Abstract
Bioactivity-guided fractionation of F. drupacea Thunb. extract revealed that the water fraction (FDWF) increased pH of the artificial gastric juice from 1.2 to 5.67 ± 0.015. The gastroprotective effect of FDWF against ulcer induced by ethanol was evaluated in rats. In ulcerogenic rats, increase in the gastric juice volume and ulcer lesions, and decrease in the gastric pH were evident. However, pretreatment with FDWF (100 mg/kg b.wt., p.o.) significantly inhibited lesion index, reduced gastric juice volume by 56.09% and increased gastric pH value. When given after ethanol, the same dose of FDWF led to significant healing of the gastric ulcer, with 75.60% reduction of gastric juice volume, and increase in pH value. In both prophylactic and therapeutic-treated groups, the level of superoxide dismutase and reduced glutathione in gastric homogenate were increased, while that of malondialdehyde was decreased. Also, the levels of succinate dehydrogenase and lactate dehydrogenase were increased, while that of acid phosphatase was decreased. In addition, the inflammatory markers; IL-10 and PGE2 were significantly increased. The histopathological results confirmed the above findings and indicated that the antiulcer effect of FDWF is mediated, at least in part, through antioxidant and anti-inflammatory mechanisms. Twenty-three compounds were tentatively identified in FDWF using UPLC−PDA−ESI–MS/MS and most of them were found to be phenolic acid derivatives. FDWF was standardized to contain 23.66 ± 2.62 mg/g and 8.86 ± 0.29 mg/g of quinic acid and chlorogenic acid, respectively. Accordingly, FDWF is a potential natural product that could increase the healing of gastric mucosal injury and prevents the development of ethanol-induced gastric mucosal injury in rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric mucosal injury remains one of the most common diseases of GIT. The long-term use of nonsteroidal anti-inflammatory drugs, alcohol consumption, H. pylori infection, increased secretion of hydrochloric acid and pepsin, and stressful conditions are among the most aggressive factors for developing PU (Sowndhararajan and Kang 2013, Aboul Naser et al. 2020).
For gastric and duodenal ulcers, several drugs have been described, including anticholinergics, histamine H2-receptor antagonists, antacids, and proton pump inhibitors (Sowndhararajan and Kang 2013). Unfortunately, a series of adverse effects may result from prolonged use of these drugs (Sheen and Triadafilopoulos 2011). Recently, there is an increasing interest in developing plant-based agents for the effective management of PU (Patel et al. 2012).
Plants of genus Ficus are globally tropical ornamental trees, parts of which are used in folk medicine (Yessoufou et al. 2015), while the fruits of others, particularly F. sycamorus L. and F. carica L. are used as food in Egypt since ancient time (Manniche 1989).
Some Ficus spp. were studied for their antiulcer activity; the ethanolic extract of F. carica leaves exhibited antiulcer activity against chemical (HCl−Alcohol) and pylorus ligation-induced ulcers (Patil and Patil 2011). Moreover, the aqueous extract of F. deltoidea whole plant protect against ethanol-induced gastric ulcer (Fatimah et al. 2009) and the ethanolic extract of F. religiosa leaves decreases ulcer area and the volume of acidic secretion in a dose-dependent manner (Gregory et al. 2013). On the other hand, F. drupacea was used in folk medicine to treat sinusitis, malaria, and lung fluke. Moreover, the plant was used in the pharmaceutical industry as a source of α-glucosidase inhibitors (Phan et al. 2013). However, its protective effect against ethanol-induced gastric mucosal injury was not previously reported.
One of the animal models commonly used to investigate new antiulcer drugs is the ethanol-induced gastric ulcer (Arab et al. 2015; Sistani Karampour et al. 2019). Ethanol administration lowers the release of bicarbonate, gastric mucus, and nitric oxide while also causing stomach necrosis and subsequent infiltration of inflammatory cells. In addition, ethanol decreases stomach blood flow and causes oxidative stress by lowering glutathione production and elevating malondialdehyde formation (Aboul Naser et al. 2020).
This study was designed to evaluate the potential of F. drupacea extract/fractions as protective agent against ethanol-induced gastric mucosal injury in rat model. Ulcer indices, count, and pH value were recorded. The oxidative stress targets; reduced glutathione (GSH), superoxide dismutase (SOD), and malondialdehyde (MDA), inflammatory markers; interleukin-10 (IL-10) and prostaglandin E2 (PGE2), and relevant enzymes, such as succinate dehydrogenase (SDH), lactate dehydrogenase (LDH) and alkaline phosphatase (AP) were assessed. Histological features of the stomach were investigated. The metabolic profile and standardization of the bioactive fraction were performed using UPLC−PDA−ESI–MS/MS.
Materials and methods
Plant material
Sample from the leaves of Ficus drupacea Thunb., was collected from Al Zohriya botanical garden Cairo, Egypt in August 2020. The plant material was authenticated by Mrs. Trease Labib, Consultant of Plant Taxonomy at Ministry of Agriculture and the former director of El-Orman botanic garden. A voucher specimen (No. 2.10.2022. III) was deposited at the herbarium of the Department of Pharmacognosy, Faculty of Pharmacy, Cairo University. The plant material was dried in shade, powdered, and kept in closed container till use.
Fractionation of the total extract of F. drupacea leaves
A total of 1.5 kg of powdered leaves was macerated with 70% ethanol (5 × 3L) for 5 days. The solution obtained was evaporated using Rotavapor® (Heidolph, Germany) to yield 80 g of dry extract. Part (40 g) of the extract was suspended in water (100 mL) and applied on the top of a Diaion HP20 (Sigma Aldrich, Supelco, USA) column (6 cm × 125 cm). Elution was started with water (5 L), 25% methanol/water (3 L), 50% methanol/water (3 L), 100% methanol (2.5 L), and dichloromethane (1 L). Fractions were evaporated under reduced pressure to yield 26, 4.8, 2.6, 1.0, and 0.5 g of dry residue, respectively.
Determination of the neutralizing effect on artificial gastric acid
100 mg of each fraction or extract was weighed and dissolved in 250 ml of distilled water. The standards used in the proposed study were the antacid-1 (sodium bicarbonate) and antacid-2 (a combination of aluminum hydroxide and magnesium hydroxide). The freshly prepared plant fractions (in 90 mL) or distilled water (90 mL) is added separately to the artificial gastric juice (100 mL) at pH 1.2. The pH values are determined to examine the neutralizing effects on artificial gastric juice.
For preparation of artificial gastric juice, two grams of NaCl and 3.2 mg of pepsin are dissolved in 500 mL distilled water. Hydrochloric acid (7.0 mL) and adequate water are added to make a 1000 mL solution. The pH of the solution is adjusted to 1.2 (Panda et al. 2017). A pH meter is connected continuously to monitor the changes of pH, and pH values were determined in triplicates.
Antioxidant activity
The antioxidant activity of F. drupacea extract and FDWF was evaluated using DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) free radical scavenging assay (Shen et al. 2010). Samples were screened at 500 µg/ml using 0.1 mM DPPH dissolved in methanol. After an incubation for 30 min in the dark at room temperature, the absorbance was measured at 517 nm and a reference wavelength of 690 nm. Ascorbic acid was used as positive control at different concentration ranging from 11–2.7 µg/ml. The DPPH/methanol mixture was used as a negative control. The DPPH scavenging activity of samples was calculated according to the following equation:
where x indicates the absorbance of sample and av indicates the average absorbance of control and NC indicates the absorbance of negative control.
In vivo evaluation of gastroprotective effect of FDWF
Chemicals
All chemicals used in this study were supplied by Sigma Co. (Saint Louis, USA). ELISA kits for interleukin-10 (IL-10) and prostaglandin E2 (PGE2) were provided by Cloud Clone Corp (Saint Louis, USA), and famotidine was a generous gift from Amon Co., Egypt.
Acute toxicity study
Forty male Wistar albino rats (100–120 g) were divided into four groups to assess acute toxicity at different plant concentrations (50, 100, and 200 mg/kg b.wt). For 15 days, the animals were monitored. There were no dead rats observed during the experiment, indicating that the extract was safe. As a result, a dose of 100 mg/kg b.wt was chosen for the in vivo study.
Experimental design
Male Wistar albino rats (100–120 g) were obtained from the Animal House at Egypt's National Research Centre. All animals were kept in an air and temperature-controlled environment with access to water and standard pellets. Animal handling and their termination were carried out in accordance with the Medical Ethical Committee of the National Research Centre, Giza, Egypt. (Approval No. 20114).
Thirty-six male rats were divided into six groups (each of six rats). Rats in group 1 received distilled water (negative control group), while rats in group 2 received a daily dose of the FDWF (100 mg/kg b.wt, p.o) for one week. Rats in group 3 (the ulcerogenic rats) received a single dose of absolute ethanol (5 mL/kg b.wt, intragastric gavage) on a 24 h empty stomach, and sacrificed 1 h later (Mard et al. 2008; Eskander et al. 2021). Group 4 rats (the protective group) were given FDWF for 1 week prior to ulcer induction, while group 5 rats (the treatment group) were given a single oral dose of absolute ethanol on an empty stomach for 24 h and then after 1 h a daily dose of FDWF for 1 week. Group 6 rats were given absolute ethanol on an empty stomach for 24 h before receiving a daily dose of the reference antiulcer drug famotidine (20 mg/kg b.wt, p.o) for one week (Sen et al. 2013). After the last administration, all animals were fasted for 24 h, anesthetized by 1 ml of diethyl ether on piece of cotton (by inhalation) and sacrificed by decapitation.
Measurement of gastric content volume, pH, and number of gastric lesions
Just after anesthesia, the stomach contents of each rat were collected and centrifuged at 300 g for 15 min. The pH and volume of the supernatant were measured. After the stomach content was removed, the stomach was opened from the long curvature, washed with saline, expanded, and the number of lesions counted using a magnifying lens.
Tissue homogenate preparation
Longitudinal sections of each stomach weighing 0.5 g were homogenized in 5 mL of phosphate buffered saline (pH 7.4). The homogenate was centrifuged for 10 min at 300 g at 4 °C, and the supernatant was collected and kept at −20 °C for ongoing studies.
Determination of biochemical markers
The stomach oxidative stress targets were determined in all groups of animals using previously reported methods; GSH (Moron et al. 1979), MDA (Buege and Aust 1978), and SOD (Nishikimi et al. 1972). Also, level of enzymes such as SDH (mitochondria marker) (Shelton and Rice 1957), LDH (cytoplasm marker) (Babson and Babson 1973), and AP (lysosome marker) (Wattiaux and De Duve 1956) was measured. The ELISA technique was used to determine the inflammatory index in gastric tissue. PGE2 and IL-10 were measured using a solid phase competitive ELISA kit (Abcam, ab100765, Cambridge, USA), and a colorimetric assay was used to determine total protein in the stomach (Bradford 1976).
Histopathological examination
The stomach tissue slices were embedded in paraffin wax blocks after being fixed in 4% paraformaldehyde. Pathological changes were examined under a light microscope using 5-mm thick sections stained with hematoxylin and eosin (H&E) (Hirsch et al. 1997).
Statistical analysis
The data were presented as the mean ± standard deviation (SD) of six rats in each group. For statistical analysis, one-way analysis of variance (ANOVA), Costat Software Computer Program, with post hoc test at least significance difference (LSD) between groups at p < 0.05, were used.
UPLC−PDA−ESI–MS/MS analysis of F. drupacea active fraction (FDWF)
The analysis was performed on a MaXis-4G instrument. (Bruker Daltonics, Bremen, Germany) linked to an Ultimate 3000 HPLC (Thermo Fisher Scientific). UPLC condition were performed as described before by (Hegazi et al. 2020). The parameters used for the tandem MS follows (Garg et al. 2015). Sodium formate was used as a calibrant, and the acquired data was calibrated using a Bruker-developed script.
Data analysis
Compass Data Analysis 4.4 (Bruker Daltonics®, Germany) was used for raw data visualization, while Metaboscape 3.0 (Bruker Daltonics®) was used for molecular feature selection, raw data treatment, and preprocessing. The T-ReX 3D (Time aligned Region Complete eXtraction) algorithm was used to detect and combine isotopes, adducts, and fragments innate to the same compound into a single feature. After that, a bucket table was created that contained all of the detected features as well as their retention time, measured m/z, molecular weight, and detected ions (Olmo‐García et al., 2019). The catalogued ions table was created with a negative ionization mode intensity threshold of 10e3, a retention time range of 1 to 40 min, and a restricted mass range m/z of 120–1800 Da.
Molecular networking
The MS2 data were independently uploaded as an mgf file to the publicly accessible Global Natural Product Social molecular networking (GNPS) platform (http://gnps.ucsd.edu, accessed on 31 July 2022) running the online feature-based molecular networking workflow (Wang et al. 2016).
The data were analyzed with a parent mass tolerance of 0.05 Da and an MS/MS fragment ion tolerance of 0.05 Da to generate consent spectra. A network with a cosine score of 0.7 and more than 6 matched peaks between two consensus mass spectra linked by an edge was then built. The network was compared to GNPS spectral libraries (NIST13, MassBank, and Respect). The molecular network and parameters can be found at the following link: https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=8a25fad020c6409c8a577cbe9fdecb59.
Consequently, the molecular network that was built was enhanced with a MolNetEnhancer to provide a more comprehensive chemical overall view of metabolomics data. (Ernst et al. 2019). Cytoscape was used to visualize and analyze the output molecular network (3.9.1) (Shannon et al. 2003).
Total phenolic content (TPC)
Phenolic compounds (TPC) of F. drupacea extract and FDWF were determined by Folin−Ciocalteu reagent according to the method of (Antolovich et al. 2002) with minor modifications. In brief, 20 μL of samples were mixed with 100 μl of 1:10 Folin−Ciocalteu reagent, then Na2CO3 (80 L, 7.5%) was added. After incubating at room temperature for 2 h in the dark, the absorbance at 690 nm was measured in a microplate. The standard reference was gallic acid. TPC (total phenolic content) was measured in mg of gallic acid equivalents per g of dried extract (mg GAE g−1) (gallic acid equivalent).
Quantification of quinic acid and chlorogenic acid
The sample was analyzed with liquid chromatography−electrospray ionization−tandem mass spectrometry (LC–ESI–MS/MS) using an Exion LC AC system for separation and a SCIEX Triple Quad 5500 + MS/MS system with ESI for detection. For the separation, a ZORBAX Eclipse Plus C18 Column (4.6100 mm, 1.8 m) was used. The mobile phase was 0.1% formic acid in water (solvent A) and acetonitrile (solvent B) with the following gradient: 2% B from 0–1 min, 2–60% B from 1–8 min, 60% B from 8–12 min, and 2% B from 12.01–15 min. The flow rate was 0.8 mL/min and the injection volume was 3 µL. The selected polyphenols were analyzed using the negative ionization mode using Multiple Reaction Monitoring (MRM). The curtain gas was 25 psi, the ion spray voltage was 4500, the source temperature was 400 °C, the ion source gases 1 and 2 were 55 psi with a declustering potential of 50, the collision energy was 25 eV and the collision energy spread was 10 eV.
Results
Bioactivity-guided fractionation of total extract of F. drupacea leaves
Fractionation of the total extract using Diaion@ HP20 column afforded 5 fractions. Their acid neutralizing effects revealed that the water fraction (FDWF) has the most neutralizing effect (1.96 ± 0.015), followed by the 75% methanol fraction (1.81 ± 0.01) (Table 1). The antacids-1 and 2 showed neutralizing effects of 1.81 ± 0.01 and 1.66 ± 0.01, respectively). Therefore, FDWF was selected for the in vivo study.
Antioxidant activity
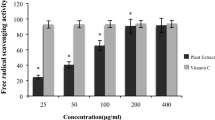
FDWF showed higher DPPH radical scavenging activity [IC50 value of 231 ± 0.074 µg/ml] compared with total extract [IC50 value of 516 ± 0.086 ug/ml].
In vivo study of FDWF on ethanol-induced gastric mucosal injury in rats
Effect on ulcer index
The volume of gastric juice in normal rats was found to be 0.17 ± 0.05 mL However, a drastic increase in the gastric juice volume to 2.05 ± 0.42 mL (+ 1105.88%) was noted in the ulcerogenic group rats (Table 2). When compared with that of ulcerogenic rats, a marked decline in gastric volume by 56.09 and 75.60%, respectively, was evident after administration of FDWF before (prophylactic group, G-4) or after ethanol (therapeutic-treated group, G-5) to rats, with respective amelioration percentages of 676 and 911%. In comparison to the control group, the pH value of the gastric juice in ulcerogenic rats was reduced by 27.47% (Table 2). When compared with the ulcerogenic group, both FDWF-prophylactic and therapeutic-treated rats, as well as the famotidine-treated group, showed insignificant increase of 12.77, 24.45, and 19.04%, respectively. As a result, the healing improvement values were 7.84, 17.7, and 13.81%, respectively.
Furthermore, the ulcerogenic rats had seven lesions on average per stomach. When compared with the ulcerogenic rats, those in the FDWF-prophylactic and therapeutic-treated groups, as well as the famotidine-treated group showed significant decrease of 44.42, 65.51, and 72.41%, respectively (Fig. 1).
Effect on cell organelles marker enzymes
The levels of SDH, LDH, and AP in mucosal tissues of rats in the control group before (G-1) or after administration of FDWF (G-2) were insignificantly different (Table 3).
However, a significant decrease in the levels of both SDH and LDH (65.41 and 61.15%, respectively), and a significant increase in AP level (140.96%) were observed in the ulcerogenic rats (G-3), when compared with the control group. On the other hand, a significant improvement in the level of SDH was observed after administration of FDWF [for protection (G-4) or treatment (G-5)], or famotidine (G-6); improvement percentages of 16.85, 29.96, and 20.22%, respectively. Similarly, LDH level was improved in the FDWF or famotidine groups (with amelioration values of 27.12, 29.34, and 26.51%, respectively), when compared with the ulcerogenic rats. However, a reduction in AP level in ulcerogenic rats (G-3) was observed in both the prophylactic and therapeutic-treated groups, with improving values of 63.27, 107.04, and 97.68%, respectively (Table 3).
Effect on oxidative stress markers
There were no significant changes in oxidative stress markers or total protein content after administering FDWF to normal rats (Table 4). Rats with stomach ulcers had a 72.09% reduction in GSH level when compared to the control group, whereas those with ulcers who were protected or treated with FDWF or famotidine had a significant increase of 91.18, 123.18, and 142.70%, respectively, when compared to the ulcerogenic group. This corresponded to a level of improvement of 25.44, 34.37, and 39.82%, respectively. Similarly, the ulcerogenic rats had a 70.49% decrease in SOD levels when compared to the healthy group. The administration of the FDWF (for protection or treatment) or famotidine to ulcerogenic rats resulted in an increase of SOD level (78.02, 127.82, and 123.64%, respectively), when compared with the ulcerogenic group. In contrast, ulcerogenic rats had a significant increase in MDA level by 477.98% when compared to normal rats, whereas those exposed to both protection and treatments had a significant decrease in MDA level by 27.34, 53.19, and 56.64%, respectively, when compared to ulcerogenic rats. As a result, MDA recorded improvement values of 158.02, 307.45, and 324.88%, respectively. In comparison to the control group, ethanol caused a 219.60% increase in total protein levels. When compared with the ulcerogenic rats, it showed a reduction level of 25.19, 60.55, and 49.10% after protection and treatments with FDWF or famotidine, respectively. As a result, the improvement values were 80.48, 139.49, and 156.91%, respectively.
Effect on inflammatory markers
The administration of FDWF to normal rats resulted in insignificant changes in the level of IL-10 and PGE2. Rats given ethanol had a 33.12% decrease in IL-10 level when compared with the control group, whereas those with ulcers and exposed to either protection or treatments with FDWF or famotidine had an increase of 12.33, 23.40, and 31.53%, respectively, when compared with the ulcerogenic group. These observations revealed a level of improvement of 8.42, 15.65, and 21.09%, respectively. Similarly, the ulcerogenic rats showed a 41.63% reduction in PGE2 level when compared to the control group. PGE2 level was increased by 45.22, 54.09, and 62.42% after administration of FDWF for either protection or treatment, as compared to the ulcerogenic group; with improvement levels of 26.39, 31.57, and 36.43% in the prophylactic, therapeutic treated, and reference groups, respectively. (Table 5).
Histopathological observations
Both control and normal rats given FDWF showed intact gastric mucosa, normally distributed gastric glands lined by mucus-secreting cells with rounded nuclei, and normal lamina propria (see Fig. 1A, 1B, as well as 2A, B).
Photomicrographs of the gastric mucosa of control and FDWF-treated rats A, B showing normal stomach layers; note the normal mucosal (*), and submucosal epithelium and intact basement membrane (arrows), C ulcerogenic group showing ulcers; note the ulcer formation represented by deep mucosal layer (*) and destruction reaching the submucosal layer (black arrow), D prophylactic group with FDWF showing gastric erosion; note the superficial mucosal layer destruction with intact basement membrane (black arrows). E Treated group with FDWF showing normal stomach layers with absence of ulcer and inflammatory cells (yellow arrow), and some blood congestion (black arrow), (F) The famotidine-treated group had a healed ulcer with a moderately developed mucosal lining and less thickening than the control group (black arrows). All specimens were stained with haematoxylin and eosin (H& E) and viewed at a magnification of × 400
Hemorrhage and necrosis were observed in the gastric mucosa of ulcerogenic rats, resulting in severe submucosal odema and epithelial cell injury. At the ulcer's base, polymorphous lymphocytes were observed, and hyperplastic gastric glands surrounded the ulcer. The lamina propria, as well as fibrotic tissues, contained lymphocytes and polymorphonuclear leucocytes (see Figs. 1C, 2C)
Gastric erosion as well as superficial mucosal layer destruction in the presence of an intact basement membrane were observed in the FDWF-prophylactic group (Figs. 1D, 2D).
Ulcerogenic rats treated with FDWF showed completely healing of ulcer with well-developed and normal thickened mucosal membrane (Figs. 1F, 2F).
UPLC−MS metabolic profile of FDWF
A total of 23 metabolites were tentatively identified in FDWF based on their retention time (rt), molecular formula, and fragmentation pattern (Fig. 3; Table 6). Phenolic acid derivatives were dominating FDWF with quinic acid as the major metabolite (Fig. 4).
Quinic acid derivatives grouped in clusters A and G were identified as coumaroylquinic acid (15, 20), chlorogenic acid (16, 19) and feruloylquinic acid (18); their MS2 spectra shared the same fragment ion at m/z 191 amu for quinic acid moiety. Dihydroxybenzoic acid derivatives grouped in cluster B were annotated as dihydroxybenzoic acid hexoside (6), dihydroxybenzoic acid pentoside hexoside (7), dihydroxybenzoic acid pentoside (9) and dihydroxybenzoic acid dipentoside (10). Derivatives of ferulic acid grouped in cluster F were identified as ferulic acid malate (22) and ferulic acid (23). Cluster D represents amino acids, E represents coumarins and derivatives, and H represents hydrolysable tannins. Hydroxycinnamic acid glycosides are selflooped annotated as caffeic acid hexoside (12) and coumaroyl hexoside (14).
Total phenolic content (TPC)
The contents of phenolics in the total extract of F. drupacea extract and FDWF were found to be 42 ± 0.55 and 52.3 ± 1.12, respectively.
Quantification of quinic acid and chlorogenic acid using LCMS
The content of quinic acid (QA) in the total extract of F. drupacea extract and FDWF was found to be 21.12 ± 2.19 and 23.66 ± 2.62 mg/g (Fig. 5), while that of chlorogenic acid (CGA) was 6.30 ± 3.09 and 8.86 ± 0.29 mg/g, respectively.
Discussion
Ethanol-induced gastric mucosal injury is one of the most frequently used experimental models for evaluating the cytoprotective and antioxidant effects of antiulcer agents (Lahiri and Palit 2012). This model mimics many aspects of acute human gastric mucosal injury condition rats develop sever ulcers, degraded gastric mucosa, increased mucosal permeability, and sometimes bleeding (Aboul Naser et al., 2020). Histopathological and macroscopical examination of the gastric mucosa of ulcerogenic rats revealed severe internal bleeding, as evidenced by severe congestion in the lamina propria submucosa and inter-villus extravasation of RBCs among the gastric mucosal villi (Lustenberger et al. 2011). Also, severe coagulative necrosis was found in some areas of the gastric mucosa (Li et al. 2013). This ulcerogenic condition led to an increase in the levels of inflammatory markers (such as MPO), proinflammatory cytokines (such as TNF-α) and reactive oxygen species (ROS), and a decrease in the levels of the anti-inflammatory cytokines (such as IL-10), PGE2, mucosal enzymes (LDH and SDH), and cellular antioxidants (Brzozowski et al. 2005; Adinortey et al. 2013). AlRashdi et al. 2012 and Kan et al. 2017 reported that increased ROS production and antioxidant depletion are associated with the pathogenesis and progression of ethanol-induced PU, and the accumulated ROS cause lipid peroxidation (Yu et al. 2017).
In this study, ethanol administration increased the level of MDA in the gastric tissues of ulcerogenic rats, but reduced the levels of the antioxidant enzymes SOD and GSH, which is in line with previous findings (Sidahmed et al. 2013).
Pretreatment of ulcerogenic rats with FDWF prevented the ROS-mediated oxidative damage by increasing the activity of the SOD enzyme, restoring the depleted GSH, and decreasing MDA level. It was reported that PGE2 regulates gastric mucus secretion, stimulates blood flow and bicarbonate production, and accelerates healing of ulcers. However, reduced level of PGEs is a relevant marker of mucosal ulceration (Tsuge et al. 2019). The gastroprotective effect of FDWF is mediated, at least in part through increase in the production of gastric IL-10 and PGE2, as supported by the histopathological examination, which revealed reduced inflammatory responses (lower ulcer index). On the other hand, the levels of LDH and SDH were significantly increased after treatment with FDWF or famotidine.
It was reported that the increase in hydrogen ion concentration lowers pH of the gastric juice and promotes gastric damage. FDWF treatment significantly improved gastric pH while decreasing gastric secretion when compared to the ulcerogenic group (Lüllmann et al. 2000). Furthermore, the gastric pH of FDWF-treated or famotidine-treated rats were found to be the same and suggested that FDWF has a strong ability to decrease stomach acid production and neutralize its acidity.
FDWF used in the present study was prepared from the total extract of F. drupacea aerial parts. FDWF was rich in phenolic acids (52.3 ± 1.12) and QA and demonstrates promising antioxidant activity (231 ± 0.074 ug/ml). Taking into consideration that ROS are linked to ulcer formation, these constituents could play a role in the anti-ulcer effect of FDWF (Panda and Suresh 2015; Elshamy et al. 2020). Among the phenolic acids identified in FDWF p-coumaric acid, caffeic acid, and ferulic acid were reported to increase PGE2 content and mucus formation in the gastric mucosa (De Barros et al. 2008). Also, sinapic acid reduced the severity of ethanol-induced injury of the gastric mucosa through reduction of the gastric acid juice volume and acidity, and increase of PGE2 and NO2 levels. These effects were exactly equivalent to those seen for omeprazole. Sinapic acid was also reported to suppress gastric inflammation by lowering MPO, TNF-α, and IL-6, inhibiting lipid peroxidation (MDA), and restoring depleted GSH and CAT activity (Raish et al. 2021). On the other hand, CGA was found to be effective in treating and preventing ethanol/HCl-induced gastric lesions by inhibiting neutrophil migration and restoring the levels of GPx, SOD, CAT, GSH, and TBARS in mice, and prevented the rise of TNF-α and leukotriene B4 (Shimoyama et al. 2013). While QA and its derivatives were reported to exert anti-inflammatory effect by inhibiting the pro-inflammatory markers (Zeng et al. 2009; Sheng et al. 2005).
Accordingly, the gastroprotective and ulcer healing activities of FDWF in this model could be attributed to the antioxidant and anti-inflammatory effects of phenolic acids and QA and its derivatives.
Conclusion
Gastric mucosal injury is one of the most prevalent gastrointestinal conditions which affect significant segment of the global population. Therefore, developing potent and safe antiulcer medications is needed. For the first time, a phenolic acids-rich fraction (FDWF) was prepared from F. drupacea aerial parts and was found to exert a pronounced effect in protecting and treating ethanol-induced gastric mucosal injury in rats. FDWF was standardized and its metabolic profile was clarified using HPLC–ESI–MS/MS. In summary, FDWF is a promising natural product that can be further developed for the management of gastric mucosal injury.
Data Availability
Data supporting findings are presented within the manuscript. Inquiries about data availability should be directed to the authors.
References
Abou-zaid MM, Nozzolilloc C (1999) 1-O-galloyl-α-l-rhamnose from Acer rubrum. Phytochemistry 52:1629–1631. https://doi.org/10.1016/S0031-9422(99)00236-8
Aboul NA, Younis E, El-feky A et al (2020) Management of Citrus sinensis peels for protection and treatment against gastric ulcer induced by ethanol in rats. Biomarkers 25:349–359. https://doi.org/10.1080/1354750X.2020.1759693
Adinortey MB, Ansah C, Galyuon I et al (2013) In vivo models used for evaluation of potential antigastroduodenal ulcer agents. Ulcers. https://doi.org/10.1155/2013/796405
Alrashdi AS, Salama SM, Alkiyumi SS et al (2012) Mechanisms of gastroprotective effects of ethanolic leaf extract of Jasminum sambac against HCl/ethanol-induced gastric mucosal injury in rats. Evid Based Complement Altern Med. https://doi.org/10.1155/2012/786426
Ammar S, Del Contreras M, Belguith-hadrich O et al (2015) New insights into the qualitative phenolic profile of Ficus carica L. fruits and leaves from Tunisia using ultra-high-performance liquid chromatography coupled to quadrupole-time-of-flight mass spectrometry and their antioxidant activity. RSC Adv 5:20035–20050. https://doi.org/10.1039/C4RA16746E
Antolovich M, Prenzler PD, Patsalides E et al (2002) Methods for testing antioxidant activity. Analyst 127:183–198. https://doi.org/10.1039/B009171P
Arab HH, Salama SA, Omar HA et al (2015) Diosmin protects against ethanol-induced gastric injury in rats: novel anti-ulcer actions. PLoS ONE 10:e0122417. https://doi.org/10.1371/journal.pone.0122417
Babson AL, Babson SR (1973) Kinetic colorimetric measurement of serum lactate dehydrogenase activity. Clin Chem 19:766–769. https://doi.org/10.1093/CLINCHEM/19.7.766
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Brzozowski T, Konturek PC, Drozdowicz D et al (2005) Grapefruit-seed extract attenuates ethanol-and stress-induced gastric lesions via activation of prostaglandin, nitric oxide and sensory nerve pathways. World J Gastroenterol 11:6450. https://doi.org/10.3748/wjg.v11.i41.6450
BuegE JA, Aust SD (1978) [30] Microsomal lipid peroxidation. Methods in enzymology. Elsevier, Amsterdam
Chansriniyom C, Nooin R, Nuengchamnong N et al (2021) Tandem mass spectrometry of aqueous extract from Ficus dubia sap and its cell-based assessments for use as a skin antioxidant. Sci Rep 11:1–13. https://doi.org/10.1038/s41598-021-96261-3
De Barros MP, Lemos M, Maistro EL et al (2008) Evaluation of antiulcer activity of the main phenolic acids found in Brazilian Green Propolis. J Ethnopharmacol 120:372–377. https://doi.org/10.1016/j.jep.2008.09.015
Elshamy AI, Farrag ARH, Mohamed SH et al (2020) Gastroprotective effects of ursolic acid isolated from Ochrosia elliptica on ethanol-induced gastric ulcer in rats. Med Chem Res 29:113–125. https://doi.org/10.1007/S00044-019-02465-8
Ernst M, Kang KB, Caraballo-rodríguez AM et al (2019) MolNetEnhancer: enhanced molecular networks by integrating metabolome mining and annotation tools. Metabolites 9:144. https://doi.org/10.3390/metabo9070144
Eskander DM, Aziz WM, Nassar MI et al (2021) Isolation and characterization of flavonoid compounds from Stachytarpheta jamaicensis (L.) Vahl and its role as anti-gastro ulcerative agent in rats. Biomarkers 26:606–616. https://doi.org/10.1080/1354750X.2021.1950210
Fang N, Yu S et al (2002) LC/MS/MS characterization of phenolic constituents in dried plums. J Agric Food Chem 50:3579–3585. https://doi.org/10.1021/jf0201327
Farag MA, Abdelfattah MS, Badr SE et al (2014) Profiling the chemical content of Ficus lyrata extracts via UPLC-PDA-qTOF-MS and chemometrics. Nat Prod Res 28:1549–1556. https://doi.org/10.1080/14786419.2014.926353
Fatimah Z, Mahmood A, Hapipah M et al (2009) Anti-ulcerogenic activity of aqueous extract of Ficus deltoidea against ethanol-induced gastric mucosal injury in rats. Res J Med Sci 3:42–46
Garg N, Kapono CA, Lim YW et al (2015) Mass spectral similarity for untargeted metabolomics data analysis of complex mixtures. Int J Mass Spectrom 377:719–727. https://doi.org/10.1016/j.ijms.2014.06.005
Gregory M, Divya B, Mary RA et al (2013) Anti–ulcer activity of Ficus religiosa leaf ethanolic extract. Asian Pac J Trop Biomed 3:554–556. https://doi.org/10.1016/S2221-1691(13)60112-4
Hegazi NM, Radwan RA, Bakry SM et al (2020) Molecular networking aided metabolomic profiling of beet leaves using three extraction solvents and in relation to its anti-obesity effects. J Adv Res 24:545–555. https://doi.org/10.1016/j.jare.2020.06.001
Hirsch C, Zouain C, Alves J et al (1997) Induction of protective immunity and modulation of granulomatous hypersensitivity in mice using PIII, an anionic fraction of Schistosoma mansoni adult worm. Parasitology 115:21–28. https://doi.org/10.1017/s0031182097001078
Jin J, Lao J, Zhou R et al (2018) Simultaneous identification and dynamic analysis of saccharides during steam processing of rhizomes of Polygonatum cyrtonema by HPLC–QTOF–MS/MS. Molecules 23:2855. https://doi.org/10.3390/molecules23112855
Kan J, Hood M, Burns C et al (2017) A novel combination of wheat peptides and fucoidan attenuates ethanol-induced gastric mucosal damage through anti-oxidant, anti-inflammatory, and pro-survival mechanisms. Nutrients 9:978. https://doi.org/10.3390/nu9090978
Lahiri S, Palit G (2012) An overview of the current methodologies used for evaluation of gastric and duodenal anti-ulcer agents. Pharmacologia 3:249–257. https://doi.org/10.17311/pharmacologia.2012.249.257
Li W, Huang H, Niu X et al (2013) Protective effect of tetrahydrocoptisine against ethanol-induced gastric ulcer in mice. Toxicol Appl Pharmacol 272:21–29. https://doi.org/10.1016/j.taap.2013.05.035
Lüllmann H, Mohr K, Hein L et al (2000) Color atlas of pharmacology. Thieme, New York
Lustenberger T, Inaba K, Barmparas G et al (2011) Ethanol intoxication is associated with a lower incidence of admission coagulopathy in severe traumatic brain injury patients. J Neurotrauma 28:1699–1706. https://doi.org/10.1089/neu.2011.1866
Manniche L (1989) An ancient Egyptian herbal. University of Texas Press, Austin
Mard S, Bahari Z, Eshaghi N et al (2008) Antiulcerogenic effect of Securigera securidaca L. seed extract on various experimental gastric ulcer models in rats. Pak J Biol Sci 11:2619. https://doi.org/10.3923/pjbs.2008.2619.2623
Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochimica et biophysica acta (BBA)-general subjects 582:67–78. https://doi.org/10.1016/0304-4165(79)90289-7
Nishikimi M, Rao NA, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46:849–854. https://doi.org/10.1016/s0006-291x(72)80218-3
Olmo-garcía L, Wendt K, Kessler N (2019) Exploring the capability of LC-MS and GC-MS multi-class methods to discriminate virgin olive oils from different geographical indications and to identify potential origin markers. Eur J Lipid Sci Technol 121:1800336. https://doi.org/10.1002/ejlt.201800336
Panda V, Shinde P, Deora J et al (2017) A comparative study of the antacid effect of some commonly consumed foods for hyperacidity in an artificial stomach model. Complement Ther Med 34:111–115. https://doi.org/10.1016/j.ctim.2017.08.002
Panda V, Suresh S (2015) Gastro-protective effects of the phenolic acids of Macrotyloma uniflorum (horse gram) on experimental gastric ulcer models in rats. Food Biosci 12:34–46. https://doi.org/10.1016/j.fbio.2015.07.004
Patel R, Jawaid T, Gautam P et al (2012) Herbal remedies for gastroprotective action: a review. Int J Phytopharm 2:64–67. https://doi.org/10.7439/ijpp.v2i2.388
Patil VV, Patil VR (2011) Evaluation of anti-inflammatory activity of Ficus carica Linn. leaves. Indian J Nat Prod Resour 2:151–155
Phan VK, Chau VM, Nguyen XN et al (2013) Chemical constituents of Ficus drupacea leaves and their α-glucosidase inhibitory activities. Bull Korean Chem Soc 34:263–266. https://doi.org/10.5012/bkcs.2013.34.1.263
Raish M, Shahid M, Binjardan YA et al (2021) Gastroprotective effect of sinapic acid on ethanol-induced gastric ulcers in rats: Involvement of Nrf2/HO-1 and NF-κB signaling and antiapoptotic role. Front Pharmacol 12:101. https://doi.org/10.3389/fphar.2021.622815
Schütz K, Kammerer DR, Carle R et al (2005) Characterization of phenolic acids and flavonoids in dandelion (Taraxacum officinale WEB. ex WIGG.) root and herb by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom 19:179–186. https://doi.org/10.1002/rcm.1767
Sen S, Asokkumar K, Umamaheswari M et al (2013) Antiulcerogenic effect of gallic acid in rats and its effect on oxidant and antioxidant parameters in stomach tissue. Indian J Pharm Sci 75:149
Shannon P, Markiel A, Ozier O et al (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504. https://doi.org/10.1101/gr.1239303
Sheen E, Triadafilopoulos G (2011) Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci 56:931–950. https://doi.org/10.1007/s10620-010-1560-3
Shelton E, Rice ME (1957) Activity of tissue homogenates? J Nat Cancer Inst 18:117
Shen CZ, Jun HY, Choi SH (2010) Evaluation of antioxidant activities and active compounds separated from water soluble extracts of Korean black pine barks. Bull Korean Chem Soc 31:3567–3572. https://doi.org/10.5012/bkcs.2010.31.12.3567
Sheng Y, Åkesson C, Holmgren K et al (2005) An active ingredient of Cat’s claw water extracts: identification and efficacy of quinic acid. J Ethnopharmacol 96:577–584. https://doi.org/10.1016/j.jep.2004.10.002
Shimoyama AT, Santin JR, Machado ID et al (2013) Antiulcerogenic activity of chlorogenic acid in different models of gastric ulcer. Naunyn-Schmiedeberg’s Archiv Pharmacol 386:5–14. https://doi.org/10.1007/s00210-012-0807-2
Sidahmed HM, Hashim NM, Amir J et al (2013) Pyranocycloartobiloxanthone A, a novel gastroprotective compound from Artocarpus obtusus Jarret, against ethanol-induced acute gastric ulcer in vivo. Phytomedicine 20:834–843. https://doi.org/10.1016/j.phymed.2013.03.002
Sistani Karampour N, Arzi A, Rezaie A, Pashmforoosh M, Kordi F (2019) Gastroprotective effect of zingerone on ethanol-induced gastric ulcers in rats. Medicina 55:64. https://doi.org/10.3390/medicina55030064
Sowndhararajan K, Kang SC (2013) Protective effect of ethyl acetate fraction of Acacia ferruginea DC. against ethanol-induced gastric ulcer in rats. J Ethnopharmacol 148:175–181. https://doi.org/10.1016/j.jep.2013.04.007
Tsuge K, Inazumi T, Shimamoto A et al (2019) Molecular mechanisms underlying prostaglandin E2-exacerbated inflammation and immune diseases. Int Immunol 31:597–606. https://doi.org/10.1093/intimm/dxz021
Wang M, Carver JJ, Phelan VV et al (2016) Sharing and community curation of mass spectrometry data with global natural products social molecular networking. Nat Biotechnol 34:828–837. https://doi.org/10.1038/nbt.3597
Wattiaux R, De duve C (1956) Tissue fractionation studies. Release of bound hydrolases by means of Triton X-100. Biochem J 63:606. https://doi.org/10.1042/bj0630606
Yessoufou K, Elansary HO, Mahmoud EA et al (2015) Antifungal, antibacterial and anticancer activities of Ficus drupacea L. stem bark extract and biologically active isolated compounds. Ind Crops Prod 74:752–758. https://doi.org/10.1016/j.indcrop.2015.06.011
Yu T, Yang Y, Kwak YS et al (2017) Ginsenoside Rc from Panax ginseng exerts anti-inflammatory activity by targeting TANK-binding kinase 1/interferon regulatory factor-3 and p38/ATF-2. J Ginseng Res 41:127–133. https://doi.org/10.1016/j.jgr.2016.02.001
Zeng K, Thompson KE, Yates CR et al (2009) Synthesis and biological evaluation of quinic acid derivatives as anti-inflammatory agents. Bioorg Med Chem Lett 19:5458–5460. https://doi.org/10.1016/j.bmcl.2009.07.096
Acknowledgements
Authors are thankful to Dr. Hamada Saad department of pharmaceutical biology, pharmaceutical institute, Eberhard Karls University of Tubingen for performing UPLC-PDA-ESI-MS/MS analysis.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This work was supported by National Research Centre (NRC, Ph.D. fund no. 2/4/4). The authors received no financial support for the research publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflicts of interest.
Ethics approval
This study was performed in National Research Centre, Giza, Egypt and approved with the Medical Ethical Committee of National Research Centre according to reference number 20114.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bakry, S.M., Naser, A.F.A., Negoumy, S.I.E. et al. Phenolic acids-rich fraction from Ficus drupacea leaves for the prevention and treatment of ethanol-induced gastric mucosal injury in rats. Inflammopharmacol 31, 1423–1436 (2023). https://doi.org/10.1007/s10787-023-01158-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-023-01158-4