Abstract
Temporary rivers are aquatic ecosystems that alternate periods of water flow with dry periods. Diatoms are a group of unicellular microalgae with a high colonizing ability, but little is known about their responses to drying. We carried out different resistance and resilience experiments to evaluate temporal and spatial dispersal capacity of diatoms during the dry period. The resistance was tested experimentally by rehydrating dried biofilms and sediments from temporary rivers, whereas resilience was tested by installing artificial mesocosms along a dry river section. Disconnected pools were also sampled to evaluate their propagule emission capacity. In turn, dogs from the area were sampled to test potential zoochory dispersal capacity. In the resistance experiment, we found living diatoms in all the rehydrated sediments but not in biofilms. Diatoms with mobility traits, high ecological plasticity, and resistance spores presented high, along with typical soil diatoms. In the resilience experiment, all mesocosms hosted living diatoms, which were low-profile, pioneering, and small species. Diatoms found in the mesocosms were also common in the disconnected pools, underscoring the potential role of the latter as a propagule emission zone. Dogs' paws also had living diatoms, which evidences that wild fauna could potentially act as passive diatom vectors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Temporary rivers are highly dynamic river ecosystems that alternate flowing and non-flowing phases (Datry et al., 2014). They are distributed throughout the world and are the most common river type in the Mediterranean Basin (Larned et al., 2010). Furthermore, it is believed that their number will increase as a result of global change (Döll & Schmied, 2012). Freshwater species that inhabit these streams might have developed resistance and resilience strategies to cope with drying (Bogan et al., 2017b). Thus, while resistance is based on developing different tolerance mechanisms in situ, resilience focuses on dispersal to other temporary habitats and subsequent recolonization after disturbance events (Bogan et al., 2017b).
Resistance strategies are considered a type of temporal dispersal and include adaptations such as diapause and dormancy (Robson et al., 2011). Some organisms can also survive in disconnected pools, where the level of dissolved oxygen is low and temperatures are high (Bogan et al., 2017b). Others even choose to move to deeper areas of the dry river bed where the humidity is higher (Bogan et al., 2017a). In contrast, resilience strategies are a form of spatial dispersal that involves active or passive movements in space to avoid disturbance (e.g. Jiguet et al., 2006; Alerstam, 2011). The existence of disconnected pools in drying riverbeds or nearby flowing reaches can act as temporary refuges during the dry period (Miller & Golladay, 1996; Boulton & Lake, 2008). Therefore, resilience requires that organisms have a strong dispersal capacity that allows a fast recolonization of riverbeds from refuges when flow resumes (Bogan et al., 2017a; Sheldon et al., 2010).
Regardless of the type of strategy, several factors are involved in the reestablishment of freshwater communities after the dry period. For example, the high frequency and intensity of drying in desert areas results in a more slowly richness recovery than in other climatic areas (Bogan et al., 2015, 2017b). The presence and connectivity to perennial refuges, as well as the type of taxonomic group are also key factors (Baguette et al., 2013). In alluvial rivers with short drying periods mayflies or amphipods are able to recolonize rehydrated aquatic environments very quickly, whereas fish usually take longer because they depend on the hydrological connectivity of the stream for their dispersion (Bogan et al., 2017a).
Diatoms have an important role as primary producers, and are present in practically all types of waters, even in humid terrestrial environments (Kooistra et al., 2007). Diatoms are known as rapid colonizers in temporary rivers, but little is known about their resistance and resilience strategies to cope with drying (Robson & Matthews, 2004). The studies based on resistance show that diatoms are capable of persisting in dry biofilms during the dry period (Robson, 2000), because the mucilage of biofilms can retain enough moisture to protect them (Peterson, 1987; Hawkins et al., 2014). They can also resist in moist refuges, such as leaf litter, leaves, seepage, woody debris, or sediments (Robson, 2008; Davis, 1972; Falasco et al., 2020). Regarding resilience strategies, drift is considered an important recolonization mechanism in perennial streams (McCormick & Stevenson, 1991; Biggs, 1996; Robson et al., 2008). However, the use of vectors that passively transport diatoms should also be considered. For example, waterfowl can carry diatoms in their plumage over long distances (Manning, 2019), and wind can also detach and move diatoms across the landscape (Agwu et al., 2005; Chrisostomou et al., 2009). In spite of the relevance of these processes for structuring diatom communities, there is little information about the importance of both strategies to cope with drying in temporary rivers (Robson et al., 2008).
In this study, the ability of diatoms to cope with drying through resistance and resilience strategies was studied. Resistance strategies were assessed by analyzing dry sediments and dry biofilms. We hypothesized (H1) that some species should resist drying in both sediment and biofilm, thanks to the presence of protective mucilage and their ability to find refuge in humid areas (Robson & Matthews, 2004; Sabater et al., 2016). Resilience strategies were assessed by analyzing the colonization of new freshwater habitats (mesocosms) and passive dispersal vectors (wind and zoochory). We hypothesized (H2) that colonization should be fast and that, in the absence of drift, dispersal should be mostly mediated by wind or mammal vectors (Kristiansen, 1996; Chrisostomou et al., 2009), and thus explained by the distance to the closest permanent refuge or by the presence of diatoms in mammal fur, respectively.
Material and methods
Study area
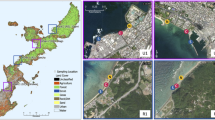
The study was carried out in Sant Llorenç del Munt i l’Obac Natural Park and its surroundings, located in the province of Barcelona (northeast of Spain) (Fig. 1). The Park has an area of almost 14,000 hectares and a Mediterranean climate. Summers are very dry, and rains are usually torrential and occur in spring and autumn (Paül & Pérez, 2002; Rieradevall et al., 1999). Five streams were sampled: Vall d'Horta (hereinafter Horta; 41° 40′ 38.5ʺ N 2° 01′ 46.0" E), Riera de Rellinars (Rellinars; 41° 37′ 51.5" N 1° 56′ 03.2ʺ E), Riera de Santa Creu (Santa Creu, 41° 41′ 48.9ʺ N 1° 53′ 07.7ʺ E), Torrent dels Oms (Sanana, 41° 37′ 14.5ʺ N 1° 54′ 40.0ʺ E), and Riera de Talamanca (Talamanca, 41° 44′ 21.8ʺ N 1° 58′ 56.5ʺ E). These streams showed a wide degree of flow permanence (%) (i.e. percentage of days with surface water during the study period), from 86.87% in Sanana to 44.46% in Santa Creu.
Study area and sampling points. a Location of Sant Llorenç del Munt i l’Obac Natural Park (Catalonia, Spain). b Diatom sampling points in the Natural Park and its surroundings. The arrows indicate the exact sampling point. The colored circles refer to the different experiments carried out for resistance and resilience for each of the rivers (biofilm, sediments, disconnected pools, mesocosms and zoochory). In the case of disconnected pools, although they could be considered as a resistance refuge, they are considered in the resilience experiment because they can act as a source of diatoms for the mesocosms
Resistance experiment
Nine calcareous stones (approx. 19 × 13 x 2 cm) were placed for diatom colonization in Sanana, Talamanca, and Santa Creu streams in May 2019, when all of them had been flowing (Fig. 2a). All stones were placed facing up (horizontal position) and submerged during the whole experiment. Stones were retrieved two months after their installation and samples from crusts growing on the bedrock were collected. Two months were assumed as enough time to get a mature biofilm, as suggested in Bogan et al. (2017a). Stones were left to dry outdoors at the University of Barcelona during summer to simulate the dry period under natural conditions. In September 2019, when rewetting occurred in the streams, stones and crusts were randomly placed in 54 closed plastic containers (27 with stones and 27 with crusts) with dechlorinated tap water and oxygenators. Random diatoms samples were taken by scraping the surface of stones and crusts using a toothbrush every 20–30 days, and until November 2019. Samples were fixed in 4% formaldehyde.
A set of images of the resistance and resilience experiments. Resistance experiment: a installation of stones in the river for the colonization of biofilm, and b plastic containers with rehydrated sediment. Resilience experiment: c mesocosm (Meso. 7), d disconnected pool (Pool. 2), and e dog sampling process
Additionally, dry sediments of 5–20 cm depth were collected in September 2021 from Horta, Rellinars, and Sanana, which were dry during ca. 3 months. Three sediment samples from each stream were randomly placed in plastic closed containers (21 × 18 x 8 cm) and transported to the University of Barcelona, where they were exposed outdoors. Furthermore, four microscope slides were embedded vertically in all the sediments of each sample to facilitate diatom colonization. The long edge of the slide (76 mm) was placed in the sediment, whereas the short edge (26 mm) was placed on top of the sediment. A few days later, sediments were rehydrated with dechlorinated tap water and oxygenators were installed (Fig. 2b). All sediments and slides were submerged to an average depth of 4 cm. To detect possible air contamination, three control plastic containers were placed with water but without sediment (control samples). One month after rehydration (Time A: October 2021), the first sampling of rehydrated sediments and control samples was carried out. One blade was used for two slides and two sides of the plastic containers. The entire content was preserved in 75% ethanol. Two months after rehydration (Time B: November 2021), another sampling was performed on the other slides and walls of each remaining plastic container. In each sampling, 9 sediment samples and 3 control samples were collected, obtaining a total of 24 samples at the end of the experiment.
Resilience experiment
Eight mesocosms (119 × 79 × 42 cm) were placed along the dry riverbed in Horta stream in September 2021. Each mesocosm contained 115 L of mineral water (with similar composition to the streams of the area, Supplementary Table S1) and four sterile stones (Fig. 2c). Mesocosms (Meso.) were placed along two dry stream reaches between disconnected pools (Pool.) (Fig. 2d). Thus, the established order from downstream to upstream was as follows: Pool.1, Meso.1, Meso.2, Meso.3, Meso.4, Pool.2, Meso.5, Meso.6, Meso.7, Meso.8. Both disconnected pools were sampled at the beginning of the experiment (Time A). Although pools could be also considered as a resistance refuge, they were used in the resilience experiment to demonstrate that they can act as a source of diatoms for mesocosms. The mesocosms at Time A were sterile, so the total absence of diatoms was assumed. The average distance between each mesocosm and between each pool and its adjacent mesocosms was 74 m (SD: 45). However, the distance between Meso.2 and Meso.3 was 404 m. This allowed differentiating a downstream section (Pool.1, Meso.1 and Meso.2) from an upstream section (Meso.3, Meso.4, Pool.2, Meso.5, Meso.6, Meso.7 and Meso.8). One month after the installation, all mesocosms and pools were sampled (Time B). A toothbrush was used to scrape the stone surfaces of each mesocosm. In total, 8 samples of the mesocosms and 4 of the disconnected pools were obtained. All samples were fixed with ethanol (75%).
To test zoochory, three dogs found in the area close to the mesocosms were sampled in Time A. According to their owners, the dogs had bathed in pools, ponds, and other water bodies near the study area the same day or less than two days ago. With the permission of their owners, the four paws of each dog were carefully placed into gloves cleaned with sterile water (Fig. 2e). Massages were performed on the lower part, sole of the foot and fingers to collect as many diatoms as possible. The upper part of the four legs was also massaged with water, collecting the contents in jars. Sterile water and not ethanol was used in these massages to avoid itching or stinging upon contact with a possible wound or any other side effect in the dogs. The entire content of the gloves and jars were poured into several bottles and fixed with ethanol (75%). Samples were let to settle during five days before being processed. Additionally, in order to assess whether the mesocosms were visited by wild mammals, a camera trap was installed near one of the mesocosms (Meso.2) and retrieved at the end of the mesocosm experiment.
Samples treatment and species identification
All samples were kept cold after collection. Two subsamples were taken from both the resistance and resilience experiments: one to check for living cells (frustules with chloroplasts) and the other for taxonomic identification. For the first subsample set, a minimum of 200 diatoms were counted under a Zeiss microscope to check. The second subsample set was treated with hydrogen peroxide, to remove organic matter, and rinsed with distilled water to obtain a clean frustule suspension. Drops of hydrochloric acid were added to remove calcium carbonate. Permanent slides were mounted on Naphrax© resin. All subsamples were identified to species level using diatom monographs (Hofmann et al., 2011; Bey & Ector, 2013; Lange-Bertalot & Krammer, 2000–2011) under a Zeiss microscope at × 1000 magnification with DIC and camera. A minimum of 400 diatom valves were counted. In the case of the zoochory experiment, it was necessary to make several subsamples due to the difficulty of finding the minimum number of diatom valves.
Statistical analysis
The average number of living cells from untreated samples and their standard deviation were calculated for each experiment. The rest of the statistical analyses of each experiment were carried out based on the results of the taxonomic identifications made from the relative abundance of the treated sample.
Firstly, for the resistance experiment, richness and Shannon diversity were calculated for each stream (Horta, Rellinars, and Sanana) and sampling time (A and B). An Analysis of Variance (ANOVA) was carried out to test for differences among all these factors on both diversity metrics. Tukey's test was used for significant post hoc comparisons by treatment pairs (Sun et al., 2022). Normality and homogeneity of variance were tested by Shapiro–Wilk and Levene tests, respectively. The number of common and exclusive species of each stream were represented using a Venn diagram. To compare the composition of the communities, a permutational analysis of variance (PERMANOVA) was used according to each sediment sample (Horta, Rellinars, and Sanana) and its sampling time (A and B). PERMANOVA test was based on Bray–Curtis distances with log(x + 1) transformation (Mangadze et al., 2017; Gething et al., 2020). Post hoc comparisons of pairwise differences were also performed with Bonferroni corrections to account for multiple comparisons at P < 0.05 (Arbizu, 2019; Gething et al., 2020). Finally, diatom communities were represented using non-metric multidimensional scaling (NMDS) using Bray–Curtis distances. The use of NMDS is considered a good tool for ecological data with many zero values in its matrix (Dexter et al., 2018).
Similarly, for the resilience experiment, richness and Shannon diversity were calculated for each mesocosm, pool, and dog, and analyzed as for the resistance experiment for mesocosms, disconnected pools, and dogs. Finally, linear models and Mantel tests were built to analyze richness and Bray–Curtis distances, respectively, to the geographical distances with disconnected pools, and separated for the lower part (Pool.1, Meso.1 and Meso.2) and the upper part of the river section (Meso.3, Meso.4, Pool.2, Meso.5, Meso.6, Meso.7 and Meso.8).
Results
Resistance experiment
For the resistance experiment, no living diatoms appeared after rehydration in any of the biofilm samples (stones or crusts, Fig. 3), although Cyanobacteria and other photosynthetic organisms were observed. Although no living diatoms were found, frustules without chloroplasts did appear. This shows that developed diatom communities existed before desiccation.
Percentage of living cells with their standard deviation for each experiment. The resistance experiment is made up of rehydrated sediment samples from different streams (Horta, Rellinars, and Sanana) and the rehydrated biofilm. The resilience experiment included the sampling of mesocosms, disconnected pools, and dogs. For both experiments, there may be two sampling moments marked as Time A (light gray) and B (dark gray)
For the second resistance experiment, living diatoms were found in all sediment samples after rehydration, along with other algae, such as Cyanobacteria and charophytes. The average percentage of living cells in each sample was 58.40% (Time A, SD: 21.24) and 58.55 (Time B, SD: 28.89) in Horta, 49.20% (Time A, SD: 5.52) and 50.30% (Time B, SD: 3.40) in Rellinars, and 58.70 (Time A, SD: 1.41), and 56.80% (Time B, SD: 1.86) in Sanana (Fig. 3). No diatoms were recorded in any of the control samples. From the treated samples, the average of the 10 most abundant species and their standard deviation (SD) are shown in Table 1. For example, Nitzschia palea var. debilis (Kützing) Grunow was dominant in Horta and Sanana, whereas Cyclotella distinguenda Hustedt was the most abundant species in the dry sediments of Rellinars. Other abundant species were Hantzschia amphioxys (Ehrenberg) Grunow and Cymbella excisa Kützing in Horta, Luticola mutica (Kützing) D.G.Mann and Achnanthidium minutissimum (Kützing) Czarnecki in Rellinars, and Navicula veneta Kützing and Craticula ambigua (Ehrenberg) D.G.Mann in Sanana.
No significant differences in richness were found among streams (P = 0.0713), between times (P = 0.795) or their interaction (P = 0.966). Significant differences for the Shannon index from rehydrated samples were found among streams (P = 0.011), but not between times (Time A and B) (P = 0.541) and their interaction (P = 0.812). According to Tukey's test Rellinars had higher Shannon values than Sanana (P = 0.008). Regarding community composition of the rehydrated samples, 48 species were totally or partially shared between the different rivers, whereas 26 taxa were exclusively found in Horta, 15 in Rellinars, and 13 in Sanana (Fig. 4a). Regarding the composition of the community, significant differences among streams were detected with the PERMANOVA analysis (P = 0.001), which are also shown in the NMDS (Fig. 4b). Post hoc analysis with Bonferroni correction indicated significant differences between all pairs of streams: Horta-Rellinars (P = 0.003), Horta-Sanana (P = 0.012), and Rellinars-Sanana (P = 0.009).
Resistance experiment results. a Venn diagram for the composition of diatoms showing the number of exclusive and common species, and its percentage, in the sediments of Horta, Rellinars, and Sanana streams. b NMDS with samples separated by river (Horta, Rellinars, and Sanana) and sampling time (A and B)
Resilience experiment
For the resilience experiment, living diatoms were found in all mesocosms (Fig. 3). The average percentage of living cells in mesocosms was 86.38 (Time A, SD: 12.04). Macroinvertebrates (e.g. Baetidae, Chironomidae, Coleoptera, Heteroptera and Mollusca) were also found. Pools presented living diatoms at both sampling times, with 71.70% (SD: 13.37) at Time A and 69.35% (SD: 9.82) at Time B. Living diatoms were found on dogs, with a percentage of living cells of 88.88 (Time A, SD: 2.07). Other types of propagules (pollen, fungal spores, fungi, etc.) also appeared.
From the treated samples, the average of the 10 most abundant species and their standard deviation (SD) are shown in Table 1. In all type of samples (i.e. mesocosms, pools, and dogs), the most dominant species was Achnanthidium minutissimum. Other abundant species were Planothidium frequentissimum (Lange-Bertalot) Lange-Bertalot and Mayamaea permitis (Hustedt) K.Bruder & Medlin in mesocosms, Gomphonema lateripunctatum E.Reichardt & Lange-Bertalot and Achnanthidium pyrenaicum (Hustedt) H.Kobayasi in pools, and Cymbella excisa and Hantzschia amphioxys on dogs. No significant differences were found among the type of samples for richness (P = 0.146) or Shannon diversity (P = 0.265). Regarding the composition of the community, 27 exclusive species were found in mesocosms, 10 in pools, and 12 on dogs (Fig. 5a). Another 51 species were partially or completely shared between mesocosms, pools, and dogs.
Resilience experiment results. a Venn diagram for the composition of diatoms found in the resilience experiment showing the number of exclusive and common species, and its percentage, in mesocosms, pools, and dogs. b NMDS with samples separated by type (mesocosms, pool, and dog) and sampling time (A and B)
Significant differences were found among the type of samples for community composition (P = 0.002). The post hoc analysis with the Bonferroni adjustment only highlighted differences between mesocosms and pools (P = 0.002), but not for mesocosm-dog (P = 0.141) or pool-dog (P = 0.081) comparisons. Actually, samples from dogs were placed in an intermediate position between mesocosm and pools in the NMDS (Fig. 5b). The NMDS also showed a differentiation between the two pools but not between the sampling times (A and B). Most mesocosms clustered together, but showed no apparent order based on their location along the stream. Only the mesocosm closest upstream of each pool showed a different community from the rest of the mesocosms (Meso.1 and Meso.5).
Linear models and Mantel tests did not detect any significant effect of the geographical distance to the pool to explain the mesocosms’ richness and composition (Supplementary Fig. 1). Finally, the camera trap installed near the mesocosms (Meso. 2) recorded several species of mammals: a field mouse (Apodemus sylvaticus), a squirrel (Sciurus vulgaris), a beech marten (Martes foina) and a wild boar (Sus scrofa) (Supplementary Fig. 2). The camera only photographed each animal once, ruling out false activations due to wind and light changes. In addition, during the days of monitoring the experiment, a weasel (Mustela nivalis) was found swimming in Pool.2 (Supplementary Fig. 2) and a roe deer (Capreolus capreolus) was also reported near the experimental setup.
Discussion
In temporary rivers, aquatic communities have developed different resistance and resilience strategies to cope with drying (Bogan et al., 2017b). Assessing these strategies is essential to understand how the recolonization and establishment of communities in these rivers occur (Robson et al., 2008). Our results show that diatoms have a combination of both strategies, demonstrating that they potentially have a wide variety of adaptive traits to drying that would be interesting to study in future works.
In agreement with our first hypothesis (resistance experiment), living diatoms were found in the dry sediments after rehydration, demonstrating that the sediments served as refugia during drying in temporary rivers. As other studies have shown, diatoms and other aquatic groups can persist in humid habitats when superficial water is absent (Peterson, 1987; Bogan et al., 2017a), where they find more stable and less harsh environmental conditions (Davis, 1972; del Rosario & Resh, 2000; DiStefano et al., 2009; Stubbington, 2012). We found a strong differentiation of diatom communities in the sediments depending on the stream, probably related to differences in the pre-desiccation communities. Many microorganism communities from temporary rivers are often composed of subsets of the communities existing during the flowing phase (Sabater et al., 2016). Thus, diatom communities in each river would be able to develop resistant cells to desiccation just before the dry phase.
In relation to resistant diatoms, there is evidence that certain species, and even genera, can cope with desiccation due to the presence of one or more distinctive traits (Falasco et al., 2021). Some of these traits are the production of mucilage (e.g., Cymbella sp. and Gomphonema sp.) and the ability to move to humid areas (Navicula sp., Nitzschia sp. and Surirella sp.) (Sabater et al., 2017; Falasco et al., 2021), or to produce resting cells (Fragilaria sp. and Diatoma sp.) (Sicko-Goad et al., 1989). In our experiment, Nitzschia palea var. debilis was the most abundant species resisting desiccation. This species is known to move within the substrate and to have high ecological plasticity (Rimet & Bouchez, 2012; Machado et al., 2016), which allows rapid population development (Trobajo et al., 2009). Therefore, Nitzschia palea var. debilis seems to be a species highly adapted to drying in temporary rivers thanks to its resistance strategies. In agreement with this, Falasco et al. (2020) found a significant abundance of this species during the transitions between hydrological phases of a temporary alpine river, specifically in the lentic phase (prior to drying). Another important form of resistance is the production of resting sexual spores (Souffreau et al., 2013), a trait that may be present in Cyclotella distinguenda, an abundant species in our rehydrated sediment Rellinars samples. This ability is typical of centric diatoms and has been previously documented in marine species of the genus Cyclotella (Montresor et al., 2013). These spores would allow the development of populations of C. distinguenda, despite being a non-motile planktic species. Other abundant species in our study were Hantzschia amphioxys and Luticola mutica, both also adapted to terrestrial habitats (Souffreau et al., 2013). It is possible that these species may increase during the dry season as a result of ecological succession, as occurs with aquatic and terrestrial macroinvertebrates (Bogan et al., 2017a). If so, freshwater diatoms would displace these soil diatoms during flow resumption. However, studying these communities in closed containers (without aerial dispersion, drift or vectors) makes it impossible to know the development of the communities with flow resumption in each river.
In the dried biofilm, contrary to our expectations, no living diatoms were found after rehydration. This contradicts the idea that biofilms serve as refugia during dry periods (Robson & Matthews, 2004; McKew et al., 2011; Sabater et al., 2016; Sarremejane et al., 2020). However, it is known that diatoms do not resist complete desiccation, unlike other algal groups such as the Rhodophyta (Acuña et al., 2005; Sabater et al., 2016). In our case, the presence of diatom frustules without chloroplasts in the biofilm confirmed the existence of diatom communities prior to drying. Therefore, the resistance structures of diatoms located on rock surfaces may not be sufficient to withstand solar radiation during the dry season of Mediterranean climate, and alternative resistance mechanisms should be used. For example, many diatoms with mobile traits are capable of migrating from the shallowest to the deepest sediments as a consequence of the effects of flow intermittence (Mckew et al., 2011). Therefore, further studies based on the actual capacity of biofilms to support diatom communities at different time intervals and solar intensities would be required. Despite this, the appearance of Cyanobacteria, together with other photosynthetic organisms, reaffirmed the ability of other algae to withstand the dry period in biofilms (Davis, 1972; Dodds et al., 1996; Robson, 2000). In fact, many Cyanobacteria react extremely quickly to humidity and flow resumption, withstanding much better flow intermittence than diatoms (Sabater, 2000; Sabater et al., 2016).
In agreement with our second hypothesis (resilience experiment), living diatoms were found in all mesocosms one month after being filled with water, confirming their high colonization capacity, even in the absence of drift (Larned et al., 2007). Algal biofilms are one the first biological communities recovering after flow resumption in temporary rivers (Bogan et al., 2017a). Regarding colonizing diatoms, there is evidence that species of small size and low-profile traits (e.g. for Achnanthidium sp.) are often the first colonizers in rivers and lakes (Cyr, 2016; Falasco et al., 2020). Other genera (e.g. Navicula sp. and Nitzschia sp.) also have a high colonization rate through their passive transport by wind (Chrisostomou et al., 2009). In our experiment, although each mesocosm presented its own community, Achnanthidium minutissimum was the most abundant species in general. This small diatom with high reproduction rates is considered an r-species, and low-profile traits (characterized by short stature, resistant to physical disturbances, slow moving) (Rimet & Bouchez, 2012; de Oliveira & Ferragut, 2023). For this reason, it usually appears quickly in colonization processes of benthic algal communities, and is often the most abundant (Cyr, 2016). A. minutissimum also presents other traits that can facilitate colonization, such as the ability to develop short stems for adhesion to the substrate (Rimet & Bouchez, 2012). Other abundant species in our mesocosms were Planothidium frequentissimum and Mayamaea permitis. In this case, although only P. frequentissimum presents low-profile traits, both are very small species (Rimet & Bouchez, 2012). The small size of these diatoms could increase the probability of being passively dispersed by wind over greater distances compared to others.
Although it was not directly measured, disconnected pools can potentially behave as important propagule refugia and emission during the dry period (Robson, 2008; Davis, 1972; Bogan et al., 2017a). Our analysis seems to confirm this, since the pools contained living diatoms in all samples and shared many species with the mesocosms. The pools were dominated by A. minutissimum, Gomphonema lateripunctatum, and Achnanthidium pyrenaicum, all of them being attached to the substrate by peduncles and stalks (Rimet & Bouchez, 2012). Therefore, it is possible that these attachment traits also confer some advantage for resistance in pools. However, contrary to our expectations, the geographical distance of these pools from the mesocosms did not show any trend in community diversity, suggesting that wind had a potentially low or random effect on diatom dispersal in our system.
Besides wind, in the absence of sediment (rocks and sterile mesocosms) and drift (no aquatic flow connectivity between pools and mesocosms), zoochory has been suggested as a potentially significant mechanism of passive transport of organisms (Kristiansen, 1996). In our study, the presence of living diatoms on dogs, many of them found in pools and mesocosms, confirmed the role of these mammals as dispersal vectors. Among the species potentially dispersed by dogs, A. minutissimum was very abundant, which reaffirms the high colonizing capacity of this species in aquatic environments (Cyr, 2016). In fact, it is known that the most abundant or widely distributed species in pre-drying streams tend to be the first to recolonize rewetted reaches (Detenbeck et al., 1992; Whitney et al., 2015). Other abundant species in the fur of dogs were Cymbella excisa (whose hook shape possibly facilitates its transport) and Hantzschia amphioxys (a common soil diatom possibly associated with the terrestrial way of life of mammals). In addition to dogs, our phototrapping cameras also revealed the frequent passage of wild mammals between mesocosms, adding evidence to the use of dry riverbeds as corridors (Kukielka et al., 2013; Valera et al., 2011). Besides wild mammals, birds may also have played an important role in transporting living diatoms in their plumage and droppings (Kristiansen, 1996; Atkinson, 1980). For this reason, the use of animal vectors is increasingly being recognized in the passive transport of diatoms (Leone et al., 2014; Manning, 2019; Donato-Rondon et al., 2018; Leone et al., 2014). However, in general, the success of algae dispersal depends on many other factors, such as the distance between sites, the size of their population, the harshness of desiccation, and other types of abiotic stresses (Chrisostomou et al., 2009).
Conclusion
In conclusion, we show that diatoms present resistance and resilience strategies to cope with drying in temporary rivers. On the one hand, whereas resistance in the sediment seems to be related to traits of mobility, ecological plasticity, and the development of resistance spores, these traits do not seem to confer resistance to drying in biofilms. On the other hand, colonization through resilience seems to select species with low-profile traits, small-size and pioneering. Unlike macroinvertebrates, where resilience clearly stands out over resistance in our studied streams (Pineda-Morante et al., 2022), both strategies seem possible for diatoms. In fact, although there are species highly adapted to a specific strategy, they are not exclusive since we have found diatoms with both strategies at the same time (e.g. Cymbella excisa, Hantzschia amphioxys and Achnanthidium minutissimum). Therefore, we suggest that a combination of resistance and resilience strategies allow diatoms to cope with drying in temporary rivers and to quickly develop new viable populations with flow resumption.
Data availability
Data available as supplementary material.
Code availability
Code available from the authors upon request.
References
Acuña, V., I. Muñoz, A. Giorgi, M. Omella, F. Sabater & S. Sabater, 2005. Drought and postdrought recovery cycles in an intermittent Mediterranean stream: structural and functional aspects. Journal of the North American Benthological Society 24(4): 919–933. https://doi.org/10.1899/04-078.1.
Agwu, C., R. Njokuocha & O. Mezue, 2005. The study of airborne pollen and spores circulating at “Head Level” In Nsukka Environment. Bio-Research. https://doi.org/10.4314/br.v2i2.28552.
Alerstam, T., 2011. Optimal bird migration revisited. Journal of Ornithology 152(1): 5–23. https://doi.org/10.1007/s10336-011-0694-1.
Arbizu, P. M. 2019. pairwiseAdonis: pairwise multilevel comparison using adonis. https://github.com/pmartinezarbizu/pairwiseAdonis. Accessed 6 Oct 2022.
Atkinson, K. M., 1980. Experiments in dispersal of phytoplankton by ducks. British Phycological Journal 15(1): 49–58. https://doi.org/10.1080/00071618000650061.
Baguette, M., S. Blanchet, D. Legrand, V. M. Stevens & C. Turlure, 2013. Individual dispersal, landscape connectivity and ecological networks. Biological Reviews 88(2): 310–326. https://doi.org/10.1111/brv.12000.
Bey, M.Y. & L. Ector, 2013: Atlas des diatomees des cours d'eau de la region Rhone-Alpes. Tome 1. Centriques, Monoraphidees. Tome 2. Araphidees, Brachyraphidees. Tome 3. Naviculacees: Naviculoidees. Tome 4. Naviculacees: Naviculoidees. Tome 5. Naviculacees: Cymbelloidees, Gomphonematoidees. Tome 6. Bacillariacees, Rhopalodiacees, Surirellacees. Direction regionale de l'Environnement, de l'Amenagement et du Logement Rhone-Alpes, Lyon, 1182.
Biggs, B. J. F., 1996. Patterns in benthic algae of streams. Algal Ecology: Freshwater Benthic Ecosystems. https://doi.org/10.1016/b978-012668450-6/50031-x.
Bogan, M. T., K. S. Boersma & D. A. Lytle, 2015. Resistance and resilience of invertebrate communities to seasonal and supraseasonal drought in arid-land headwater streams. Freshwater Biology 60(12): 2547–2558. https://doi.org/10.1111/fwb.12522.
Bogan, M. T., E. T. Chester, T. Datry, A. L. Murphy, B. J. Robson, A. Ruhi, R. Stubbington & J. E. Whitney, 2017a. Resistance, resilience, and community recovery in intermittent rivers and ephemeral streams. Intermittent Rivers and Ephemeral Streams. https://doi.org/10.1016/b978-0-12-803835-2.00013-9.
Bogan, M. T., J. L. Hwan, K. Cervantes-Yoshida, J. Ponce & S. M. Carlson, 2017b. Aquatic invertebrate communities exhibit both resistance and resilience to seasonal drying in an intermittent coastal stream. Hydrobiologia 799(1): 123–133. https://doi.org/10.1007/s10750-017-3205-4.
Boulton, A. J. & P. S. Lake, 2008. Effects of drought on stream insects and its ecological consequences. Aquatic Insects: Challenges to Populations. https://doi.org/10.1079/9781845933968.0081.
Chrisostomou, A., M. Moustaka-Gouni, S. Sgardelis & T. Lanaras, 2009. Air-dispersed phytoplankton in a Mediterranean River-Reservoir System (Aliakmon-Polyphytos, Greece). Journal of Plankton Research 31(8): 877–884. https://doi.org/10.1093/plankt/fbp038.
Cyr, H., 2016. Wind-driven thermocline movements affect the colonisation and growth of Achnanthidium minutissimum, a ubiquitous benthic diatom in lakes. Freshwater Biology 61(10): 1655–1670. https://doi.org/10.1111/fwb.12806.
Datry, T., S. T. Larned, K. M. Fritz, M. T. Bogan, P. J. Wood, E. I. Meyer, A. N. Santos & A, 2014. Broad-scale patterns of invertebrate richness and community composition in temporary rivers: effects of flow intermittence. Ecography 37(1): 94–104. https://doi.org/10.1111/j.1600-0587.2013.00287.x.
Davis, J. S., 1972. Survival records in the algae, and the survival role of certain algal pigments, fat, and mucilaginous substances. Biologist 54: 52–93.
de Oliveira, R. & C. Ferragut, 2023. Periphyton responses to enrichment and nutrient dilution in two mesocosm experiments in a shallow hypereutrophic reservoir. Limnetica, 42(1), 69–84. https://doi.org/10.23818/limn.42.06
del Rosario, R. B. & V. H. Resh, 2000. Invertebrates in intermittent and perennial streams: is the hyporheic zone a refuge from drying? Journal of the North American Benthological Society 19(4): 680–696. https://doi.org/10.2307/1468126.
Detenbeck, N. E., P. W. DeVore, G. J. Niemi & A. Lima, 1992. Recovery of temperate-stream fish communities from disturbance: a review of case studies and synthesis of theory. Environmental Management 16(1): 33–53. https://doi.org/10.1007/bf02393907.
Dexter, E., G. Rollwagen-Bollens & S. M. Bollens, 2018. The trouble with stress: a flexible method for the evaluation of nonmetric multidimensional scaling. Limnology and Oceanography: Methods 16(7): 434–443. https://doi.org/10.1002/lom3.10257.
DiStefano, R. J., D. D. Magoulick, E. M. Imhoff & E. R. Larson, 2009. Imperiled crayfishes use hyporheic zone during seasonal drying of an intermittent stream. Journal of the North American Benthological Society 28(1): 142–152. https://doi.org/10.1899/08-072.1.
Dodds, W. K., R. E. Hutson, A. C. Eichem, M. A. Evans, D. A. Gudder, K. M. Fritz & L. Gray, 1996. The relationship of floods, drying, flow and light to primary production and producer biomass in a prairie stream. Hydrobiologia 333(3): 151–159. https://doi.org/10.1007/bf00013429.
Döll, P. & H. M. Schmied, 2012. How is the impact of climate change on river flow regimes related to the impact on mean annual runoff? A Global-Scale Analysis. Environmental Research Letters 7(1): 014037. https://doi.org/10.1088/1748-9326/7/1/014037.
Donato-Rondon, JCh., J. D. González-Trujillo, B. Romero & M. I. Castro-Rebolledo, 2018. Diatom assemblages associated with turtle carapaces in the Neotropical region. Revista De Biología Tropical 66(4): 1362.
Falasco, E., A. Doretto, S. Fenoglio, E. Piano & F. Bona, 2020. Supraseasonal drought in an Alpine river: effects on benthic primary production and diatom community. Journal of Limnology. https://doi.org/10.4081/jlimnol.2020.1933.
Falasco, E., F. Bona, A. M. Risso & E. Piano, 2021. Hydrological intermittency drives diversity decline and functional homogenization in benthic diatom communities. Science of the Total Environment 762: 143090. https://doi.org/10.1016/j.scitotenv.2020.143090.
Gething, K. J., M. C. Ripley, K. L. Mathers, R. P. Chadd & P. J. Wood, 2020. The influence of substrate type on macroinvertebrate assemblages within agricultural drainage ditches. Hydrobiologia 847(20): 4273–4284. https://doi.org/10.1007/s10750-020-04416-6.
Hawkins, M. L., F. Faÿ, K. Réhel, I. Linossier & M. A. Grunlan, 2014. Bacteria and diatom resistance of silicones modified with PEO-silane amphiphiles. Biofouling 30(2): 247–258. https://doi.org/10.1080/08927014.2013.862235.
Hofmann, G., M. Werum. & H. Lange-Bertalot, 2011. Diatomeen im Süßwasser-Benthos von Mitteleuropa: Bestimmungsflora Kieselalgen für die ökologische Praxis:über 700 der häufigsten Arten und ihre Ökologie. ARG Gantner.
Jiguet, F., R. Julliard, C. D. Thomas, O. Dehorter, S. E. Newson & D. Couvet, 2006. Thermal range predicts bird population resilience to extreme high temperatures. Ecology Letters 9(12): 1321–1330. https://doi.org/10.1111/j.1461-0248.2006.00986.x.
Kooistra, W. H., R. Gersonde, L. K. Medlin & D. Mann, 2007. The origin and evolution of the diatoms: their adaptation to a planktonic existence. Evolution of Primary Producers in the Sea. https://doi.org/10.1016/b978-012370518-1/50012-6.
Kristiansen, J., 1996. Dispersal of freshwater algae—a review. Biogeography of Freshwater Algae. https://doi.org/10.1007/978-94-017-0908-8_15.
Kukielka, E., J. A. Barasona, C. E. Cowie, J. A. Drewe, C. Gortazar, I. Cotarelo & J. Vicente, 2013. Spatial and temporal interactions between livestock and wildlife in South Central Spain assessed by camera traps. Preventive Veterinary Medicine 112(3–4): 213–221. https://doi.org/10.1016/j.prevetmed.2013.08.008.
Lange-Bertalot, H. & K. Krammer, (ed.) 2000–2011. Diatoms of Europe: Diatoms of the European Inland Waters and Comparable Habitats. Several volumes. A.R.G. Gantner Verlag K.G.
Larned, S. T., T. Datry & C. Robinson, 2007. Invertebrate and microbial responses to inundation in an ephemeral river reach in New Zealand: effects of preceding dry periods. Aquatic Sciences 69(4): 554–567. https://doi.org/10.1007/s00027-007-0930-1.
Larned, S. T., T. Datry, D. B. Arscott & K. Tockner, 2010. Emerging concepts in temporary-river ecology. Freshwater Biology 55(4): 717–738. https://doi.org/10.1111/j.1365-2427.2009.02322.x.
Leone, P. B., J. Cerda, S. Sala & B. Reid, 2014. Mink (Neovison vison) as a natural vector in the dispersal of the diatom Didymosphenia geminata. Diatom Research 29(3): 259–266. https://doi.org/10.1080/0269249x.2014.890957.
Machado, M., M. Bromke, A. P. Domingues Júnior, M. G. M. V. Vaz, R. M. Rosa, C. C. Vinson, J. S. Sabir, D. I. Rocha, M. A. Martins, W. L. Araújo, L. Willmitzer, J. Szymanski & A. Nunes-Nesi, 2016. Comprehensive metabolic reprograming in freshwater Nitzschia palea strains undergoing nitrogen starvation is likely associated with its ecological origin. Algal Research 18: 116–126. https://doi.org/10.1016/j.algal.2016.06.003.
Mangadze, T., R. J. Wasserman & T. Dalu, 2017. Use of diatom communities as indicators of conductivity and ionic composition in a small austral temperate river system. Water, Air, & Soil Pollution. https://doi.org/10.1007/s11270-017-3610-3.
Manning, F, 2019. An experimental assessment of potential freshwater diatom dispersal via waterfowl feathers (Doctoral dissertation, University of British Columbia).
McCormick, P. V. & R. J. Stevenson, 1991. Mechanisms of benthic algal succession in lotic environments. Ecology 72(5): 1835–1848. https://doi.org/10.2307/1940982.
McKew, B. A., J. D. Taylor, T. J. McGenity & G. J. C. Underwood, 2011. Resistance and resilience of benthic biofilm communities from a temperate saltmarsh to desiccation and rewetting. The ISME Journal 5(1): 30–41. https://doi.org/10.1038/ismej.2010.91.
Miller, A. M. & S. W. Golladay, 1996. Effects of spates and drying on macroinvertebrate assemblages of an intermittent and a perennial prairie stream. Journal of the North American Benthological Society 15(4): 670–689. https://doi.org/10.2307/1467815.
Montresor, M., C. Di Prisco, D. Sarno, F. Margiotta & A. Zingone, 2013. Diversity and germination patterns of diatom resting stages at a coastal Mediterranean site. Marine Ecology Progress Series 484: 79–95. https://doi.org/10.3354/meps10236.
Paül, V. & L. M. Pérez, 2002. Episodis extraordinaris de precipitació i ordenació territorial a Sant Llorenç del Munt i voltants. V Trobada d’Estudiosos de Sant Llorenç del Munt i l’Obac, Diputació de Barcelona. Barcelona.
Peterson, C. G., 1987. Influences of flow regime on development and desiccation response of lotic diatom communities. Ecology 68(4): 946–954. https://doi.org/10.2307/1938366.
Pineda-Morante, D., J. M. Fernández-Calero, S. Pölsterl, D. Cunillera-Montcusí, N. Bonada & M. Cañedo-Argüelles, 2022. Local hydrological conditions and spatial connectivity shape invertebrate communities after rewetting in temporary rivers. Hydrobiologia 849(6): 1511–1530. https://doi.org/10.1007/s10750-022-04799-8.
Rieradevall, M., N. Bonada & N. Prat, 1999. Community structure and water quality in the Mediterranean streams of a natural park (St. Llorenç del Munt, NE Spain). Limnetica, 17(1), 45–56. https://doi.org/10.23818/limn.17.05
Rimet, F. & A. Bouchez, 2012. Life-forms, cell-sizes and ecological guilds of diatoms in European rivers. Knowledge and Management of Aquatic Ecosystems 406: 01. https://doi.org/10.1051/kmae/2012018.
Robson, B. J., 2000. Role of residual biofilm in the recolonization of rocky intermittent streams by benthic algae. Marine and Freshwater Research 51(7): 725. https://doi.org/10.1071/mf00012.
Robson, B. J. & T. G. Matthews, 2004. Drought refuges affect algal recolonization in intermittent streams. River Research and Applications 20(7): 753–763. https://doi.org/10.1002/rra.789.
Robson, B. J., T. G. Matthews & P. R. & N. A. Thomas, 2008. Pathways for algal recolonization in seasonally-flowing streams. Freshwater Biology 53(12): 2385–2401. https://doi.org/10.1111/j.1365-2427.2008.02061.x.
Robson, B. J., E. T. Chester & C. M. Austin, 2011. Why life history information matters: drought refuges and macroinvertebrate persistence in non-perennial streams subject to a drier climate. Marine and Freshwater Research 62(7): 801. https://doi.org/10.1071/mf10062.
Sabater, S., 2000. Structure and architecture of a stromatolite from a Mediterranean stream. Aquatic Microbial Ecology 21: 161–168. https://doi.org/10.3354/ame021161.
Sabater, S., X. Timoner, C. Borrego & V. Acuña, 2016. Stream biofilm responses to flow intermittency: from cells to ecosystems. Frontiers in Environmental Science 4: 14. https://doi.org/10.3389/fenvs.2016.00014.
Sabater, S., X. Timoner, G. Bornette, M. De Wilde, J. C. Stromberg & J. C. Stella, 2017. The Biota of Intermittent Rivers and Ephemeral Streams: Algae and Vascular Plants. Intermittent Rivers and Ephemeral Streams. https://doi.org/10.1016/b978-0-12-803835-2.00016-4.
Sarremejane, R., J. England, C. E. M. Sefton, S. Parry, M. Eastman & R. Stubbington, 2020. Local and regional drivers influence how aquatic community diversity, resistance and resilience vary in response to drying. Oikos 129(12): 1877–1890. https://doi.org/10.1111/oik.07645.
Sheldon, F., S. E. Bunn, J. M. Hughes, A. H. Arthington, S. R. Balcombe & C. S. Fellows, 2010. Ecological roles and threats to aquatic refugia in arid landscapes: dryland river waterholes. Marine and Freshwater Research 61(8): 885. https://doi.org/10.1071/mf09239.
Sicko-Goad, L., E. F. Stoermer & J. P. Kociolek, 1989. Diatom resting cell rejuvenation and formation: time course, species records and distribution. Journal of Plankton Research 11(2): 375–389. https://doi.org/10.1093/plankt/11.2.375.
Souffreau, C., P. Vanormelingen, K. Sabbe & W. Vyverman, 2013. Tolerance of resting cells of freshwater and terrestrial benthic diatoms to experimental desiccation and freezing is habitat-dependent. Phycologia 52(3): 246–255. https://doi.org/10.2216/12-087.1.
Stubbington, R., 2012. The hyporheic zone as an invertebrate refuge: a review of variability in space, time, taxa and behaviour. Marine and Freshwater Research 63(4): 293. https://doi.org/10.1071/mf11196.
Sun, X., N. Wu, G. Hörmann, C. Faber, B. Messyasz, Y. Qu & N. Fohrer, 2022. Using integrated models to analyze and predict the variance of diatom community composition in an agricultural area. Science of the Total Environment 803: 149894. https://doi.org/10.1016/j.scitotenv.2021.149894.
Trobajo, R., E. Clavero, V. A. Chepurnov, K. Sabbe, D. G. Mann, S. Ishihara & E. J. Cox, 2009. Morphological, genetic and mating diversity within the widespread bioindicator Nitzschia palea (Bacillariophyceae). Phycologia 48(6): 443–459. https://doi.org/10.2216/08-69.1.
Valera, F., C. Díaz-Paniagua, J. A. Garrido-García, J. Manrique, J. M. Pleguezuelos & F. Suárez, 2011. History and adaptation stories of the vertebrate fauna of southern Spain’s semi-arid habitats. Journal of Arid Environments 75(12): 1342–1351. https://doi.org/10.1016/j.jaridenv.2011.05.004.
Whitney, J. E., K. B. Gido, E. C. Martin & K. J. Hase, 2015. The first to arrive and the last to leave: colonisation and extinction dynamics of common and rare fishes in intermittent prairie streams. Freshwater Biology 61(8): 1321–1334. https://doi.org/10.1111/fwb.12668.
Acknowledgements
This study was carried out in the FEHM research group (Freshwater Ecology, Hydrology and Management) and supported by the MECODISPER (CTM2017-89295-P) and the DRY-Guadalmed projects (PID2021-126143OB-C21) funded by the Spanish “Ministerio de Ciencia, Innovación y Universidades – Agencia Estatal de Investigación'' and by “ERDF A way of making Europe”. Núria Bonada and Daniel von Schiller are Serra Húnter Fellows. Thanks also to the dogs and their owners for participating in this project, and the two anonymous reviewers for their constructive comments.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. Funding was provided by the MECODISPER (CTM2017-89295-P) and the DRY-Guadalmed projects (PID2021-126143OB-C21) funded by the Spanish “Ministerio 662 de Ciencia, Innovación y Universidades – Agencia Estatal de Investigación’’ and by “ERDF A way of making Europe
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Not applicable.
Ethical approval
The three dogs used in this study did not infringe the provisions of the Spanish Animal Welfare Act (“Ley 7/2023, de 28 de marzo, de protección de los derechos y el bienestar de los animals”). As only their paws were cleaned with distilled water, no ethics committee approval was needed.
Additional information
Handling editor: Judit Padisak
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Quevedo-Ortiz, G., Fernández-Calero, J.M., Cañedo-Argüelles, M. et al. An experimental study to assess resistance and resilience strategies of freshwater diatoms to cope with drying in Mediterranean temporary rivers. Hydrobiologia (2024). https://doi.org/10.1007/s10750-024-05585-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10750-024-05585-4