Abstract
Primate encounter rates often vary throughout the year due to seasonal differences in activity, ecology, and behaviour. One notably extreme behaviour is continuous hibernation. Although a rare adaptation in primates, the dwarf lemurs of Madagascar (genus Cheirogaleus) enter obligate hibernation each year during the dry season, after spending the wet season consuming high-energy foods. Whilst seasonal changes in activity in some Cheirogaleus populations are well-known, many species remain little-studied, and there is no specific information on their encounter rates, nor when they enter and emerge from hibernation. This uncertainty critically affects reliable calculation of population density estimates for these highly threatened lemurs. In this study, we assessed how encounter rates of the fat-tailed dwarf lemur (C. medius) vary seasonally in the transitional forests of the Sahamalaza-Iles Radama National Park, northwest Madagascar, during a 4-year period. We established a system of line transects (N = 60) throughout our study area, on which we conducted distance sampling of C. medius. We then used our distance sampling data to calculate encounter rate and population density data. We found encounter rates of C. medius to be significantly higher during the wet season compared with the dry season. Furthermore, encounter rates of C. medius were particularly low from May–August. These results provide some evidence to suggest the time period that C. medius hibernate in Sahamalaza-Iles Radama National Park. These findings underpin the importance of careful study design when surveying threatened species with seasonal differences in activity, such as those that hibernate. This study also demonstrates the importance of species-specific behavioural data for accurate population density assessment, which is required to inform conservation action.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primate encounter rates often vary profoundly between seasons (Branch, 1983; van Schaik & Brockman, 2005). This is because seasonal differences in primate activity levels (Erkert & Kappeler, 2004), feeding ecology (Hanya & Chapman, 2013; Poulsen et al., 2001), ranging (Reyna-Hurtado et al., 2018), and social behavior (Heldstab et al., 2021) can affect our ability to observe and detect wild primates at different times of the year. Whilst primate encounter rate data can be collected for the whole year to calculate the most reliable population density estimates, this often is not possible due to the remote location of many primate populations and the limited financial resources available to conduct such studies (Cowlishaw & Dunbar, 2021; Turner & Corlett, 1996). Because accurate and reliable data on primate population densities are required for their conservation, primate studies need to be carefully planned to avoid periods when primate observation and detection rates are likely to be lower due to certain behaviors.
Torpor, the act of entering into a state of physical inactivity, is one such adaptation that impacts seasonal detection rates in some primate species (Miller et al., 2018). Seasonal torpor is a rare and extreme adaptation among primates (Lymann et al., 1982; Schülke & Ostner, 2007), and this is primarily because most species of primates live in the tropics where seasonal variations in temperature are less profound (Lovegrove, 2003). With the exception of the small-bodied tamarins (Moynihan, 1970), all documented cases of primate torpor occur in nocturnal strepsirrhine species that have a body mass of less than 600 g (Dausmann & Warnecke, 2016). Many primate taxa that undergo torpor employ only daily torpor in times of low food and water availability (Dausmann & Warnecke, 2016; Perret & Aujard, 2001). This is a well-known trait in mouse lemurs (genus Microcebus) (Kobbe et al., 2011; Lovegrove et al., 2014; Ortmann et al., 1997; Schmid, 2000), but it also occurs in the Javan slow loris (Nycticebus javanicus), the African lesser bushbaby (Galago moholi), the hairy-eared dwarf lemur (Allocebus trichotis), and possibly Coquerel’s giant mouse lemur (Mirza coquereli) (Dausmann, 2014; Dausmann & Warnecke, 2016; Nowack et al., 2010). However, some strepsirrhines, such as the dwarf lemurs of the Cheirogaleus genus (Dausmann & Warnecke, 2016), the pygmy slow loris (N. pygmaeus) (Ruf et al., 2015), Goodman’s mouse lemur (M. lehilahytsara) (Andriambeloson et al., 2020), the reddish-gray mouse lemur (M. griseorufus) (Kobbe et al., 2011), and the brown mouse lemur (M. rufus) (Atsalis, 1999) enter torpor for prolonged periods of several days or months (hibernation) if they are able to store enough energy reserves. Studying such species is therefore difficult, because they are inactive and undetectable for long periods of the year.
The dwarf lemurs (genus Cheirogaleus) are a group of nine, small, omnivorous, primate species from Madagascar (Groves, 2016; McLain et al., 2017) and are the sole group of primates that enter obligate hibernation (Dausmann, 2008; Dausmann & Blanco, 2016; Wright & Martin, 1995). To do this, Cheirogaleus spp. spend much of their active periods feeding and building up reserves of fat in order to survive periods of food and water shortages during the dry season (Dausmann & Blanco, 2016). Cheirogaleus are distributed throughout Madagascar and are present in a range of habitat types, including dry forest (e.g., the fat-tailed dwarf lemur (C. medius): Dausmann et al., 2005), humid forest (e.g., the greater dwarf lemur (C. major), the Montagne d'Ambre dwarf lemur (C. andysabini): Lahann, 2007; Dausmann, 2013; Lei et al., 2015), central highland and montane forest (e.g., Crossleyi’s dwarf lemur (C. crossleyi), Sibreei’s dwarf lemur (C. sibreei): Blanco et al., 2008; Blanco & Godfrey, 2014), and transitional forests that have characteristics of both humid and dry forests (e.g., C. crossleyi, Sheth’s dwarf lemur (C. shethi): Hending et al., 2017a). They also have been observed in agroecosystems (Hending et al., 2018; Webber et al., 2020). Habitat structure and resource availability vary considerably among these habitat types, and differences in encounter rates, behavior, ecology, and hibernation length have been described for different Cheirogaleus species from different forest types and between different bioclimatic zones (Dausmann & Blanco, 2016). Information on Cheirogaleus is generally lacking, and our knowledge of species-specific encounter rates and hibernation duration is generally restricted to just a few studies from only one or two study locations (e.g., C. major in eastern Madagascar: Lahann, 2007; Blanco et al., 2018). Because some species of Cheirogaleus have large geographic distributions in multiple habitat types and climatic zones (Frasier et al., 2016), we therefore have no knowledge of when many Cheirogaleus populations enter and emerge from hibernation. As Cheirogaleus encounter rates are certain to vary seasonally due to hibernation periods, this limits our ability to study Cheirogaleus behavioural ecology and constrains the period in which we can reliably carry out population assessments.
With a seasonally fluctuating mean body size of 150–270 g, Cheirogaleus medius is one of the smallest Cheirogaleus (Fietz & Ganzhorn, 1999), and, like other Cheirogaleus, it has the ability to store fat reserves as adipose tissue in its tail. This adaptation allows C. medius to hibernate for periods of up to 8 months, the longest for any primate (Dausmann & Blanco, 2016). C. medius has a very large, discontinuous geographic distribution, and it inhabits the dry forests of the west of Madagascar and the transitional forest of the Sambirano Domain in the northwest. The hibernation duration of this species has thus far been studied in only dry and littoral forest habitat (Dausmann & Blanco, 2016; Dausmann et al., 2005; Lahann & Dausmann, 2011), and differences in hibernation length between these two habitat types have been documented, suggesting that this species has flexible life-history traits in relation to its environment (Lahann & Dausmann, 2011).
In this study, we investigated how encounter rates of C. medius vary among the wet and dry season in transitional forests of the Sahamalaza-Iles Radama National Park, northwest Madagascar, to gain insight into the hibernation schedule of this species. We also aimed to improve survey methods and study-planning for C. medius to obtain reliable density estimates. As the hibernation schedule of this species is likely to affect detection rates, and therefore population density estimates, we predicted that detection rates would vary significantly between the wet and dry seasons. As hibernation duration differences have already been observed between dry and littoral forest due to climatic differences (Dausmann & Blanco, 2016; Lahann & Dausmann, 2011), we also predicted that C. medius hibernation would be different in transitional forest in comparison to the other two habitat types.
Methods
Ethical Note
All research complied with UK Home Office policies when working with animals and all research adhered to the legal requirements of Madagascar. Research in the Sahamalaza-Iles Radama National Park was permitted by Madagascar National Parks (Permit numbers 245/19 and 124/22 – MEEF/SG/DGGE/DAPRNE/SCBE.Re). We consulted the Code of Best Practices for Field Primatology when planning all methods undertaken in this study.
Data Availability
The datasets used during this study are available from the corresponding author on reasonable request.
Conflict of Interest
The authors declare that they have no conflict of interest.
Study Site
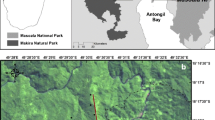
Sahamalaza-Iles Radama National Park (hereafter referred to as SIRNP) is located in North West Madagascar on the Sahamalaza peninsula, between 13°52’S – 14°37’S and 45°38’E – 48°04’E (Rode et al., 2013). SIRNP is a UNESCO Biosphere Reserve and gained full National Park status in 2007 (Schwitzer et al., 2007), yet despite this protection, it has undergone heavy deforestation in recent years (Seiler et al., 2014). SIRNP now has only two remaining forests: the 1,169 ha Anabohazo continuous forest block and the highly fragmented 1,020 ha Ankarafa forest (Volampeno, 2009). Whilst a few smaller, highly degraded forest fragments do remain, scattered throughout the National Park, much of the area is now characterised by disturbed, anthropogenic savannah, grassland, and some remnants of scrub and gallery forest (Volampeno et al., 2011). We conducted our study in both the forests of Ankarafa (14°22’S, 47°45E) and Anabohazo (14°19’S, 47°54’E) (Fig. 1).
The Sahamalaza-Iles Radama National Park, Madagascar, and the location of Ankarafa forest (A) and Anabohazo forest (B). Figure created in ArcMap, with a scale of 1:7,000,000 for Madagascar and a scale of 1:350,000 for the zoomed panel. The two red lines surrounding protected areas represent the protected area (inner) and a 3-km buffer zone (outer).
SIRNP is located in the Sambirano Domain, an area of Madagascar that is characterized by subhumid transitional forest; the forests of SIRNP therefore encompass a mixture of deciduous and evergreen tree species, many of which are endemic to the Sambirano Domain (Du Puy & Moat, 1996; Koechlin, 1972). The climate of SIRNP is highly seasonal, with a separate hotter wet season (November–April) and a cooler dry season (May–October) (Mandl et al., 2018). Mean temperature range in SIRNP is 20.6–32.0 °C, with recorded extreme temperatures of 13.2–39.1 °C (Hending et al., 2017b; Volampeno et al., 2011) and mean annual precipitation rates of approximately 1,600 mm (Schwitzer et al., 2007).
Distance Sampling
Between June 9, 2018 and May 13, 2022, we conducted line-transect distance sampling along several transects, a method regularly used for arboreal primates (Buckland et al., 2010), to assess the encounter rates of Cheirogaleus medius in SIRNP. We established a total of 60 line transects in the forests of Ankarafa (N = 40, mean length = 1,021 m, range = 525–1,525 m) and Anabohazo (N = 20, mean length = 1,124 m, range = 500–1,500 m). Due to the large number of transects, we did not walk all transects each month. The Ankarafa transects were in 40 separate forest fragments (one transect per forest fragment). We began each transect walk at approximately 19:00, when C. medius would be actively feeding (Fietz & Ganzhorn, 1999; Müller, 1999). During each transect walk, two researchers and one guide walked along the transect at a steady and continuous pace of approximately 1 km/h. The same three participants conducted all transects to avoid observational bias (Buckland et al., 2001). We used high-lumen handheld torches (EC20, Nitecore, Guangzhou, China) and headlamps (Tikkina and Tikka + , Petzl, Crolles, France) to spot any C. medius individuals that were present along the transect based on eye-shine. For any sighted C. medius individuals, we recorded their geographic position using a handheld GPS (eTrex 30, Garmin, Olathe, KS), the date and time of the observation, and we measured the perpendicular distance from the transect line to the tree in which the lemur was positioned using a handheld laser measure (80 m, XCSource, San Francisco, CA). Despite the high density of the vegetation at the study site, we were able to distinguish C. medius from the sympatric Sahamalaza sportive lemur (Lepilemur sahamalaza) by their pelage colouration and size differences (Mittermeier et al., 2008). Furthermore, we could distinguish C. medius from the sympatric northern giant mouse lemur (Mirza zaza) and the Sambirano mouse lemur (Microcebus sambiranensis) by their size and movement; locomotion of C. medius is slower than that of M. zaza (Müller & Thalmann, 2002; Rode-Margono et al., 2016) and C. medius is larger than M. sambiranensis (Mittermeier et al., 2008). Although our study took place over almost 4 years, we did not conduct any transect surveys during December due to the inaccessibility of the site around this time.
Encounter Rates and Population Density Estimates
We calculated monthly mean encounter rates for this study, by dividing the number of observed individuals for each transect by the total transect distance (N/km). We then calculated mean C. medius encounter rate for each transect across all repeat walks, for both forests individually and for SIRNP as whole. We also calculated mean C. medius encounter rates for each month across transects. We submitted our C. medius count data, transect length values and perpendicular distance values (Supplementary File 1) to the Distance 7.5 software (Thomas et al., 2010) to quantify transect survey effort (estimated strip width: ESW—the area under the distance function from its left-truncation limit to its right-truncation limit) and to calculate C. medius population size and density, with lower and upper 95% confidence limits, for SIRNP. We used the Buckland method to calculate our population densities (Buckland et al., 2001), as it has been used successfully in other lemur population studies (Axel & Maurer, 2011; Quémére et al., 2010) and because it accounts for the decreasing probability of observing our study species as its perpendicular distance from the transect increases. We used instance detection functions, and we truncated our datasets with a 5% threshold to remove very distant observations. We also used Distance to estimate the population size for C. medius with lower and upper 95% confidence limits using our population density values and the area of each surveyed forest. Forest area was determined before this study with GPS waypoint data obtained during perimeter walks; forest area was later calculated using ArcGIS (Version 10.6.1, Esri, Redlands, CA).
Extraction of Temperature and Precipitation Variables
We used R Studio (R.Studio, Inc., Boston, MA) packages “raster” (Hijmans, 2019) and “rgdal” (Bivand et al., 2019) to download average monthly temperature and precipitation variable data for SIRNP for our study period (2018–2022) directly from the WorldClim global climate database (Fick & Hijmans, 2017). Specifically, we downloaded mean monthly data for average temperature, minimum temperature, maximum temperature, and precipitation. When specifying the parameters of our variable extraction, we used the GPS point corresponding to the midpoint of our study area (latitude = − 14.3881, longitude = 47.8540), and we used the highest possible raster resolution (1-km2 tiles) to obtain the most accurate and localized data possible (Fig. 2).
Precipitation and temperature of the Sahamalaza-Iles Radama National Park, Madagascar, for February 2018–May 2022. Precipitation represents the total rainfall for each month. Figure created using data downloaded from the Worldclim database (Fick & Hijmans, 2017).
Data Analysis
We performed all statistical analyses in R Studio with an α-level of 0.05. Our monthly encounter rate data for each transect was of normal distribution (Shapiro–Wilk normality test: w = 0.884, P = 0.116), so we compared how C. medius encounter rates vary between months by using a chi-square test. To investigate the relationship between C. medius encounter rate and temperature and precipitation, we first subjected the temperature and precipitation variables (independent variables) to tests of residual normality and homoscedasticity with the “olsrr” package (Hebbali, 2018). We investigated the relationship between C. medius activity levels and temperature and precipitation with individual Spearman’s rank correlation tests. To compare C. medius population density and size between the wet season (November–April) and dry season (May–October), we created a separate dataset of mean encounter rates for only the transects that we surveyed during both seasons (we removed transects surveyed in only the wet or dry season and excluded them from this dataset). The mean encounter rates dataset did not meet the assumptions of parametric analyses (Shapiro–Wilk normality test: w = 0.776, P = 0.005), and so we compared mean encounter rates between the two sampling periods (wet season transects N = 84, dry season transects N = 160) with a paired Wilcoxon test (one mean encounter rate value per transect for both the wet and dry season).
Results
Encounter Rates
We performed a total of 264 transect surveys across our 60 established transect lines (mean = 4.40 walks per transect, range = 1–10), with a total survey distance of 275.30 km. During our study, we observed a total of 130 Cheirogaleus medius individuals, a mean of 0.49 individuals per transect walk (0.47 per km). The encounter rates of C. medius were significantly uneven among the months that were surveyed (chi-square test: χ2 = 504.91, df = 207, P < 0.001; Fig. 3). Specifically, we observed the lowest encounter rates of C. medius from May to August.
The mean encounter rate of C. medius was significantly positively correlated with monthly mean temperature (S (test statistic) = 69.658, ρ (correlation coefficient) = 0.683, P = 0.020) and mean maximum temperature (S = 50.000, ρ = 0.773, P = 0.008). However, C. medius encounter rate did not correlate significantly with monthly mean minimum temperature (S = 135.030, ρ = 0.386, P = 0.241) and precipitation (S = 148.000, ρ = 0.327, P = 0.327; Fig. 4).
Population Density and Size Estimates
The population density and size estimates for C. medius were profoundly different between the data collected for the wet and dry seasons (Fig. 3; Table I). Our statistical analysis of transect mean encounter rates also show a significant difference between the two periods (paired Wilcoxon test: V = 66, P = 0.034), with encounter rate being higher during the wet than dry season.
Discussion
Seasonal Differences in Encounter Rates
Although we observed Cheirogaleus medius individuals throughout the whole study period, our encounter rates were significantly higher in the wet season than in the dry season (Fig. 3). These findings are certainly due to lower activity levels of C. medius during the dry season, as many individuals would have been hibernating, and these observations are important for the planning and organisation of future studies into this species at this site. As has been suggested for the populations of C. medius in dry forest habitat (Dausmann et al., 2005), C. medius activity may be lower during the dry season due to lower water and food availability and lower temperatures; we also observed C. medius less frequently during times of the year with low precipitation (although this difference was not significant) and lower temperatures in this study, as demonstrated by our correlation analyses (Fig. 4). However, our C. medius encounter rates were high in September and October (Fig. 3) when rainfall was still very low at our study site (Fig. 2). C. medius encounter rate therefore may not be fully dependent on climatic factors. The lack of significant correlation also may be related to inaccurate information on weather from the WorldClim datasets, because often the number of weather stations from which this information is collected are very limited in tropical regions. Species-specific behavioural ecology and the habitat-related and environmental causes for the onset and offset of hibernation are likely to provide a fuller explanation of why C. medius encounter rates vary between months and between seasons. As these factors often are site-specific and species-specific (Dausmann & Blanco, 2016), further research into C. medius ecology and natural history in SIRNP is required to provide a more comprehensive answer to our research question.
Insights into Cheirogaleus medius Hibernation
Encounter rates of C. medius were very low during the period May–August (Fig. 3), and these observations may indicate the period when C. medius enters hibernation in SIRNP. This period coincides with the lowest monthly rates of precipitation and the lowest annual temperatures (Fig. 2), which is supported by our Spearman’s correlation analyses of temperature (Fig. 4). This potential hibernation period of four months is much shorter than that of C. medius in dry forest habitat (6–8 months: Dausmann & Blanco, 2016) but similar to that of the littoral forest habitat medius-like (Thomas’s dwarf lemur, C. thomasi) populations (4–5 months: Lahann & Dausmann, 2011). As decreasing temperatures, low water availability, and food scarcity influence the hibernation patterns of C. medius (Dausmann et al., 2005), these differences among habitat types are expected as the transitional forests of North West Madagascar have a higher annual rainfall than dry forest habitat (Donque, 1972). Although we observed six C. medius individuals during the period May–August, these lemurs may have been juveniles that were not able to accumulate the necessary fat reserves for longer hibernation (Blanco & Rahalinarivo, 2010). As Cheirogaleus have flexible life history traits (Lahann & Dausmann, 2011), it is likely that the climate of SIRNP has influenced the timing of when the resident C. medius populations enter hibernation.
C. medius is considered to have the highest hibernation duration of any of the Cheirogaleus species (Dausmann & Blanco, 2016). However, our encounter rate data for SIRNP suggest that some populations of C. medius may hibernate for shorter periods than the rainforest species C. crossleyi (3.5–5.5 months) and C. sibreei (4.5–7 months) (Blanco & Godfrey, 2014). These differences in hibernation duration may be due to the actual cause of the hibernation itself; hibernation expression in sympatric C. crossleyi and C. sibreei for example is interspecific due to species-specific ecology (Blanco & Rahalinarivo, 2010; Dausmann, 2013). This further supports the theory of flexible life history traits in relation to hibernation among Cheirogaleus (Dausmann & Blanco, 2016).
Population Density and Size
The population density of C. medius in SIRNP (Table 1) was similar to that reported in other studies of this species from the dry forest (20–200 individuals/km2: Hladik et al., 1980; Fietz, 1999). C. medius population density also varied between the two forest sites that we surveyed in SIRNP (Table 1). Cheirogaleus may be sensitive to habitat degradation and edge-effects (Lehman et al., 2006; but see Hending, 2021), and the population density of C. medius in SIRNP may be limited by the ongoing forest degradation that is typical of the region (Seiler et al., 2010); this also may explain the differences in density between Anabohazo (a continuous forest) and Ankarafa (a highly-degraded and fragmented forest). Furthermore, C. medius lives sympatrically with the northern giant mouse lemur (Mirza zaza), the Sambirano mouse lemur (Microcebus sambiranensis), and the Sahamalaza sportive lemur (Lepilemur sahamalaza) in SIRNP (Hending et al., 2017c, 2022a). Although niche separation exists between these species, all four of these taxa require secure sleeping sites. Well-insulated tree holes are particularly sought after by these species, because they provide cover from predators and insulation from the cold (Hending et al., 2017c; Rode et al., 2013; Schmid, 1998; Seiler et al., 2013). Tree holes are particularly important for C. medius, and the high competition for these sleeping sites also may limit C. medius numbers in the forests of SIRNP, which has been observed in other cheirogaleid lemurs (Lutermann et al., 2010).
Although all Cheirogaleus are obligate hibernators (Dausmann & Blanco, 2016), many surveys of their population structure and density have occurred partly or entirely in Madagascar’s dry season, a period during which they are likely hibernating (Herrera et al., 2011; Lehman et al., 2006; Müller, 1998; Müller et al., 2000). We found significant differences in our encounter rate of C. medius between the wet and dry seasons. From these findings, it is clear that results of surveys that included either wholly or partly the hibernation period do not represent accurate population density values and are serious underestimates of total population size. Such underestimates would be detrimental for accurate IUCN Red List assessments for Cheirogaleus, considering that their forest habitat is highly threatened by deforestation and unmitigated climate change (Hending et al., 2022b; Vieilledent et al., 2018). Our results underpin the importance of a sound understanding of the activity periods of Cheirogaleus populations at specific sites and in specific habitats so that population surveys can be carried out at the correct time to calculate accurate and reliable population assessments.
Summary and Future Directions
This study provides an overview of seasonal activity level, hibernation and population density of C. medius in a transitional forest habitat. However, we still know nothing of the behavioural ecology of this species in transitional forest and how it compares to that of the C. medius populations in dry forest. To better understand C. medius ecology and hibernation and how it varies among habitat types and bioclimatic regions, the thermoregulatory physiology and hibernaculum choice of C. medius should be investigated for the populations in SIRNP, because these determine Cheirogaleus ecology and hibernation expression (Dausmann & Blanco, 2016). Furthermore, a more detailed knowledge of C. medius feeding ecology is needed and its implications for hyperphagy (the prehibernation “fattening” period) need to be explored (Fietz & Ganzhorn, 1999). Generally, the ecology and natural history of C. medius is still little known despite the large geographic distribution of this species. Such datasets may provide answers as to how and why lemurs have evolved a survival strategy as extreme as obligatory hibernation.
Change history
14 March 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10764-023-00357-8
References
Andriambeloson, J. B., Greene, L. K., & Blanco, M. B. (2020). Prolonged torpor in Goodman’s mouse lemur (Microcebus lehilahytsara) from the high-altitude forest of Tsinjoarivo, central-eastern Madagascar. Folia Primatologica, 91, 697–710.
Atsalis, S. (1999). Seasonal fluctuations in body fat and activity levels in a rain-forest species of mouse lemur, Microcebus rufus. International Journal of Primatology, 20, 883–910.
Axel, A. C., & Maurer, B. A. (2011). Lemurs in a complex landscape mapping species density in subtropical dry forests of southwestern Madagascar using data at multiple levels. American Journal of Primatology, 73, 38–52.
Bivand, R., Keitt, T., & Rowlingson, B. (2019). rgdal: Bindings for the 'Geospatial' Data. Abstraction Library. R package version 1.4–8.
Blanco, M. B., Dausmann, K. H., Faherty, S. L., & Yoder, A. D. (2018). Tropical heterothermy is “cool”: The expression of daily torpor and hibernation in primates. Evolutionary Anthropology: Issues, News, and Reviews, 27(4), 147–161.
Blanco, M. B., & Godfrey, L. R. (2014). Hibernation patterns of dwarf lemurs in the high altitude forest of eastern Madagascar. In N. B. Grow, S. Gursky-Doyen, & A. Krzton (Eds.), High Altitude Primates (pp. 23–42). Springer.
Blanco, M. B., Godfrey, L. R., Rakotondratsima, M., Rahalinarivo, V., Samonds, K. E., Raharison, J. L., & Irwin, M. T. (2008). Discovery of sympatric dwarf lemur species in the high-altitude rain forest of Tsinjoarivo, Eastern Madagascar: Implications for biogeography and conservation. Folia Primatologica, 80, 1–17.
Blanco, M. B., & Rahalinarivo, V. (2010). First direct evidence of hibernation in an eastern dwarf lemur species (Cheirogaleus crossleyi) from the high-altitude forest of Tsinjoarivo, central-eastern Madagascar. Naturwissenschaften, 97, 945–950.
Branch, L. C. (1983). Seasonal and habitat differences in the abundance of primates in the Amazon (Tapajós) National Park, Brazil. Primates, 24(3), 424–431.
Buckland, S. T., Anderson, D. R., Burnham, K. P., Laake, J. L., Borchers, D. L., & Thomas, L. (2001). Introduction to Distance Sampling. Oxford University Press.
Buckland, S. T., Plumptre, A. J., Thomas, L., & Rexstad, E. A. (2010). Design and analysis of line transect surveys for primates. International Journal of Primatology, 31(5), 833–847.
Cowlishaw, G., & Dunbar, R. I. (2021). Primate Conservation Biology. University Press.
Dausmann, K. H. (2008). Hypometabolism in primates: torpor and hibernation. In: B. G. Lovegrove, A. E. McKechnie (Eds.), Hypometabolism in Animals: Torpor, Hibernation and Cryobiology. University of Kwazulu-Natal.
Dausmann, K. H. (2013). Spoilt for choice: Selection of hibernacula by Cheirogaleus medius. In J. Masters, M. Gamba, & F. Génin (Eds.), Leaping Ahead: Advances in Prosimian Biology (pp. 205–214). Springer.
Dausmann, K. H. (2014). Flexible patterns in energy savings: Heterothermy in primates. Journal of Zoology, 292, 101–111.
Dausmann, K. H., & Blanco, M. B. (2016). Possible causes and consequences of different hibernation patterns in Cheirogaleus species: Mitovy fatsy sahala. In S. Lehman, U. Radespiel, & E. Zimmermann (Eds.), Dwarf and Mouse Lemurs of Madagascar: Biology, Behavior and Conservation Biogeography of the Cheirogaleidae (pp. 335–349). University Press.
Dausmann, K. H., Glos, J., Ganzhorn, J. U., & Heldmaier, G. (2005). Hibernation in the tropics: Lessons from a primate. Journal of Comparative Physiology B, 175, 147–155.
Dausmann, K. H., & Warnecke, L. (2016). Primate torpor expression: Ghost of the climatic past. Physiology, 31(6), 398–408.
Donque, G. (1972). The climatology of Madagascar. In R. Battistini & G. Richard-Vindard (Eds.), Biogeography and Ecology in Madagascar (pp. 87–144). Springer.
Du Puy, D. J., & Moat, J. (1996). A refined classification of the primary vegetation of Madagascar based on the underlying geology: using GIS to map its distribution and to assess its conservation status. Biogéographie de Madagascar, 1996, 205–218.
Erkert, H. G., & Kappeler, P. M. (2004). Arrived in the light: diel and seasonal activity patterns in wild Verreaux’s sifakas (Propithecus v. verreauxi; Primates: Indriidae). Behavioral Ecology and Sociobiology, 57(2), 174–186.
Fick, S. E., & Hijmans, R. J. (2017). WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. International Journal of Climatology, 37(12), 4302–4315.
Fietz, J. (1999). Demography and floating males in a population of Cheirogaleus medius. In B. Rakotosamimanana, H. Rasaminanana, J. U. Gaznhorn, & S. M. Goodman (Eds.), New Directions in Lemur Studies (pp. 159–172). Springer.
Fietz, J., & Ganzhorn, J. U. (1999). Feeding ecology of the hibernating primate Cheirogaleus medius: How does it get so fat? Oecologia, 121(2), 157–164.
Frasier, C. L., Lei, R., McLain, A. T., Taylor, J. M., Bailey, C. A., Ginter, A. L., Nash, S. D., Randriamampionona, R., Groves, C. P., Mittermeier, R. A., & Louis, E. E. (2016). A new species of dwarf lemur (Cheirogaleidae: Cheirogaleus medius group) from the Ankarana and Andrafiamena-Andavakoera Massifs, Madagascar. Primate Conservation, 30, 59–72.
Groves, C. (2016). The taxonomy of Cheirogaleidae: An ever-expanding species list. In S. Lehman, U. Radespiel, & E. Zimmermann (Eds.), Dwarf and Mouse Lemurs of Madagascar: Biology, Behavior and Conservation Biogeography of the Cheirogaleidae (pp. 21–53). University Press.
Hanya, G., & Chapman, C. A. (2013). Linking feeding ecology and population abundance: A review of food resource limitation on primates. Ecological Research, 28(2), 183–190.
Hebbali, A. (2018). olsrr: Tools for Building OLS Regression Models. R package version 0.5.2.
Heldstab, S. A., van Schaik, C. P., Müller, D. W., Rensch, E., Lackey, L. B., Zerbe, P., Hatt, J. M., Clauss, M., & Matsuda, I. (2021). Reproductive seasonality in primates: Patterns, concepts and unsolved questions. Biological Reviews., 96(1), 66–88.
Hending, D. (2021). Environmental drivers of Cheirogaleidae population density: Remarkable resilience of Madagascar’s smallest lemurs to habitat degradation. Ecology and Evolution, 11(11), 5874–5891.
Hending, D., Andrianiaina, A., Rakotomalala, Z., & Cotton, S. (2017a). Range extension and behavioural observations of the recently described Sheth’s dwarf lemur (Cheirogaleus shethi). Folia Primatologica, 88(5), 401–408.
Hending, D., Andrianiaina, A., Rakotomalala, Z., & Cotton, S. (2018). The use of vanilla plantations by lemurs: Encouraging findings for both lemur conservation and sustainable agroforestry in the Sava Region, Northeast Madagascar. International Journal of Primatology, 39(1), 141–153.
Hending, D., Holderied, M., & McCabe, G. (2017b). The use of vocalizations of the Sambirano mouse lemur (Microcebus sambiranensis) in an acoustic survey of habitat preference. International Journal of Primatology, 38(4), 732–750.
Hending, D., Holderied, M., McCabe, G., & Cotton, S. (2022a). Effects of future climate change on the forests of Madagascar. Ecosphere, 13(4), e4017.
Hending, D., McCabe, G., & Holderied, M. (2017c). Sleeping and ranging behavior of the Sambirano Mouse Lemur, Microcebus sambiranensis. International Journal of Primatology, 38(6), 1072–1089.
Hending, D., Randrianarison, H., Andriamavosoloarisoa, N. N. M., Ranohatra-Hending, C., McCabe, G., Cotton, S., & Holderied, M. (2022b). A new population of mouse lemurs (Microcebus sp.) from North-western Madagascar, with population size and density estimates. Primate Conservation, 36, 103–111.
Herrera, J. P., Wright, P. C., Lauterbur, E., Ratovonjanahary, L., & Taylor, L. L. (2011). The effects of habitat disturbance on lemurs at Ranomafana National Park. Madagascar. International Journal of Primatology, 32(5), 1091–1108.
Hijmans, R. J. (2019). raster: Geographic Data Analysis and Modeling. R package version 3.0–7.
Hladik, C. M., Charles-Dominique, P., & Petter, J. J. (1980). Feeding strategies of five nocturnal prosimians in the dry forest of the west coast of Madagascar. In P. Charles-Dominique, H. M. Cooper, A. Hladik, C. M. Hladik, E. Pages, G. F. Pariente, A. Petter-Rousseaux, J. J. Petter, & A. Schilling (Eds.), Nocturnal Malagasy Primates: Ecology, Physiology, and Behavior (pp. 41–73). Academic Press.
Kobbe, S., Ganzhorn, J. U., & Dausmann, K. H. (2011). Extreme individual flexibility of heterothermy in free-ranging Malagasy mouse lemurs (Microcebus griseorufus). Journal of Comparative Physiology B, 181(1), 165–173.
Koechlin, J. (1972). Flora and vegetation of Madagascar. In R. Battistini & G. Richard-Vindard (Eds.), Biogeography and Ecology in Madagascar (pp. 145–190). Springer.
Lahann, P. (2007). Biology of Cheirogaleus major in a littoral rain forest in southeast Madagascar. International Journal of Primatology, 28, 895–905.
Lahann, P., & Dausmann, K. H. (2011). Live fast, die young: Flexibility of life history traits in the fat-tailed dwarf lemur (Cheirogaleus medius). Behavioural Ecology and Sociobiology, 65, 381–390.
Lehman, S. M., Rajaonson, A., & Day, S. (2006). Edge effects on the density of Cheirogaleus major. International Journal of Primatology, 27(6), 1569–1588.
Lei, R., McLain, A. T., Frasier, C. L., Taylor, J. M., Bailey, C. A. S., Engberg, S. E., Ginter, A. L., Nash, S. D., Randriamampionona, R., Groves, C. P., Mittermeier, R. A., & Louis, E. E. (2015). A new species in the genus Cheirogaleus (Cheirogaleidae). Primate Conservation, 29, 1–12.
Lovegrove, B. G. (2003). The influence of climate on the basal metabolic rate of small mammals: A slow-fast continuum. Journal of Comparative Physiology B, 173, 87–112.
Lovegrove, B. G., Canale, C. I., Levesque, D. L., Fluch, G., Reháková-Petru, M., & Ruf, T. (2014). Are tropical small mammals physiologically vulnerable to Arrhenius effects and climate change? Physiological and Biochemical Zoology, 87, 30–45.
Lutermann, H., Verburgt, L., & Rendigs, A. (2010). Resting and nesting in a small mammal: Sleeping sites as a limiting resource for female grey mouse lemurs. Animal Behaviour, 79(6), 1211–1219.
Lymann, C. P., Willis, J. S., Malan, A., & Wang, L. C. H. (1982). Hibernation and Torpor in Mammals and Birds. Academic Press.
Mandl, I., Holderied, M., & Schwitzer, C. (2018). The effects of climate seasonality on behavior and sleeping site choice in Sahamalaza sportive lemurs, Lepilemur sahamalaza. International Journal of Primatology, 39(6), 1039–1067.
McLain, A. T., Lei, R., Frasier, C. L., Taylor, J. M., Bailey, C. A., Robertson, B. A. D., Nash, S. D., Randriamanana, J. C., Mittermeier, R. A., & Louis, E. E. (2017). A new Cheirogaleus (Cheirogaleidae: Cheirogaleus crossleyi group) species from southeastern Madagascar. Primate Conservation, 31, 27–36.
Miller, A., Mills, H., Ralantoharijaona, T., Volasoa, N. A., Misandeau, C., Chikhi, L., Bencini, R., & Salmona, J. (2018). Forest type influences population densities of nocturnal lemurs in Manompana, northeastern Madagascar. International Journal of Primatology, 39(4), 646–669.
Mittermeier, R. A., Ganzhorn, J. U., Konstant, W. R., Glander, K., Tattersall, I., Groves, C. P., Rylands, A. B., Hapke, A., Ratsimbazafy, J., Mayor, M. I., & Louis, E. E. (2008). Lemur diversity in Madagascar. International Journal of Primatology, 29(6), 1607–1656.
Moynihan, M. (1970). Some behaviour patterns of platyrrhine monkeys II. Saguinus geoffroyi and some other tamarins. Smithsonian Contributions to Zoology, 28, 1–77.
Müller, A. E. (1998). A preliminary report on the social organisation of Cheirogaleus medius (Cheirogaleidae; Primates) in north-west Madagascar. Folia Primatologica, 69(3), 160–166.
Müller, A. E. (1999). Social organization of the fat-tailed dwarf lemur (Cheirogaleus medius) in northwestern Madagascar. In B. Rakotosamimanana, H. Rasamimanana, J. U. Ganzhorn, & S. M. Goodman (Eds.), New Directions in Lemur Studies (pp. 139–157). Springer.
Müller, A. E., & Thalmann, U. (2002). Biology of the fat‐tailed dwarf lemur (Cheirogaleus medius E. Geoffroy 1812): new results from the field. Evolutionary Anthropology: Issues, News, and Reviews, 11(S1), 79-82.
Müller, P., Velo, A., Raheliarisoa, E. O., Zaramody, A., & Curtis, D. J. (2000). Surveys of sympatric lemurs at Anjamena, north-west Madagascar. African Journal of Ecology, 38(3), 248–257.
Nowack, J., Mzilikazi, N., & Dausmann, K. H. (2010). Torpor on demand: Heterothermy in the non-lemur primate Galago moholi. PLoS ONE, 5, e10797.
Ortmann, S., Heldmaier, G., Schmid, J., & Ganzhorn, J. U. (1997). Spontaneous daily torpor in Malagasy mouse lemurs. Naturwissenschaften, 84, 28–32.
Perret, M., & Aujard, F. (2001). Daily hypothermia and torpor in a tropical primate: Synchronization by 24-h light-dark cycle. American Journal of Physiology, 281(6), 1925–1933.
Poulsen, J. R., Clark, C. J., & Smith, T. B. (2001). Seasonal variation in the feeding ecology of the grey-cheeked mangabey (Lophocebus albigena) in Cameroon. American Journal of Primatology., 54(2), 91–105.
Quémére, E., Champeau, J., Besolo, A., Rasolondraibe, E., Rabarivola, C., Crouau-Roy, B., & Chikhi, L. (2010). Spatial variation in density and total size estimates in fragmented primate populations: The golden-crowned sifaka (Propithecus tattersalli). American Journal of Primatology, 72, 72–80.
Reyna-Hurtado, R., Teichroeb, J. A., Bonnell, T. R., Hernández-Sarabia, R. U., Vickers, S. M., Serio-Silva, J. C., Sicotte, P., & Chapman, C. A. (2018). Primates adjust movement strategies due to changing food availability. Behavioral Ecology, 29(2), 368–376.
Rode, E. J., Nekaris, K. A. I., Markolf, M., Schliehe-Diecks, S., Seiler, M., Radespiel, U., & Schwitzer, C. (2013). Social organisation of the northern giant mouse lemur Mirza zaza in Sahamalaza, north western Madagascar, inferred from nest group composition and genetic relatedness. Contributions to Zoology, 82(2), 71–83.
Rode-Margono, E. J., Schwitzer, C., & Nekaris, K. A. I. (2016). Morphology, behaviour, ranging patterns and habitat use of the northern giant mouse lemur Mirza zaza in Sahamalaza, northwestern Madagascar. In S. Lehman, U. Radepsiel, & E. Zimmermann (Eds.), The Dwarf and Mouse Lemurs of Madagascar: Biology, Behavior and Conservation Biogeography of the Cheirogaleidae (pp. 235–254). University Press.
Ruf, T., Streicher, U., Stalder, G. L., Nadler, T., & Walzer, C. (2015). Hibernation in the pygmy slow loris (Nycticebus pygmaeus): Multiday torpor in primates is not restricted to Madagascar. Scientific Reports, 5, 17392.
Schmid, J. (1998). Tree holes used for resting by gray mouse lemurs (Microcebus murinus) in Madagascar: Insulation capacities and energetic consequences. International Journal of Primatology, 19(5), 797–809.
Schmid, J. (2000). Daily torpor in the gray mouse lemur (Microcebus murinus) in Madagascar: Energetic consequences and biological significance. Oecologia, 123, 175–183.
Schülke, O., & Ostner, J. (2007). Physiological ecology of cheirogaleid primates: Variation in hibernation and torpor. Acta Ethologica, 10(1), 13–21.
Schwitzer, N., Randriatahina, G. H., Kaumanns, W., Hoffmeister, D., & Schwitzer, C. (2007). Habitat utilization of blue-eyed black lemurs, Eulemur macaco flavifrons (Gray, 1867), in primary and altered forest fragments. Primate Conservation, 22, 79–87.
Seiler, M., Holderied, M., & Schwitzer, C. (2013). Effects of habitat degradation on sleeping site choice and use in Sahamalaza sportive lemurs (Lepilemur sahamalazensis). International Journal of Primatology, 34(2), 260–280.
Seiler, M., Holderied, M., & Schwitzer, C. (2014). Habitat selection and use in the Critically Endangered Sahamalaza sportive lemur Lepilemur sahamalazensis in altered habitat. Endangered Species Research, 24(3), 273–286.
Seiler, M., Randriatahina, G. H., & Schwitzer, C. (2010). Ongoing threats to lemurs and their habitat inside the Sahamalaza-Iles Radama National Park. Lemur News, 15, 7–9.
Thomas, L., Buckland, S., Rexstad, E. A., Laake, J. L., Strindberg, S., Hedley, S. L., Bishop, J. R., Marques, T. A., & Burnham, K. (2010). Distance software: Design and analysis of distance sampling surveys for estimating population size. Journal of Applied Ecology, 47, 5–14.
Turner, I. M., & Corlett, R. T. (1996). The conservation value of small, isolated fragments of lowland tropical rain forest. Trends in Ecology and Evolution, 11(8), 330–333.
Van Schaik, C. P., & Brockman, D. K. (2005). Seasonality in primate ecology, reproduction, and life history. In D. K. Brockman & C. P. van Schaik (Eds.), Seasonality in Primates (pp. 3–20). University Press.
Vieilledent, G., Grinand, C., Rakotomalala, F. A., Ranaivosoa, R., Rakotoarijaona, J. R., Allnutt, T. F., & Achard, F. (2018). Combining global tree cover loss data with historical national forest cover maps to look at six decades of deforestation and forest fragmentation in Madagascar. Biological Conservation, 222, 189–197.
Volampeno, M. S. N. (2009). Reproductive behaviour and habitat use in the blue-eyed black lemur (Eulemur flavifrons, Gray, 1867) at the Sahamalaza Peninsula, National Park Madagascar. PhD thesis, University of KwaZulu–Natal, Pietermaritzburg, South Africa.
Volampeno, M. S. N., Masters, J. C., & Downs, C. T. (2011). Home range size in the blue-eyed black lemur (Eulemur flavifrons): A comparison between dry and wet seasons. Mammalian Biology, 76(2), 157–164.
Webber, A. D., Solofondranohatra, J. S., Razafindramoana, S., Fernández, D., Parker, C. A., Steer, M., Abrahams, M., & Allainguillaume, J. (2020). Lemurs in cacao: Presence and abundance within the shade plantations of Northern Madagascar. Folia Primatologica, 91(2), 96–107.
Wright, P. C., & Martin, L. B. (1995). Predation, pollination and torpor in two nocturnal prosimians: Cheirogaleus major and Microcebus rufus in the rainforest of Madagascar. In L. Alterman, G. A. Doyle, & M. K. Izard (Eds.), Creatures of the Dark: The Nocturnal Prosimians (pp. 45–60). Plenum Press.
Acknowledgements
The authors thank the field guides, Raumiald, Mahatsara, Avitsara, Hervé, Aristide, and Jacquis, and cooks, Klariny, Klarice, and Marceline, for all their help and enthusiasm during our time together in the field. We also express our sincere gratitude to the people of Ambinda, Betsimpoaka, Marovato, Maropapango, Antanandava, Antanambao Manambaro, Antafiabe, Maromandia, and Ambolobozo for their logistical assistance. We are grateful to MICET and MNP for their facilitation services and for kindly allowing us to conduct research within the Sahamalaza-Iles Radama National Park (permit numbers 245/19 and 124/22—MEEF/SG/DGGE/DAPRNE/SCBE.Re). We thank National Geographic Society, Global Wildlife Conservation, Primate Conservation Incorporated, La Vallée des Singes, Idea Wild, Primate Society of Great Britain, AEECL, the University of Bristol, and many private donors for kindly funding this fieldwork. Finally, thanks to the handling editor and two anonymous reviewers whose comments enabled us to improve the quality of this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Addisu Mekonnen
The original online version of this article was revised: There are author names that have appeared twice. Full information regarding the corrections made can be found in the erratum/correction for this article.
Supplementary Information
Below is the link to the electronic supplementary material.
10764_2023_353_MOESM1_ESM.docx
Supplementary File 1. Summary of the perpendicular distances at which Cheirogaleus medius individuals were observed from the transects. (DOCX 688 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hending, D., Randrianarison, H., Andriamavosoloarisoa, N.N.M. et al. Seasonal Differences in the Encounter Rate of the Fat-Tailed Dwarf Lemur (Cheirogaleus medius) in the Transitional Forests of Northwest Madagascar: Implications for Reliable Population Density Assessment. Int J Primatol 44, 482–498 (2023). https://doi.org/10.1007/s10764-023-00353-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-023-00353-y