Abstract
Rheumatoid arthritis (RA) stands as an autoimmune disorder characterized by chronic joint inflammation, resulting in profound physiological alterations within the body. Affecting approximately 0.4–1.3% of the global population, this condition poses significant challenges as current therapeutic approaches primarily offer symptomatic relief, with the prospect of complete recovery remaining elusive. This review delves into the contemporary advancements in understanding the pathophysiology, diagnosis, and the therapeutic potential of herbal medicine in managing RA. Notably, early diagnosis during the initial stages emerges as the pivotal determinant for successful recovery post-treatment. Utilizing tools such as Magnetic Resonance Imaging (MRI), anti-citrullinated peptide antibody markers, and radiography proves crucial in pinpointing the diagnosis of RA with precision. Unveiling the intricate pathophysiological mechanisms of RA has paved the way for innovative therapeutic interventions, incorporating plant extracts and isolated phytoconstituents. In the realm of pharmacological therapy for RA, specific disease-modifying antirheumatic drugs have showcased commendable efficacy. However, this conventional approach is not without its drawbacks, as it is often associated with various side effects. The integration of methodological strategies, encompassing both pharmacological and plant-based herbal therapies, presents a promising avenue for achieving substantive recovery. This integrated approach not only addresses the symptoms but also strives to tackle the underlying causes of RA, fostering a more comprehensive and sustainable path towards healing.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic, inflammatory, systemic, autoimmune disease affecting approximately 0.4–1.3% of the world population with unknown etiology. In RA, synovitis, structural and joint bone damages followed by pain and disability are the cascade of immune reactions produced by both genetic and environmental factors (Ahmed et al. 2019). It initiates with inflammation of joint and synovial tissue proliferation, followed by massive cell infiltration and surplus formation of inflammatory mediators, finally resulting in cartilage and bone devastation (Baker et al. 2022) RA is also termed as “immortal cancer,” causing huge burdens on patients and society due to its higher incidence of RA(Cabrales-Rico et al. 2017; Chen et al. 2017; Littlejohn and Monrad 2018). Over the last 30 years, there has been no permanent cure for the patients suffering from RA as these only reduce pain and inflammation, decelerate the disease progression and prevent joint damage (Barnabe 2020; Conigliaro et al. 2019). The commonly used drugs for RA consist of non-steroidal anti-inflammatory drugs (NSAIDs), disease-modifying antirheumatic drugs (DMARDs), corticosteroids, biologics, and their combinations (Zhao et al. 2021a, b). These therapeutic agents do not provide persistent diminution but may cause immunity suppression, leading to severe complications. In case of infections, pathogenic cells are eliminated by antigen-specific therapies, utilizing existing autoantigens like heat shock proteins, and their derived peptides, leaving the immune system of the body unaffected (Cabrales-Rico et al. 2017). Due to the side effects linked with these therapeutic agents, including hepatotoxicity, cardiovascular complications, and gastrointestinal lesions, 30–50% of patients do not respond effectively to the standard therapies, thereby decreasing the inclusive benefits of the treatment on a long-term basis.
Herbal products have been the source of many bioactive compounds with therapeutic potential, of which many eventually have been developed into drugs that are consumed worldwide for diverse disorders, including inflammatory and autoimmune diseases. Furthermore, a variety of herbal products belonging to the traditional systems of medicine are either already being used by patients with autoimmune diseases, including RA, with or without the primary physician’s knowledge, or are under investigation for their therapeutic potential (Venkatesha et al. 2016). Thus, the utilization of phytoconstituents which are effective in relieving the inflammation and thereby alleviate the chances of occurrence of side effects associated with allopathic drugs, could be a potential alternative such as sinomenine, total glucosides of paeony, and triptolide (Doss et al. 2016; Han et al. 2016; Jia et al. 2016; Li et al. 2017; Voon et al. 2017). Thus, there is an urgent requirement to quest for more effective, safe, and economical drugs for relieving pain in joints and improving the quality of life of RA patients.
Natural plant products belonging to the traditional systems of medicine, such as traditional Chinese medicine and Indian Ayurvedic medicine, offer a vast and promising resource in this regard. With the number of RA patients increasing yearly, medicinal researchers are actively looking for cheap and effective alternative drugs with fewer side effects to treat RA. Recently, herbal medicines have been given more and more attention for their remarkable curative effects and fewer side effects (Luo et al. 2019; Newman and Cragg 2016). Herbal medicines have been used for the clinical management of RA for thousands of years, and their efficacy and safety have been proved by their long-term clinical application (Pu et al. 2016). Herbal medicines can act in multiple pathways to prevent and treat RA through numerous components, including flavonoids, alkaloids, phenylpropanins, terpenes, etc. The main pharmacological effects are related to pain relief, improvement of inflammation, immune function regulation, cartilage protection, reduction of pannus formation, inhibition of synovial hyperplasia, etc. (Zhang et al. 2019).
Methodology
The qualitative systematic review analyzed globally accepted databases, including indexed and peer-reviewed journals from PubMed, Scopus, Medline, Google Scholar, and Research Gate. Papers from between 2001 and 2023 were included. The search was made using keywords such as rheumatoid arthritis, pathogenesis of rheumatoid arthritis, therapy of rheumatoid arthritis, herbal medicine for rheumatoid arthritis. The botanical names and families of the plants used for RA were mentioned after verification from published literature and databases. Data selection criteria are in accordance to botanical features, phytoconstituents, in vitro and in vivo models used, and clinical studies of RA. The inclusion criteria of the studies/reports in the present review are (i) plants reported for anti-arthritic activity, (ii) plants used in traditional systems and various polyherbal preparations, (iii) plants native to India and other areas such as America, Africa or Europe, and (iv) plants use under different models such as Complete Freud’s Adjuvant (CFA) and monosodium iodoacetate (MIA)-induced RA rat models.
Etiology of rheumatoid arthritis
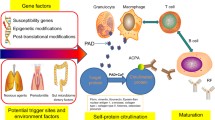
The etiology behind RA is still not precisely defined; however, specific research findings have provided evidence in favor of the involvement of various factors, as shown in Fig. 1 (Alam et al. 2017; Littlejohn and Monrad 2018; Daikh 2022).
Pathophysiology of rheumatoid arthritis
In the pathogenesis of RA (Fig. 2), T cells, CD147, interleukins (ILs), and tumor necrosis factor-alpha (TNF-α) play crucial roles (Christman and Gu 2020). It has been described as follows:
Function of T cells
Various reports advocated the connection of T cells in the pathogenesis of RA as it has been found in synovial tissue and fluids. There exists a relationship between specific major histocompatibility complex (MHC) class II alleles, an abundance of T cells, and this disorder (Alam et al. 2017; Melet et al. 2013). The helper T cell type 17 (Th17), through the production and discharge of TNF-α, IL-17F, IL-17, IL-22, and IL-21, has been considered as a cell adaptive immune response in RA (Quesniaux et al. 2013). T cells are triggered and produced due to the release of several cytokines (IL-32, IL-6, IL-15, IL-12, IL-18), co-stimulatory, and HLA class II molecules. The release of these intermediates has been linked to the abundance of myeloid cells such as macrophages, monocytes, basophils, neutrophils, erythrocytes, eosinophils, megakaryocytes or platelets, dendritic cells, and plasmacytoid dendritic cells in the synovium (Lebre et al. 2008), validating the use of abatacept, which blocks the interaction between T cells and dendritic cells or B cells through CD28 on the T cells with CD86 or CD80 on B cell or the dendritic cell, i.e., co-stimulatory signal. Autoreactive T cells have also been found against citrullinated self-proteins (Cantaert et al. 2009). The dendritic cells and macrophages are responsible for producing cytokines such as TGF-β, IL-6, IL-1b, IL-23, and IL-21, which provide an environment for differentiation of Th17 and suppress the differentiation of regulatory T cells (Genovese et al. 2014). In RA patients, the functional ability of regulatory T cells decreases, which is regulated by Foxp3 (forkhead box P3, master regulator) and TNF-α. In antigen nonspecific T-cell mediated activation, there is activation of macrophages and fibroblast through the interaction of ICAM-1 & LFA-1, CD200 & CD200L, and CD40 & CD40L (Noack and Miossec 2014).
Role of CD147
The CD147 (EMMPRIN-an extracellular matrix metalloproteinase inducer) is a member of the immunoglobulin super family. CD147 induces matrix metalloproteinases (MMPs) expression. In the synovial membrane of patients suffering from RA, it has been found that cellular response to a molecular stimulus increases due to a rise in the number of receptors of CD147 on peripheral blood CD14þ monocytes. Reports have suggested that in the RA patients, CD147 expression on monocytes/ macrophages increases, and could be responsible for the enhanced secretion of MMP, cyclophilin A-mediated migration of the cell into the joints of the patient, and cell invasion, which is ultimately responsible for bone and cartilage destruction in the patients. The monoclonal antibody CD147/HAb18 reduces inflammatory cytokines, inhibits MMPs, and alleviates synovitis as well as cartilage erosion in immuno-deficient mice engrafted with human rheumatoid synovium tissue and cartilage. All these outcomes advised that CD147 can be a novel target for the treatment of RA (Guo et al. 2019).
Role of TNF-α and interleukins
The TNF-α has a very vital function in RA in controlling the inflammatory response. There is an elevated TNF-α level in the synovium, blood, and synovial fluid of RA patients. The TNF-α acts both on fibroblast-like synoviocytes (FLS) and macrophages, facilitating the release of MMPs that enable the destruction of the tissue. The MMPs are involved in both pathologic destructions of the tissue and physiologic connective tissue remodeling. All the important structural proteins in the cartilage matrix are degraded by at least 19 human MMPs extracellularly (Huang et al. 2013; Roeleveld and Koenders 2015). The FLS is an important class of cells and chief contributor of the hyperplasia of synovium, which is involved in the pathogenesis and progression of RA by regulating the secretion of inflammatory mediators like TNF-α, IL-6, IL-1α, chemo-attractant protein-1 (MCP-1), IL-17, monocytes, cyclooxygenase-2 (COX-2), and IL-1β-inducible nitric oxide synthase (iNOS). It has been suggested that intracellular signaling molecules such as nuclear factor кB (NF-кB) and activator protein-1 (AP-1) are very activated in RA-FLS and contribute to the pathogenesis of RA. In basic cellular processes, like proliferation, apoptosis, differentiation, and immunity, NF-кB, and AP-1 are the chief transcription factors regulating gene expression.
TNF-α, NF-кB, IL-1b, AP-1, and Mitogen-activated protein kinases (MAPKs) intermediates get activated after receiving the pro-inflammatory stimuli and release pro-inflammatory chemokines, MMPs, inflammatory enzymes, adhesion molecules, and angiogenic factors resulting in neo-vascularization, arthritic joint destruction and chronic inflammation. Furthermore, it has been revealed during several pre-clinical and clinical trials that RA-FLS plays a significant role in RA through the manufacturing of receptor activator of NF-кB ligand (RANKL), which results in bone erosion through enhanced stimulation of osteoclast formation (Alam et al. 2017; Cantaert et al. 2009; Chen et al. 2017; Doss et al. 2016; Ganesan et al. 2016; Gao et al. 2017; Genovese et al. 2014; Guo et al. 2019; Huang et al. 2013; Lebre et al. 2008; McInnes and Schett 2017; Melet et al. 2013; Noack and Miossec 2014; Quesniaux et al. 2013; Roeleveld and Koenders 2015).
Role of mast cells
Mast cells and macrophages act by releasing interleukins (IL-1, IL-6, IL-12, IL-15, IL-18, IL-23), TNF-α, reactive nitrogen and oxygen species, phagocytosis and antigen presentation, production of matrix-degrading enzymes and prostanoids (Shichita et al. 2012) and cause synovitis in RA through interaction with the Toll-like receptors (TLR), suppression of tumorigenicity 2 (ST2), Fc receptor ϵ and γ through innate immune system activation (Hueber et al. 2010; Németh and Mócsai 2012; Nigrovic and Shin 2015).
Role of B cells
Due to the occurrence of different factors like proliferation-inducing ligand (APRIL), chemokines and B-lymphocyte stimulator (B LyS), synovial B cells get localized as T cell–B cell aggregates or tertiary lymphoid follicles in synovium in RA (Ohata et al. 2005). The pathogenic involvement of CD20+ B cells has been verified by rituximab used in patients suffering from RA. Moreover, clinical observations show that B cells are engaged in the RA pathogenesis beyond autoantibody production. The cytokine production and autoantigen, e.g., lymphotoxin-b or TNF-c, IL-6, and TNF-α, contribute to adaptive humoral immunity. Plant-derived natural products offer a vital and promising resource for new therapeutic agents for RA and other autoimmune diseases. Practitioners of the traditional systems of medicine prefer to use herbal extracts, either singly or in a formulation using multiple herbs. The principal mechanisms of RA prevention and symptom alleviation are the reduction of inflammatory mediators and inhibition of specific signaling pathways involved in the inflammatory response in RA.
Diagnostic techniques of rheumatoid arthritis
The RA can be diagnosed by two methods, i.e., Clinical diagnostic methods and Radiographic diagnostic techniques, as discussed in Tables 1 and 2, respectively (Ebel and O’Dell 2021; Alghuweri et al. 2012; Deodhar 2015; Gotthardt et al. 2010; Haville and Deane 2022; Karnati et al. 2013; Kashyap et al. 2015; Kim et al. 2014; Llopis et al. 2017; Maharaj and Daikh 2022; Patil et al. 2011; Rezaei et al. 2013; Rosa Neto et al. 2009; Suter et al. 2011; Verma et al. 2002; Wakefield et al. 2005).
Therapy of rheumatoid arthritis
The treatment for RA is a mixed avenue of allopathic and non-allopathic strategies. Allopathic medications and surgical interventions are the most popular treatments for RA. Currently available drugs either used alone or in combination for the treatment of RA include Glucocorticoids (GCs), NSAIDs, and DMARDs of both biological origin (such as TNF Inhibitors, IL-1, IL-6, and B cell depleting drugs) as well as synthetic (conventional like Methotrexate (MTX) and targeted like JAK-Inhibitors) (Akram et al. 2021; Zago et al. 2020; Burmester and Pope 2017). Phytoconstituents are also taken by large populations (Swathi et al. 2021). Alternative therapies like physiotherapy, acupuncture, yoga, and massage are also popular among RA patients.
Medication
Synthetic drugs like corticosteroids, DMARDs, and NSAIDs reduce swelling, pain, and associated symptoms of RA (Fig. 3) (Zhao et al. 2021a, b; Wysham et al 2022). These drugs can either be used alone or in combination to diminish inflammation caused due to the activation of RA mediators.
Non-steroidal anti-inflammatory drugs
These drugs are COX enzyme inhibitors, which decrease pain and inflammation. The COX occurs in two natural forms, i.e., COX-1 and COX-2. During the arachidonic acid pathway, the COX or prostaglandin synthase produces prostaglandin E2 (PGE2), which is accountable for synovium inflammation and articular degradation of cartilage. NSAIDs inhibit COX-2 and COX-1 and, decrease synovial inflammation in RA (Stańczyk and Kowalski 2001). The NSAIDs are preliminary drugs, usually prescribed in patients without RA risk factors like multiple swollen joints, RF, mobility loss, and bone erosion. For better recovery, these can be given in combination with a low dose of glucocorticoids (Ebel and O’Dell 2021). However, some side effects such as renal malfunction, gastrointestinal disturbance, and increased cardiovascular risk are associated with NSAIDs. Some of the important NSAIDs used clinically are shown in Fig. 3A.
Disease modifying antirheumatic drugs
Based on their characteristic mechanism in RA, these drugs are classified as biological DMARDs and standard synthetic DMARDs. The action of synthetic DMARDs is intracellular (Kiely and Nikiphorou 2018). The first-line standard drug used in RA is MTX and about 40% of the RA patients are sensitive to it (Peres et al. 2015). Some of the important synthetic DMARDs are shown in Fig. 3B. Synthetic DMARDs such as MTX, Hydroxychloroquine (HCQ), Sulfasalazine (SSZ), Clofazimine (CLO), Leflunomide (LEF), Actarit, and Azathioprine show various side effects including myelosuppression, hepatic cirrhosis, interstitial pneumonitis, and gastrointestinal disorders (Zhao et al. 2021a, b).
Adalimumab and infliximab are the most commonly used biological DMARDs, which mimic the effects of natural biological agents generated by the immune system of the body by acting as TNF-α inhibitors (Barrios et al. 2017; Rein and Mueller 2017; Schoellnast et al. 2006). These frequently target the CD molecules of antigen-presenting cells, soluble cytokines, and B cells. The mechanism of action of biological DMARDs involves antagonism of TNF-α, IL-1 or IL-6 receptor. These agents reduce the formation of rheumatoid factors and antibodies, downregulate T cells activation and deplete B cells. Although these agents are effective, their usage is limited due to various associated side effects like hypersensitivity, renal disease, hypertension, cardiac complications, gastrointestinal damage, respiratory difficulty, ulcer, and immune suppression, leading to opportunistic infections. Some of the important biological agents useful in RA are mentioned in Table 3 (Alten et al. 2011; Bredemeier et al. 2014; Emery et al. 2009; Flouri et al. 2014; von Kempis et al. 2012; Koike et al. 2007; Kurrasch et al. 2013; Lee et al. 2014a, b; Nam et al. 2014; Schiff et al. 2014; Smolen et al. 2015; Takeuchi et al. 2016; van Vollenhoven et al. 2015; Weinblatt et al. 2014; Deane et al. 2023).
Corticosteroids
When glucocorticoid secretion from the adrenal gland is insufficient, stress and inflammation intervene. Corticosteroids, the analogs of cortisol, are used for anti-inflammatory and immune-regulatory functions. These constitute second-line therapy. These are prescribed for symptomatic relief as they reduce the inflammatory intermediates in the body like prostaglandins and leukotrienes to reduce inflammation. The mechanism of action of corticosteroids involves the prevention of phospholipid release, anti-inflammation, and immune suppression. Glucocorticoids like Dexamethasone, Betamethasone, Methylprednisolone acetate, Prednisolone phosphate, and Budesonide show many side effects such as hyperadrenocorticism, insulin resistance, atherosclerosis, hypertension, osteonecrosis and osteoporosis, obesity, and inhibition of wound repair. These are usually combined with other synthetic drugs when they are ineffective for RA separately (Gupta and Singh 2014). Corticosteroids used in the RA therapy are displayed in Fig. 3C.
Phytoconstituents
Before developing synthetic drugs, mankind was reliant on medicinal plants to get relief from a specific disorder. Regardless of the accessibility of numerous synthetic drugs for rheumatoid joint pain, the side effects restrict the utilization of the remedial treatment, and the likelihood of use of medicinal plants for the treatment of rheumatoid joint inflammation has expanded. A considerable number of plants have potential anti-arthritic constituents, and a few formulations are likewise endorsed by professionals in place of synthetic drugs (Kapoor et al. 2017). Engineered remedial medications act by one of the accompanying systems, i.e., a) modulating a specific cell, b) augmenting inflammatory mediator, and c) inhibiting tissue debasing enzymes. Yet, restoration of the immune system of the patient is not achieved. Such disadvantages can be overcome by using natural phytoconstituents, which are considered superior to allopathic medications and surgical treatments in various terms. Phytoconstituents are non-harmful and multifocal on the malady pathway. The rundown of some anti-arthritic plants, their phytoconstituents are portrayed in Table 4 (Ahmed 2010; Ali et al. 2007; Anilkumar 2010; Bang et al. 2009; Gupta and Singh 2014; Haleagrahara et al. 2017; Lee et al. 2013; Patil et al. 2011; Stańczyk and Kowalski 2001; Sun et al. 2006; Voon et al. 2017; Zaka et al. 2017; Zhou et al. 2017).
Bartogenic acid
Bartogenic acid (BA) isolated from Barringtonia racemosa was evaluated in arthritic rats in which arthritis was induced by Complete Freud’s Adjuvant (CFA). The results indicated that BA exhibited a protective role in rats against the haematological perturbations, body weight changes as well as primary and secondary arthritic lesions induced by CFA (doses 2, 5, and 10 mg kg−1 day−1, p.o.) with remarkable reduction in the rheumatoid factor and C-reactive protein (CRP), which are distinct serum markers of arthritis and inflammation. This result establishes the potency of BA in arthritis. However, further clinical investigations are mandatory to demonstrate the efficiency of BA in the treatment of several immuno-inflammatory ailments (Ali et al. 2007; Patil et al. 2011; Sun et al. 2006).
Quercetin
Quercetin (QU), a natural flavonoid, is found in hundreds of herbs, vegetables, and fruits. QU has numerous pharmacological potentials, such as anti-inflammation, anti-platelet aggregation, expanding coronary arteries, antitumor, lowering blood pressure, anti-allergy, etc. For a single oral dose of 4 g or 500 mg twice a day, no side effects were reported after continuous administration for a month (Haleagrahara et al. 2017). When analyzed on adjuvant-induced arthritis in mice, it exhibited anti-inflammatory action by lowering the production of macrophage inflammatory mediators by regulating NF-κB activity, and finally resulted in the inhibition of inflammatory cytokine expression and inflammatory reactions. QU can inhibit the activity of MMP-2, thereby inhibiting the degradation of the basement membrane and indirectly inhibiting angiogenesis and cartilage damage (Haleagrahara et al. 2017; Ji et al. 2013).
Askari and colleagues studied the effect of flavonoid QU (500 mg/day) supplementation in healthy male non-professional athletes for 2 months, with regular exercise. The results showed a remarkable lowering in the levels of CRP (Askari et al. 2012). QU having the ability to inhibit xanthine oxidase prevented the uric acid accumulation, which benefited the gout patients (Anand David et al. 2016).
In a pathological condition, QU did not show any significant alteration in CRP levels in women with RA (Javadi et al. 2017). The QU dosage continuously for 8 weeks significantly reduced after-activity pain, morning pain, and EMS (p < 0.05). In comparison to the placebo, hs-plasma TNF-α level, Health Assessment Questionnaire (HAQ) scores, DAS-28, and the number of patients were decreased in the QU group with active disease (p < 0.05). Thus, there were notified improvements in clinical symptoms, hs-TNFα, disease activity, and HAQ after 500 mg/day QU dose intake in women suffering from RA for 8 weeks (Javadi et al. 2017).
Epigallocatechin-3-gallate
Epigallocatechin-3-gallate (EGCG) is abundant in green tea and can be an effective adjunct therapy for the treatment. It has been reported to inhibit inflammatory mediators like PGE2, COX-2, NF-κB, iNOS, interferon-γ, TNF-α in arthritic joints collagen type II-induced arthritis (CIA) in pre-clinical studies (Ahmed 2010). Experiments conducted by Ahmed and colleagues have shown that pre-treatment of human chondrocytes with EGCG significantly prohibited the activities and expression of MMP-13 and MMP-1 (IC50 values 16.5 and 27 mM, respectively) in vitro in a dose-dependent manner. The EGCG was found to be effective in inhibiting IL-1b-induced MMP-1, MMP-3, and MMP-13 in human tendon fibroblasts (Ahmed et al. 2005; Ahmed 2010).
The EGCG suppresses pro-inflammatory cytokines and chemokines induced by TLR9 agonists in prostate cancer cells (Mukherjee et al. 2014). The anti-inflammatory and anti-cancer properties of EGCG are mediated by folate cycle disruption, adenosine release, and NF-kB suppression (Navarro-Perán et al. 2008). The EGCG of green tea suppresses autoimmune arthritis through Indoleamine-2,3-dioxygenase expressing dendritic cells and the nuclear factor, Erythroid 2 of the antioxidant pathway (Min et al. 2015).
Boswellic acid
Boswellic acid (BA) (Boswellia serrata, Burseraceae), pentacyclic terpene, is found in plants in various derivatives such as acetyl-11-keto-BA and 11-keto-BA. BA and its derivatives are therapeutically used in multiple ailments such as ulcerative colitis, cancer, hepatitis, inflammation, pain, cough, bacterial infection, and osteoarthritis. In pre-clinical evaluation, BA was reported to reduce cartilage loss, synovitis, and osteophyte formation and hence has a beneficial role in osteoarthritis and other joint disorder.
Clinical investigation of B. serrata extract provided statistically substantial improvement in patients suffering osteoarthritis and was well-tolerated with minor gastric disturbance. The anti-arthritic activity of BA is due to the inhibition of NF-ĸB, COX-2, LOX-5 (Anilkumar 2010).
The BA inhibited microsomal prostaglandin E synthase1 (mPGES-1), a terminal enzyme of PGE2 biosynthesis (IC50 = 3–30 μM) in IL-1beta-stimulated human A549 cells. Some other triterpene acids (i.e., tircuallic, lupeolic, and roburic acids) isolated from frankincense were also found to suppress mPGES-1 with increased potencies. 3α-Acetoxy-7,24-dienetirucallic acid and 3α-acetoxy-8,24-dienetirucallic acid inhibited mPGES-1 activity (IC50 = 0.4 μM, each). Docking simulations and structure–activity relationship (SAR) studies established structure-related interactions with mPGES-1 and its co-substrate glutathione (Verhoff et al. 2014). Acyl derivatives of BA also act as inhibitors of NF-κB and STATs (signal transducer and activator of transcription protein) determined against human HL60 cells after 48 h by MTT assay (Kumar et al. 2012).
Mangostin
Lee and colleagues have shown that mangostin extract possesses immunomodulatory activity due to the presence of α-, β- and γ-mangostin in the extract. These inhibited the secretion of TNF-α (IC50 = 2–11 μg/mL), and IL-6 in mice (ED50 < 100 mg/kg). It reduced the arthritis score in the CIA rats and the paw oedema in the carrageenan-induced rats (Lee et al. 2013).
Piperine
Bang and colleagues conducted the study on piperine and reported that it inhibited the expression of MMP-13 and IL-6 in a dose-dependent manner and reduced the production of PGE2 (concentrations 10–100 μg/mL). Piperine suppressed the migration of activator protein-1 (AP-1) into the nucleus in IL1β-treated synoviocytes. On days 4 and 8, piperine significantly reduced arthritic and nociceptive symptoms in rats. Histological staining revealed the anti-inflammatory effect of piperine especially in the ankle joints (Bang et al. 2009).
Sinomenine
The studies conducted by Zhou and colleagues and Feng and colleagues showed that the treatment with Sinomenine (SIN) decreased the occurrence and severity of anti-CII IgG and the antigen-specific splenocyte proliferation and carrageenan-induced arthritis. It was observed during the study that secretion of cytokines such as IL-5, IFN-γ, and generation of all isotypes of antibodies including anti-CII IgG1, IgE and IgG2a were found to be suppressed along with increased TGF-β secretion and with no effect on IL-10 production. These inferences proposed that anti-arthritic activity can be possible due to the suppression of Th1 and Th2 immune responses (Feng et al. 2007; Zhou et al. 2017). The anti-inflammatory effects of sinomenine may occur via the inhibition of pro-inflammatory cytokine and COX-2 production through the inhibition of MAPKs and NF-kB pathway activation by phorbol 12-myristate-13-acetate (PMA) along with A23187 stimulation in HMC-1 cells (human mast cells) (Oh et al. 2012).
Cardamonin
Boesenbergia rotunda (L) Mansf is the natural source of cardamonin (2′,4′-dihydroxy-6′-methoxychalcone). The study conducted by Voon and colleagues showed that cardamonin inhibited the release of the pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β, in vitro. This study was conducted in RA-induced rat model to determine the anti-arthritic properties of cardamonin, specifically on the pain and inflammatory response of RA. The anti-arthritic activity was evaluated using four different doses of cardamonin (5.0, 2.5, 1.25, and 0.625 mg/kg), through responses to the CFA-induced paw oedema, thermal hyperalgesia, and mechanical allodynia. Histological, behavioral, and biochemical studies in rats demonstrated that cardamonin exhibited substantial inhibition of RA-induced pain, inflammation, and advancement of joint destruction. In cardamonin-treated RA rats, results from ELISA showed significant inhibition in IL-6, IL-1β, and TNF-α levels in plasma. Overall, cardamonin showed noteworthy anti-arthritic potential in CFA-induced RA rat model (Voon et al. 2017).
In another study, the anti-inflammatory property of cardamonin was examined in IFN-γ/LPS-stimulated microglial cells. In this investigation, cardamonin demonstrated promising anti-inflammatory activity in microglial cell line BV2 by inhibiting the secretion of pro-inflammatory mediators, including TNF-α, PGE2, IL-6, IL-1β, and nitric oxide (NO). The inhibition of NO and PGE2 by cardamonin has resulted from the reduced expression of iNOS and COX-2, respectively (Chow et al. 2012).
Withanolide
Zaka and colleagues showed that anti-arthritic actions of withanolides can be because of the inhibition of stimulation of NF-kB and gene expression regulated by NF-kB (Zaka et al. 2017). Rasool and Varalakshmi examined W. somnifera root powder in MSU crystal-induced arthritis in rats. A substantial increase in paw volume and the levels of lysosomal enzymes in serum were observed in rats with arthritis, due to enhanced lactate dehydrogenase and β-glucuronidase levels in MSU incubated polymorpho-nuclear leucocytes. The levels of lactate dehydrogenase and β-glucuronidase reverted to near normal after treatment (500 to 1000 mg/kg). Thus, these have shown potent analgesic and antipyretic effects at different doses, without any sign of gastric damage. These inferences provide substantial proof for the suppressive action of withanolides present in W. somnifera root powder on arthritis through reduction of propagation of the inflammatory response, without producing any gastric damage (Rasool and Varalakshmi 2007).
Withaferin A
Sultana and colleagues targeted the synovial macrophages by injecting steroidal lactone withaferin A containing mannosylated liposomes (ML-WA) into adjuvant-induced arthritic (AIA) rats to develop an improved therapeutic method to treat RA through internalization of ML-WA in the primarily isolated synovial macrophages. It was observed that targeting the synovial macrophages via ML-WA reduced the severity of inflammation of the paw oedema, bone resorption, and oxidative stress (ROS and NO) in AIA rats through repolarization of M1 to M2 macrophage (Sultana et al. 2017; Zaka et al. 2017). The clinical evidence for bee venom acupuncture (BVA) for RA has also been reported (Lee et al. 2014a, b).
To check the safety and efficacy of traditional Chinese medicine Paeoniflorin (PAE) plus cervus and cucumis polypeptide injection (CCPI), a double-blinded study was conducted using control leflunomide (LEF) and MTX. Patients were randomly assigned to one of the three groups: PAE + CCPI, MTX + LEF, and MTX + LEF + CCPI. The CCPI groups responded better to the ACR20 during early treatment. After 6 months, ACR20 was similar in the three treatment groups. The maximum improvement in the two DMARD groups was higher than that in the PAE + CCPI group (p < 0.01). CCPI decreased the onset action of the DMARD therapy 4.6 times. The PAE + CCPI had significantly lower adverse event incidences than two DMARD groups. Thus, PAE + CCPI was observed to be an acceptable alternative to DMARDs and can also be used in adjunct with DMARDs to increase the rate of onset of action, when necessitated. Although not as effective as DMARDs, PAE can be a safer option to substitute DMARDs for long-term RA treatment in case of DMARD toxicity (Chen et al. 2013). Withaferin A down-regulated lipopolysaccharide (LPS)-induced COX-2 expression and PGE2 production by inhibiting STAT1/3 activation in microglial cells (Kyoung-Jin et al. 2011). In another study, Withaferin A inhibited iNOS expression and NO production by Akt inactivation and down-regulation of LPS-induced activity of NF-kB in RAW 264.7 cells (Jung et al. 2008). It also inhibited monosodium urate crystal-induced inflammation (Evan et al. 2008).
Resveratrol
Resveratrol, a natural active compound present in Polygonum cuspidatum has been reported to show promising curative effects on RA symptoms of CIA rats (Peng et al. 2013). It can induce the primary cultured FLS (rFLS) from CIA rats via up-regulation of Caspase-8 and down-regulation of FLICE inhibitory protein (FLIP). This inhibitory protein is a vital anti-apoptotic protein, which can suppress the death receptors ligands (such as TNF-α, FasL, and TRAIL) induced apoptosis in FLS of RA patients (RA-FLS) (Gu and Jin 2015).
Plant extract
Apart from single active compounds derived from plants, various studies have explored the effects of plant extracts in the treatment of RA. From the pharmacological perspective, natural plant extracts or mixed herbal compounds effectively regulate the immune system to alleviate RA by inhibiting pro-inflammatory cytokines (Zhao et al. 2021a, b).
In this context, Maresca and colleagues have evaluated the pharmacological activity of dried 50% hydroalcoholic extract (50%HA) of Astragalus (Astragali radix) using two different in vivo models of articular damage resembling RA and osteoarthritis. The authors demonstrated that administration of 300 mg/Kg per os of 50%HA significantly decreased both CFA-induced pain and monosodium iodoacetate (MIA)-induced pain (96% and 78% pain relief, respectively). In CFA model, 50%HA decreased the plasma level of pro-inflammatory cytokines IL-1β, TNF-α, and joint diameter (Maresca et al. 2017).
In a recent study, cinnamaldehyde (CA) in cinnamon (Cinnamomum cassia Presland) extract showed anti-inflammatory effects in in vitro experiments using activated macrophages (Raw246.7 cells) and in a rat model of adjuvant arthritis (AA) in vivo (Liu et al. 2020). Maresca and colleagues studied the acute effect of Capparis spinosa root extract on rat articular pain in osteoarthritis and RA rat models. Post 14th day CFA or MIA injection, different doses of C. spinosa, i.e., 300, 100, and 300 mg/kg of powdered roots, decoction, and hydroethanolic extract, respectively, were administered p.o. Significantly reduced hypersensitivity to noxious mechanical stimuli and spontaneous pain were evaluated as hind limb bearing alterations in both models. The methylene chloride (CH2Cl2) extract of C. spinosa and its correlated aqueous residue (30 mg/kg) were found to be the most effective in reducing the sensitivity to the pain (Maresca et al. 2016).
Clinical studies on plant extracts and formulations containing plant extracts
In 1989, a placebo-controlled double-blind study was performed on an herbaceous plant of Southern China origin, Tripterygium wilfordii, involving 70 RA patients. A significant improvement was observed in the patients treated with the herbal drug compared to placebo after 3 months, in all the studied parameters like morning stiffness, swelling count, tenderness score, and grip strength at the end of treatment (Soeken et al. 2003).
In a randomized double-blind (RDB), single-center, placebo-controlled, parallel efficacy, drug trial in phase II, RA-1(a standardized formulation), composing purified plant extracts of Zingiber officinale, B. serrata, C. longa, and W. somnifera, was evaluated on 182 patients with active-on-chronic RA for 16 weeks. As a rescue analgesic, oral paracetamol and prednisolone (a fixed-dose not exceeding 7.5 mg daily) were permitted. This study was significant in terms of (i) enhanced patient percentage with a 50% decrease in the swollen joint count and swollen joint score, (ii) improved blood haemoglobin, (iii) a reduced RF titre. The ACR (American College of Rheumatology) 20% improvement index response was observed in 39% of the RA-1 group versus 30% of placebo. Only minor side-effects were reported in treatment groups with no report of drug toxicity.
A significant improvement in all ACR core efficacy variables and a modified version of HAQ were observed at weeks 32 and 54. Thus, this herbal formulation containing plant extracts was established as a good DMARD with a significant safety profile (Chopra et al. 2010).
A multicenter, RDB placebo-controlled phase III drug trial was conducted for 3 months on 130 patients, followed by a single-centre, open-label phase trial of 9-months, involving IRA-01 with extracts of C. longa (Turmeric), B. serrata (Salai Guggul), Camellia sinensis (Green tea), Linum usitatissimum (Flaxseed), Trigonella foenum-graecum (Fenugreek), Piper nigrum (Black pepper) and Tribulus terrestris (Gokshur). Prednisolone, DMARDs, or NSAIDs, were not allowed during this study period. Only paracetamol was qualified as a rescue analgesic. During the RDB phase, in comparison to the placebo, the above extract formulation showed better improvement in all efficacy measures, but achieved significance only in the case of physician global assessment of disease activity (Mann Whitney, Z = 2.18; 95% CI of change − 1.15, − 0.01). Only minor side-effects were recorded without significant adverse effects on metabolic parameters, biochemistry (renal and hepatic), or routine hematology during the entire study period. After 3 months, 58 patients went on to complete a 1-year follow-up, and 70 patients entered the open-label phase. A significant improvement in all efficacy variables, including joint pain and swelling, was observed. Here, 80% and 40% patients showed ACR 20 and 50 improvement response, respectively (Chopra et al. 2010).
In a study, 125 patients with joint pain were screened. Eighty-six patients satisfied inclusion criteria and took Ashwagandha powder (5 g, 2 times a day for 3 weeks) with lukewarm water or milk. SidhMakardhwaj (100 mg/day) with honey was given for successive 4 weeks. The patients were tested positive for increased ESR level and rheumatoid factor.
There was a significant reduction in RA factor after treatment with the above two herbal drugs along with the drastic change in scores of swollen joint counts, patient global assessment score, tender joint counts, patient self-assessed disability index score, physician global assessment score, pain assessment score ESR level in comparison to baseline.
In 56.4% of patients, ACR20 response and in 39.74% patients’ moderate response [European League against Rheumatism (EULAR) criteria] was observed. Normal kidney and liver function tests and increased urinary mercury levels were observed in the treated patients after 7 weeks (Kumar et al. 2015).
An investigator-blind, multicentre, parallel efficacy, three-arm (two Ayurvedic and Hydroxychloroquine sulfate (HCQS)) drug trial study was conducted on 121 patients for 24 weeks with active moderately severe RA (ACR 1988 classified) using polyherb (Z. officinale and Tinospora cordifolia) and monoherb (Semecarpus anacardium). The study measures included pain visual analog scale, joint counts (pain/tenderness and swelling), health assessment questionnaire, and global disease assessments. In the polyherb, monoherb, and HCQS arms, 44%, 36%, and 51%, respectively, improved ACR 20 index.
In the HCQS and polyherb groups, there was an improvement in efficacy measures no difference among the groups (significant P values). But the polyherb was observed better than monoherb. There were no differences between the groups with only mild adverse events (skin and gut, and none of them withdrew) were reported. Thus in this study, in comparison to HCQS, standardized Ayurvedic polyherb drug was found to be safe and effective in active RA patients (Chopra et al. 2012).
Conclusion and future aspects
Rheumatoid arthritis manifests as an enduring, dynamic, and debilitating autoimmune condition, marked by persistent inflammation affecting bone joints and leading to damage in the surrounding ligaments. This disease extends its impact beyond the joints, affecting internal organs such as the lungs, heart, and eyes. Despite the prevalent use of synthetic drugs in the standard treatment of rheumatoid arthritis, the therapeutic journey is often marred by side effects. Regrettably, a definitive curative solution for rheumatoid arthritis remains elusive within contemporary medicine. While current medications effectively manage symptoms, offering relief from pain and joint inflammation, a true cure remains absent. Alternative treatments, including herbal remedies, various forms of massage therapy, gene therapy, yoga, and acupuncture, can be explored in conjunction with conventional approaches to alleviate pain and inflammation in the joints. However, the challenge persists in refining evaluation methodologies and introducing novel modalities to mitigate potential adverse reactions. The quest for more effective and targeted therapeutic interventions continues, seeking not only to manage symptoms but to address the root causes of rheumatoid arthritis for a more comprehensive and lasting relief.
Data availability
Enquiries about data availability should be directed to the authors.
References
Ahmed KSZ, Ahmed SSZ, Thangakumar A, Krishnaveni R (2019) Therapeutic effect of Parmotrema tinctorum against complete Freund’s adjuvant-induced arthritis in rats and identification of novel isophthalic ester derivative. Biomed Pharmacother 112:108646
Ahmed S (2010) Green tea polyphenol epigallocatechin 3-gallate in arthritis: progress and promise. Arthritis Res Ther 12(2):208
Ahmed S, Anuntiyo J, Malemud CJ, Haqqi TM (2005) Biological basis for the use of botanicals in osteoarthritis and rheumatoid arthritis: a review. J Evid Based Complement Altern Med 2(3):301–308
Akram M, Daniyal M, Sultana S, Owais A, Akhtar N, Zahid R, Said F, Bouyahya A, Ponomarev E, Shariat MA, Thiruvengadam M (2021) Traditional and modern management strategies for rheumatoid arthritis. Clin Chim Acta 512:142–155
Alam J, Jantan I, Bukhari SNA (2017) Rheumatoid arthritis: Recent advances on its etiology, role of cytokines and pharmacotherapy. Biomed Pharmacother 92:615–633
Alghuweri A, Marafi A, Alhiary M (2012) Use of serological markers for evaluation patients with rheumatoid arthritis. Int J Biol Med Res 3(1):1397–1398
Ali AM, Muse R, Mohd NB (2007) Anti-oxidant and anti-inflammatory activities of leaves of Barringtonia racemosa. J Med Plants Res 1(5):095–102
Alten R, Gomez-Reino J, Durez P, Beaulieu A, Sebba A, Krammer G, Preiss R, Arulmani U, Widmer A, Gitton X, Kellner H (2011) Efficacy and safety of the human anti-IL-1beta monoclonal antibody canakinumab in rheumatoid arthritis: results of a 12-week, Phase II, dose-finding study. BMC Musculoskelet Disord 12(1):153
Anand David AV, Arulmoli R, Parasuraman S (2016) Overviews of biological importance of quercetin: a bioactive flavonoid. Pharmacogn Rev 10(20):84–89
Anilkumar M (2010) Ethnomedicinal plants as anti-inflammatory and analgesic agents. J Ethnomed 267–293
Askari G, Ghiasvand R, Feizi A, Ghanadian SM, Karimian J (2012) The effect of quercetin supplementation on selected markers of inflammation and oxidative stress. J Res Med Sci 17(7):637
Baker R, Narla R, Baker JF, Wysham KD (2022) Risk factors for osteoporosis and fractures in rheumatoid arthritis. Best Pract Res Clin Rheumatol 36(3):101773
Bang JS, Choi HM, Sur BJ, Lim SJ, Kim JY, Yang HI, Yoo MC, Hahm DH, Kim KS (2009) Anti-inflammatory and anti-arthritic effects of piperine in human interleukin 1β-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res Ther 11(2):R49
Barnabe C (2020) Disparities in rheumatoid arthritis care and health service solutions to equity. Rheum Dis Clin 46(4):685–692
Barrios CBC, Ibargüen JER, Quintana-López G (2017) Biological therapy in early rheumatoid arthritis: efficacy in disease remission. Rev Colomb Reumatol (eng Ed) 24(3):164–176
Bredemeier M, de Oliveira FK, Rocha CM (2014) Low- versus high-dose rituximab for rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res (hoboken) 66(2):228–235
Burmester GR, Pope JE (2017) Novel treatment strategies in rheumatoid arthritis. Lancet 389:2338–2348
Cabrales-Rico A, Ramos Y, Besada V, del Carmen DM, Lorenzo N, García O, Alexis J, Prada D, Reyes Y, López AM (2017) Development and validation of a bioanalytical method based on LC–MS/MS analysis for the quantitation of CIGB-814 peptide in plasma from Rheumatoid Arthritis patients. J Pharm Biomed Anal 143:130–140
Cantaert T, Brouard S, Thurlings RM, Pallier A, Salinas GF, Braud C, Klarenbeek PL, de Vries N, Zhang Y, Soulillou JP (2009) Alterations of the synovial T cell repertoire in anti-citrullinated protein antibody-positive rheumatoid arthritis. Arthritis Rheum 60(7):1944–1956
Chen L, Li J, Ke X, Qu W, Zhang J, Feng F, Liu W (2017) The therapeutic effects of Periploca forrestii Schltr. Stem extracts on collagen-induced arthritis by inhibiting the activation of Src/NF-κB signaling pathway in rats. J Ethnopharmacol 202:12–19
Chen L, Qi H, Jiang D, Wang R, Chen A, Yan Z, Xiao J (2013) The new use of an ancient remedy: a double-blinded randomized study on the treatment of rheumatoid arthritis. Am J Chin Med 41(2):263–280
Chopra A, Saluja M, Tillu G (2010) Ayurveda–modern medicine interface: a critical appraisal of studies of Ayurvedic medicines to treat osteoarthritis and rheumatoid arthritis. J Ayurveda Integr Med 1(3):190
Chopra A, Saluja M, Tillu G, Venugopalan A, Narsimulu G, Handa R, Bichile L, Raut A, Sarmukaddam S, Patwardhan B (2012) Comparable efficacy of standardized Ayurveda formulation and hydroxychloroquine sulfate (HCQS) in the treatment of rheumatoid arthritis (RA): a randomized investigator-blind controlled study. Clin Rheumatol 31(2):259–269
Chow YL, Lee KH, Vidyadaran S, Lajis NH, Akhtar MN, Israf DA, Syahida A (2012) Cardamonin from Alpinia rafflesiana inhibits inflammatory responses in IFN-γ/LPS-stimulated BV2 microglia via NF-κB signalling pathway. Int Immunopharmacol 12(4):657
Christman LM, Gu L (2020) Efficacy and mechanisms of dietary polyphenols in mitigating rheumatoid arthritis. J Funct Foods 71:104003
Conigliaro P, Triggianese P, De Martino E, Fonti GL, Chimenti MS, Sunzini F, Viola A, Canofari C, Perricone R (2019) Challenges in the treatment of rheumatoid arthritis. Autoimmun Rev 18(7):706–713
Daikh DI (2022) Rheumatoid arthritis: evolving recognition of a common disease. Best Pract Res Clin Rheumatol 36(1):101740
Deane KD et al (2023) Rheumatoid arthritis. In: Stone JH (ed) A clinician’s pearls & myths in rheumatology. Springer
Deodhar A (2015) Dual energy X-ray absorptiometry in rheumatoid arthritis, atlas of rheumatoid arthritis. Springer, pp 157–173
Doss HM, Ganesan R, Rasool M (2016) Trikatu, an herbal compound ameliorates rheumatoid arthritis by the suppression of inflammatory immune responses in rats with adjuvant-induced arthritis and on cultured fibroblast like synoviocytes via the inhibition of the NFκB signaling pathway. Chem Biol Interact 258:175–186
Ebel AV, O’Dell JR (2021) Clinical features, diagnosis, and treatment of rheumatoid arthritis. Physician Assist Clin 6(1):41–60
Emery P, Fleischmann RM, Moreland LW, Hsia EC, Strusberg I, Durez P, Nash P, Amante EJB, Churchill M, Park W (2009) Golimumab, a human anti–tumor necrosis factor-α monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty- four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum 60(8):2272–2283
Evan PS, Sonal C, Mahaboob KR (2008) Inhibition of monosodium urate crystal-induced inflammation by Withaferin A. J Pharm Pharm Sci 11(4):46–55
Feng H, Yamaki K, Takano H, Inoue K, Yanagisawa R, Yoshino S (2007) Effect of sinomenine on collagen-induced arthritis in mice. J Autoimmun 40(7):532–539
Flouri I, Markatseli TE, Voulgari PV, Boki KA, Papadopoulos I, Settas L, Zisopoulos D, Skopouli FN, Iliopoulos A, Bertsias GK (2014) Comparative effectiveness and survival of infliximab, adalimumab, and etanercept for rheumatoid arthritis patients in the Hellenic Registry of Biologics: low rates of remission and 5-year drug survival. Semin Arthritis Rheum 43(4):447–457
Ganesan R, Doss HM, Rasool M (2016) Majoon ushba, a polyherbal compound ameliorates rheumatoid arthritis via regulating inflammatory and bone remodeling markers in rats. Cytokine 77:115–126
Gao S, Wang Q, Tian XH, Li HL, Shen YH, Xu XK, Wu GZ, Hu ZL, Zhang WD (2017) Total sesquiterpene lactones prepared from Inula helenium L. has potentials in prevention and therapy of rheumatoid arthritis. J Ethnopharmacol 196:39–46
Genovese MC, Greenwald M, Cho CS, Berman A, Jin L, Cameron GS, Benichou O, Xie L, Braun D, Berclaz PY (2014) A phase II randomized study of subcutaneous ixekizumab, an anti–interleukin-17 monoclonal antibody, in rheumatoid arthritis patients who were naive to biologic agents or had an inadequate response to tumor necrosis factor inhibitors. Arthritis Rheumatol 66(7):1693–1704
Gotthardt M, Bleeker-Rovers CP, Boerman OC, Oyen WJ (2010) Imaging of inflammation by PET, conventional scintigraphy, and other imaging techniques. J Nucl Med 51(12):1937–1949
Gu YY, Jin X (2015) Effect of Resveratrol on proliferation and apoptosis of rheumatoid arthritis fibroblast-like synoviocytes. Jiangsu Med J 41:2182–2184
Guo N, Ye S, Zhang K, Yu X, Cui H, Yang X, Lin P, Lv M, Miao J, Zhang Y, Han Q, Zhang R, Chen Z, Zhu P (2019) A critical epitope in CD147 facilitates memory CD4(+) T-cell hyper-activation in rheumatoid arthritis. Cell Mol Immunol 16(6):568–579
Gupta A, Singh S (2014) Evaluation of anti-inflammatory effect of Withania somnifera root on collagen-induced arthritis in rats. Pharm Biol 52(3):308–320
Haleagrahara N, Miranda-Hernandez S, Alim MA, Hayes L, Bird G, Ketheesan N (2017) Therapeutic effect of quercetin in collagen-induced arthritis. Biomed Pharmacother 90:38–46
Han X, Su D, Xian X, Zhou M, Li X, Huang J, Wang J, Gao H (2016) Inhibitory effects of Saussurea involucrata (Kar. et Kir.) Sch. Bip. on adjuvant arthritis in rats. J Ethnopharmacol 194:228–235
Haville S, Deane KD (2022) Pre-RA: Can early diagnosis lead to prevention? Best Pract Res Clin Rheumatol 36(1):101737
Huang J, Xie B, Li Q, Xie X, Zhu S, Wang M, Peng W, Gu J (2013) Infliximab reduces CD147, MMP-3, and MMP-9 expression in peripheral blood monocytes in patients with active rheumatoid arthritis. Eur J Pharmacol 698(1–3):429–434
Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, Antoni C, Draelos Z, Gold MH, Durez P, Tak PP, Gomez-Reino JJ, Foster CS, Kim RY, Samson CM, Falk NS, Chu DS, Callanan D, Nguyen QD, Rose K, Haider A, Di Padova F (2010) Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis and uveitis. Sci Transl Med 2(52):52–72
Javadi F, Ahmadzadeh A, Eghtesadi S, Aryaeian N, Zabihiyeganeh M, Rahimi Foroushani A, Jazayeri S (2017) The effect of quercetin on inflammatory factors and clinical symptoms in women with rheumatoid arthritis: a double-blind, randomized controlled trial. J Am Coll Nutr 36(1):9–15
Ji JJ, Lin Y, Huang SS, Zhang HL, Diao YP, Li K (2013) Quercetin: a potential natural drug for adjuvant treatment of rheumatoid arthritis. Afr J Tradit Complement Altern Med 10(3):418–421
Jia N, Chu W, Li Y, Ding L, Duan J, Cui J, Cao S, Zhao C, Wu Y, Wen A (2016) Iridoid glycosides from the flowers of Gentiana macrophylla Pall. ameliorate collagen-induced arthritis in rats. J Ethnopharmacol 189:1–9
Jung HO, Tae-Jin L, Jong-Wook P, Taeg KK (2008) Withaferin A inhibits iNOS expression and nitric oxide production by Akt inactivation and down-regulating LPS-induced activity of NF-kappaB in RAW 264.7 cells. Eur J Pharmacol 599(1–3):11–17
Kapoor B, Gupta R, Gupta M (2017) Natural products in treatment of rheumatoid arthritis. Int J Green Pharm 11(03):S356–S363
Karnati M, Chandra RH, Veeresham C, Kishan B (2013) Anti-arthritic activity of root bark of Oroxylum indicum (L.) vent against adjuvant-induced arthritis. Pharmacog Res 5(2):121
Kashyap B, Tiwari U, Garg A, Kaur IR (2015) Diagnostic utility of anti-CCP antibodies and rheumatoid factor as inflammatory biomarkers in comparison with C-reactive protein and TNF-α in rheumatoid arthritis. Trop J Med Res 18(1):5
Kiely PD, Nikiphorou E (2018) Management of rheumatoid arthritis. Medicine 46(4):216–221
Kim JY, Cho SK, Han M, Choi YY, Bae SC, Sung YK (2014) The role of bone scintigraphy in the diagnosis of rheumatoid arthritis according to the 2010 ACR/EULAR classification criteria. J Korean Med Sci 29(2):204–209
Koike R, Takeuchi T, Eguchi K, Miyasaka N (2007) Update on the Japanese guidelines for the use of infliximab and etanercept in rheumatoid arthritis. Mod Rheumatol 17(6):451–458
Kumar A, Shah BA, Singh S, Hamid A, Singh SK, Sethi VK, Saxena AK, Singh J, Taneja SC (2012) Acyl derivatives of boswellic acids as inhibitors of NF-κB and STATs. Bioorg Med Chem Lett 22(1):431–435
Kumar G, Srivastava A, Sharma SK, Rao TD, Gupta YK (2015) Efficacy & safety evaluation of Ayurvedic treatment (Ashwagandha powder & Sidh Makardhwaj) in rheumatoid arthritis patients: a pilot prospective study. Indian J Med Res 141(1):100–106
Kurrasch R, Brown JC, Chu M, Craigen J, Overend P, Patel B, Wolfe S, Chang DJ (2013) Subcutaneously administered ofatumumab in rheumatoid arthritis: a phase I/II study of safety, tolerability, pharmacokinetics, and pharmacodynamics. J Rheumatol 40(7):1089–1096
Kyoung-Jin M, Kyounghwa C, Taeg KK (2011) Withaferin A down-regulates lipopolysaccharide-induced cyclooxygenase-2 expression and PGE2 production through the inhibition of STAT1/3 activation in microglial cells. Int Immunopharmacol 11(8):1137–1142
Lebre MC, Jongbloed SL, Tas SW, Smeets TJ, McInnes IB, Tak PP (2008) Rheumatoid arthritis synovium contains two subsets of CD83-DC-LAMP-dendritic cells with distinct cytokine profiles. Am J Pathol 172(4):940–950
Lee EB, Fleischmann R, Hall S, Wilkinson B, Bradley JD, Gruben D, Koncz T, Krishnaswami S, Wallenstein GV, Zang C, Zwillich SH, van Vollenhoven RF (2014a) Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 370(25):2377–2386
Lee JA, Son MJ, Choi J, Jun JH, Kim JI, Lee MS (2014b) Bee venom acupuncture for rheumatoid arthritis: a systematic review of randomised clinical trials. BMJ Open 4(11):e006140
Lee LT, Tsai YF, Hu NY, Wang CW, Huang KK, Hsiao JK, Shih YC, Munekazu I (2013) Anti-arthritis effect of mangostins from G. Mangostana. Biomed Prev Nutr 3(3):227–232
Li R, Guo LX, Li Y, Chang WQ, Liu JQ, Liu LF, Xin GZ (2017) Dose-response characteristics of Clematis triterpenoid saponins and clematichinenoside AR in rheumatoid arthritis rats by liquid chromatography/mass spectrometry-based serum and urine metabolomics. J Pharm Biomed Anal 136:81–91
Littlejohn EA, Monrad SU (2018) Early diagnosis and treatment of rheumatoid arthritis. Prim Care 45(2):237–255
Liu P, Wang J, Wen W, Pan T, Chen H, Fu Y, Wang F, Huang JH, Xu S (2020) Cinnamaldehyde suppresses NLRP3 derived IL-1β via activating succinate/HIF-1 in rheumatoid arthritis rats. Int Immunopharmacol 84:106570
Llopis E, Kroon HM, Acosta J, Bloem JL (2017) Conventional radiology in rheumatoid arthritis. Radiol Clin 55(5):917–941
Luo C, Xu X, Wei X, Feng W, Huang H, Liu H, Xu R, Lin J, Han L, Zhang D (2019) Natural medicines for the treatment of fatigue: Bioactive components, pharmacology, and mechanisms. Pharmacol Res 148:104409
Maharaj AB, Daikh DI (2022) Diagnosing rheumatoid arthritis: challenges and opportunities. Best Pract Res Clin Rheumatol 36(1):101743
Maresca M, Micheli L, Cinci L, Bilia AR, Ghelardini C, Di Cesare ML (2017) Pain relieving and protective effects of Astragalus hydroalcoholic extract in rat arthritis models. J Pharm Pharmacol 69(12):1858–1870
Maresca M, Micheli L, Mannelli LDC, Tenci B, Innocenti M, Khatib M, Mulinacci N, Ghelardini C (2016) Acute effect of Capparis spinosa root extracts on rat articular pain. J Ethnopharmacol 193:456–465
McInnes IB, Schett G (2017) Pathogenetic insights from the treatment of rheumatoid arthritis. The Lancet 389(10086):2328–2337
Melet J, Mulleman D, Goupille P, Ribourtout B, Watier H, Thibault G (2013) Rituximab-induced T cell depletion in patients with rheumatoid arthritis: association with clinical response. Arthritis Rheum 65(11):2783–2790
Min SY, Yan M, Kim SB, Ravikumar S, Kwon SR, Vanarsa K, Kim HY, Davis LS, Mohan C (2015) Green tea epigallocatechin-3-gallate suppresses autoimmune arthritis through indoleamine-2,3-dioxygenase expressing dendritic cells and the nuclear factor, erythroid 2-like 2 antioxidant pathway. J Inflamm 12:53
Mukherjee S, Siddiqui MA, Dayal S, Ayoub YZ, Malathi K (2014) Epigallocatechin-3-gallate suppresses pro-inflammatory cytokines and chemokines induced by Toll-like receptor 9 agonists in prostate cancer cells. J Inflamm Res 7:89–101
Nam JL, Ramiro S, Gaujoux-Viala C, Takase K, Leon-Garcia M, Emery P, Gossec L, Landewe R, Smolen JS, Buch MH (2014) Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2013 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 73(3):516–528
Navarro-Perán E, Cabezas-Herrera J, Sánchez-Del-Campo L, García-Cánovas F, Rodríguez-López JN (2008) The anti-inflammatory and anti-cancer properties of epigallocatechin-3-gallate are mediated by folate cycle disruption, adenosine release and NF-kappaB suppression. Inflamm Res 57(10):472–478
Németh T, Mócsai A (2012) The role of neutrophils in autoimmune diseases. Immunol Lett 143(1):9–19
Newman DJ, Cragg GM (2016) Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 79(3):629–661
Nigrovic PA, Shin K (2015) Evaluation of synovial mast cell functions in autoimmune arthritis, mast cells. Springer, pp 423–442
Noack M, Miossec P (2014) Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev 13(6):668–677
Oh YC, Kang OH, Kim SB, Mun SH, Park CB, Kim YG, Kim YI, Lee YS, Han SH, Keum JH, Shin DW, Ma JY, Kwon DY (2012) Anti-inflammatory effect of sinomenine by inhibition of pro-inflammatory mediators in PMA plus A23187-stimulated HMC-1 cells. Eur Rev Med Pharmacol Sci 16(9):1184–1191
Ohata J, Zvaifler NJ, Nishio M, Boyle DL, Kalled SL, Carson DA, Kipps TJ (2005) Fibroblast-like synoviocytes of mesenchymal origin express functional B cell-activating factor of the TNF family in response to pro-inflammatory cytokines. J Immunol 174(2):864–870
Patil KR, Patil CR, Jadhav RB, Mahajan VK, Patil PR, Gaikwad PS (2011) Anti-arthritic activity of bartogenic acid isolated from fruits of Barringtonia racemosa Roxb (Lecythidaceae). Evid Based Complement Alternat Med 785245
Peng W, Qin RX, Li XL, Zhou H (2013) Botany, phytochemistry, pharmacology, and potential application of Polygonum cuspidatum Sieb.et Zucc.: a review. J Ethnopharmacol 148:729–745
Peres RS, Liew FY, Talbot J, Carregaro V, Oliveira RD, Almeida SL, França RF, Donate PB, Pinto LG, Ferreira FI (2015) Low expression of CD39 on regulatory T cells as a biomarker for resistance to methotrexate therapy in rheumatoid arthritis. Proc Natl Acad Sci 112(8):2509–2514
Pu J, Fang FF, Li XQ, Shu ZH, Jiang YP, Han T, Peng W, Zheng CJ (2016) Matrine exerts a strong anti-arthritic effect on type II collagen-induced arthritis in rats by inhibiting inflammatory responses. Int J Mol Sci 17(9):1410
Quesniaux V, Ryffel B, Di Padova F (2013) IL-17, IL-22 and their producing cells: role in inflammation and autoimmunity, 2nd edn. Springer
Rasool M, Varalakshmi P (2007) Protective effect of Withania somnifera root powder in relation to lipid peroxidation, antioxidant status, glycoproteins and bone collagen on adjuvant-induced arthritis in rats. Fundam Clin Pharmacol 21(2):157–164
Rein P, Mueller RB (2017) Treatment with biologicals in rheumatoid arthritis: An overview. Rheumatol Ther 4(2):247–261
Rezaei H, Saevarsdottir S, Geborek P, Petersson IF, van Vollenhoven RF, Forslind K (2013) Evaluation of hand bone loss by digital X-ray radiogrammetry as a complement to clinical and radiographic assessment in early rheumatoid arthritis: results from the SWEFOT trial. BMC Musculoskelet Disord 14(1):79
Roeleveld DM, Koenders MI (2015) The role of the Th17 cytokines IL-17 and IL-22 in rheumatoid arthritis pathogenesis and developments in cytokine immunotherapy. Cytokine 74(1):101–107
Rosa Neto NS, de Carvalho JF, Shoenfeld Y (2009) Screening tests for inflammatory activity: applications in rheumatology. Mod Rheumatol 19(5):469–477
Schiff M, Weinblatt ME, Valente R, van der Heijde D, Citera G, Elegbe A, Maldonado M, Fleischmann R (2014) Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: two-year efficacy and safety findings from AMPLE trial. Ann Rheum Dis 73(1):86–94
Schoellnast H, Deutschmann HA, Hermann J, Schaffler GJ, Reittner P, Kammerhuber F, Szolar DH, Preidler KW (2006) Psoriatic arthritis and rheumatoid arthritis: findings in contrast-enhanced MRI. AJR Am J Roentgenol 187(2):351–357
Shichita T, Ago T, Kamouchi M, Kitazono T, Yoshimura A, Ooboshi H (2012) Novel therapeutic strategies targeting innate immune responses and early inflammation after stroke. J Neurochem 123(2):29–38
Smolen JS, Emery P, Ferraccioli GF, Samborski W, Berenbaum F, Davies OR, Koetse W, Purcaru O, Bennett B, Burkhardt H (2015) Certolizumab pegol in rheumatoid arthritis patients with low to moderate activity: the certain double-blind, randomised, placebo-controlled trial. Ann Rheum Dis 74(5):843–850
Soeken KL, Miller SA, Ernst E (2003) Herbal medicines for the treatment of rheumatoid arthritis: a systematic review. Rheumatology (oxford) 42(5):652–659
Stańczyk J, Kowalski M (2001) The role of cyclooxygenase and prostaglandins in the pathogenesis of rheumatoid arthritis. Pol Merkur Lekarski 11(65):438–443
Sultana F, Neog MK, Rasool M (2017) Withaferin-A, a steroidal lactone encapsulated mannose decorated liposomes ameliorates rheumatoid arthritis by intriguing the macrophage repolarization in adjuvant-induced arthritic rats. Colloids Surf B Biointerfaces 155:349–365
Sun HY, Long LJ, Wu J (2006) Chemical constituents of mangrove plant Barringtonia racemosa. Zhong Yao Cai 29(7):671–672
Suter LG, Fraenkel L, Braithwaite RS (2011) Role of magnetic resonance imaging in the diagnosis and prognosis of rheumatoid arthritis. Arthritis Care Res (hoboken) 63(5):675–688
Swathi KP, Jayaram S, Sugumar D, Rymbai E (2021) Evaluation of anti-inflammatory and anti-arthritic property of ethanolic extract of Clitoria ternatea. Chin Herb Med 13(2):243–249. https://doi.org/10.1016/j.chmed.2020.11.004
Takeuchi T, Tanaka Y, Ishiguro N, Yamanaka H, Yoneda T, Ohira T, Okubo N, Genant HK, van der Heijde D (2016) Effect of denosumab on Japanese patients with rheumatoid arthritis: a dose–response study of AMG 162 (Denosumab) in patients with rheumatoid arthritis on methotrexate to Validate inhibitory effect on bone Erosion (DRIVE)-a 12-month, multicentre, randomised, double-blind, placebo-controlled, phase II clinical trial. Ann Rheum Dis 75(6):983–990
Van Vollenhoven RF, Wax S, Li Y, Tak PP (2015) Safety and efficacy of atacicept in combination with rituximab for reducing the signs and symptoms of rheumatoid arthritis: a phase II, randomized, double-blind, placebo-controlled pilot trial. Arthritis Rheumatol 67(11):2828–2836
Venkatesha SH, Astry B, Nanjundaiah SM, Kim HR, Rajaiah R, Yang Y, Tong L, Yu H, Berman BM, Moudgil KD (2016) Control of autoimmune arthritis by herbal extracts and their bioactive components. Asian J Pharm 11(2):301–307
Verhoff M, Seitz S, Paul M, Noha SM, Jauch J, Schuster D, Werz O (2014) Tetra-and pentacyclic triterpene acids from the ancient anti-inflammatory remedy frankincense as inhibitors of microsomal prostaglandin E(2) synthase-1. J Nat Prod 77(6):1445–1451
Verma UN, Misra R, Singh RR, Agarwal S, Naik S (2002) Serological correlates of inflammation in rheumatoid arthritis: usefulness of acute phase reactants in monitoring disease activity. J Indian Rheumatol Assoc 10:1–4
von Kempis J, Dudler J, Hasler P, Kyburz D, Tyndall A, Zufferey P, Villiger PM (2012) Use of abatacept in rheumatoid arthritis. Swiss Med Wkly 142:w13581
Voon FL, Sulaiman MR, Akhtar MN, Idris MF, Akira A, Perimal EK, Israf DA, Ming-Tatt L (2017) Cardamonin (2’,4’-dihydroxy-6’-methoxychalcone) isolated from Boesenbergia rotunda (L.) Mansf. inhibits CFA-induced rheumatoid arthritis in rats. Eur J Pharmacol 794:127–134
Wakefield RJ, Balint PV, Szkudlarek M, Filippucci E, Backhaus M, D’Agostino MA, Sanchez EN, Iagnocco A, Schmidt WA, Bruyn GA, Kane D, O’Connor PJ, Manger B, Joshua F, Koski J, Grassi W, Lassere MN, Swen N, Kainberger F, Klauser A, Ostergaard M, Brown AK, Machold KP, Conaghan PG, Group OSI (2005) Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol 32(12):2485–2487
Weinblatt ME, Genovese MC, Ho M, Hollis S, Rosiak-Jedrychowicz K, Kavanaugh A, Millson DS, Leon G, Wang M, van der Heijde D (2014) Effects of fostamatinib, an oral spleen tyrosine kinase inhibitor, in rheumatoid arthritis patients with an inadequate response to methotrexate: results from a phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheumatol 66(12):3255–3264
Wysham KD, Baker JF, Narla R (2022) Osteoporosis evaluation and treatment recommendations in rheumatoid arthritis. Best Pract Res Clin Rheumatol 36(3):101757
Zago BA, Priyadharshini A, Vijayakumar TM (2020) Safety and efficacy of newer biologics DMARDs in the management of rheumatoid arthritis: A systematic review. Osteoarthr Cartil 2:100116
Zaka M, Sehgal SA, Shafique S, Abbasi BH (2017) Comparative in silico analyses of Cannabis sativa, Prunella vulgaris and Withania somnifera compounds elucidating the medicinal properties against rheumatoid arthritis. J Mol Graph Model 74:296–304
Zhang Q, Peng W, Wei S, Wei D, Li R, Liu J, Peng L, Yang S, Gao Y, Wu C, Pu X (2019) Guizhi-Shaoyao-Zhimu decoction possesses anti-arthritic effects on type II collagen-induced arthritis in rats via suppression of inflammatory reactions, inhibition of invasion & migration and induction of apoptosis in synovial fibroblasts. Biomed Pharmacother 118:109367
Zhao J, Chen X, Ho KH, Cai C, Li CW, Yang M, Yi C (2021a) Nanotechnology for diagnosis and therapy of rheumatoid arthritis: evolution towards theranostic approaches. Chin Chem Lett 32:66–86
Zhao X, Kim YR, Min Y, Zhao Y, Do K, Son YO (2021b) Natural plant extracts and compounds for rheumatoid arthritis therapy. Medicina 57(3):266
Zhou H, Liu JX, Luo JF, Cheng CS, Leung EL, Li Y, Su XH, Liu ZQ, Chen TB, Duan FG, Dong Y, Zuo YH, Li C, Lio CK, Li T, Luo P, Xie Y, Yao XJ, Wang PX, Liu L (2017) Suppressing mPGES-1 expression by sinomenine ameliorates inflammation and arthritis. Biochem Pharmacol 142:133–144
Acknowledgements
Declared none.
Funding
No funding is associated with this work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaur, C., Mishra, Y., Kumar, R. et al. Pathophysiology, diagnosis, and herbal medicine-based therapeutic implication of rheumatoid arthritis: an overview. Inflammopharmacol 32, 1705–1720 (2024). https://doi.org/10.1007/s10787-024-01445-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-024-01445-8