Abstract
The artificial pulsed-flows impact associated with hydropower production on the downstream biological and physical processes has been extensively addressed, showing that it may cause fish drift while changing fish habitat selection toward lower water velocity patches, acting as refuge areas. We aimed to evaluate the attraction efficiency of two flow-refuges differing in their approaching angles, for Luciobarbus bocagei at an indoor experimental flume. We tested two flow-refuge insertion angles (45° and 70°), and two flow events (base 7 L/s and pulsed-flow 60 L/s) for each. To analyze flow-refuge efficiency, we quantified fish individual and group patterns, flow-refuge use, and permanence time, while measuring glucose and lactate responses. The results showed that the individual frequency of use during simulated pulsed-flow was higher, especially on the 45° flow-refuge. The simulated pulsed-flow condition did not disrupt group behavior for both approaching angles. Glucose and lactate analysis did not trigger physiological responses in the tested trials. The 45° flow-refuge was used more often than the 70°. The narrowing of the channel caused by the 45° flow-refuge, and the consequent higher flow homogeneity increased the attractiveness of the flow-refuge. Therefore, the flow-refuge insertion angle should be a decisive criterion in the construction and installation of flow-refuges for cyprinids, and be considered a potential indirect mitigation measure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydropower is a highly efficient electricity source, responding almost instantly to the market demand by changing the turbine discharges. Due to its flexibility and affordability, hydropower generates approximately 16% of worldwide electricity (IHA, 2022). However, the consequent artificial pulsed-flows associated with hydropower production, i.e., hydropeaking, disturb the biological, morphological, and hydrological processes of the downstream river system (Schmutz et al., 2015; Alp et al., 2020; Sukhbaatar et.al., 2020), impairing its ecological integrity. Fish represent one of the most impacted biological groups by such events, often experiencing negative effects such as downstream displacements, stranding, obstruction of migration routes, and degradation (Marmulla, 2001). These, together with habitat loss, will affect key life-cycle needs, namely migration, spawning, feeding, and sheltering (Birnie-Gauvin et al., 2020).
In recent years, the amount of research addressing hydropeaking has grown substantially (Almeida et al., 2002; Scruton et al., 2003, 2005; Alexandre et al., 2015; Costa et al., 2019a, 2019b; Boavida et al., 2020a, 2020b; Oliveira et al., 2020; Boavida et al., 2023) and have been mostly focused on salmonids. However, few studies have proposed habitat enhancement solutions to reduce its impacts based on fish behavior (Costa et al., 2019a, 2019b). For example, Iberian barbel (Luciobarbus bocagei, hereafter L. bocagei) was tested using lateral refuges as a flow-refuge against simulated hydropeaking (Costa et al., 2018, 2019a, 2019b; Moreira et al., 2020). T-shaped (Vehanen et al., 2000) and lateral refuges (Ribi et al., 2014) were also subjected to simulated hydropeaking by brown trout (Salmo trutta trutta). These studies, conducted at indoor and outdoor flumes, demonstrated that changing particular features in artificial habitat design can trigger distinct behavioral changes, dictating their efficiency.
However, there is still a gap in existing solutions to protect cyprinids, which are the most abundant fish family in European rivers, overall, as well as in Iberian. According to Goodwin et al. (2014), fish behavior may be influenced due to their capacity to feel hydraulic signals in their immediate vicinity that could be linked to their migrations and aggregation. In fact, Katopodis & Gervais (2016) mentioned that, normally, the lower velocity at the entrance of the channel will reduce the fish attraction. Katopodis et al. (2019) also referred that fish behavior and physiology, as well as hydraulic conditions, are directly linked to their capacity to be attracted. Additionally, the insertion angle of flow-refuges in relation to the flow due to the water exchange between them, is an important factor for their efficiency (use by fish), although it is still poorly studied (Ribi et al., 2014).
The main goal of the present study was to evaluate the use of two types of flow-refuge by L. bocagei at an experimental flume, during simulated pulsed-flow conditions on movement behavior and stress physiology. An integrated approach was conducted where behavioral metrics and blood physiology were quantified, in addition to the characterization of the hydraulic environment. The two flow-refuges differed in the insertion angle to the flume wall, i.e., 45° and 70° and they were considered based on previous studies (e.g., Moreira et al., 2020; Boavida et al., 2023) to improve the fish capacity on the flow-refuge utilization. The following hypotheses were tested: (i) the flow-refuge use, assessed by the metrics frequency of use and permanence time, will differ between the different insertion angles during base- and pulsed-flows; (ii) the different flow-refuge insertion angles will trigger fish physiological adjustments during base- and pulsed-flow events; (iii) the two insertion angles will create distinct hydraulic conditions in the vicinity of flow-refuges.
Methods
Ethical statement
All procedures involving animal manipulation, from capture in their natural environment to holding in the laboratory, were carried out according to European norms CEN EN 14011:2003 (CEN, 2003), and Portuguese legislation (Decreto-Lei, 2013, 2019) and guidelines (INAG, 2008). For this, a permit was issued by the Portuguese licensing entity, the Instituto da Conservação da Natureza e Florestas (ICNF). All the researchers involved with the direct manipulation of fish are authorized to carry out and design procedures and scientific projects involving animal experimentation.
The EcoPeak4Fish project is authorized by the governmental organization Direção Geral de Alimentação e Veterinária (DGAV). The Laboratory of Hydraulics of Instituto Superior Técnico is certified by DGAV as a bioterium to perform trials with live fish for conservation and scientific purposes while complying with ethical and legal rules. No fish were sacrificed for this study. The principle of replacement, reduction, and refinement (3Rs) was followed while developing the experimental protocol.
Experimental facilities
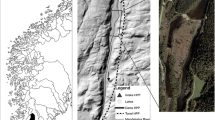
The experiments were conducted at an indoor flume located at the Hydraulics Laboratory of Instituto Superior Técnico, University of Lisbon (Fig. 1).
The flume, with a rectangular cross-section (8.0 m long, 0.7 m wide, 0.8 m high), is built on a steel frame with glass panels on both sides and it has a mobile floor that allows the placement of different structures. The usable area of the flume for fish was 6.5 m long × 0.7 m wide, delimited upstream and downstream by perforated metallic panels. The maximum discharge was 60 L/s, with a maximum water depth of 0.22 m, controlled by a downstream flap gate and an upstream sluice gate.
Two plywood flow-refuge types were constructed, differing in the angle of insertion to the flume wall, i.e., 45° or 70° (Fig. 1). For each type, two aligned flow-refuges were installed, with a 2.30 m spacing between each other. Both flow-refuge types were 0.25 m wide and 0.20 m high, differing in length, i.e., 0.45 m and 0.29 m for the 45° and the 70°- insertion angles, respectively.
Fish capture and holding
Largely spread among the northern and central basins in Portugal (Oliveira et al., 2020), the L. bocagei was the selected species to evaluate the use of two types of flow-refuge at an indoor flume. It is an endemic Iberian potamodromous cyprinid, occurring in most of the Iberian rivers, with a widespread presence in the middle and lower rivers reaches. L. bocagei is a rheophilic and other bottom-oriented species (Branco et al., 2013; Costa et al., 2019a) have their diet adapted according to their necessity, primarily consuming plant material and benthic invertebrates. Nevertheless, out of the spawning season, the adults tend to be limnophilic, showing their preferences for lower water velocities and deep available refuges (Costa et al., 2019a, b). Therefore, for the present study, young adults of L. bocagei were used because of their capacity to support fast-moving flow conditions and such phase represents the most susceptible phase of their life.
Fish sampling took place in the Sorraia River (39.011376° N, − 8.357126° W), a tributary of the lower Tagus River (central Portugal). The sampling site is not affected by artificial pulsed-flows, which makes it an appropriate site to capture fish that will be subjected to simulated pulsed-flows. Fish capture occurred on the 5th and 12th of November of 2021, through electrofishing performed with a low-voltage (400 V) unit (Hans Grassl IG-200). This period coincides with the upstream movements from this and other potamodromous species for refuge, feeding, and exploratory purposes (Benitez et al., 2015), after the reproductive season (Santos et al., 2011). In total, 125 young-adult L. bocagei were captured (Table 1), never exceeding 80 individuals per sampling occasion. After the sampling procedure, fish were placed in a permanently aerated tank (Linn Thermoport 190 L) and transported to the experimental facilities. Once in the laboratory, fish were allocated in two different 900 L tanks covered with a ventilated lid, with continuous aeration, and water filtered biologically (Fluval Canister Filters FX5 and FX6). Clay roof tiles and PVC pipes were placed at the bottom of each tank to provide sheltering areas and minimize stress.
Fish acclimated under these conditions, at room temperature and natural photoperiod for a 72 h period. Using a multiparametric probe (YSI 556 MPS), the tanks water quality (mean ± SD) was monitored daily for pH (7.47 ± 0.17), conductivity (200.8 ± 17.38 μS/cm), dissolved oxygen (8.23 ± 0.59 mg/l) and temperature (19.75 ± 1.6 °C), and twice a week, for nitrites (0.35 ± 0.24 mg/l), and ammonia (0.01 ± 0 mg/l) using colorimetric methods. The water quality in the flume was monitored twice a day for pH (7.59 ± 0.11), conductivity (141 ± 9.47 μS/cm), dissolved oxygen (7.99 ± 0.82 mg/l), and temperature (21.34 ± 1.38 °C). A commercial diet for benthic fish species was administrated every night to avoid food deprivation and minimize stress. Feeding took place during the acclimation period and stopped 24 h before the experiments (Costa et al., 2019b). All fish were returned to their natural habitat after each week of experiments.
Experimental trials
Two flow events were tested, a base-flow (BF) and a pulsed-flow (PF). The BF event consisted of a continuous 7 L/s discharge, while the PF consisted of a continuous 60 L/s discharge, both events lasted 40 min. Before each event, the fish acclimated to the flume for 30 min at 7 L/s.
To create a PF event, the upstream gate was closed to fill the flume reservoir, and then, manually, the discharge was controlled until it reaches the constant 60 L/s throughout trials (maximum discharge). Afterward, the gate was rapidly opened to 12° to release the maximum flow until reaching the target flow. For the 60 L/s discharge, and with the downstream gate fixed at a 82° angle, the maximum water level was 0.22 m for both flow-refuges. The combination of flow-refuge types (i.e., 45°, and 70°) with the flow events (i.e., BF and PF) resulted in four different sets of trials: BF45, PF45, BF70, and PF70 (Table 1), each one being replicated five times, giving a total of 20 trials. For each trial, a school of five L. bocagei was tested, and each fish was tested only once. The experimental design was outlined in accordance with previous studies (Amaral et al., 2016; Costa et al., 2018; Moreira et al., 2020), which found the sample size to be representative, thereby the need for a large number of captures and, complying to the 3R’s policy (Brønstad & Berg. 2011).
To evaluate fish use of each flow-refuge insertion angle (i.e., 45º and 70º) to attract L. bocagei and provide shelter under pulsed-flows, frequency (absolute) and time of permanence were quantified. The absolute frequency corresponded to the number of times that a single fish (I), or a group of fish—two to five individuals (G) were successfully attracted to the flow-refuge. A successful use was considered when an individual or a group of individuals were observed in the downstream area outside the flow-refuge (30 cm) (I_Down; G_Down) or inside (I_Ins; G_Ins) the flow-refuge.
The Down and Ins permanence time was quantified considering the time spent on same area/distance as for the absolute frequency. The frequency of flow-refuge use was visually accessed by three trained observers. The permanence time was registered by three GoPro cameras that were installed facing the flume glass walls, covering the whole flume area (downstream, middle, and upstream reaches). The recordings were analyzed using the BORIS software (Friard & Gamba, 2016).
Physiological responses
To analyze potential physiological adjustments between flow-refuge types (i.e., 45º and 70º) and flow events (BF and PF), the concentrations of blood glucose and lactate were measured. Both are secondary-level responses of the stress axis and are expected to increase when the organism is subjected to an external disturbance (Pankhurst, 2011). Lactate is particularly interesting to address the impacts of pulsed-flows because it is associated with fatigue (Costa et al., 2019a, b), whereas glucose is a proxy for stress (Costa et al., 2018). To collect blood samples, fish were dip-netted from the flume after each trial and transferred to a bucket with continuously oxygenated water. Immediately after, the fish were transferred to a V-shaped structure in a supine position and, a blood sample (0.1–0.5 ml) was collected via caudal puncture using 23 G or 25 G pre-heparinized needles (Costa et al., 2018). This procedure was completed in less than 3 min, assuring that it did not influence primary-level responses (e.g., cortisol). The glucose and lactate levels were immediately measured using the portable meters Accu-check Aviva (Roche) and Lactate Plus (Nova Biomedical UK) respectively (Costa et al., 2019b).
Flow-field characterization
The flow-field characterization was performed using an Acoustic Doppler Velocimeter (ADV; Nortek-AS Vectrino 10 MHz). The ADV allows orthogonal measurements (x, y, z) through a four-beam down-looking probe attached to a fixed structure (Lohrmann et al., 1994; Baladron et al., 2021). Velocity measurements were conducted following a grid of 290 and 288 measuring points for the 45° and 70° flow-refuges respectively. The perpendicular direction had a spacing interval of 0.10 m, with varying spacing in the longitudinal direction (0.10 m in the refuge area and 0.15 between areas). The selected grid allowed a more detailed characterization of the flow-field over the flow-refuge area.
The velocity measurements were carried out at each point using a 100 Hz sampling rate and a sampling period of 180 s for BF and 90 s for PF, which have been considered adequate (Buffin‐Bélanger & Roy, 2005; Silva et al., 2011).
A two-way distance-based multivariate analysis with permutations (using the Euclidian distance and 999 permutations) (Oksanen, 2007) was applied to analyze if the tested trials (i.e., BF45, BF70, PF45, PF70) triggered an effect on the frequency and permanence time of the flow-refuge use. This method accepts small sample sizes (Walters & Coen, 2006) and it does not require assumptions of parametric tests (Anderson, 2001), being suitable for continuous and factor predictors (Oksanen, 2007). The R-package vegan (Oksanen, et al., 2017) was used for this analysis. When an effect was detected a Kruskal–Wallis analysis with a posthoc Nemenyi test (pairwise contrasts between trials) (Pohlert, 2014) was conducted for each metric of flow-refuge use and permanence time. To verify if there were significant physiological adjustments in L. bocagei among trials a Kruskall-Wallis followed by a posthoc Nemenyi test was conducted (Pohlert, 2014). These analyses were performed using R-Package PMCMR (Pohlert, 2014).
Water velocity data were interpolated between the points, to go from a discontinuous to a quasi-continuous. The Ordinary Kriging spatial interpolation, through Krige function from the GSTAT package (Gräler et al., 2016) was chosen, due to the best results obtained. Velocity maps were completed via the plot.grid function (R Core Team, 2021). All treatment and pre-processing of the data were performed in the RStudio software (R Core Team, 2021). The average speed ADV was plotted on a hydraulic map with each point having its reference. The calculation of the mean value was performed for each measurement and this difference was considered in the script, calculating the negative mean speed for each measurement of that line.
Results
Flow-refuge use
Overall, the absolute frequency of fish use (sum of mean ± sum of SD) at the 45º flow- refuge (96.35 ± 56.77) was higher than the 70º (69.00 ± 28.90), when pooling both “Downs” and “Ins” fish use data.
The multivariate analysis showed a significant effect of the flow on the flow-refuge use (F = 5.235; P = 0.008). There were significant differences in the frequency of individual use, inside (I_Ins; χ2(3) = 9.7968, P = 0.02) and downstream (I_Down; χ2 (3) = 11.159, P = 0.01) of the area of the flow-refuge, among events. Rank comparisons demonstrated that I_Ins was higher (25.20 ± 15.04) for fish when subjected to PF45 in comparison with BF70 (05.40 ± 2.19) (P = 0.035) (Fig. 2A). Similarly, the downstream location of the flow-refuge used by L. bocagei was higher individually during PF45 (14.20 ± 06.14) in comparison with BF45 (03.75 ± 04.99) and BF70 (03.40 ± 01.67) (P = 0.041 and P = 0.047, respectively) (Fig. 2A). The group use of the flow-refuge, did not show a significant effect in both, Inside (G_Ins; χ2(3) = 6.4573, P = 0.09) and Downstream (G_Down; χ2(3) = 6.4443, P = 0.09) locations of the flow-refuge.
A Frequency use for the 45º and 70º approaching angle structures use by L. bocagei (n = 100) for base-flow (BF) and pulsed-flow (PF) trials at the two structures areas: Downstream (Down_I and Down_G) and Inside (Ins_I and Ins_G) approaches (I: Individual; G: Group) (B) Flow-refuge time use, in total, per trial
The permanence time (minutes) in refuge within the four trials tested is presented in Fig. 2B.
Similar to the frequency of use (Fig. 2A), the permanence time in the flow-refuge for the L. bocagei on the 45º flow-refuge (10′12″ ± 13′08″) was higher than 70º (09′08″ ± 09′52″) (Fig. 2B). On average, the individual use, on the inside area, fish were present more frequently on the 45º flow-refuge (05′27″ ± 09′00″) than 70º (04′25″ ± 07′34″) flow-refuge (Fig. 2B) (Table 2). Group use also showed the same results where 45º flow-refuge (05′21″ ± 06′41″) was higher than the 70º (04′42″ ± 04′54″). The statistical analysis did not show a significant effect of the flow on permanence time (F = 2.7747; P = 0.053).
Physiological responses
The mean (± SD) levels of blood glucose in L. bocagei were 49.27 ± 12.85 mg/l (BF45), 42.10 ± 11.09 mg/l (PF45), 47.32 ± 12.56 mg/l (BF70) and 51.73 ± 18.72 mg/l (PF70) (Fig. 3). Even though glucose levels were higher in PF70, such differences were not statistically significant among trials (χ2 (3) = 3.8787, P = 0.2749). The mean (± SD) levels of blood lactate in L. bocagei were 3.88 ± 1.04 mM (BF45) 3.68 ± 1.35 mM (PF45), 3.00 ± 1.14 mM (BF70), and 3.64 ± 1.06 mM (PF70) (Fig. 3). Lactate levels did not indicate significant differences (χ2 (3) = 5.7665, P = 0.1235).
Boxplots with the variation of blood glucose (mg/dL) and lactate (mM) levels for L. bocagei subjected to trials: BF45 and BF70, which represent the base-flow event (7L/s) for the 45° and 70° flow-refuge, respectively, and PF45 and PF70, which represent the pulsed-flow (60 L/s) events for the 45° and 70° flow-refuge respectively. The asterisk corresponds to the mean value of the physiological indicator of each trial
Flow-field
The maximum velocities for pulsed-flow (60 L/s) found were 0.914 m/s (PF45) and 0.781 m/s (PF70). Those velocities were found in the narrowest area, i.e., between the edge of the flow-refuge and the flume wall (0.35 m for the 45° and 0.42 m for the 70º) (Fig. 4). Downstream and inside areas of the flow-refuge, are characterized by lower velocities, in both configurations. As expected, the lowest velocities were observed during BF trials and, they were found in the immediate area downstream of the flow-refuge (i.e., less than 0.30 m far from the flow-refuge), for the two tested discharges (BF45—0.001 m/s and BF70—0.002 m/s) and inside of the flow-refuge 0.000 m/s
Discussion
The efficiency of flow-refuges varying in the approaching angles to the flume wall to assess their attraction potential was evaluated through the quantification of behavioral and physiological responses of L. bocagei, during simulated base- and pulsed-flow conditions.
An experimental design was conducted through a multidisciplinary approach, combining behavioral and physiological responses, while correlating them with the flow-field. The approach for the flow-refuge use (frequency and permanence time) by L. bocagei differed between the different insertion angles during base- and pulsed-flows. The different flow-refuge insertion angles triggered physiological adjustments in BF and PF events.
The flow-refuge use indicated that fish behavior changed according to the flow conditions and the insertion angle. L. bocagei used the inside area of the flow-refuge more often than the downstream area for both the BF and PF conditions. Such evidence was more prominent for the 45° insertion angle than for the 70° one. Although L. bocagei used more frequently these flow-refuges (inside and downstream), this distinction was clear in the PF45 compared to the BF trials. The individual frequency of use was higher during BF45 and PF45 flow-refuge than the BF70 and PF70. Such a result was more evident during pulsed-flow events. A similar result was found by Costa et al. (2018), where, during simulated discharges conditions, L. bocagei used the flow-refuge individually more often than in groups. Although dissimilar results between trials were found in the group use frequency, the statistical analysis did not demonstrate significant differences, as mentioned before. This may be understood as a non-disruption of group behavior, which means that the simulated pulsed-flow conditions may not have affected the group behavior. In fact, schooling behavior could generate benefits, such as the frequency increase of the tail beat by the leading fish (Liao, 2007) or the reduction of the energy cost during spawning season (Standen et al., 2002; Wang & Chanson, 2018), or under turbulent flows (Enders et al., 2005). Also, according to Costa et al. (2019a, b) findings, the frequency of use in group could be explained by the fact that lower discharge and pressure magnitudes can generate stability in the group behavior. Even though the time spent within the flow-refuge areas was longer, the statistical analysis did not show any significant difference (P = 0.053).
The absence of physiological adjustments among trials, together with the characterization of the flow-field, suggests that the conditions created were not harsh enough to trigger a stress response. The connection between flow variability and stress response is difficult to identify (Costa et al., 2017) and may be justified by the type of external stimulus or the severity of the stressor (Pankhurst, 2011). Thus, the presence of both types of flow-refuge and the created hydraulic conditions favored flow-refuging (Flodmark et al., 2002; Krimmer et al., 2011; Costa et al., 2019a, b) and avoided physiological adjustments.
According to our experimental settings, the two insertion angles create distinct flow-field conditions, with higher velocities found in the narrowest distance (distance between the edge of the refuge and the channel wall) due to the refuge locations. Thus, it is possible to select the most efficient flow-refuge based on its potential to attract L. bocagei. This may be explained by the fact that the 45º flow-refuge, due to the insertion on the flume channel, has the narrowest distance (0.35 m) when compared to the 70º flow-refuge (0.42 m), creating then higher velocities, and leading to a higher attraction. This shortest distance seemed to be sufficient to trigger the rheophilic behavior of L. bocagei and increase the attractiveness of the 45° flow-refuge. According to Goodwin et al. (2014), fish detection of hydraulic signals could trigger aggregation and migration behavior. A similar result was found by Ribi et al. (2014), in their experimental study in an indoor flume simulating hydropeaking event equipped with a lateral refuge, when they compared the capability of brown trout juveniles to find different entrances installed laterally in their facility. According to their results, as long as they have flow cues toward the entrances, fish could be attracted.
The presence of the flow-refuges, under different flow conditions (BF and PF) created a differential use of flow-refuges by fish. The ADV results demonstrated that the flow-field was changed by such structures (i.e., different velocity ranges within the flume).
The collected data also allowed identifying the best flow-refuge for fish. The 45° flow-refuge demonstrated to be the most suitable flow-refuge during pulsed-flow conditions throughout the study, as it displayed the highest flow-refuge use and permanence time. Thus, considering the effects of pulsed-flows (e.g., loss of suitable habitat areas, Moreira et al., 2020), flow-refuges that create water deflection given their insertion angles are highly recommended to create suitable flow-refuging areas.
As demonstrated in this and other studies (Costa et al., 2018, 2019a, b; Moreira et al., 2020) fish can benefit from these habitat enhancement solutions. However, each approach is unique, thus, a previous analysis should consider the river morphology and hydrodynamic patterns (Schmutz et al., 2015; Costa et al., 2019a, b), as well as the fish’s ecological requirements. Thus, such an instream structure may be considered as a potential indirect mitigation measure to artificial pulsed-flows due to hydropower production.
Conclusion
This study provided novel insight regarding the attraction efficiency of two alternative flow-refuges use for the potamodromous cyprinid L. bocagei. The two approaching angles tested (i.e., 45° and 70°) created distinct flow-field conditions at the entrance of the flow-refuges and evidenced that the flow-refuge with the lowest insertion angle to the flume wall (i.e., 45°) was more used by L. bocagei in comparison with the widest angle (70°). Flume studies may not replicate natural conditions but provide knowledge regarding fish interactions under a controlled environment, which are essential for the development of more efficient solutions and their application in the field. These indirect mitigation measures can be used by cyprinids for flow-refuges during pulsed-flows associated with hydropower production. Future studies should consider extended tests with different ramping rates, different ranges of flow rates (higher ones), or even other fish guilds (e.g., water-column species).
Data availability
Data will be available on reasonable request.
References
Alexandre, C. M., S. Sales, M. T. Ferreira & P. R. Almeida, 2015. Food resources and cyprinid diet in permanent and temporary Mediterranean rivers with natural and regulated flow. Ecology of Freshwater Fish 24(4): 629–645.
Almeida, P. R., B. R. Quintella & N. M. Dias, 2002. Movement of Radio-Tagged Anadromous Sea Lamprey During the Spawning Migration in the River Mondego (Portugal). In Thorstad, E. B., I. A. Fleming & T. F. Næsje (eds), Aquatic Telemetry Springer, Dordrecht: 1–8.
Alp, A., A. Akyüz & S. Kucukali, 2020. Ecological impact scorecard of small hydropower plants in operation: an integrated approach. Renewable Energy 162: 1605–1617.
Amaral, S. D., P. Branco, A. T. da Silva, C. Katopodis, T. Viseu, M. T. Ferreira, A. N. Pinheiro & J. M. Santos, 2016. Upstream passage of potamodromous cyprinids over small weirs: the influence of key-hydraulic parameters. Journal of Ecohydraulics. 1: 79–89.
Anderson, D. R., 2001. The need to get the basics right in wildlife field studies. Wildlife Society Bulletin 2001: 1294–1297.
Baladron, A., M. J. Costa, M. D. Bejarano, A. Pinheiro & I. Boavida, 2021. Can vegetation provide shelter to cyprinid species under hydropeaking? Science of the Total Environment 769: 145339.
Benitez, J. P., B. Nzau Matondo, A. Dierckx & M. Ovidio, 2015. An overview of potamodromous fish upstream movements in medium-sized rivers, by means of fish passes monitoring. Aquatic Ecology 49: 481–497.
Birnie-Gauvin, K., J. Nielsen, S. B. Frandsen, H. M. Olsen & K. Aarestrup, 2020. Catchment-scale effects of river fragmentation: a case study on restoring connectivity. Journal of Environmental Management 264: 110408.
Boavida, I., F. Ambrósio, M. J. Costa, A. Quaresma, M. M. Portela, A. Pinheiro & F. Godinho, 2020a. Habitat use by Pseudochondrostoma duriense and Squalius carolitertii downstream of a small-scale hydropower plant. Water 12(9): 2522.
Boavida, I., L. Caetano & A. N. Pinheiro, 2020b. E-flows to reduce the hydropeaking impacts on the Iberian barbel (Luciobarbus bocagei) habitat: an effectiveness assessment based on the COSH tool application. Science of the Total Environment 699: 134209.
Boavida, I., M. J. Costa, M. M. Portela, F. Godinho, J. Tuhtan & A. Pinheiro, 2023. Do cyprinid fish use lateral flow-refuges during hydropeaking? River Research and Applications 39(3): 554–560.
Branco, P., J. M. Santos, C. Katopodis, A. N. Pinheiro & M. T. Ferreira, 2013. Effect of flow regime hydraulics on passage performance of Iberian chub (Squalius pyrenaicus) (Gu¨ nther, 1868) in an experimental pool-andweir fishway. Hydrobiologia. 714: 145–154. https://doi.org/10.1007/s10750-013-1532-7.
Brønstad, A. & A. G. T. Berg, 2011. The role of organizational culture in compliance with the principles of the 3Rs. Lab Animal 40(1): 22–26.
Buffin-Bélanger, T. & A. G. Roy, 2005. 1 min in the life of a river: selecting the optimal record length for the measurement of turbulence in fluvial boundary layers. Geomorphology 68(1–2): 77–94. https://doi.org/10.1016/j.geomorph.2004.09.032.
CEN, 2003. Water Quality: Sampling of Fish with Electricity, European Committee for Standardization, Brussels:
Costa, M. J., R. J. Lennox, C. Katopodis & S. J. Cooke, 2017. Is there evidence for flow variability as an organism-level stressor in fluvial fish?. Journal of Ecohydraulics 2: 68–83. https://doi.org/10.1080/24705357.2017.1287531.
Costa, M. J., I. Boavida, V. Almeida, S. J. Cooke & A. N. Pinheiro, 2018. Do artificial velocity refuges mitigate the physiological and behavioural consequences of hydropeaking on a freshwater Iberian cyprinid? Ecohydrology 11(7): e1983.
Costa, M. J., M. T. Ferreira, A. N. Pinheiro & I. Boavida, 2019a. The potential of lateral refuges for Iberian barbel under simulated hydropeaking conditions. Ecological Engineering 127: 567–578.
Costa, M. J., J. F. Fuentes-Pérez, I. Boavida, J. A. Tuhtan & A. N. Pinheiro, 2019b. Fish under pressure: examining behavioural responses of Iberian barbel under simulated hydropeaking with instream structures. PloS One 14(1): e0211115.
Decreto-Lei (2013) Diário da República no 151/2013, Série I de 2013-08-07. no 113/2013. https://data.dre.pt/eli/dec-lei/113/2013/08/07/p/dre/pt/html. Accessed 15 Dec 2020.
Decreto-Lei no 1/2019 (2019) Diário da República no 7/2019, Série I de 2019-01-10. https://data.dre.pt/eli/dec-lei/1/2019/01/10/p/dre/pt/html. Accessed 15 Dec 2020.
Enders, E. C., D. Boisclair & A. G. Roy, 2005. A model of total swimming costs in turbulent flow for juvenile Atlantic salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences 62(5): 1079–1089.
Flodmark, L. E. W., H. A. Urke, J. H. Halleraker, J. V. Arnekleiv, L. A. Vøllestad & A. B. S. Poléo, 2002. Cortisol and glucose responses in juvenile brown trout subjected to a fluctuating flow regime in an artificial stream. Journal of Fish Biology 60(1): 238–248.
Friard, O. & M. Gamba, 2016. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods in Ecology and Evolution 7(11): 1324–1330. https://doi.org/10.1111/2041-210X.12584.
Goodwin, R. A., Lai, Y. G., Taflin, D. E., Smith, D. L., McQuirk, J., Trang, R., & Reeves, R. (2023). Predicting nearterm, out-of-sample fish passage, guidance, and movement across diverse river environments by cognitively relating momentary behavioral decisions to multiscale memories of past hydrodynamic experiences. Frontiers in Ecology and Evolution, 11: 703946
Gräler, B., E. Pebesma & G. Heuvelink, 2016. Spatio-temporal interpolation using gstat. The R Journal 8: 204–218.
IHA (International Hydropower Association). 2022 Hydropower Status Report; IHA: London, UK, 2022.
INAG IP, 2008. Manual Para a Avaliação Biológica da Qualidade da Água em Sistemas Fluviais Segundo a Directiva Quadro da Água—Protocolo de Amostragem e Análise Para a Fauna Piscícola, Ministério do Ambiente, do Ordenamento do Terrritório e do Desenvolvimento Regional. Instituto da Água, Lisboa:
Katopodis, C., & Gervais, R. (2016). Fish swimming performance database and analyses (p. 550). Canadian Science Advisory Secretariat (CSAS).
Katopodis, C., Cai, L., & Johnson, D. (2019). Sturgeon survival: The role of swimming performance and fish passage research. Fisheries Research, 212: 162–171
Krimmer, A. N., A. J. Paul, A. Hontela & J. B. Rasmussen, 2011. Behavioural and physiological responses of brook trout Salvelinus fontinalis to midwinter flow reduction in a small ice-free mountain stream. Journal of Fish Biology 79: 707–725.
Liao, J. C., 2007. A review of fish swimming mechanics and behaviour in altered flows. Philosophical Transactions of the Royal Society b: Biological Sciences 362(1487): 1973–1993.
Lohrmann, A., Cabrera, R., Kraus, N. C. 1994. Acoustic-doppler velocimeter (ADV) for laboratory use. Proceedings Conference on Fundamentals and Advancements in Hydraulic Measurements and Experimentation. American Society of Civil Engineers, Buffalo, NY (USA).
Marmulla, G. (2001). Dams, fish and fisheries. Opportunities, challenges and conflict resolution.
Moreira, M., M. J. Costa, J. Valbuena-Castro, A. N. Pinheiro & I. Boavida, 2020. Cover or velocity: what triggers Iberian Barbel (Luciobarbus bocagei) refuge selection under experimental hydropeaking conditions? Water 12(2): 317.
Oksanen, J., 2007. Multivariate Analysis of Ecological Communities in R: Vegan Tutorial, Univ. of Oulu, Oulu:
Oksanen, F. J., et al. 2017. Vegan: Community Ecology Package. R package Version 2.4–3. https://CRAN.R-project.org/package=vegan
Oliveira, I. C., C. M. Alexandre, B. R. Quintella & P. R. Almeida, 2020. Impact of flow regulation for hydroelectric production in the movement patterns, growth and condition of a potamodromous fish species. Ecohydrology 13(8): e2250.
Pankhurst, N. W., 2011. The endocrinology of stress in fish: an environmental perspective. General and Comparative Endocrinology 170(2): 265–275.
Pohlert, T. 2014. The pairwise multiple comparison of mean ranks package (PMCMR). R package. http://cran.r-project.org/package=PMCMR
R Core Team, 2021. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna:
Ribi, J. M., J. L. Boillat, A. Peter & A. J. Schleiss, 2014. Attractiveness of a lateral shelter in a channel as a refuge for juvenile brown trout during hydropeaking. Aquatic Sciences 76: 527–541.
Santos, J. M., Reino, L., Porto, M., Oliveira, J., Pinheiro, P., Almeida, P. R., ... & Ferreira, M. T. 2011. Complex size-dependent habitat associations in potamodromous fish species. Aquatic sciences, 73: 233–245.
Schmutz, S., T. H. Bakken, T. Friedrich, F. Greimel, A. Harby, M. Jungwirth, et al., 2015. Response of fish communities to hydrological and morphological alterations in hydropeaking rivers of Austria. River Research and Applications. 31: 919–930.
Scruton, D. A., L. M. N. Ollerhead, K. D. Clarke, C. Pennell, K. Alfredsen, A. Harby & D. Kelley, 2003. The behavioural response of juvenile Atlantic salmon (Salmo salar) and brook trout (Salvelinus fontinalis) to experimental hydropeaking on a Newfoundland (Canada) river. River Research and Applications 19(5–6): 577–587.
Scruton, D. A., C. J. Pennell, M. J. Robertson, L. M. N. Ollerhead, K. D. Clarke, K. Alfredsen & R. S. McKinley, 2005. Seasonal response of juvenile Atlantic salmon to experimental hydropeaking power generation in Newfoundland, Canada. North American Journal of Fisheries Management 25(3): 964–974.
Silva, A. T., J. M. Santos, M. T. Ferreira, A. N. Pinheiro & C. Katopodis, 2011. Effects of water velocity and turbulence on the behaviour of Iberian barbel (Luciobarbus bocagei, Steindachner 1864) in an experimental pool-type fishway. River Research and Applications 27: 360–373. https://doi.org/10.1002/rra.1363.
Sloman, K. A., K. M. Gilmour, A. C. Taylor & N. B. Metcalfe, 2000. Physiological effects of dominance hierarchies within groups of brown trout, Salmo trutta, held under simulated natural conditions. Fish Physiology and Biochemistry 22(1): 11.
Standen, E. M., S. G. Hinch, M. C. Healey & A. P. Farrell, 2002. Energetic costs of migration through the Fraser River Canyon, British Columbia, in adult pink (Oncorhynchus gorbuscha) and sockeye (Oncorhynchus nerka) salmon as assessed by EMG telemetry. Canadian Journal of Fisheries and Aquatic Sciences 59(11): 1809–1818.
Sukhbaatar, C., T. Sodnom & C. Hauer, 2020. Challenges for hydropeaking mitigation in an ice-covered river: a case study of the Eg hydropower plant, Mongolia. River Research and Applications 36(8): 1416–1429.
Vehanen, T., P. L. Bjerke, J. Heggenes, A. Huusko & A. Mäki-Petäys, 2000. Effect of fluctuating flow and temperature on cover type selection and behaviour by juvenile brown trout in artificial flumes. Journal of Fish Biology 56(4): 923–937.
Walters, K. & L. D. Coen, 2006. A comparison of statistical approaches to analyzing community convergence between natural and constructed oyster reefs. J Exp Mar Bio Ecol. 330: 81–95. https://doi.org/10.1016/j.jembe.2005.12.018.
Wang, H. & H. Chanson, 2018. Modelling upstream fish passage in standard box culverts: interplay between turbulence, fish kinematics, and energetics. River Research and Applications 34(3): 244–252.
Acknowledgments
The authors would like to thank Anthony Merianne and Francisco Almeida for his valuable assistance during the fieldwork and laboratory experiments. This study received funding from Fundação para a Ciência e a Tecnologia (FCT) through the EcoPeak4Fish project—an integrated approach to support self-sustaining fish populations downstream hydropower plants (PTDC/EAM-AMB/4531/2020). Electrofishing permits were issued by the Institute for Conservation of Nature and Forests (ICNF) (permit numbers 257/2021/CAPT, 258/2021 /CAPT, and 260/2021/CAPT). Forest Research Centre (CEF) is a research unit funded by FCT, Portugal (UIDB/00239/2020). The authors are also grateful to FCT for its support through funding UIDB/04625/2020 from the research unit Civil Engineering Research and Innovation for Sustainability (CERIS). Renan Leite was supported by a grant from FCT, given through the River Restoration and Management Doctoral Programme (FLUVIO) Portugal (grant UI/BD/151419/2021). Finally, the authors would like to thank two anonymous reviewers for their comments and suggestions that improved an early draft of this manuscript.
Funding
Open access funding provided by FCT|FCCN (b-on). Funding was provided by Fundação para a Ciência e a Tecnologia (Grant Nos. UI/BD/151419/2021, UIDB/04625/2020, UIDB/00239/2020, PTDC/EAM-AMB/4531/2020).
Author information
Authors and Affiliations
Contributions
Conceptualization, Renan Leite, Maria João Costa, Isabel Boavida, José Maria Santos; Methodology, Renan Leite, Isabel Boavida, José Maria Santos and Maria Joao Costa; Validation, Renan Leite, Isabel Boavida, Maria Joao Costa and José Maria Santos; Formal analysis, Renan Leite, Isabel Boavida, Maria Joao Costa; Investigation, Renan Leite, Isabel Boavida, Maria Joao Costa, and Daniel Mameri Resources, Isabel Boavida, Maria Joao Costa, José Maria Santos and António Pinheiro; Data curation, Renan Leite and Maria Joao Costa; Writing-original draft preparation, Renan Leite; Writing-review and editing, Renan Leite, Maria Joao Costa, Daniel Mameri, Fernando Afonso, António Pinheiro, José Maria Santos and Isabel Boavida; Visualization, Renan Leite, Isabel Boavida, José Maria Santos and Maria Joao Costa; Supervision, Isabel Boavida, José Maria Santos and Maria Joao Costa; Project Administration, Isabel Boavida and Maria Joao Costa; Funding acquisition, Isabel Boavida.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent for publication
All authors have read and agreed to the published version of the manuscript.
Additional information
Handling editor: Louise Chavarie
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Leite, R., Costa, M.J., Mameri, D. et al. The hide-and-seek effect of pulsed-flows in a potamodromous cyprinid fish. Hydrobiologia (2024). https://doi.org/10.1007/s10750-024-05575-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10750-024-05575-6