Abstract
This study aimed to achieve a better understanding of the features of zooplankton assemblages in small water bodies and their biodiversity and composition in relation to the presence of fish and accompanying environmental characteristics. This study was conducted in 16 mid-field ponds. Compositional and biodiversity indexes and ordination methods were used to analyze the relationship between zooplankton assemblages in unstable fishless and stable fish ponds. A total of 121 zooplankton taxa were identified. Compositional indicators revealed significant differences in zooplankton assemblages between fish ponds (FPs) and fishless ponds (FLPs). Canonical correspondence analysis indicated that variation in zooplankton assemblages depended on the ponds’ features. Most of the high trophic state indicator species were present only in FPs or occurred sporadically in FLPs. Rarefaction and extrapolation indicated a higher number of zooplankton taxa in FPs than in FLPs. The stability of the ecosystem was essential for maintaining the high species richness of zooplankton. Diversity indices were not influenced by variations in species composition or environmental differences among ponds. Diversified pond types are necessary to maintain the heterogeneity of mid-field ponds, which support the high regional biodiversity of zooplankton assemblages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Small water bodies are important local biodiversity hot spots in anthropogenic landscapes (Scheffer et al., 2006; Kuczyńska‐Kippen & Joniak, 2010; Florencio et al., 2020), but they are endangered by drought, global warming, and pollution. Small water bodies are highly diversified, which greatly impacts the formation of zooplankton assemblages (Céréghino et al., 2008). Ponds features such as environmental conditions and biotic interactions function as filters imposed on the regional species pool (Diaz et al., 1998). These filters result in the removal of species that lack traits necessary for persistence under specific conditions (Keddy, 1992). An increase in species richness and biodiversity of zooplankton can be expected in larger ponds and those with diverse microhabitats (Mimouni et al., 2018; Kuczyńska-Kippen et al., 2021). However, in the case of small water bodies, their depth and surface area undergoes fluctuations, sometimes resulting in water scarcity or desiccation of the water bodies. Temporary ponds, irrespective of their surface area, display a unique ecological characteristic—namely, the absence of large predators, specifically fishes (Collinson et al., 1995; Wellborn et al., 1996).
Mid-field water bodies are more prone to nutrient enrichment than water bodies of different types of land use (e.g., seminatural or natural areas) (Soranno et al., 2015), which accelerates eutrophication processes, enabling intensive macrophyte growth. The depth of ponds is an important factor that determines the coverage of macrophytes (Rea et al., 1998). Shallow ponds undergo rapid development of macrophytes, which can serve as additional habitat for microinvertebrates, thereby reducing pressure from fish (Lampert & Sommer, 2007; Kuczyńska‐Kippen & Joniak, 2010; Quirino et al., 2021).
The presence of fish is presumed to have a limiting effect on zooplankton due to fish selective predation regarding larger zooplankton species and the dominance of smaller species in such a system (Brooks & Dodson, 1965; Irvine et al., 1989; Lampert & Sommer, 2007). A size shift in large-bodied species, such as Daphnia, was observed in the absence of fish (Irvine et al., 1989). Considering the hydrological conditions in ponds and related water parameters such as oxygen concentrations, most of the fish species have higher habitat requirements than invertebrates; therefore, many zooplankton taxa could survive and develop in small ephemeral or very shallow fishless ponds (FLPs) (Collinson et al. 1995; Florencio et al., 2016, 2020; Brendonck et al., 2017; Karpowicz et al., 2020a). Moreover, some zooplankton species are perfectly adapted to highly fluctuating hydrological features of ephemeral ponds (Florencio et al., 2016; Franch-Gras et al., 2017). The lack of fish in such a habitat promotes the establishment of plankton assemblages imposed by less pronounced top-down regulation by herpetofauna or macroinvertebrates (Cobbaert et al., 2010; Zokan & Drake, 2015). Therefore, factors such as the size of the water body, hydroperiod, macrophyte cover, or presence of fish often act conversely regarding species richness and biodiversity, which hinders finding clear patterns in these small ecosystems.
Microinvertebrates are indicators of the trophic state in ecological assessments (Jeppesen et al., 2011; Ejsmont-Karabin, 2012; Ejsmont-Karabin & Karabin, 2013; Ochocka & Pasztaleniec, 2016; Karpowicz et al., 2020a, b). Microinvertebrates as indicators are often used in the assessment of the ecological status of lakes with a strong top-down regulation by fish and a highly stable water level (Gutkowska et al., 2013; Haberman & Haldna, 2014; Dembowska et al., 2015; Krupa et al., 2020). To use microinvertebrates in the ecological assessment of small water bodies, it is necessary to understand their performance in different types of ponds.
To better understand the features of zooplankton assemblages in small water bodies and their biodiversity and composition, we hypothesize that the stability of conditions offered by FPs is more important for maintaining high species richness and biodiversity than habitat diversity (macrophytes) and lack of fish predation pressure in FLPs. We assumed that fishless ponds would be more homogeneous compared to fishponds, anticipating that hydrologically not stable ponds would favor the survival of a reduced number of zooplankton species. Additionally, we aimed to verify if the habitat instability of FLPs affects the usefulness of zooplankton assemblages in ecological assessment.
Material and methods
Study area
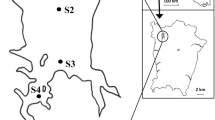
This study was conducted in the drainage of Oder River, northwestern Poland, in 16 mid-field ponds (Fig. 1, Table 1). The size of the water bodies varied from 0.2 to 2.8 ha, and their drainage varied from 1.2 to 54 ha. In all the ponds with fish, the top predators were Esox lucius Linnaeus, 1758 and/or Perca fluviatilis Linnaeus, 1758; however, in pond no. 4, juvenile individuals of pike and tench were observed only once (Table 1). Fish ponds (FPs) were dominated by Ceratophyllum demersum L., Lemna gibba L., Nuphar lutea (L.) Sibth. & Sm., Persicaria amphibia (L.) Gray, and Potamogeton crispus L., whereas in fishless ponds (FLPs), C. demersum, Elodea canadensis Michx., L. gibba, and Sagittaria sagittifolia L. were most prevalent (Table 1). There were significant changes in water levels in the surveyed ponds, with deficits of water recorded only at FLPs. Some FLPs were dry during the summer seasons of these years: 2016, pond no. 16; 2017, ponds no. 2 and 3; 2018, ponds no. 7 and 9. Samples were not collected under such conditions. For calculations, absent values were not considered zero values. For each analysis description, we indicate whether the data for the analyses are sample-based or refer to cumulative data.
Sample collection and characteristics of ponds
All the water bodies studied in this work could be categorized as ponds according to Richardson et al. (2022). Zooplankton assemblages were collected once a year from each not-dried-out pond in summer (July) from 2016 to 2018. In temperate regions, zooplankton assemblages are most stable in summer; hence, summer is considered the best season to assess the ecological status of water bodies (Mimouni et al., 2018; Ejsmont-Karabin, 2012; Ejsmont-Karabin & Karabin, 2013). At each pond, 10 L of water was collected from five randomly selected open water habitats (in total 50 L from each pond). Water from the surface to the bottom was collected using a van Dorn sampler (3 L). Samples were pooled into one vessel and filtered through a plankton net with a mesh size of 35 μm. The samples were then concentrated to 100 mL and fixed in a 4–5% formalin solution. The contents of the samples were counted using a Sedgewick-Rafter counting chamber and identified using a Nikon Eclipse 50i microscope (Nikon, Tokyo, Japan). Finally, the species were identified using taxonomic keys (Nogrady & Segers, 2002; Mirabdullayev et al., 2003; Bielańska-Grajner et al., 2015; Błędzki & Rybak, 2016).
Maximum depth (m) was measured in situ (Table 2). Pond surface area (ha) was measured using QGIS (QGIS Development Team, 2021) with satellite maps. Physical and chemical measurements were taken directly in the field. Temperature (°C), conductivity (µS/cm), pH, water oxygenation (mg/L) and chlorophyll a (µg/L) were measured with a Hydrolab DS5 multiparameter sensor (Hach, USA). The concentration of ammonium (mg/L), nitrates (mg/L), and orthophosphates (mg/L) were determined colorimetrically using an automatic flow analyzer produced by Skalar (Breda, The Netherlands). The percentage of individual patches of vegetation was calculated on the basis of panchromatic photogrammetric images from drone raids (Buczyńska et al., 2023). The mosaic of photographs was combined into one image using AgiSoft software. The identified surfaces thus obtained were digitized to an SHP format vector file. The individual polygons in meters used for the ellipsoid were then calculated.
During each year surveyed, fish were captured using electric fishing gear in each pond (Hans Grassl ELT60 II, Germany) and released. Catches were carried out in accordance with the EN 14011 European Committee for Standardization procedure. Based on these catches, ponds were categorized into FPs and FLPs. This classification reflects the fluctuating nature of fishless ponds, with two exceptions, namely ponds no. 8 and no. 15, where we did not record fish, and we did not observe water shortages during the survey period.
Data analyses
The taxonomical similarity between the ponds was calculated using the Jaccard index (presence–absence data) (Chao et al., 2005) using cumulative data from the three study years for each pond. Dendrogram was created in MVSP version 3.2. using the best grouping method, i.e., the farthest neighbor method (FN). Apart from FN, we also utilized nonmetric multidimensional scaling (NMDS), which generated an ordination of cumulative data from ponds based on Bray–Curtis distance (abundance data) in the CANOCO, version 5 (ter Braak & Šmilauer, 2012).
To avoid bias toward ponds with a higher abundance of microinvertebrates, the calculations were based on the number of samples (Colwell et al., 2012). The Shannon diversity index (mean among runs, natural logarithms) and the Simpson (inverse) diversity index (mean among runs) were calculated (Magurran, 2004). Rarefaction and extrapolation were used (Colwell et al., 2012) to calculate the species number and biodiversity indexes for the data from the FPs and FLPs (EstimateS version 9.1.0.).
To calculate Local Contribution to Beta Diversity (LCBD) and Species Contribution to Beta Diversity (SCBD) values, we used abundance data and subsequently calculated the total beta diversity (BD total) (Legendre & De Cáceres, 2013). LCBD represents the ecological uniqueness of a site and SCBD indicates contribution of a species to overall beta diversity. Legendre and De Cáceres (2013) state that large LCBD or SCBD values suggest a site’s or species’ strong significance to the overall beta diversity. Beta diversity analyses were performed with R (version 4.3.2; R CoreTeam 2023) and adespatial package (version 0.3-23; Dray et al., 2023).
Welch’s test, which considers heterogeneity of variance and is robust to deviations from the normal distribution of the data, was employed to compare mean values across environmental parameters and biodiversity indices. To compare zero-inflated microinvertebrate abundance data between the pond types, the Wald test for the Tweedie distribution was used (STATISTICA version 13.0, StatSoft). Before analyzing those data sets, we checked the data distribution using descriptive statistics and histograms, and similarly assessed the homogeneity of variances with Levene’s test.
The effects of environmental parameters on the composition of microcrustaceans (sample-based data) were assessed using the CANOCO software package, version 5 (ter Braak & Šmilauer, 2012). Zooplankton distribution patterns in relation to habitat variables were determined using canonical correspondence analysis (CCA) and detrended correspondence analysis, which detected the unimodal structure of the data. Variables that were strongly correlated with other explanatory variables were excluded from the model. The results of the stepwise forward selection of variables revealed their statistical significance. To select the explanatory variables for analysis, we utilized correlations and variance inflation factor (VIF) (Table S1). A complete CCA was performed using the following seven variables: presence of fish, depth, surface area, macrophyte cover, concentrations of nitrates, dissolved oxygen, and orthophosphates. In addition, the statistical significance of each variable was calculated (p). The LOESS model was used to fit the number of species in relation to both CCA axes (ter Braak & Šmilauer, 2012).
Trophic state indicators
In order to assess the efficacy of bioindicators, we employed indices developed for lakes. Indicators of the high trophic state in the lakes of the temperate European region include small rotifers such as Anuraeopsis fissa Gosse, 1851, Brachionus spp., Filinia longiseta (Ehrenberg, 1834), Keratella tecta (Gosse, 1851), Keratella quadrata (Müller, 1786), Pompholyx sulcata Hudson, 1885, Trichocerca pusilla (Jennings, 1903), and the planktonic crustaceans Bosmina longirostris (O.F. Müller, 1785), Chydorus sphaericus (O.F. Müller, 1776), Diaphanosoma brachyurum (Liévin, 1848), Mesocyclops leuckarti (Claus, 1857) and Thermocyclops oithonoides (G.O. Sars, 1863) (Ejsmont-Karabin, 2012; Ejsmont-Karabin & Karabin, 2013). However, other small rotifers as Ascomorpha ecaudis Perty, 1850, Ascomorpha ovalis (Bergendahl, 1892), Conochilus hippocrepis (Schrank, 1803), Gastropus stylifer (Imhof, 1891), Polyarthra major Burckhardt, 1900 are considered be indicative of low trophic status.
Results
Environmental background
Based on the results of the Welch’s test, surface area, depth, and the concentration of dissolved oxygen (p < 0.0001) were found to be significantly higher in FPs than in FLPs (Table 2). Macrophyte cover values were significantly higher in FLPs (p < 0.0001) than in FPs. No significant differences were observed for other variables (p > 0.05).
Compositional distinctiveness
Both Jaccard with FN clustering and Bray–Curtis distance applied in NMDS were useful for separating the zooplankton assemblages of FLPs and FPs. In the FN, only one pond (pond no. 4) was not clustered in their group (Fig. 2a). NMDS effectively separated the FPs from the FLPs, as indicated by a stress value of 0.0889 (Fig. 2b).
Jaccard’s similarity coefficient dendrograms based on presence–absence data using the farthest neighbor grouping method (a) and Bray Curtis distance based on abundance data using nonmetric multidimensional scaling (NMDS) (b). Both data sets are cumulative for the three years of sampling. Numbers indicate ponds. F Fish ponds, FL Fishless ponds
Biodiversity
A total of 121 taxa (mainly species) were identified (the complete list with author citations is provided in Supplementary file 2). The most diverse were Rotifera (71 taxa), followed by Cladocera (26 taxa) and Copepoda (24 taxa). Considering sample-based species richness, Welch’s test (t = 1.93; df = 39.6; p = 0.0598) did not identify significant differences between FPs (n = 27; mean = 18.4; SD = 7.00) and FLPs (n = 16; mean = 14.9; SD = 4.91). The abundance of zooplankton assemblages was not significantly different (t = 1.32; df = 41; p = 0.1942) between FPs (n = 27; mean = 265; SD = 28) and FLPs (n = 16; mean = 179; SD = 14). For the Simpson index (Simpson index 1-D), the Welch’s test (t = 0.07; df = 29.3; p = 0.9400) did not identify significant differences between FPs (n = 27; mean = 0.744; SD = 0.19) and FLPs (n = 16; mean = 0.740; SD = 0.21). For the Shannon index, the test (t = 0.56; df = 33.7; p = 0.5737) did not identify significant differences between FPs (n = 27; mean = 2.020; SD = 0.69) and FLPs (n = 16; mean = 1.901; SD = 0.64).
Rarefaction and extrapolation indicated a higher number of zooplankton taxa in FPs, than in FLPs (Fig. 3a). The asymptote of the extrapolation curve for FPs and FLPs showed 118 (131, 95% CI upper bound) and 95 species (111, 95% CI upper bound), respectively. The Simpson (inverse) index and the Shannon index did not indicate considerable differences in biodiversity between the two groups of ponds (Fig. 3b).
Partitioning beta diversity
According to the beta diversity analysis (abundance-based), replacement contributed more than richness to the total beta diversity (Fig. 4a). The share of replacement and richness was similar in both pond types (Fig. 4b). We also examined beta diversity indices separately in the pond groups (Fig. 4c, d). Specifically, ponds no. 14, no. 6, no. 12, and no. 13 exhibited the highest values of LCBD replacement in fish ponds. Pond no. 4 was distinct from the rest of FPs regarding LCBR replacement, representing the lowest beta diversity values. Ponds no. 7, no. 8, no. 2, and no. 16 were the primary contributors in FLPs. Pond no. 15 was distinct from the rest of FPs regarding LCBR replacement, representing the lowest beta diversity value. Moreover, ponds no. 4, no. 10, no. 1, and no. 11 played a predominant role in LCBD richness in fish ponds, whereas ponds no. 15, no. 3, and no. 16 were the major contributors in fishless ponds.
Total beta diversity (BD) in fish (F), fishless (FL) and all ponds (F + FL) with partitioning to replacement (Repl) and richness (Rich) (a). Contribution of replacement and richness to total beta diversity (b). Divergence in local contribution to beta diversity (LCBD) regarding replacement (c), and richness difference (d) between two pond types. Horizontal lines indicate the medians, boxes indicate the first and third quantiles, whiskers indicate range of non-outlier, and dots indicate real values. The numbers on charts c and d refer to the pond number (see Table 1)
The highest SCBD values (abundance-based) in FPs were calculated for A. fissa (14.2%), Keratella cochlearis (Gosse, 1851) (11.4%), and B. longirostris (4%) (Fig. 5a). In FLPs, the top contributors to the values of SCBD were A. fissa (17.3%), Scaridium longicaudum (Müller, 1786) (9.1%), Ceriodaphnia reticulata (Jurine, 1820) (6.1%), and Trichocerca weberi (Jennings, 1903) (4.3%) (Fig. 5b).
Frequency of occurrence
In all ponds (sample-based calculations), the following were the most frequently observed taxa: Bdelloidea Hudson, 1884 (67%), Polyarthra vulgaris Carlin, 1943 (58%), Simocephalus exspinosus (De Geer, 1778) (51%), C. sphaericus (47%), Lepadella ovalis (Müller, 1786) (46%), M. leuckarti (44%), A. fissa (44%), Scapholeberis mucronata (O.F. Müller, 1776) (41%) and Lecane closterocerca (Schmarda, 1859) (41%).
Among the species that were observed at a high frequency in one group of ponds (sample-based calculation) (> 20%), F. longiseta, K. cochlearis, Polyarthra remata Skorikov, 1896, T. pusilla, Sida crystallina (O.F. Müller, 1776), and Thermocyclops crassus (Fischer, 1853) were found exclusively in FPs (Table 3). Asplanchna priodonta Gosse, 1850, K. quadrata, P. vulgaris, B. longirostris, and S. mucronata showed significantly higher (p < 0.05) abundance in FPs, whereas Ceriodaphnia reticulata (Jurine, 1820), S. exspinosus, and Megacyclops viridis (Jurine, 1820) showed higher abundance (p < 0.05) in FLPs.
Species distinctiveness
Nine rotifers, two cladocerans, and seven copepod taxa were recorded only in FLPs (Table S4). Twenty eight rotifers, ten cladocerans, and four copepod taxa were found only in FPs.
Assemblages’ relationship to the environment
The results of CCA showed that all the variables accounted for 22.65% of the total variance in the zooplankton data (Table S2). The canonical axes were significant as tested by the unrestricted Monte Carlo permutation test (F = 2.6; p = 0.002). Seven variables were statistically significant (p < 0.05) (Table S4). The highest total variance was observed for macrophyte cover (5.5%) and the presence of fish (5.5%). Other significant factors were the concentration of dissolved oxygen (5.2%), depth (5.0%), the concentration of orthophosphates (4.0%), concentration of nitrates (4.0%), and surface area (3.6%). According to the ordination diagram (Fig. 6a), some of the taxa (A. priodonta, B. longirostris, F. longiseta, K. cochlearis, K. quadrata, P. remata, P. vulgaris, S. mucronata, S. crystallina, T. crassus, and T. pusilla) were associated with the presence of fish, and the highest values of depth, concentration of dissolved oxygen, and surface area, in contrast to macrophyte cover and concentration of orthophosphates which were favorable for other taxa (C. reticulata, S. exspinosus, M. viridis). The diagram also showed (Fig. 6b) the ordination of ponds according to the presence of fish and accompanying environmental factors. The highest species richness was observed in the presence of fish and the highest values of depth, concentration of dissolved oxygen, and surface area (Fig. 7).
Zooplankton composition in relation to environmental factors: CCA ordination diagram of taxa abundance (the diagram shows species with the highest frequency of occurrence and species for which the frequency in one pond group was at least 20%—see Table 3; analysis was based on a complete data set) with environmental variables (a) and ordination of ponds from 2016 to 2018 (b). The red and blue color indicates species and ponds related to the fish and fish presence, respectively. Taxa: Afi, A. fissa; Apr, A. priodonta; Bde, Bdelloidea; Blo, B. longirostris; Csp, C. sphaericus; Cre, C. reticulata; Flo, F. longiseta; Kco, K. cochlearis; Kte, K. tecta; Kqu, K. quadrata; Lcl, L. closterocerca; Lov, L. ovalis; Mvi, M. viridis; Mle, M. leuckarti; Pre, P. remata; Pvu, P. vulgaris; Smu, S. mucronata; Scr, S. crystallina; Sex, S. exspinosus; Tcr, T. crassus; Tpu, T. pusilla. Environmental variables: DEP, maximum depth (m); FP, fish presence; MAC, macrophyte cover (%); NO3, nitrates (mg/L); O2, concentration of dissolved oxygen (mg/L); PO4, orthophosphates (mg/L); SUR, pond surface area (ha).
Diagrams representing the number of taxa in zooplankton assemblages and ordination of environmental variables along the first two CCA. Summary of the fitted LOESS model: residual SE = 6.13, R2 [%] = 31.3. DEP, maximum depth (m); FP, fish presence; MAC, macrophyte cover (%); NO3, nitrates (mg/L); O2, concentration of dissolved oxygen (mg/L); PO4, orthophosphates (mg/L); SUR, pond surface area (ha)
Discussion
The composition of zooplankton assemblages
We observed zooplankton assemblage divergence in unstable FLPs compared to stable FPs. The use of various tools for grouping FLPs and FPs helped reveal details that would not have been possible to show with a single method. Although NMDS based on quantitative data completely separated the studied ponds, grouping based on presence–absence data and dendrogram clustering allowed the identification of one pond (no. 4) that did not fit the classification of ponds adopted by us. This is because pond no. 4 was constantly inhabited only by Carassius; hence, top-down regulation was not functional in this pond, thus developing zooplankton assemblages that were similar to those of FLPs. However, NMDS analysis was resistant to these qualitative differences between communities, resulting in a complete separation of the FPs from FLPs.
The lack of fish in a pond provides good conditions for the development of large microinvertebrates, especially pelagic crustaceans. In contrast, in fish ponds, small rotifers and fast-moving copepods have the best chance of survival, whereas the most threatened are large cladocerans living in open water (Meester et al., 1993; Vrba et al., 2023). Previous studies on the fish–zooplankton relationship in experimental ponds have revealed that a lack of fish leads to the domination of large Daphniidae, with Daphnia being prominent in ponds without macrophytes and Simocephalus being prominent in ponds with a high biomass of macrophytes (Irvine et al., 1989). In the present study, FLPs were abundantly overgrown with macrophytes; therefore, their zooplankton assemblages were dominated by Simocephalus, whereas Daphnia species (D. curvirostris Eylmann, 1887, D. longispina (O.F. Müller, 1776), and D. pulex Leydig, 1860) were rare. The presence of large cladocerans leads to the suppression of soft-shell rotifers (Gilbert, 1988); therefore, the type of water body (the presence or absence of fish) had a significant impact on trophic food webs and the composition of aquatic organisms (Brysiewicz et al., 2017). Thus, the presence of fish considerably affects the entire zooplankton community composition, contributing to the development of specific zooplankton assemblages.
Size is one of the essential traits of microinvertebrates necessary to explain the taxonomic differences between FPs and FLPs, whereas other traits are primarily related to features responsible for the survival of species under harsh environmental conditions. The largest zooplankton species can take over regulatory roles in ponds without pressure from fish. Studies on the predation efficiency of common copepod species in Central Europe have identified Megacyclops gigas (Claus, 1857) and M. viridis as exhibiting the highest predatory efficiency (Früh et al., 2019). Nevertheless, as per the aforementioned authors, intra-specific variation in the body size of M. viridis was not found to be correlated with predation efficiency. In general, FLPs are inhabited by large copepods such as M. viridis, and large cladocerans such as S. exspinosus, but also small cladocerans such as C. reticulata. These species were also found to be the most stable components of small temporal water bodies in southern Portugal (Caramujo & Boavida, 2010). M. viridis is commonly found in deeper layers of water bodies but can migrate to well-oxygenated areas under harsh conditions (Tinson & Laybourn-Parry, 1985). In addition, it can undergo diapause during summer and therefore can survive water shortages. In turn, the time of resting egg production and the physiological adaptation to survival in the fluctuating environment of small water bodies seem to be crucial for the survival of the cladocerans often found in FLPs. Large S. exspinosus and small C. reticulata occupy different ecological niches and therefore coexist in small water bodies.
A vast number of species were observed in FPs, and traits that are related to these species were also related to body size, life span, and visibility to predators. A total of 28 rotifer species were unique to FPs, primarily comprising small species commonly found in lakes in northwest Poland (Sługocki & Czerniawski, 2018). Among the ten unique cladoceran species in FPs, there were species associated with the littoral zone and pelagic species frequently found in lakes. Additionally, all four copepod species unique to fish ponds were littoral species (Błędzki & Rybak, 2016). Out of the nine unique rotifer species, two cladoceran species, and seven copepod species in FLPs, the majority were littoral species (Błędzki & Rybak, 2016). A clear increase in rotifer and small cladoceran diversity was observed in FPs, while copepod diversity increased in FLPs. This is because FLPs provide a favorable habitat for many copepod species, whereas FPs support small rotifers and cladoceran species.
Among frequently occurred species, F. longiseta, K. cochlearis, P. remata, and T. pusilla were observed only in FPs. These species have a short life span (Cieplinski et al., 2018) and feed mainly on detritus and bacteria (Bielańska-Grajner et al., 2015). These species were frequently observed in FPs, where the cycle of matter and resuspension from the bottom is accelerated by fish (Zheng et al., 2021). The same trend was observed for K. quadrata and P. vulgaris, which were observed more frequently and with a higher abundance in FPs. Similarly, B. longirostris was frequently observed in FPs. These rotifers and Bosmina are associated with open water, and large cladocerans that can survive the pressure of fish are associated with the littoral. The same trend was observed for filter-feeding Sida attached to plants (Fairchild, 1981) and neustonic S. mucronata. A. priodonta is a large-bodied omnivorous rotifer, but its transparency makes it invisible to large predators. It requires high dissolved oxygen concentrations (Bielańska-Grajner et al., 2015); therefore, it requires stable hydrological conditions as observed in the FPs.
It is difficult to find universal traits that are specific to certain water bodies; instead, a combination of multiple traits is observed. Small water bodies are affected by many strong antagonistic and synergic factors; therefore, reliable explanations and modeling of assemblages could be difficult. The changes observed could be also considered an indicator of different habits, supporting the existence of fish.
Biodiversity
This survey showed that small water bodies provide a large heterogeneity of habitats, essential for the diversity of microinvertebrates and the total biodiversity in the landscape. The crucial role of ponds in shaping the local and regional biodiversity of microinvertebrates has also been highlighted in recent studies (Pinel-Alloul & Mimouni, 2013; Kuczyńska-Kippen & Pronin, 2018; Ramos et al., 2021). For example, Mimouni et al. (2018) found 90 zooplankton taxa (60 rotifers, 24 cladocerans, and 6 copepods) in 19 urban water bodies (three fishless ponds and the others with diverse fish community) on the Island of Montréal (Canada) during summer. The present study also focused only on the summer season, but mid-field ponds provide seminatural habitat favoring biodiversity; hence, the number of species observed in mid-field ponds is actually higher than the species richness in urban ponds. Kuczyńska-Kippen & Pronin (2018) found 134 taxa (94 Rotifera, 24 Cladocera, and 16 Copepoda) in a year-round study of six mid-field ponds in central Poland. Year-round research allows for the observation of cold-water species we did not observe in this study.
With respect to species richness and sample-based data, no significant differences were observed, but over the whole study period a larger number of taxa were accumulated in FPs. Kuczyńska-Kippen & Pronin (2018) obtained contrasting results and observed a higher species diversity in FLPs than in FPs. They attributed these results to the domination of macrophytes in FLPs, which support a high diversity of microinvertebrates. Studies from different climatic zones have also identified macrophytes as a factor that increases habitat complexity and enhances predator avoidance for microinvertebrates, ultimately contributing to higher species richness (Meerhoff et al., 2007; Dos Santos et al., 2020; Quirino et al., 2021). The results of rarefaction and extrapolation in present study suggested that the higher number of species in FPs is not only affected by the number of samples in the groups studied, but also is instead a general trend. This could be explained by the larger environment and the higher complexity of food webs (MacArthur, 1965; Briand & Cohen, 1984; Jordan, 2009; Fahrig, 2013). Lower species richness in FLPs could be related to the type of habitat. Most of the FLPs were sporadically dry (not in the same year) during summer. This probably leads to the disappearance of fish fauna and some zooplankton taxa. However, recent studies also indicate significant variability in invertebrate communities in a fluctuating environment (Ruhí et al., 2017), suggesting that, within the scope of just 3 years of research, we may not have captured the full range of variability among communities.
The beta indexes and biodiversity indexes (Shannon and Simpson) showed that both pond types had a similar structure of zooplankton assemblages. This proves that none of the pond types was characterized by the dominance of single species, which could be a sign of the ponds’ poor ecological condition. Therefore, despite the qualitatively different types of ponds, where pelagic species predominate in FPs and macrophyte-related species predominate in FLPs, they seem to be similar in terms of ecosystem quality.
Slight differences in beta diversity values in FPs and FLPs most likely result from the relatively small research area and the homogeneous land use within the watershed. In such conditions, we expected a greater contribution of replacement to shaping beta diversity, as a small area can accumulate a limited number of individuals (Fahrig, 2013). Under these circumstances, the decisive factor influencing invertebrate composition is the local environmental conditions, which undergo natural fluctuations over time. We speculate that, with the consideration of a larger spatial unit (continental scale), richness will play a more significant role in shaping beta diversity.
The replacement and richness indicators of beta diversity allowed for the identification of distinct ponds within the studied groups of FPs and FLPs. This applies to pond no. 4 among FPs and pond no. 15 among FLPs. Both ponds had the highest contribution to shaping LCBD richness, while they had a minimal impact on LCBD replacement. The distinctiveness of pond no. 4 has been discussed earlier, on the other hand, pond no. 15, despite the fact that it was fishless and very shallow, we found water in it every time during the three survey years. Thus, their dissimilarity in the studied groups is due to local environmental conditions. Beta diversity partitioning allowed for a more in-depth examination of differences between pond groups, highlighting the utility of this method. Biodiversity data and spatial information, particularly beta diversity, are vital for comprehending the processes shaping assemblages (Whittaker, 1960; Legendre & De Cáceres, 2013). These metrics offer insights into components of biodiversity, facilitating the identification of ecological mechanisms influencing zooplankton communities (Napiórkowski et al., 2019; Brito et al., 2020). Pieńkowski (2000) reported that 59% of the mid-field ponds of the Pleistocene landscape of Pomerania (Poland) disappeared completely within one century (1888–1980). Hydrological changes due to human activities and climate change pose a great threat to this type of water body (Dinka, 2022; Querner et al., 2022), which has a substantial effect on the regional biodiversity of microinvertebrates and the functioning of ecosystems of small water bodies.
Indicative properties
The presence of fish was related to the frequent occurrence of species considered trophic indicators (Ejsmont-Karabin, 2012; Ejsmont-Karabin & Karabin, 2013) in the temperate waters of Europe. Species that are considered trophic indicators (Ascomorpha ovalis, Brachionus angularis, B. calyciflorus, Filinia longiseta, Keratella cochlearis, Trichocerca pusilla, and Diaphanosoma brachyurum) were present only in FPs. Those species were absent in the case of fishless ponds, despite the fact that these ponds were eutrophic as indicated by the presence of macrophytes and high orthophosphate values. Therefore, we suggest caution when using commonly used metrics in relation to ponds that do not have fish as a top predators and/or are ephemeral. Anuraeopsis fissa, Chydorus sphaericus, Keratella tecta and Mesocyclops leuckarti are among the indicative species that occurred with high frequency in both pond types. The size of specimens is another promising indicator of ecological status (Karpowicz et al., 2020b) that could be applied in frequently occurring taxa. It seems that Daphnia or Simocephalus are less suitable as indicators of ecological status in fish ponds because their size in mainly affected by top-up forces. In turn, in ponds inhabited by fish, zooplankton species that are subject to less pronounced fish pressure should be used.
Conclusions
The stability of the ecosystem was more important for maintaining the high species richness of zooplankton. Diversity indices were not influenced by variations in species composition or environmental differences among ponds. The presence of fish in stable mid-field ponds influences zooplankton composition. The distinctiveness of habitats promotes the occurrence of species characterized by certain traits. In general, FLPs support large-bodied crustaceans and species that can withstand harsh environmental conditions, whereas FPs support small species and large species associated with macrophytes, but also transparent species. This is attributable to a combination of habitat characteristics and the top-up regulation by fish. Due to their larger size and more complex food webs, FPs are characterized by a higher species richness of microinvertebrates. Nevertheless, diversified pond types are necessary to maintain the heterogeneity of mid-field ponds, which supports the high regional biodiversity of zooplankton assemblages. The absence of fish in unstable ponds impacted the indicative properties of zooplankton assemblages commonly used in lake ecosystem assessments. Therefore, assessments of ecosystem functioning in fishless ponds should rely on distinct models and species.
Data availability
Data are available from the authors upon reasonable request.
References
Bielańska-Grajner, I., J. Ejsmont-Karabin, & S. Radwan, 2015. Rotifers: Rotifera Monogononta. Łódź University Press.
Błędzki, L. A. & J. I. Rybak, 2016. Freshwater Crustacean Zooplankton of Europe: Cladocera & Copepoda (Calanoida, Cyclopoida) Key to species identification, with notes on ecology, distribution, methods and introduction to data analysis, Springer:
Brendonck, L., T. Pinceel & R. Ortells, 2017. Dormancy and dispersal as mediators of zooplankton population and community dynamics along a hydrological disturbance gradient in inland temporary pools. Hydrobiologia 796: 201–222. https://doi.org/10.1007/s10750-016-3006-1.
Briand, F. & J. E. Cohen, 1984. Community food webs have scale-invariant structure. Nature 307: 264–267. https://doi.org/10.1038/307264a0.
Brito, M. T. D. S., J. Heino, U. M. Pozzobom & V. L. Landeiro, 2020. Ecological uniqueness and species richness of zooplankton in subtropical floodplain lakes. Aquatic Sciences 82(2): 43. https://doi.org/10.1007/s00027-020-0715-3.
Brooks, J. L. & S. I. Dodson, 1965. Predation, body size, and composition of Plankton: The effect of a marine planktivore on lake plankton illustrates theory of size, competition, and predation. Science 150(3692): 28–35. https://doi.org/10.1126/science.150.3692.28.
Brysiewicz, A., Ł Sługocki, P. Wesołowski & R. Czerniawski, 2017. Zooplankton community structure in small ponds in relation to fish community and environmental factors. Applied Ecology and Environmental Research 15: 929–941. https://doi.org/10.15666/aeer/1504_929941.
Buczyńska, A., J. Blachowski & N. Bugajska-Jędraszek, 2023. Analysis of post-mining vegetation development using remote sensing and spatial regression approach: A Case study of former Babina Mine (Western Poland). Remote Sensing 15(3): 719. https://doi.org/10.3390/rs15030719.
Caramujo, M. J. & M. J. Boavida, 2010. Biological diversity of copepods and cladocerans in Mediterranean temporary ponds under periods of contrasting rainfall. Journal of Limnology 69: 64. https://doi.org/10.3274/JL10-69-1-06.
Céréghino, R., J. Biggs, B. Oertli & S. Declerck, 2008. The ecology of European ponds: defining the characteristics of a neglected freshwater habitat. Hydrobiologia 597: 1–6. https://doi.org/10.1007/978-90-481-9088-1_1.
Chao, A., R. L. Chazdon, R. K. Colwell & T.-J. Shen, 2005. A new statistical approach for assessing compositional similarity based on incidence and abundance data. Ecology Letters 8: 148–159. https://doi.org/10.1111/j.1461-0248.2004.00707.x.
Cieplinski, A., U. Obertegger & T. Weisse, 2018. Life history traits and demographic parameters in the Keratella cochlearis (Rotifera, Monogononta) species complex. Hydrobiologia 811: 325–338. https://doi.org/10.1007/s10750-017-3499-2.
Cobbaert, D., S. E. Bayley & J. L. Greter, 2010. Effects of a top invertebrate predator (Dytiscus alaskanus; Coleoptera: Dytiscidae) on fishless pond ecosystems. Hydrobiologia 644: 103–114. https://doi.org/10.1007/s10750-010-0100-7.
Collinson, N. H., J. Biggs, A. Corfield, M. J. Hodson, D. Walker, M. Whitfield & P. J. Williams, 1995. Temporary and permanent ponds: an assessment of the effects of drying out on the conservation value of aquatic macroinvertebrate communities. Biological Conservation 74(2): 125–133. https://doi.org/10.1016/0006-3207(95)00021-U.
Colwell, R. K., A. Chao, N. J. Gotelli, S.-Y. Lin, C. X. Mao, R. L. Chazdon & J. T. Longino, 2012. Models and estimators linking individual-based and sample-based rarefaction, extrapolation, and comparison of assemblages. Journal of Plant Ecology 5: 3–21. https://doi.org/10.1093/jpe/rtr044.
De Meester, L. D., S. Maas, K. Dierckens & H. J. Dumont, 1993. Habitat selection and patchiness in Scapholeberis: horizontal distribution and migration of S. mucronata in a small pond. Journal of Plankton Research 15: 1129–1139. https://doi.org/10.1093/plankt/15.10.1129.
Dembowska, E. A., P. Napiórkowski, T. Mieszczankin & S. Józefowicz, 2015. Planktonic indices in the evaluation of the ecological status and the trophic state of the longest lake in Poland. Ecological Indicators 56: 15–22. https://doi.org/10.1016/j.ecolind.2015.03.019.
Diaz, S., M. Cabido & F. Casanoves, 1998. Plant functional traits and environmental filters at a regional scale. Journal of Vegetation Science 9(1): 113–122. https://doi.org/10.2307/3237229.
Dinka, M. O., 2022. Statistical analysis of groundwater quality at Wonji Shoa Sugar Estate. Journal of Water and Land Development 53: 45–50. https://doi.org/10.24425/jwld.2022.140778.
Dos Santos, N. G., L. R. Stephan, A. Otero, C. Iglesias & M. S. M. Castilho-Noll, 2020. How free-floating macrophytes influence interactions between planktivorous fish and zooplankton in tropical environments? An in-lake mesocosm approach. Hydrobiologia 847: 1357–1370. https://doi.org/10.1007/s10750-020-04194-1.
Dray S., D. Bauman, G. Blanchet, D. Borcard, S. Clappe, G. Guénard, T. Jombart, G. Larocque, P. Legendre, N. Madi, H.H. Wagner, 2023. adespatial: Multivariate Multiscale Spatial Analysis. R package version 0.3–23. https://CRAN.R-project.org/package=adespatial
Ejsmont-Karabin, J., 2012. The usefulness of zooplankton as lake ecosystem indicators: rotifer trophic state index. Polish Journal of Ecology 60: 339–350.
Ejsmont-Karabin, J. & A. Karabin, 2013. The suitability of zooplankton as lake ecosystem indicators: Crustacean trophic state index. Polish Journal of Ecology 61: 561–573.
Fahrig, L., 2013. Rethinking patch size and isolation effects: the habitat amount hypothesis. Journal of Biogeography 40(9): 1649–1663. https://doi.org/10.1111/jbi.12130.
Fairchild, G. W., 1981. Movement and microdistribution of Sida crystallina and other littoral microcrustacea. Ecology 62: 1341–1352. https://doi.org/10.2307/1937297.
Florencio, M., C. Díaz-Paniagua & L. Serrano, 2016. Relationships between hydroperiod length, and seasonal and spatial patterns of beta-diversity of the microcrustacean assemblages in Mediterranean ponds. Hydrobiologia 774: 109–121. https://doi.org/10.1007/s10750-015-2515-7.
Florencio, M., R. Fernández-Zamudio, M. Lozano & C. Díaz-Paniagua, 2020. Interannual variation in filling season affects zooplankton diversity in Mediterranean temporary ponds. Hydrobiologia 847: 1195–1205. https://doi.org/10.1007/s10750-019-04163-3.
Franch-Gras, L., E. M. Garcia-Roger, M. Serra & M. Jose Carmona, 2017. Adaptation in response to environmental unpredictability. Proceedings of the Royal Society b: Biological Sciences 284(1868): 20170427. https://doi.org/10.1098/rspb.2017.0427.
Früh, L., H. Kampen, G. A. Schaub & D. Werner, 2019. Predation on the invasive mosquito Aedes japonicus (Diptera: Culicidae) by native copepod species in Germany. Journal of Vector Ecology 44(2): 241–247. https://doi.org/10.1111/jvec.12355.
Gilbert, J. J., 1988. Suppression of rotifer populations by Daphnia: a review of the evidence, the mechanisms, and the effects on zooplankton community structure. Limnology and Oceanography 33: 1286–1303. https://doi.org/10.4319/lo.1988.33.6.1286.
Gutkowska, A., E. Paturej & E. Kowalska, 2013. Rotifer trophic state indices as ecosystem indicators in brackish coastal waters. Oceanologia 55: 887–899. https://doi.org/10.5697/oc.55-4.887.
Haberman, J. & M. Haldna, 2014. Indices of zooplankton community as valuable tools in assessing the trophic state and water quality of eutrophic lakes: long term study of Lake Võrtsjärv. Journal of Limnology 73: 263–273. https://doi.org/10.4081/jlimnol.2014.828.
Irvine, K., B. Moss & H. Balls, 1989. The loss of submerged plants with eutrophication II. Relationships between fish and zooplankton in a set of experimental ponds, and conclusions. Freshwater Biology 22: 89–107. https://doi.org/10.1111/j.1365-2427.1989.tb01086.x.
Jeppesen, E., P. Noges, T. A. Davidson, J. Haberman, T. Noges, K. Blank & S. L. Amsinck, 2011. Zooplankton as indicators in lakes: a scientific-based plea for including zooplankton in the ecological quality assessment of lakes according to the European Water Framework Directive (WFD). Hydrobiologia 676: 279–297. https://doi.org/10.1007/s10750-011-0831-0.
Jordan, F., 2009. Keystone species and food webs. Philosophical Transactions of the Royal Society B: Biological Sciences 364(1524): 1733–1741. https://www.jstor.org/stable/40485950
Karpowicz, M., J. Ejsmont-Karabin, J. Kozłowska, I. Feniova & A. R. Dzialowski, 2020a. Zooplankton community responses to oxygen stress. Water 12(3): 706. https://doi.org/10.3390/w12030706.
Karpowicz, M., Ł Sługocki, J. Kozłowska, A. Ochocka & C. López, 2020b. Body size of Daphnia cucullata as an indicator of the ecological status of temperate lakes. Ecological Indicators 117: 106585. https://doi.org/10.1016/j.ecolind.2020.106585.
Keddy, P. A. 1992. Assembly and response rules: two goals for predictive community ecology. Journal of Vegetation Science 3(2): 157–164. https://doi.org/10.2307/3235676.
Krupa, E., S. Barinova, S. Romanova, M. Aubakirova & N. Ainabaeva, 2020. Planktonic invertebrates in the assessment of long-term change in water quality of the sorbulak wastewater disposal system (Kazakhstan). Water 12: 3409. https://doi.org/10.3390/w12123409.
Kuczyńska-Kippen, N., 2014. Environmental variables of small mid-field water bodies and the presence of Rotifera groups of different ecological requirements. Polish Journal of Environmental Studies 23: 373–378.
Kuczyńska-Kippen, N., 2018. The use of bdelloids in reference to rotifer biocoenotic indices as an indicator of the ecological state of small field water bodies: The effect of macrophytes, shading and trophic state of water. Ecological Indicators 89: 576–583. https://doi.org/10.1016/j.ecolind.2018.02.046.
Kuczyńska-Kippen, N. & T. Joniak, 2010. The impact of water chemistry on zooplankton occurrence in two types (field versus forest) of small water bodies. International Review of Hydrobiology 95: 130–141. https://doi.org/10.1002/iroh.200911166.
Kuczyńska-Kippen, N. & M. Pronin, 2018. Diversity and zooplankton species associated with certain hydroperiods and fish state in field ponds. Ecological Indicators 90: 171–178. https://doi.org/10.1016/j.ecolind.2018.03.016.
Kuczyńska-Kippen, N., M. Špoljar, M. Mleczek & C. Zhang, 2021. Elodeids, but not helophytes, increase community diversity and reduce trophic state: Case study with rotifer indices in field ponds. Ecological Indicators 128: 107829. https://doi.org/10.1016/j.ecolind.2021.107829.
Lampert, W. & U. Sommer, 2007. Limnoecology: the ecology of lakes and streams, Oxford University Press:
Legendre, P. & M. De Cáceres, 2013. Beta diversity as the variance of community data: dissimilarity coefficients and partitioning. Ecology Letters 16(8): 951–963. https://doi.org/10.1111/ele.12141.
MacArthur, R. H., 1965. Patterns of species diversity. Biological Reviews 40: 510–533. https://doi.org/10.1111/j.1469-185X.1965.tb00815.x.
Magurran, A. E., 2004. Measuring biological diversity, Blackwell:
Meerhoff, M., C. Iglesias, F. T. De Mello, J. M. Clemente, E. Jensen, T. L. Lauridsen & E. Jeppesen, 2007. Effects of habitat complexity on community structure and predator avoidance behaviour of littoral zooplankton in temperate versus subtropical shallow lakes. Freshwater Biology 52(6): 1009–1021. https://doi.org/10.1111/j.1365-2427.2007.01748.x.
Mimouni, E. A., B. Pinel-Alloul, B. E. Beisner & P. Legendre, 2018. Summer assessment of zooplankton biodiversity and environmental control in urban waterbodies on the Island of Montréal. Ecosphere 9: e02277. https://doi.org/10.1002/ecs2.2277.
Mirabdullayev IM, Reid JW, & Ueda H (2003) Genus Thermocyclops Kiefer, 1927. In Ueda, H, & J. W. Reid (eds), Copepoda: Cyclopoida. Genera Mesocyclops and Thermocyclops: 213–302. (Backhuys Publishers, Leiden).
Napiórkowski, P., M. Bąkowska, N. Mrozińska, M. Szymańska, N. Kolarova & K. Obolewski, 2019. The effect of hydrological connectivity on the zooplankton structure in floodplain lakes of a regulated large river (the Lower Vistula, Poland). Water 11(9): 1924. https://doi.org/10.3390/w11091924.
Nogrady, T. & H. Segers, 2002. Rotifera 6: Asplanchnidae, Gastropodidae, Lindiidae, Microcodidae, Synchaetidae, Trochosphaeridae and Filinia. Guides to the Identification of the Microinvertebrates of the Continental Waters of the World 18. Backhuys.
Ochocka, A. & A. Pasztaleniec, 2016. Sensitivity of plankton indices to lake trophic conditions. Environmental Monitoring and Assessment 188: 1–16. https://doi.org/10.1007/s10661-016-5634-3.
Pieńkowski, P., 2000. Disappearance of ponds in the younger pleistocene landscapes of Pomerania. Journal of Water and Land Development 4: 55–68.
Pinel-Alloul, B. & E. A. Mimouni, 2013. Are cladoceran diversity and community structure linked to spatial heterogeneity in urban landscapes and pond environments? Hydrobiologia 715: 195–212. https://doi.org/10.1007/s10750-013-1484-y.
QGIS Development Team, 2021. QGIS Geographic Information System. Open Source Geospatial Foundation. http://qgis.org
Querner, E., J. den Besten, R. van Veen & H. Jager, 2022. A scenario analysis of climate change and adaptation measures to inform Dutch policy in The Netherlands. Journal of Water and Land Development 54: 177–183. https://doi.org/10.24425/jwld.2022.141570.
Quirino, B. A., F. Teixeira de Mello, S. Deosti, C. C. Bonecker, A. L. P. Cardozo, K. Y. Yofukuji & R. Fugi, 2021. Interactions between a planktivorous fish and planktonic microcrustaceans mediated by the biomass of aquatic macrophytes. Journal of Plankton Research 43(1): 46–60. https://doi.org/10.1093/plankt/fbaa061.
R Core Team, 2023. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Ramos, E. D. A., A. T. Ramos Okumura, A. G. Silva, T. L. Pereira & N. R. Simões, 2021. Alpha and beta diversity of planktonic microcrustaceans are associated with environmental heterogeneity in the Frades River Basin Brazil. Studies on Neotropical Fauna and Environment. https://doi.org/10.1080/01650521.2021.1933702.
Rea, T. E., D. J. Karapatakis, K. K. Guy, J. E. Pinder III. & H. E. Mackey Jr., 1998. The relative effects of water depth, fetch and other physical factors on the development of macrophytes in a small southeastern US pond. Aquatic Botany 61(4): 289–299. https://doi.org/10.1016/S0304-3770(98)00069-2.
Richardson, D. C., M. A. Holgerson, M. J. Farragher, K. K. Hoffman, K. King, M. B. Alfonso & J. N. Sweetman, 2022. A functional definition to distinguish ponds from lakes and wetlands. Scientific Reports 12: 1–13. https://doi.org/10.1038/s41598-022-14569-0.
Ruhí, A., T. Datry & J. L. Sabo, 2017. Interpreting beta-diversity components over time to conserve metacommunities in highly dynamic ecosystems. Conservation Biology 31(6): 1459–1468. https://doi.org/10.1111/cobi.12906.
Scheffer, M., G. J. Van Geest, K. Zimmer, E. Jeppesen, M. Søndergaard, M. G. Butler & L. De Meester, 2006. Small habitat size and isolation can promote species richness: Second-order effects on biodiversity in shallow lakes and ponds. Oikos 112: 227–231. https://doi.org/10.1111/j.0030-1299.2006.14145.x.
Sługocki, Ł., & R. Czerniawski, 2018. Trophic state (TSISD) and mixing type significantly influence pelagic zooplankton biodiversity in temperate lakes (NW Poland). PeerJ 6: e5731. https://doi.org/10.7717/peerj.5731.
Soranno, P. A., K. S. Cheruvelil, T. Wagner, K. E. Webster & M. T. Bremigan, 2015. Effects of land use on lake nutrients: The importance of scale, hydrologic connectivity, and region. PloS One 10: e0135454. https://doi.org/10.1371/journal.pone.0135454.
ter Braak, C. J. F. & P. Šmilauer, 2012. Canoco reference manual and user's guide: software for ordination (version 5.0). Ithaca, New York, Microcomputer Power.
Tinson, S. & J. Laybourn-Parry, 1985. The behavioural responses and tolerance of freshwater benthic cyclopoid copepods to hypoxia and anoxia. Hydrobiologia 127: 257–263. https://doi.org/10.1007/BF00024230.
Vrba, J., M. Šorf, J. Nedoma, Z. Benedová, L. Kröpfelová, J. Šulcová, B. Tesařová, M. Musil, L. Pechar, J. Potužák & J. Regenda, 2023. Top-down and bottom-up control of plankton structure and dynamics in hypertrophic fishponds. Hydrobiologia. https://doi.org/10.1007/s10750-023-05312-5.
Wellborn, G. A., D. K. Skelly & E. E. Werner, 1996. Mechanisms creating community structure across a freshwater habitat gradient. Annual Review of Ecology and Systematics 27(1): 337–363. https://doi.org/10.1146/annurev.ecolsys.27.1.337.
Whittaker, R. H., 1960. Vegetation of the Siskiyou mountains, Oregon and California. Ecological Monographs 30: 279–338. https://doi.org/10.2307/1943563.
Zheng, X., K. Zhang, T. Yang, Z. He, L. Shu, F. Xiao & Q. Yan, 2021. Sediment resuspension drives protist metacommunity structure and assembly in grass carp (Ctenopharyngodon idella) aquaculture ponds. Science of the Total Environment 764: 142840. https://doi.org/10.1016/j.scitotenv.2020.142840.
Zokan, M. & J. M. Drake, 2015. The effect of hydroperiod and predation on the diversity of temporary pond zooplankton communities. Ecology and Evolution 5: 3066–3074. https://doi.org/10.1002/ece3.1593.
Acknowledgements
We are grateful for the comments provided by the editors and anonymous reviewers, which significantly enhanced the quality of this manuscript.
Funding
This work was co-financed by the Institute of Technology and Life Sciences—National Research Institute and by the Minister of Science under the “Regional Excellence Initiative” program for 2024–2027.
Author information
Authors and Affiliations
Contributions
Conceptualization was done by ŁS and AB; methods were developed by ŁS and AB; the research was conducted by ŁS and AB; data analysis was done by ŁS; data interpretation was done by ŁS; figures and tables were prepared by ŁS; writing was done by ŁS.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling Editor: Margarita Patricia Florencio Díaz
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sługocki, Ł., Brysiewicz, A. Divergence of zooplankton assemblages in unstable fishless and stable fish ponds. Hydrobiologia (2024). https://doi.org/10.1007/s10750-024-05544-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10750-024-05544-z