Abstract
Objectives
Pulmonary fibrosis (PF) is a chronic and refractory interstitial lung disease with limited therapeutic options. 4-octyl itaconate (4-OI), a cell-permeable derivative of itaconate, has been shown to have anti-oxidative and anti-inflammatory properties. However, the effect and the underlying mechanism of 4-OI on PF are still unknown.
Methods
WT or Nrf2 knockout (Nrf2−/−) mice were intratracheally injected with bleomycin (BLM) to establish PF model and then treated with 4-OI. The mechanism study was performed by using RAW264.7 cells, primary macrophages, and conditional medium-cultured MLE-12 cells.
Results
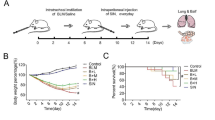
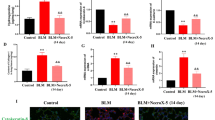
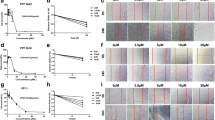
4-OI significantly alleviated BLM-induced PF and EMT process. Mechanism studies have found that 4-OI can not only directly inhibit EMT process, but also can reduce the production of TGF-β1 by restraining macrophage M2 polarization, which in turn inhibits EMT process. Moreover, the effect of 4-OI on PF and EMT depends on Nrf2.
Conclusion
4-OI ameliorates BLM-induced PF in an Nrf2-dependent manner, and its role in alleviating PF is partly due to the direct inhibition on EMT, and partly through indirect inhibition of M2-mediated EMT. These findings suggested that 4-OI has great clinical potential to develop as a new anti-fibrotic agent for PF therapy.







Similar content being viewed by others
Data availability statement
The authors will supply the relevant data in response to reasonable requests.
Abbreviations
- PF:
-
Pulmonary fibrosis
- BLM:
-
Bleomycin
- 4-OI:
-
4-Octyl itaconate
- EMT:
-
Epithelial-mesenchymal transition
- AT II:
-
Alveolar epithelial type II
- Nrf2:
-
Nuclear factor E2-related factor 2
- E-cad:
-
E-cadherin
- α-SMA:
-
α-Smooth muscle actin
- Fn:
-
Fibronectin
- MMP2:
-
Matrix metalloproteinase 2
- MTA1:
-
Metastasis-associated gene 1
- M1:
-
Classically activated macrophages
- M2:
-
Alternatively activated macrophages
- Arg-1:
-
Arginase-1
- Ym1:
-
Chitinase-like protein 3
- Fizz1:
-
Resistin-like molecule α1
- IL-4:
-
Interleukin-4
- IL-13:
-
Interleukin-13
- BALF:
-
Bronchoalveolar lavage fluid
- PBS:
-
Phosphate-buffered saline
- M-csf:
-
Macrophage-colony-stimulating factor
- Col1a1:
-
Collagen alpha1
- Keap1:
-
Kelch-like ECH-associated protein 1
References
Ng B, Dong J, D'Agostino G, Viswanathan S, Widjaja AA, Lim WW, et al. Interleukin-11 is a therapeutic target in idiopathic pulmonary fibrosis. Sci Transl Med. 2019;11:eaaw1237
Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet. 2017;389:1941–52.
Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35:821–9.
Crestani B, Huggins JT, Kaye M, Costabel U, Glaspole I, Ogura T, et al. Long-term safety and tolerability of nintedanib in patients with idiopathic pulmonary fibrosis: results from the open-label extension study, INPULSIS-ON. Lancet Respir Med. 2019;7:60–8.
Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. an update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192:e3–19.
Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med. 2018;378:1811–23.
Song Y, Li C, Jin L, Xing J, Sha Z, Zhang T, et al. RIOK2 is negatively regulated by miR-4744 and promotes glioma cell migration/invasion through epithelial-mesenchymal transition. J Cell Mol Med. 2020;24:4494–509.
Ji Y, Dou YN, Zhao QW, Zhang JZ, Yang Y, Wang T, et al. Paeoniflorin suppresses TGF-beta mediated epithelial-mesenchymal transition in pulmonary fibrosis through a Smad-dependent pathway. Acta Pharmacol Sin. 2016;37:794–804.
Zhang L, Wang Y, Wu G, Xiong W, Gu W, Wang CY. Macrophages: friend or foe in idiopathic pulmonary fibrosis? Respir Res. 2018;19:170.
Pechkovsky DV, Prasse A, Kollert F, Engel KM, Dentler J, Luttmann W, et al. Alternatively activated alveolar macrophages in pulmonary fibrosis-mediator production and intracellular signal transduction. Clin Immunol. 2010;137:89–101.
Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–40.
Zhu L, Fu X, Chen X, Han X, Dong P. M2 macrophages induce EMT through the TGF-beta/Smad2 signaling pathway. Cell Biol Int. 2017;41:960–8.
Olagnier D, Brandtoft AM, Gunderstofte C, Villadsen NL, Krapp C, Thielke AL, et al. Nrf2 negatively regulates STING indicating a link between antiviral sensing and metabolic reprogramming. Nat Commun. 2018;9:3506.
Liu G, Wu Y, Jin S, Sun J, Wan BB, Zhang J, et al. Itaconate ameliorates methicillin-resistant Staphylococcus aureus-induced acute lung injury through the Nrf2/ARE pathway. Ann Transl Med. 2021;9:712.
Li R, Yang W, Yin Y, Ma X, Zhang P, Tao K. 4-OI Attenuates carbon tetrachloride-induced hepatic injury via regulating oxidative stress and the inflammatory response. Front Pharmacol. 2021;12: 651444.
Jaiswal AK, Yadav J, Makhija S, Mazumder S, Mitra AK, Suryawanshi A, et al. Irg1/itaconate metabolic pathway is a crucial determinant of dendritic cells immune-priming function and contributes to resolute allergen-induced airway inflammation. Mucosal Immunol. 2022;15:301–13.
Tian F, Wang Z, He J, Zhang Z, Tan N. 4-Octyl itaconate protects against renal fibrosis via inhibiting TGF-beta/Smad pathway, autophagy and reducing generation of reactive oxygen species. Eur J Pharmacol. 2020;873: 172989.
Runtsch MC, Angiari S, Hooftman A, Wadhwa R, Zhang Y, Zheng Y, et al. Itaconate and itaconate derivatives target JAK1 to suppress alternative activation of macrophages. Cell Metab. 2022;34(487–501): e8.
Nie Y, Zhang D, Qian F, Wu Y. Baccatin III ameliorates bleomycin-induced pulmonary fibrosis via suppression of TGF-beta1 production and TGF-beta1-induced fibroblast differentiation. Int Immunopharmacol. 2019;74: 105696.
Wu YX, Wang YY, Gao ZQ, Chen D, Liu G, Wan BB, et al. Ethyl ferulate protects against lipopolysaccharide-induced acute lung injury by activating AMPK/Nrf2 signaling pathway. Acta Pharmacol Sin. 2021;42:2069–81.
Wu YX, Jiang FJ, Liu G, Wang YY, Gao ZQ, Jin SH, et al. Dehydrocostus lactone attenuates methicillin-resistant staphylococcus aureus-induced inflammation and acute lung injury via modulating macrophage polarization. Int J Mol Sci. 2021;22(18):9754
Andugulapati SB, Gourishetti K, Tirunavalli SK, Shaikh TB, Sistla R. Biochanin-A ameliorates pulmonary fibrosis by suppressing the TGF-beta mediated EMT, myofibroblasts differentiation and collagen deposition in in vitro and in vivo systems. Phytomedicine. 2020;78: 153298.
Artaud-Macari E, Goven D, Brayer S, Hamimi A, Besnard V, Marchal-Somme J, et al. Nuclear factor erythroid 2-related factor 2 nuclear translocation induces myofibroblastic dedifferentiation in idiopathic pulmonary fibrosis. Antioxid Redox Signal. 2013;18:66–79.
Dianat M, Radan M, Badavi M, Mard SA, Bayati V, Ahmadizadeh M. Crocin attenuates cigarette smoke-induced lung injury and cardiac dysfunction by anti-oxidative effects: the role of Nrf2 antioxidant system in preventing oxidative stress. Respir Res. 2018;19:58.
Sakai T, Takagaki H, Yamagiwa N, Ui M, Hatta S, Imai J. Effects of the cytoplasm and mitochondrial specific hydroxyl radical scavengers TA293 and mitoTA293 in bleomycin-induced pulmonary fibrosis model mice. Antioxidants. 2021;10(9):1398.
Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Prim. 2017;3:17074.
Miura Y, Ohkubo H, Niimi A, Kanazawa S. Suppression of epithelial abnormalities by nintedanib in induced-rheumatoid arthritis-associated interstitial lung disease mouse model. ERJ Open Res. 2021;7(4):00345-2021.
Bao X, Liu X, Liu N, Zhuang S, Yang Q, Ren H, et al. Inhibition of EZH2 prevents acute respiratory distress syndrome (ARDS)-associated pulmonary fibrosis by regulating the macrophage polarization phenotype. Respir Res. 2021;22:194.
Ying H, Fang M, Hang QQ, Chen Y, Qian X, Chen M. Pirfenidone modulates macrophage polarization and ameliorates radiation-induced lung fibrosis by inhibiting the TGF-beta1/Smad3 pathway. J Cell Mol Med. 2021;25:8662–75.
Peace CG, O'Neill LA. The role of itaconate in host defense and inflammation. J Clin Investig. 2022;132(2):e148548.
Mills EL, Ryan DG, Prag HA, Dikovskaya D, Menon D, Zaslona Z, et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. 2018;556:113–7.
Li R, Yang W, Yin Y, Zhang P, Wang Y, Tao K. Protective role of 4-octyl itaconate in murine LPS/D-GalN-induced acute liver failure via inhibiting inflammation, oxidative stress, and apoptosis. Oxid Med Cell Longev. 2021;2021:9932099.
Yi Z, Deng M, Scott MJ, Fu G, Loughran PA, Lei Z, et al. Immune-responsive gene 1/itaconate activates nuclear factor erythroid 2-related factor 2 in hepatocytes to protect against liver ischemia-reperfusion injury. Hepatology. 2020;72:1394–411.
Ogger PP, Albers GJ, Hewitt RJ, O'Sullivan BJ, Powell JE, Calamita E, et al. Itaconate controls the severity of pulmonary fibrosis. Sci Immunol. 2020;5(52):eabc1884.
Ke Q, Shi C, Lv Y, Wang L, Luo J, Jiang L, et al. SGLT2 inhibitor counteracts NLRP3 inflammasome via tubular metabolite itaconate in fibrosis kidney. FASEB J. 2022;36: e22078.
Fan K, Zan X, Zhi Y, Yang Y, Hu K, Zhang X, et al. Immune response gene 1 deficiency impairs Nrf2 activation and aggravates liver fibrosis in mice. Biochem Biophys Res Commun. 2022;607:103–9.
Henderson J, Dayalan Naidu S, Dinkova-Kostova AT, Przyborski S, Stratton R. The cell-permeable derivative of the immunoregulatory metabolite itaconate, 4-octyl itaconate, is anti-fibrotic in systemic sclerosis. Cells. 2021;10(8):2053.
Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Asp Med. 2011;32:234–46.
Cho HY, Reddy SP, Yamamoto M, Kleeberger SR. The transcription factor NRF2 protects against pulmonary fibrosis. FASEB J. 2004;18:1258–60.
Xin X, Yao D, Zhang K, Han S, Liu D, Wang H, et al. Protective effects of Rosavin on bleomycin-induced pulmonary fibrosis via suppressing fibrotic and inflammatory signaling pathways in mice. Biomed Pharmacother. 2019;115:108870.
Liu Y, Zhong W, Zhang J, Chen W, Lu Y, Qiao Y, et al. Tetrandrine modulates Rheb-mTOR signaling-mediated selective autophagy and protects pulmonary fibrosis. Front Pharmacol. 2021;12: 739220.
Liu Y, Lu F, Kang L, Wang Z, Wang Y. Pirfenidone attenuates bleomycin-induced pulmonary fibrosis in mice by regulating Nrf2/Bach1 equilibrium. BMC Pulm Med. 2017;17:63.
Tocmo R, Parkin K. S-1-propenylmercaptocysteine protects murine hepatocytes against oxidative stress via persulfidation of Keap1 and activation of Nrf2. Free Radic Biol Med. 2019;143:164–75.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (82100018); Natural Science Foundation of Jiangsu Province (BK20200602); China Postdoctoral Science Foundation (2020M671347); National Natural Science Foundation of China (82171704).
Author information
Authors and Affiliations
Contributions
YXW and QFP designed the study and drafted the paper; YJN participated in the design and helped draft the paper; YXW, YRZ and FJJ performed the experiments; SH and YLZ participated in the animal experiments; DC and YT performed the data analysis; YXW and YJN were involved in the discussion of the experiments. All authors read and approved the final paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Additional information
Responsible Editor: Anatolii Kubyshkin.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, Yx., Zhang, Yr., Jiang, Fj. et al. 4-OI ameliorates bleomycin-induced pulmonary fibrosis by activating Nrf2 and suppressing macrophage-mediated epithelial-mesenchymal transition. Inflamm. Res. 72, 1133–1145 (2023). https://doi.org/10.1007/s00011-023-01733-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01733-z