25 gauge vitrectomy day surgery for idiopathic epiretinal membrane for idiopathic epiretinal membrane. BMC Ophthalmol 17:188
Ito M, Tanikawa A, Shimada Y, Horiguchi M (2016) Comparison of the operative times and intraocular pressure of sutureless vitrectomy with a 27- versus 25-gauge system in eyes with epiretinal membrane. Fujita Med J 2(4):62–65
Takashina H, Watanabe A, Tsuneoka H (2017) Perioperative changes of the intraocular pressure during the treatment of epiretinal membrane by using 25- or 27-gauge vitrectomy without gas tamponade. Clin Ophthalmol 11:739–743
Holladay JT, Cravy TV, Koch DD (1992) Calculating the surgical induced refractive change following ocular surgery. J Cataract Refract Surg 18:429–443
Nishino M, Eguchi H, Iwata A et al (2009) Are topical steroids essential after an uneventful cataract surgery? J Med Invest 56:11–15
Okamoto F, Okamoto C, Sakata N et al (2007) Changes in corneal topography after 25-gauge transconjunctival sutureless vitrectomy versus after 20-gauge standard vitrectomy. Ophthalmology 114:2138–2141
Park DH, Shin JP, Kim SY (2009) Surgically induced astigmatism in combined phacoemulsification and vitrectomy; 23-G transconjuctival sutureless vitrectomy versus -20 gauge standard vitrectomy. Graefes Atrch Clin Exp Ophthalmol 247:1331–1337
Singh RP, Bando H, Brasil OF et al (2008) Evaluation of wound closure using different incision techniques with 23-gauge and 25-gauge microincision vitrectomy systems. Retina 28(2):242–248
Shimada H, Nakashizuka H, Mori R et al (2006) 25-gauge scleral tunnel transconjunctival vitrectomy. Am J Ophthalmol 142(5):871–873
Hsu J, Chen E, Gupta OP et al (2008) Hypotony after 25-gauge 25-gauge vitrectomy using oblique versus direct cannula insertions in fluid- filled eyes. Retina 28(7):937–940
Chen D, Lian Y, Cui L, Lu F et al (2010) Sutureless vitrectomy incision architecture in the immediate postoperative period evaluated in vivo using optical coherence tomography. Ophthalmology 117(10):2003–2009
Sandali O, Sanharawi M, Lecuen N et al (2011) 25-, 23-, 20-gauge vitrectomy in epiretinal membrane surgery: a comparative study of 553 cases. Graefes Arch ClinExpOphthalmol 249:1811–1819
Haas A, Seidel G, Steinbrugger I, Maier R et al (2010) Twenty three-gauge and 20-gauge vitrectomy in epiretinal membrane surgery. Retina 30:112–116
Rush RB, Simunovic MP, Aragon AV, Ysasaga JE (2014) Postoperative macular hole formation after vitrectomy with internal limiting membrane peeling for the treatment of epiretinal membrane. Retina 34:890–896
Tachi N, Hashimoto Y, Kondo M et al (1997) Vitreous surgery for macular hole followed membrane peeling. Nihon Ganka Gakkai Zasshi 101:692–697
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lubiński, W., Gosławski, W., Podborączyńska–Jodko, K. et al. Comparison of 27-gauge versus 25-gauge vitrectomy results in patients with epiretinal membrane: 6-month follow-up. Int Ophthalmol 40, 867–875 (2020). https://doi.org/10.1007/s10792-019-01250-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-019-01250-1