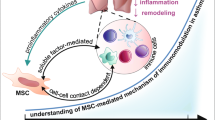
Abstract
Chronic asthma is characterized by airway hyperresponsiveness, inflammation, and remodeling. Previous studies have shown that mesenchymal stromal/stem cells (MSCs) exert anti-inflammatory effects on asthma via regulation of the immune cells. However, the therapeutic mechanism of MSCs, especially the mechanism of airway remodeling in chronic asthma, remains to be elucidated. Here, we aimed to investigate the therapeutic effect of MSCs on airway remodeling in chronic asthma and explored the mechanisms by analyzing the polarization phenotype of macrophages in the lungs. We established a mouse model of chronic asthma induced by ovalbumin (OVA) and evaluated the effect of MSCs on airway remodeling. The data showed that MSCs treatment before the challenge exerted protective effects on OVA-induced chronic asthma, i.e., decreased the inflammatory cell infiltration, Th2 cytokine levels, subepithelial extracellular matrix deposition, and transforming growth factor β (TGF-β)/Smad signaling. Additionally, we found that MSCs treatment markedly suppressed macrophage M2 polarization in lung tissue. At the same time, MSCs treatment inhibited NF-κB p65 nuclear translocation, ER stress, and oxidative stress in the OVA-induced chronic allergic airway remodeling mice model. In conclusion, these results demonstrated that MSCs treatment prevents OVA-induced chronic airway remodeling by suppressing macrophage M2 polarization, which may be associated with the dual inhibition of ER stress and oxidative stress. This discovery may provide a new theoretical basis for the future clinical application of MSCs.








Similar content being viewed by others
DATA AVAILABILITY
No datasets were generated or analysed during the current study.
References
Mims, J.W. 2015. Asthma: Definitions and pathophysiology. International Forum of Allergy & Rhinology 5 (Suppl 1): S2–S6. https://doi.org/10.1002/alr.21609.
Saradna, A., D.C. Do, S. Kumar, Q.L. Fu, and P. Gao. 2018. Macrophage polarization and allergic asthma. Translational Research 191: 1–14. https://doi.org/10.1016/j.trsl.2017.09.002.
Mattoo, H., D. Bangari, S. Cummings, Z. Humulock, D. Habiel, E. Xu, N. Pate, R. Resnick, V. Savova, G. Qian, C. Beil, E. Rao, F. Nestle, P. Bryce, and A. Subramaniam. 2023. Molecular features and stages of pulmonary fibrosis driven by type 2 inflammation. American Journal of Respiratory Cell and Molecular Biology 69: 404–421. https://doi.org/10.1165/rcmb.2022-0301OC.
Hong, S., Y. Lu, S. Chen, C. Hsu, Y. Lu, C. Wang, and K. Huang. 2023. Targeting pathogenic macrophages by the application of SHP-1 agonists reduces inflammation and alleviates pulmonary fibrosis. Cell Death & Disease 14: 352. https://doi.org/10.1038/s41419-023-05876-z.
Lee, H., J. Hur, J. Kang, C. Rhee, and S. Lee. 2021. MicroRNA-21 inhibition suppresses alveolar M2 macrophages in an ovalbumin-induced allergic asthma mice model. Allergy, Asthma & Immunology Research 13: 312–329. https://doi.org/10.4168/aair.2021.13.2.312.
Liu, L., Y. Qin, Z. Cai, Y. Tian, X. Liu, J. Li, and P. Zhao. 2021. Corrigendum to: Effective-components combination improves airway remodeling in COPD rats by suppressing M2 macrophage polarization via the inhibition of mTORC2 activity. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology 92: 153976. https://doi.org/10.1016/j.phymed.2021.153759.
Asgari Taei, A., P. Khodabakhsh, S. Nasoohi, M. Farahmandfar, and L. Dargahi. 2022. Paracrine effects of mesenchymal stem cells in ischemic stroke: opportunities and challenges. Molecular Neurobiology 59: 6281–6306. https://doi.org/10.1007/s12035-022-02967-4.
Shi, Y., Y. Wang, Q. Li, K. Liu, J. Hou, C. Shao, and Y. Wang. 2018. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nature Reviews Nephrology 14: 493–507. https://doi.org/10.1038/s41581-018-0023-5.
Wang, Y., B. Huang, T. Jin, D. Ocansey, J. Jiang, and F. Mao. 2022. Intestinal fibrosis in inflammatory bowel disease and the prospects of mesenchymal stem cell therapy. Frontiers in Immunology 13: 835005. https://doi.org/10.3389/fimmu.2022.835005.
Wang, M., N. Zhao, C. Wang, Z. Jin, and L. Zhang. 2023. Immunomodulatory properties of mesenchymal stem cells: A potential therapeutic strategy for allergic rhinitis. Allergy 78: 1425–1440. https://doi.org/10.1111/all.15729.
Abreu, S., L. Alves, L. Carvalho, D. Xisto, N. Blanco, L. Castro, P. Olsen, and J. Lapa E Silva, M. Morales, M. Lopes-Pacheco, D. Weiss, P. Rocco,. 2023. Serum from patients with asthma potentiates macrophage phagocytosis and human mesenchymal stromal cell therapy in experimental allergic asthma. Cytotherapy 25: 967–976. https://doi.org/10.1016/j.jcyt.2023.05.014.
Gholami, M., K. Ghorban, M. Sadeghi, M. Dadmanesh, N. Rouzbahani, and S. Dehnavi. 2023. Mesenchymal stem cells and allergic airway inflammation; a therapeutic approach to induce immunoregulatory responses. International Immunopharmacology 120: 110367. https://doi.org/10.1016/j.intimp.2023.110367.
Moroncini, G., C. Paolini, F. Orlando, C. Capelli, A. Grieco, C. Tonnini, S. Agarbati, E. Mondini, S. Saccomanno, G. Goteri, S. Svegliati Baroni, M. Provinciali, M. Introna, N. Del Papa, and A. Gabrielli. 2018. Mesenchymal stromal cells from human umbilical cord prevent the development of lung fibrosis in immunocompetent mice. PloS ONE 13: e01960481. https://doi.org/10.1371/journal.pone.0196048.
Dong, L., Y. Wang, T. Zheng, Y. Pu, Y. Ma, X. Qi, W. Zhang, F. Xue, Z. Shan, J. Liu, X. Wang, and C. Mao. 2021. Hypoxic hUCMSC-derived extracellular vesicles attenuate allergic airway inflammation and airway remodeling in chronic asthma mice. Stem Cell Research & Therapy 12: 4. https://doi.org/10.1186/s13287-020-02072-0.
Zhong, H., X.L. Fan, S.B. Fang, Y.D. Lin, W. Wen, and Q.L. Fu. 2019. Human pluripotent stem cell-derived mesenchymal stem cells prevent chronic allergic airway inflammation via TGF-β1-Smad2/Smad3 signaling pathway in mice. Molecular Immunology 109: 51–57. https://doi.org/10.1016/j.molimm.2019.02.017.
Pathinayake, P., D. Waters, K. Nichol, A. Brown, A. Reid, A. Hsu, J. Horvat, L. Wood, K. Baines, J. Simpson, P. Gibson, P. Hansbro, and P. Wark. 2022. Endoplasmic reticulum-unfolded protein response signalling is altered in severe eosinophilic and neutrophilic asthma. Thorax 77: 443–451. https://doi.org/10.1136/thoraxjnl-2020-215979.
Michaeloudes, C., H. Abubakar-Waziri, R. Lakhdar, K. Raby, P. Dixey, I. Adcock, S. Mumby, P. Bhavsar, and K. Chung. 2022. Molecular mechanisms of oxidative stress in asthma. Molecular Aspects of Medicine 85: 101026. https://doi.org/10.1016/j.mam.2021.101026.
Shi, B., Y. Hao, W. Li, H. Dong, M. Xu, and P. Gao. 2022. TIPE2 may target the Nrf2/HO-1 pathway to inhibit M1 macrophage-related neutrophilic inflammation in asthma. Frontiers in Immunology 13: 883885. https://doi.org/10.3389/fimmu.2022.883885.
Ning, H., H. Chen, J. Deng, C. Xiao, M. Xu, L. Shan, C. Yang, and Z. Zhang. 2021. Exosomes secreted by FNDC5-BMMSCs protect myocardial infarction by anti-inflammation and macrophage polarization via NF-κB signaling pathway and Nrf2/HO-1 axis. Stem Cell Research & Therapy 12: 519. https://doi.org/10.1186/s13287-021-02591-4.
Gu, W., R. Cui, T. Ding, X. Li, J. Peng, W. Xu, F. Han, and X. Guo. 2017. Simvastatin alleviates airway inflammation and remodelling through up-regulation of autophagy in mouse models of asthma. Respirology (Carlton, Vic.) 22: 533–541. https://doi.org/10.1111/resp.12926.
Sun, Y.Q., M.X. Deng, J. He, Q.X. Zeng, W. Wen, D.S. Wong, H.F. Tse, G. Xu, Q. Lian, J. Shi, and Q.L. Fu. 2012. Human pluripotent stem cell-derived mesenchymal stem cells prevent allergic airway inflammation in mice. Stem Cells (Dayton, Ohio) 30: 2692–2699. https://doi.org/10.1002/stem.1241.
Reddy, A.P., and M.R. Gupta. 2014. Management of asthma: The current US and European guidelines. Advances in Experimental Medicine and Biology 795: 81–103. https://doi.org/10.1007/978-1-4614-8603-9_6.
Yao, Y., X. Fan, D. Jiang, Y. Zhang, X. Li, Z. Xu, S. Fang, S. Chiu, H. Tse, Q. Lian, and Q. Fu. 2018. Connexin 43-mediated mitochondrial transfer of iPSC-MSCs alleviates asthma inflammation. Stem Cell Reports 11: 1120–1135. https://doi.org/10.1016/j.stemcr.2018.09.012.
Abreu, S.C., M.A. Antunes, D.G. Xisto, F.F. Cruz, V.C. Branco, E. Bandeira, J. Zola Kitoko, A.F. de Araújo, L. Dellatorre-Texeira, P.C. Olsen, D.J. Weiss, B.L. Diaz, M.M. Morales, and P.R.M. Rocco. 2017. Bone marrow, adipose, and lung tissue-derived murine mesenchymal stromal cells release different mediators and differentially affect airway and lung parenchyma in experimental asthma. Stem Cells Translational Medicine 6: 1557–1567. https://doi.org/10.1002/sctm.16-0398.
Choi, J., J. Hur, S. Jeon, C. Jung, and C. Rhee. 2022. Effects of human adipose tissue- and bone marrow-derived mesenchymal stem cells on airway inflammation and remodeling in a murine model of chronic asthma. Scientific Reports 12: 12032. https://doi.org/10.1038/s41598-022-16165-8.
Cui, Z., Y. Feng, D. Li, T. Li, P. Gao, and T. Xu. 2020. Activation of aryl hydrocarbon receptor (AhR) in mesenchymal stem cells modulates macrophage polarization in asthma. Journal of Immunotoxicology 17: 21–30. https://doi.org/10.1080/1547691x.2019.1706671.
Zhu, X., J. Cui, L. Yi, J. Qin, W. Tulake, F. Teng, W. Tang, Y. Wei, and J. Dong. 2020. The role of T cells and macrophages in asthma pathogenesis: A new perspective on mutual crosstalk. Mediators of Inflammation 2020: 7835284. https://doi.org/10.1155/2020/7835284.
Mantovani, A., A. Sica, S. Sozzani, P. Allavena, A. Vecchi, and M. Locati. 2004. The chemokine system in diverse forms of macrophage activation and polarization. Trends in Immunology 25: 677–686. https://doi.org/10.1016/j.it.2004.09.015.
Guo, Z., S. Li, N. Zhang, Q. Kang, and H. Zhai. 2020. Schisandra inhibit bleomycin-induced idiopathic pulmonary fibrosis in rats via suppressing M2 macrophage polarization. BioMed Research International 2020: 5137349. https://doi.org/10.1155/2020/5137349.
Wang, J., L. Xu, Z. Xiang, Y. Ren, X. Zheng, Q. Zhao, Q. Zhou, Y. Zhou, L. Xu, and Y. Wang. 2020. Microcystin-LR ameliorates pulmonary fibrosis via modulating CD206(+) M2-like macrophage polarization. Cell Death & Disease 11: 136. https://doi.org/10.1038/s41419-020-2329-z.
Lee, H.Y., I.K. Kim, H.K. Yoon, S.S. Kwon, C.K. Rhee, and S.Y. Lee. 2017. Inhibitory effects of resveratrol on airway remodeling by transforming growth factor-β/Smad signaling pathway in chronic asthma model. Allergy, Asthma & Immunology Research 9: 25–34. https://doi.org/10.4168/aair.2017.9.1.25.
Chen, M., Z. Lv, L. Huang, W. Zhang, X. Lin, J. Shi, W. Zhang, R. Liang, and S. Jiang. 2015. Triptolide inhibits TGF-β1-induced cell proliferation in rat airway smooth muscle cells by suppressing Smad signaling. Experimental Cell Research 331: 362–368. https://doi.org/10.1016/j.yexcr.2014.10.016.
Bansod, S., N. Doijad, and C. Godugu. 2020. Berberine attenuates severity of chronic pancreatitis and fibrosis via AMPK-mediated inhibition of TGF-β1/Smad signaling and M2 polarization. Toxicology and Applied Pharmacology 403: 115162. https://doi.org/10.1016/j.taap.2020.115162.
Roach, K.M., C. Feghali-Bostwick, H. Wulff, Y. Amrani, and P. Bradding. 2015. Human lung myofibroblast TGFβ1-dependent Smad2/3 signalling is Ca(2+)-dependent and regulated by KCa3.1 K(+) channels. Fibrogenesis & Tissue Repair 8: 5. https://doi.org/10.1186/s13069-015-0022-0.
Malyshev, I., and Y. Malyshev. 2015. Current concept and update of the macrophage plasticity concept: Intracellular mechanisms of reprogramming and M3 macrophage “switch” phenotype. BioMed Research International 2015: 341308. https://doi.org/10.1155/2015/341308.
Wang, Y., J. Zhu, L. Zhang, Z. Zhang, L. He, Y. Mou, Y. Deng, Y. Cao, P. Yang, Y. Su, J. Zhao, S. Zhang, Q. Yu, J. Hu, Z. Chen, Q. Ning, X. Xiang, Y. Xu, C. Wang, and W. Xiong. 2017. Role of C/EBP homologous protein and endoplasmic reticulum stress in asthma exacerbation by regulating the IL-4/signal transducer and activator of transcription 6/transcription factor EC/IL-4 receptor α positive feedback loop in M2 macrophages. The Journal of Allergy and Clinical Immunology 140: 1550–1561.e1558. https://doi.org/10.1016/j.jaci.2017.01.024.
Chen, J., J. Chen, Y. Cheng, Y. Fu, H. Zhao, M. Tang, H. Zhao, N. Lin, X. Shi, Y. Lei, S. Wang, L. Huang, W. Wu, and J. Tan. 2020. Mesenchymal stem cell-derived exosomes protect beta cells against hypoxia-induced apoptosis via miR-21 by alleviating ER stress and inhibiting p38 MAPK phosphorylation. Stem Cell Research & Therapy 11: 97. https://doi.org/10.1186/s13287-020-01610-0.
Liao, Z., R. Luo, G. Li, Y. Song, S. Zhan, K. Zhao, W. Hua, Y. Zhang, X. Wu, and C. Yang. 2019. Exosomes from mesenchymal stem cells modulate endoplasmic reticulum stress to protect against nucleus pulposus cell death and ameliorate intervertebral disc degeneration in vivo. Theranostics 9: 4084–4100. https://doi.org/10.7150/thno.33638.
Ahmed, S., L. Luo, A. Namani, X. Wang, and X. Tang. 1863. Nrf2 signaling pathway: Pivotal roles in inflammation, biochimica et biophysica acta. Molecular Basis of Disease 2017: 585–597. https://doi.org/10.1016/j.bbadis.2016.11.005.
Wu, Y., Y. Zhang, F. Jiang, S. He, Y. Zhang, D. Chen, Y. Tong, Y. Nie, and Q. Pang. 2023. 4-OI ameliorates bleomycin-induced pulmonary fibrosis by activating Nrf2 and suppressing macrophage-mediated epithelial-mesenchymal transition. Inflammation Research 72: 1133–1145. https://doi.org/10.1007/s00011-023-01733-z.
Toh, W., R. Lai, B. Zhang, and S. Lim. 2018. MSC exosome works through a protein-based mechanism of action. Biochemical Society Transactions 46: 843–853. https://doi.org/10.1042/bst20180079.
Asadirad, A., A. Ghadiri, A. Amari, M. Ghasemi Dehcheshmeh, M. Sadeghi, and S. Dehnavi. 2023. Sublingual prophylactic administration of OVA-loaded MSC-derived exosomes to prevent allergic sensitization. International Immunopharmacology 120: 110405. https://doi.org/10.1016/j.intimp.2023.110405.
Dehnavi, S., A. Khodadadi, A. Asadirad, and A. Ghadiri. 2023. Immune response modulation by allergen loaded into mesenchymal stem cell-derived exosomes as an effective carrier through sublingual immunotherapy. Immunobiology 228: 152361. https://doi.org/10.1016/j.imbio.2023.152361.
Shan, L., S. Liu, Q. Zhang, Q. Zhou, and Y. Shang. 2022. Human bone marrow-mesenchymal stem cell-derived exosomal microRNA-188 reduces bronchial smooth muscle cell proliferation in asthma through suppressing the JARID2/Wnt/β-catenin axis. Cell Cycle (Georgetown, Tex.) 21: 352–367. https://doi.org/10.1080/15384101.2021.2020432.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81770023).
Author information
Authors and Affiliations
Contributions
Dr. Haiyang Yu and Guiyin Zhu contributed to the study conduct and drafted the manuscript. Qiangqiang Qin contributed to data analysis. Dr. Xueting Wang provided language help. Dr. Wen Gu and Dr. Xuejun Guo contributed to the study design and guide. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors have no conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, H., Zhu, G., Qin, Q. et al. Mesenchymal Stromal Cell Therapy Alleviates Ovalbumin-Induced Chronic Airway Remodeling by Suppressing M2 Macrophage Polarization. Inflammation (2024). https://doi.org/10.1007/s10753-024-01977-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10753-024-01977-9