Abstract
A near-unique pair of adjacent bays in the karst region of western Ireland provided the opportunity to compare phytoplankton diversity and abundance in an area of submarine groundwater discharge (SGD) side by side with a ‘control’ region of similar size and with the same marine influences. Freshwater input to Kinvara Bay comes from the surrounding karst hinterland via a subterranean groundwater network, emerging at several sites within the inner bay as submarine groundwater discharge (SGD). Aughinish Bay in contrast has no connection to a SGD network and negligible overground fresh water input. Sampling was carried out in both bays during spring and neap tides over Winter, Spring/Summer and Summer/Autumn of 2016 and 2017. Comparison of the phytoplankton communities in the two bays showed higher diversity in Aughinish, while a bloom of Prorocentrum micans (Ehrenber, 1834), a brackish-water-tolerant marine dinoflagellate, was observed only in Kinvara. This work demonstrates how SGD can control phytoplankton community structure even in well-flushed macro-tidal bays and thus controls the biogeography at local scales.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Marine and brackish water phytoplankton provide a wide range of ecosystem services as well as having some negative impacts for humans where toxic blooms occur (Naselli-Flores and Padisák, 2022), so understanding the controls on their distribution at local scales is important. Phytoplankton assemblages are complex with intricate responses controlled by the surrounding environment, thus changes in community structure can act as a bio-indicator of the overall health status of coastal bays and estuaries (Livingston, 2002). Changes in phytoplankton community structure and asymmetric shifts in dominant species in coastal systems happen rapidly. These changes are caused by complex responses to variations in controlling factors such as salinity and nutrients, which in turn will be controlled by the hydrological status of the area (Garcés et al., 2011).
Submarine groundwater discharge (SGD) has long been shown as an important pathway to coastal waters for anthropogenically derived nutrients (Fleury et al., 2007; Moore, 2010; Liu et al., 2018), trace metals and other contaminants (Burnett et al., 2003; Slomp and Van Cappellen, 2004; Einsiedl, 2012; Knee and Paytan, 2012; Rodellas et al., 2014). More recently, it has also been shown as an important driver of carbonate chemistry in coastal waters (McGrath et al., 2019a).
SGD therefore plays a vitally important role in the transport and supply of inorganic nutrients and other constituents that control coastal phytoplankton dynamics (Fig. 1). SGD can be a key supply route of nutrients to otherwise depleted waters in summer (Paerl, 1997). Even moderate nutrient loadings (~ 25 µmol NO3, 16 µmol PO4, Van Meerssche and Pinckney, 2019) not only change the biomass but also the phytoplankton species composition. Periodic elevated levels of inorganic nutrients may lead to eutrophic conditions (Statham, 2012) and overall anoxia. These, when coupled with other climatic influences like irradiance and warmer temperatures, have been known to facilitate harmful algal bloom events (HABs, Gobler, 2020) that can have negative health and socio-economic effects. There are some positive effects from moderate nutrient enrichment, as this can support phytoplankton communities year round, helping sustain local shellfish aquaculture.

Adapted from Lecher and Mackey (2018). Dashed arrow indicates progression from initial uptake of dissolved inorganic nutrients (DIN) by diatoms through use of excreted products (DON- dissolved organic nitrogen and DOP—dissolved organic phosphate) by dinoflagellates followed by their grazing by cryptophytes. Curved arrows indicate mixing of SGD and coastal water
Pathways and effects of nutrient loading via SGD.
Temperate macro-tidal regions have highly variable weather and tidal conditions, making it difficult to replicate real-world conditions in laboratory-based experiments. Gregory et al., (2020) showed that the retention of inorganic nutrients supplied by submarine groundwater discharge over a neap tidal cycle has the potential to stimulate phytoplankton bloom conditions, including HABs. This enhanced nutrient availability would also be available to the more opportunistic phytoplankton species causing asymmetric community changes. The study by Garcés et al., (2011) on the effects of SGD enrichment on local phytoplankton communities from the Mediterranean Sea area under controlled conditions, observed a shift in community structure favouring the more opportunistic species.
In karst areas, phosphate appears to be attenuated on its pathway to the sea (Slomp and Van Cappellen, 2004; Pain et al., 2020), while nitrates and silicates are more efficiently transmitted, though some of the nitrate may be generated from remineralisation of organic matter. Much of the coloured dissolved organic carbon (CDOM) component is also likely to be transmitted efficiently since it is largely broken down by ultra-violet light on entry into the estuarine system (e.g., Kieber et al., 1990), and this is lacking in the conduit network. CDOM from SGD therefore potentially provides a slow-release source of nutrients to coastal waters, especially in temperate zones in summer when there are long hours of daylight and UV radiation is strongest. CDOM is a known and accepted indicator of fresh water input to coastal waters (e.g., Maie et al., 2006; Kratzer & Tett, 2009), and can influence primary production and bacterial activity through light limitation (e.g., Gameiro et al., 2011).
Here, we present data from two adjacent bays, Kinvara Bay (also known as Kinvara) and Aughinish Bay, Ireland, separated by a narrow isthmus only 1 km wide at its narrowest point, and whose mouths are within 8 km of each other. Many of their external controlling variables such as climate and weather conditions, tidal regime, seawater input from greater Galway Bay, underlying geology, surrounding hinterland are identical, and they have similar depths and surface areas. Both bays experience some limited urbanization stresses but the main difference is that one bay (Kinvara) has significant and well-documented SGD input (e.g., Cave & Henry, 2011; Einsiedl, 2012; Rocha et al., 2015), while the other bay (Aughinish) lacks any such source of fresh water input (Smith & Cave, 2012). Very seldom are scientists presented with such a unique study area, where a natural control area exists in such close proximity to a ‘test’ area.
This provided an opportunity to test two hypotheses: whether SGD input leads to significant differences in phytoplankton diversity and abundance in adjacent macro-tidal coastal embayments and whether SGD inorganic nutrient enrichment can drive periodic blooms of HAB species.
Methods
Site description
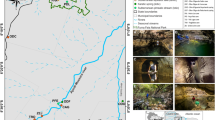
Kinvara and Aughinish bays are adjacent shallow semi-enclosed bays in the south-west corner of Galway Bay on the west coast of Ireland (Fig. 2). They are of similar size—Kinvara surface area 5.19 km2, average mean high water (MHW) depth of 4.2 m, Aughinish surface area 4.82 km2, average MHW depth of 4.1 m (Smith & Cave, 2012). They have strong tidal flows, slightly greater in Aughinish due to the narrower mouth of this bay. Kinvara bay has no riverine input but has a strong submarine groundwater input (SGD) in the inner bay whose discharge varies with precipitation in the catchment but is never absent. Aughinish in contrast has negligible freshwater inputs other than overland drainage during heavy rainfall. Flow paths for groundwater in the catchment have been demonstrated by dye tracing (Drew and Daly 1993; Boycott et al., 2003). There is some evidence that there may be a groundwater conduit feeding off the Kinvara system into Aughinish that becomes active during periods of heavy flooding such as occurred in autumn 2009 (Smith and Cave, 2012) but this is not the case in years of normal rainfall. Anthropogenic loading comes from agriculture in the Kinvara catchment (Smith & Cave, 2012) and SGD is driven by hydrostatic head pressure and controlled by precipitation-filled turlough levels (Gill et al., 2013).
Map showing the location of Kinvara Bay and Aughinish Bay in the southeast corner of Galway Bay, mid-west Ireland. Tidal sampling stations were at New Quay pier (NQ), Aughinish Bay and at Parkmore Pier (PP) in Kinvara Bay. The mooring deployment in Kinvara Bay was at location ‘X’, adjacent to boat station 4, and boat stations 1–5 were sampled during a Prorocentrum bloom (see text). The black star in inner Kinvara Bay shows the submarine groundwater discharge point, beside the local landmark of Dunguaire Castle
Both bays are designated shellfish waters of economic importance under EU Shellfish Waters Directive (2006/113/EC) and Irish Statutory Instrument (SI) No 268 of 2006. Kinvara falls within the Clarinbridge/Kinvara Bay Shellfish Area, with a total catchment of 1030 km2 (though part of this relates to the Clarin and Kilcolgan rivers to the north of Kinvara Bay, not included in Fig. 2), while Aughinish has a much smaller catchment of 170 km2. Blue mussel (Mytilus edulis L.), native oyster (Ostrea edulis L.) and Pacific oyster (Crassostrea gigas Thunberg) are grown and harvested here. Detailed reports on the catchment and shellfish waters can be found on https://www.housing.gov.ie/water/water-quality/shellfish-waters/galway. Both bays are within the Inner Galway Bay Special Protection Area (SPA).
Cave and Henry (2011) estimated a timeline of approximately three days from the onset of a rainfall event in the catchment to its influx as SGD at Kinvara, indicating a rapid pathway for water borne contaminants from land source to estuarine sink (Fig. 1). If the rate of transmission of nutrients is the same as that of water, then there is little time for attenuation or transformation within the submarine ‘estuary’ (Moore, 1999).
Tidal time series
Tidal sampling was carried out in 2016 and 2017 (Table 1) from piers at the mouth of both bays, at the narrowest point where the semi-enclosed bays meet the greater Galway Bay (Fig. 2). Boat surveys have shown that due to the topography and high rates of water movement in the mouth area, the water column is well mixed vertically (Cave & Henry, 2011). Thus, samples taken from piers near the mouths of the bays are fully representative of the water entering or leaving the bays. Ebb tide samples give a good representation of the submarine ground water effects on water parameters while also transporting phytoplankton from the inner bay to the outer bay. The effects of this tidal elution are more evident on the larger tidal fluctuations at spring tides. On a flooding tide, the samples collected will initially be a representation of a mix between the ebbing inner bay water and the flooding Galway Bay waters. As the tide approaches the highest point it is expected to represent mostly Galway Bay water and its phytoplankton communities. This provides samples that differ in temperature, salinity, nutrients and phytoplankton communities to that of the inner bay and thus allows us to distinguish between the ‘resident’ estuarine phytoplankton communities and those from the greater Galway bay waters.
A WTW Condi 197i T/S sensor was deployed logging at 5-min intervals for each sampling period. Water samples were collected from both piers using a 5L plastic bucket attached to a nylon rope, as initial attempts using a 5 l Niskin water sampling bottle were unsuccessful. Samples were collected from a depth of approximately 0.3 m below the surface. Care was taken when recovering the bucket to ensure no sample contamination occurred through collision of the bucket with the pier wall. Both sites were sampled with exactly the same procedures and both piers had similar heights above the water.
Half-hourly water samples were collected over full tidal cycles (13 h) for nutrients and chlorophyll-a, with hourly samples for phytoplankton, over both spring and neap tides for each bay. Samples were collected seasonally during March, June and August, representing late winter and early and late summer (Table 1). Winter samples allowed a distinction of the background parameters where nutrients tend to be more abundant and phytoplankton growth is at a minimum, while early and late summer samples captured the growing seasons of phytoplankton.
Transect sampling
Highly discoloured reddish/brown and turbid waters were noted during a drifter survey (see Gregory et al., 2020) carried out in Kinvara bay on 10th August 2017, Suspecting a phytoplankton bloom, opportunistic phytoplankton samples were collected from five stations by boat (1 to 5, see Fig. 2) at high tide and the following low tide. On the flood tide, approaching high tide, the samples were collected from the inner bay to the outer bay, while on the ebb approaching low tide, the transect was run from outer bay to inner. Due to the opportunistic nature of the sampling, no corresponding nutrient and Chl-a samples were collected; however, temperature and salinity data were collected (see Gregory et al., 2020).
The following day (11th August 2017) two samples were collected from Aughinish bay at New Quay pier, one at high tide and one at low tide, to investigate if the bloom was also present there.
Phytoplankton sampling and analysis
Phytoplankton samples were collected from the piers using a clean bucket to collect ~ 1 l at a time. The sample was stirred gently to ensure homogeneity and then decanted directly into sterile 50-ml polypropylene screw cap conical bottomed tubes (Starstedt©). During the boat transects, phytoplankton samples were taken directly by manually inserting the 50-ml tube into the water to a depth of 0.5 m over the side of the boat, opening the cap underwater, flooding the container and then closing the cap while still underwater. All samples were dosed with 4 or 5 drops of Lugol’s iodine to preserve the phytoplankton. The tubes were stored in a dark cooler box until taken back to the laboratory for analysis.
Identification and enumeration of phytoplankton was carried out by the Utermohl technique (Utermöhl, 1958; Edler and Elbrächter, 2010) using 10 ml sedimentation chambers with an inverted light microscope (Olympus CKX41). After inverting the sample four times, a sub-sample was removed from the 50-ml polypropylene bottle using a sterile pipette, inserted into an Utermohl chamber and allowed to settle overnight.
The phytoplankton within the samples were then identified to species level where possible, and enumerated using the inverted light microscope at either × 200 or × 400 magnification as applicable. Total phytoplankton counts for both bays and individual sampling events in 2016 and 2017 were analysed for (a) differences in species diversity using the Shannon diversity index (H) (Shannon, 1948) and the Simpson diversity index (D) (Simpson, 1949), (b) evenness using the Shannon equitability index (Eh) and (c) community similarity using Sørensen’s similarity index (Sφrensen, 1948). A non-parametric test was used to establish whether the phytoplankton diversity was different between the two bays, as the diversity indices are not normally distributed. A Mann–Whitney test was applied to the diversity indices derived from paired sampling occasions carried out < 48 h of each other. Data derived from March samplings were excluded, as nutrient levels, which are considered to be the driving variable causing differences in phytoplankton between the bays, were at their pre-spring bloom winter maximum concentrations at this time.
Chlorophyll-a sampling and analysis
A 500 ml aliquot of the sample was transferred into a rinsed plastic measuring jug, and then filtered through a GF/F 47mm diameter Whatman® filter paper. The filter paper was folded, removed and inserted into a 15-ml sterile polypropylene tube. The tube was then frozen upright in the dark at −20 °C in a portable freezer for transport to the lab for analysis. Duplicates were taken for every second sample.
For analysis of chlorophyll-a, the Welschmeyer method was followed (Welschmeyer, 1994). Samples were treated with 10 ml of 90% Acetone and refrigerated for 24 h at 5 °C in the dark to extract the chlorophyll from the filter paper. On removal from the fridge, they were inverted a number of times and the filter was pressed to the bottom of the 15-ml polypropylene tube before centrifuging for 5 min at 4000 rpm. An aliquot was poured into a cuvette and analysed before and after acidification (1–2 drops of 50% HCl) in a Turner Designs model 10 fluorimeter. Concentrations in µg ml−1 extract were obtained using the calibration approach outlined in Tett et al., (1977).
CDOM sampling and analysis
CDOM samples were collected from Kinvara at Parkmore Pier and from Aughinish at New Quay Pier during the tidal sampling sessions in June and August 2016. Aliquots of water from the filtration for chlorophyll were collected in opaque HDPE plastic bottles and refrigerated at 5 °C until analysis. Samples were analysed for absorbance between 350 and 750 nm, with 440 nm used to determine CDOM, using a Shimadzu UV-1601 UV–Visible spectrophotometer with a 10 cm cuvette (Goddjin & White, 2006; Boyd et al., 2010; Fichot et al., 2016). CDOM results are in the supplementary material including supplementary figure S3, as they simply provide supporting evidence of SGD input to Kinvara.
Nutrient sampling and analysis
Tidal series nutrient samples were collected using a bucket form the pier wall. SGD samples were collected directly in the outflow also using a bucket. All equipment used for nutrient sampling (Including filters) were acid washed with 10% HCl acid in the lab and packed into acid washed Ziploc® bags prior to sampling excursions.
Water samples were decanted directly into the nutrient dedicated filtration unit and filtered through a 0.40 µm Cyclopore track etched membrane filter (47 mm diameter, Whatman®). The collecting unit was rinsed twice prior to the final aliquot being filtered and decanted into a 50-ml polypropylene tube, which had been rinsed three times with the filtrate. The sample was frozen upright immediately at −20 °C in a portable freezer for transport to the lab. The filters were changed every two to four samples depending on the turbidity. Triplicates were taken for every sample.
Nitrate, nitrite, silicate and phosphate were determined using a Skalar San++ continuous flow analyser by standard colorimetric methods (Hydes et al., 2010) at the Marine Institute, Ireland (MI) following the methods and procedures outlined in McGrath et al., (2019b). Calibration standards covering the expected range of the samples were made daily and run at the start of each analysis, any sample outside of these values was diluted before re-analysis. To ensure accuracy, KANSO CRMs were run with each set of samples. Every sample was run twice and a relative percentage difference was calculated (RDP: difference between values/mean × 100) which should be ≤ 10 to be accepted. The Marine Institute participates in the QUASIMEME laboratory quality and control programme for nutrient analysis and operates under ISO 17025 quality control standards. Results for N are presented as total oxidised nitrogen, TOxN, i.e., the sum of NO3− and NO2−.
Groundwater discharging to Kinvara Bay is known to contain high concentrations of both silicate and nitrate (Rocha et al. 2015) indicating it is likely to be the primary source of both these nutrients to Kinvara Bay. An exploratory analysis using linear regression was applied to the TOxN and Silicate data for both bays to establish relationships, if any, between these two variables and to compare results with the relative concentrations of these two constituents measured in groundwater input. The sample sizes are small (n = 9 to 12), so there are not enough data to test that the values of Y (where Y = TOxN concentration) are normally distributed and that the variances are not driven by the magnitude of the X parameter (where X = dissolved Si concentration).
Mooring
An instrument mooring was deployed from 4th August to 14th September 2017 in Kinvara Bay at 53°09.34’ N, 8°56.65’ W (see Fig. 2). The mooring layout is shown in Supplementary Fig. S1. The instrumentation comprised two temperature and salinity sensors (HOBO® ONSET, model: U24-002-C) held at 1 m and 3.5 m above the sea bed and a third suspended 0.15 m below the sea surface. A fluorimeter (Wetlabs Ecotriplet FL3B-858) was also included at a depth 3 m above the seabed.
Rainfall
Rainfall data were taken from two weather stations, the Met Éireann station near Ardrahan (53.148°N, 8.745°W) approximately 10 km inland and due east of Kinvara bay in the central part of the catchment area, and the University of Galway weather station on the Galway campus, approximately 15 km north-west of Kinvara Bay and 1 km from the coast. Daily rainfall data from these two stations were averaged, with the assumption that this gives an accurate indication of precipitation in the Kinvara and Aughinish catchments. Historical weather station data for Ireland are available from https://www.met.ie/climate/available-data/historical-data.
Results
Total annual rainfall recorded at Ardrahan for 2016 was 1110 mm, with 1093 mm recorded at the University of Galway. The combined averaged total for June, July and August 2016 was 272 mm. 2017 was a wetter year than 2016, with a total rainfall of 1263 mm at Ardrahan, and 1334 mm at University of Galway. The combined averaged total for June, July and August 2017 was 408 mm. Daily rainfall is presented in Supplementary Fig. S2. 23% more rain fell in July 2017, 44% more rain fell in July 2017 and 33% more rain fell in August 2017 than in the respective months in 2016.
Table 1 shows the ranges in salinity values measured over full tidal cycles in Kinvara and Aughinish bays in 2016 and 2017 at the designated sampling locations towards the mouth of each bay. On each sampling occasion, the salinity of water in Kinvara bay was consistently lower, and the ranges encountered between high and low tides were correspondingly greater, than in Aughinish (Table 1). Lowest salinity values of 17.7 (Kinvara) and 26.4 (Aughinish) were measured in March, as would be expected during wetter seasons. The highest salinity of 33.8 was found at Aughinish in June 2017, when the smallest difference in salinity between high and low tide of 0.3 was observed. The tidal range in salinity was smaller in June and August when compared with March in both years, with the single exception of measurements made in August 2017 in Kinvara. On this occasion, which occurred after period of high rainfall, the salinity range was comparable to those recorded in March. As this result was not reflected in measurements made during the same August period in Aughinish, we conclude that the variability in salinity that occurred in Kinvara reflected freshwater input through SGD.
The variations in surface salinity observed through the tidal cycle are shown. Also given is the mean chlorophyll concentration measured in samples taken over a 6.5 h tidal period (high-low).
Groundwater discharges into Kinvara Bay contained elevated nutrient levels. Direct measurements at the discharge point taken between March and June 2017 showed it to contain 51–71 μmol TOxN l−1 and 38–49 μmol silicate l−1 with a molar ratio N:Si of 1.4:1 (Table 2). Phosphate concentrations in groundwater were, however, low, always less than 0.35 μmol l−1. At the tidal cycle sampling points, maximum phosphate concentrations were observed in March 2017. At Aughinish, the mean concentration recorded was 0.36 μmol l−1 (sd = 0.18) on 14 March and no variation in concentration with tide was observed. At Kinvara, phosphate concentrations generally decreased from 0.4 μmol l−1. at high tide to 0.2 μmol l−1 at low tide. The levels of phosphate recorded at high tide near the mouths of both bays are compatible with the winter phosphate concentration in Galway Bay observed in previous years (e.g., Nolan et al., 2009). In June and August of both 2016 and 2017, however, phosphate levels were ~ 0.1 μmol l−1. In March 2017, TOxN levels were of order 10 μmol l−1 (Kinvara: mean 12.1 μmol l−1 ,sd 3.2; Aughinish: mean 11.1 μmol l−1 , sd 13.3) over the tidal cycles measured at both bays, with little variation over the tidal cycle except for a single outlier value of 58 μmol l−1 measured at low tide at Aughinish (data not shown). Tidal variations in TOxN levels observed in the summer months are shown in Fig. 3. At both locations, elevations in nutrients (TOxN, Si) were evident at low tide, except for Aughinish on 20 June 2017. Usually, elevated levels were short-lived (1 h) and were represented by increases in TOxN from 0.5 μmol l−1 to 5–10 μmol l−1 . On 8 August in Kinvara, however, TOxN concentrations rose to 15 μmol l−1 2 hours before low tide and remained above baseline levels for this location for approximately 4 h (Fig. 3h).
Variations in salinity, chlorophyll-a (mg m-3) and TOxN (µmol l-1) measured in water sampled over tidal cycles in Aughinish Bay (from New Quay pier) and Kinvara Bay (from Parkmore pier), 2016–2017. X axes are time of day. a, b early June and c, d early August 2016; e, f mid-June and g, h early August. Note that chlorophyll for a–f is 2 × actual chlorophyll so variation can be seen clearly. For g and h, the scale is extended and chlorophyll is actual measured values, so that the difference between Aughinish and Kinvara is clear in this much wetter period
The molar N:Si ration is also given. Note the relatively low levels of phosphate.
There were strong positive linear relationships between TOxN and Si in samples taken from the piers in both Aughinish and Kinvara in 2017 (Fig. 4a to d).
Relationships between water constituents sampled in Aughinish Bay (from New Quay pier) and Kinvara Bay (from Parkmore pier) in 2017. a–d TOxN: Silicate relationships in June (a, b) and August (c, d) 2017; dashed lines drawn show the slope of the regression between the two constituents calculated for samples not substantially diluted by Galway Bay water. e TOxN and Si in SGD water sampled between March and June 2017 plotted with the tidal cycle samples taken at Parkmore on 8th August; the regression shown in d is reproduced here and extended. f Variations in surface salinity (solid line), chlorophyll-a and TOxN over a full ebb tide on 9th June 2017, near station 5, Kinvara Bay (for location see Fig. 2). The regression analyses should be considered exploratory due to the small sample sizes
The molar ratio of these constituents as estimated by linear regression on samples not substantially diluted by Galway Bay water ranged from 0.15 (TOxN:Si) to 1.53 (see Fig. 4). However, in 2016, a much dryer summer, only the June 2016 sample from Kinvara showed any relationship between TOxN and Si (see Supplementary Fig. S4). The slope of the regression noted for 8 August in Kinvara was 1.31 (N:Si) calculated for samples whose TOxN concentration was > 1.5 µmol l−1. . The similarity of this ratio to the mean N:Si ratio measured in SGD samples of 1.4 (Table 2; Fig. 4e) is a good indication that groundwater was the source of nutrients on this occasion. This inference is further strengthened by the inverse relationship between chlorophyll-a and TOxN over the salinity gradient in inner Kinvara Bay (Fig. 4f). N:Si ratios calculated on SGD samples from Rocha et al (2015), collected in 3 different summers, fall between 1.07 and 1.47.
Tidal variability was not observed in chlorophyll concentration in March 2016, with mean values recorded for Aughinish of 1.56 mg m−3 (sd 0.34, n = 9) and for Kinvara 1.45 mg.m−3 (sd = 0.96, n = 16). Similar values were observed over tidal cycle surveys carried out for both bays in June and August, 2016 when values ranged from 0.48–3.2 (Kinvara) and 0.34–1.90 (Aughinish) mg m−3 in June 2016 (Fig. 3a, b) and 1.30–2.40 (Kinvara) and 0.75–1.70 (Aughinish) mg m−3 in August (Fig. 3c, d). Mean chlorophyll levels over a single tide (high-low) are presented in Table 1, in order to make the two data series from the two locations directly comparable. A consistent feature of the data was that, particularly in summer, chlorophyll levels in Kinvara were significantly (P < 0.001 except for 19/20 June 2017 when P < 0.1) higher than those in Aughinish. The single exception to this occurred in March 2016 when mean chlorophyll levels were 1.5 mg m−3 in both bays, although the number of samples taken at this time was substantially less than on other sampling occasions. Chlorophyll levels were consistently low in Aughinish, where mean tidal concentrations only ranged 0.8–1.0 mg m−3 and 0.9–1.3 mg m−3 in 2016 and 2017. A tidal variability can be seen in chlorophyll data when small increases in levels at low tide coincided with elevated nutrient levels at low tide (Fig. 3). This was more marked in Kinvara Bay than in Aughinish Bay. Chlorophyll levels found in Kinvara in 2016 were up to twice those in Aughinish, and in August 2017 were substantially higher with mean values 4.0 and 5.4 mg m−3 on 3 and 8 August, respectively. Of particular note is that on the 8 August values up to 17.3 mg m−3 were recorded.
A total of 53 identifiable species or forms of phytoplankton were identified in the tidal sampling series in 2016. Of these, 39 were present in Kinvara samples, while 41 species were detected in samples from Aughinish. The equivalent number for 2017 was 50 forms, of which 40 were present in Kinvara samples and 37 in Aughinish samples. Detailed results are found in Supplementary Table 2A and B.
In March of both 2016 and 2017, phytoplankton samples from both bays were numerically dominated by the diatom Skeletonema costatum (Greville) Cleve. Cell densities in 0.1–0.5*106 cells l−1 were found in both bays in 2016, with numbers of this organism approximately one order of magnitude lower in 2017. Cryptophytes and unidentified thin pennate diatoms in densities 10–50*103 cells l−1 were also present in March 2016, with slightly higher cell densities in Kinvara than Aughinish. Pseudo-nitzschia Peragallo and other pennate diatoms were predominant in June 2016 in both bays, and diatoms were still prevalent in both bays in August of that year (Supplementary Table 2A). A small bloom of the diatom Leptocylindrus minimum Gran was found in both bays in June 2017. Samples from low tide in Kinvara also contained high numbers (0.3*106 cells l−1) of a small Chaetoceros sp. indicating a substantial population of this species inside Kinvara Bay. Samples taken in Kinvara in August were notable for the presence of the dinoflagellate Prorocentrum micans Ehrenberg, which was dominant in samples, and had a cell density of 93*103 cells l−1 in samples taken at low tide on the 8 August. There was a marked contrast between samples taken at Aughinish (8 August) and Kinvara (7 August). Samples from Aughinish had a low biomass totalling 7200 cells l−1, with a total of 19 identifiable forms of which ten species were not present in Kinvara. The three most abundant were P. micans (1840 cells l−1), an unidentified microflagellate 10–20 µm diameter (800 cells l−1) and Rhizosolenia imbricata Brightwell (720 cells l−1). Kinvara had 13 species totalling 129,000 cells l−1 of which four species were not present at Aughinish, the three most abundant species being P. micans (93,000 cells l−1), Polykrikos schwartzii Butschli (7300 cells l−1 ) and Rhizosolenia imbricata (5300 cells l−1. ). At this time, Kinvara had approximately seven times the chlorophyll concentration (7.2 mg m−3), three times the TOxN (1.1 µmol l−1 ), eight times the silicate (4.1 µmol l−1 ) and a 5 PSU difference in salinity when compared with Aughinish (Fig. 3).
The elevated cell density of P. micans observed on 8 August 2017, coupled with the sighting of discoloured water in Kinvara Bay prompted an opportunistic sampling by boat of 5 locations along the axis of Kinvara Bay at high and low tide on 10 August 2017. Cell densities of this organism in surface water samples are plotted in Fig. 5, which shows a population of 1–3*106 cells l−1. extending 2 km north from the innermost part of the bay. Further samples from Aughinish taken from the tidal monitoring location at high and low tide had cell densities of 2300 and 1800 cells l−1. , respectively, similar to the cell density of 1840 cells l−1. found here at low tide on 7 August.
P.micans counts along a transect of stations in Kinvara Bay at high and low tide on 10th August 2017. The numbers 1 to 5 refer to the sampling station locations on the map shown in Fig. 2. Samples from the inner bay (locations 1,2) contained up to 3.5 million cells L-1 at high tide. The tidal range on 10 August 2017 was 3.2m
An additional sample taken for chlorophyll and phytoplankton analysis while servicing the instrument mooring in Kinvara bay on 9 August showed that the phytoplankton was a near monoculture of P. micans with cell density 1.3*106 cells l−1, and the chlorophyll was 25.4 mg m−3. Data output from the instrument mooring (Fig. 6) suggested that the bloom gradually dispersed from its most intense on 10 August, to a low chlorophyll signal output on 16 August.
Time series plots of data output from the instrument mooring deployed in mid- Kinvara Bay near sampling station 4 (see Fig. 2) for the period of 8–16 August 2017. Chlorophyll-a and water pressure (showing tidal state) from the Ecotriplet are presented along with salinity recorded with a Hobo T/S probe located 3.5 m above the sea bed
Similarity and diversity indices for the two sites for these sample series are summarised in Table 3. Overall, there was a higher diversity and evenness in Aughinish Bay than in Kinvara in both 2016 and 2017, with the greatest differences in 2017. The similarity index, where a value of 1 equals population equivalence, showed very little change between the two years, at 0.68 (2016) and 0.70 (2017).
Diversity indices for individual sampling events showed no consistent trend in the differences in diversity between high and low tide samples for either bay for any given sampling occasion (Table 3.). The hypothesis that samples from Aughinish Bay had a higher diversity (i.e., higher H values and 1/ D values) than those from Kinvara Bay was tested using a Mann–Whitney test for evaluating two independent datasets. Only samples from the summer months (June, August) were used for this test as it is at this time of year when groundwater nutrient inputs would impact the overall nutrient balance, but included are data for other summer high and low samples (11 samples for Kinvara, 12 for Aughinish, data not shown). The test showed that the diversity in Aughinish samples was significantly higher (P < 0.5) than that in samples from Kinvara.
The Shannon equitability/evenness (Eh) for comparable periods shows Aughinish generally closer to 1 (Table 3). Hmax, the highest potential diversity, is very similar between Kinvara and Aughinish for 2016 with a steady increase as the summer progresses. The differences In Hmax for 2017 between the two bays was more apparent, with Kinvara having a general potential for higher species diversity.
1/D = Simpson’s Diversity Index (reciprocal); H = Shannon Diversity Index, Hmax = highest potential diversity (Shannon); Eh = Shannon Equitability Index.
Discussion
Despite their close geographic proximity and size, Kinvara and Aughinish bays contrast markedly in their biogeochemistry. Neither bay has a riverine input, yet Kinvara bay consistently exhibits a lower salinity than Aughinish, which is particularly noticeable after periods of heavy rainfall. The cause of this must lie in the ground water inputs which exist in Kinvara Bay but not in Aughinish (Smith & Cave, 2012).
Groundwater entering Kinvara bay is rich in nutrients, particularly nitrates and silicates. Nutrients in SGD are derived from surrounding farmland and from the overflow of domestic waste water from septic tanks into the groundwater system. The latter increases in summer due to an increase in tourism locally. Nitrate (ToxN) concentrations in SGD discharge into Kinvara from March to June 2017 were always > 50 µmol l−1 which is many times the winter concentrations in greater Galway bay of ~ 9 µmol l−1 (Smith & Cave 2012). The nitrate concentrations found in ground water are of the same order, although slightly lower, than those found by Einsiedl (2012) who found levels of 60–380 µmol l−1 nitrates in samples taken from groundwater wells in the Kinvara region in 2008/2009. Silicates in the SGD were > 30 µmol l−1, again much higher than the ~ 8 µmol l−1 routinely measured in Galway Bay on annual winter nutrient surveys by the Marine Institute (Nolan et al., 2009). Phosphate levels were, however, low (< 0.35 µmol l−1). Phosphate is removed either during transit within the groundwater conduits or within the submarine estuary, where it is adsorbed on to particles (Slomp and Van Cappellen, 2004; Pain et al., 2020). Thus, in times of discharge, water in Kinvara Bay is phosphate limited, with the N:P ratio well above the Redfield molar ratio of ca. 15:1. The catchment has a small and dispersed population, and land-use is largely pastures and agricultural areas with significant natural vegetation, so phosphate from land-based sources is low (Corine land-use data for 2018). Driven by the Nitrates Directive (91/676/EEC), a rolling series of 4-year Nitrate Action Programmes (NAP) for all natural waters in Ireland began in 2006 (S.I. No. 378/2006—European Communities (Good Agricultural Practice for Protection of Waters) Regulations 2006). These helped to somewhat improve groundwater and surface water status in respect of both nitrate and phosphate in the Kinvara catchment, with the first NAP in operation during the 2008/09 study by Einsiedl (2012) and the third NAP in operation during the period of this study. A comprehensive report on water quality status in Ireland between 2013 and 2018 was published by the Environmental Protection Agency (O'Boyle et al., 2019), finding an average of 80–161 µmol l−1 of N (5–10 mg l−1 NO3) in groundwater in the study area, but less than 0.8 µmol l−1 P (< 0.025 mg l−1). The link between freshwater (SGD) and nutrient inputs is witnessed by the strong nutrient–salinity relationships observed in Kinvara Bay, particularly after extended periods of rainfall. The link is further strengthened by the similarity between N:Si ratios of groundwater and nutrient enriched water in Kinvara bay.
The promotion of phytoplankton growth in coastal regions through nutrient additions from groundwater has been shown through experiments with mesocosms and in the field (Lecher and Mackey 2018). Field studies have usually involved measurements of radon, a natural tracer of groundwater, with chlorophyll levels (e.g., Honda et al., 2018). None of these, however, have involved a contemporaneous study including an adjacent comparable area which does not receive groundwater. This does, however, mean that the effective sample size is small, with Aughinish Bay the control and Kinvara Bay the experimental site, and differences in the phytoplankton between the bays could be caused by factors other than nutrients in SGD. This is unlikely as, aside from their geographic proximity, phytoplankton biomass, as estimated by chlorophyll measurements, for example was consistently richer in Kinvara bay than Aughinish, even in relatively dry summer periods such as occurred in 2016. This must be a consequence of groundwater input, which can be quite variable. In 2017, after an extended period of rainfall, nutrient enrichment resulted in surface chlorophyll levels of up to at least 25 mg m−3 approximately twenty times the chlorophyll levels in Aughinish at this time.
The species diversity of phytoplankton samples taken from Kinvara was significantly lower than that in samples from Aughinish. Comparisons of phytoplankton between the bays counted in samples showed that in 2016 Kinvara had a slightly lower species diversity, but more than double the total cell abundance compared to Aughinish. In 2017, which was a wetter summer Kinvara bay still had a lower diversity yet nearly five times the total cell abundance than Aughinish. The increased diversity observed in Aughinish bay, compared with Kinvara bay, is not unusual as there is a tendency for increased nutrient loading to decrease phytoplankton diversity (see e.g., Smith et al., 1999). Furthermore, a bloom of a one or two species in a bay decreases the diversity of the phytoplankton. Such an event occurred in August 2017, when an intense bloom of the armoured dinoflagellate P. micans occurred. Such a bloom was not evident at this time in Aughinish where the level of variation in salinity after the same period of rainfall was extremely small. It is perhaps surprising that the groundwater nutrient input to Kinvara did not encourage a diatom bloom, a class of phytoplankton characterised by cells with frustules of silica. Generally, diatoms have a requirement for N:Si in equimolar concentration (Brzezinski, 1985), which approximates that in the groundwater measured in this study. Species from the diatom genus Pseudo-nitzschia are known to respond favourably to SGD nutrient input (Lecher and Mackey 2018). P. micans has mainly a neritic and estuarine distribution, but occasionally occurs in oceanic waters, and is more often found in late summer-autumn. This species is commonly found throughout the Galway Bay area (Pybus, 1990) and elsewhere along the Irish coast. P. micans was able to outcompete other phytoplankton in Kinvara bay in August 2017, and grew to a population with a cell density that visibly discoloured the seawater. This was most likely as a response to environmental variables such as low salinity and minimal turbulence and dispersion, particularly at neap tides, in a sheltered semi-enclosed bay whose tidal excursion is small (Gregory et al., 2020). The species does not produce known toxins, although there are however rare reports of this species having directly caused shellfish kills in South Africa and Portugal (Pinto & Silva, 1956; Hortsman, 1981). Aquaculture kills from P. micans blooms are generally due to post-bloom deoxygenation.
There is a history of extensive Prorocentrum blooms in this region. The first record is that of Pybus (1990) who observed a dense population of P. micans in Poulnaclough Bay (Bell Harbour) which is a semi-enclosed inlet found 4 km to the west of Aughinish bay. It receives groundwater, and is of similar proportion in size to Aughinish and Kinvara, which together form the three inlets which give the southern coast of Galway Bay its characteristic coastline. Pybus (1990) noted a bloom of 11 * 106 cells l−1 in Poulnaclough Bay in 1980 which lasted for over 4 weeks. He stated that some (unknown) mechanism must exist which favours the maintenance of such high cell densities. We propose that pulses of nutrients from groundwater inputs would indeed maintain such a bloom. In Kinvara bay, phytoplankton records are now available through the monitoring of transitional and coastal waters under the EU Water Framework Directive (2000/60/EC), carried out by the Environment Protection Agency, Dublin. Records began in 2006 and these show blooms of P. micans in Kinvara Bay with cell densities of between 0.5 and 3 * 106 cells l−1 occurring between mid-July and mid-September every year up to 2021, with the exceptions of 2007, 2016, 2017, 2019 and 2020. Caution should be applied when analysing these data as the frequency of sampling was low, carried out 3–4 times per year. Nevertheless, in four out of these five years records showed populations of 100,000 to 400,000 cells l−1. Of interest are two records for 20 July 2017 in Kinvara Bay of 200,000 cells l−1 which is indicative of the forthcoming red tide observed in the current study on 8 August, and fits with a general observation of a phytoplankton bloom having a life time of the order 10 days (Smayda, 1997). Clearly, these bloom events are frequent and occur almost annually. Given the absence of red tide observations or records from Aughinish Bay, we conclude that nutrient-rich SGD discharges into Kinvara Bay cause an asymmetric shift in species abundance directed to opportunistic species such as P. micans.
Kinvara bay is an important aquaculture site and has a high cultural and economic value in tourism. Red tide blooms of P. micans or other HAB species could have long lasting detrimental effects on the intricately balanced ecosystem and hence on the local economy (e.g. Park et al., 2013) . Episodic supplies of high levels of nutrients like nitrate and silicate to Kinvara bay, linked to rainfall, help sustain communities of phytoplankton, especially in summer when nutrients are normally limited. Phosphate limitation, however, reduces the number of phytoplankton species that can take advantage of it. Estuaries are known as breeding grounds for many marine species and have commercial value in tourism and aquaculture. However, the additional pressure caused by elevated nutrient levels and larger salinity gradients driven by SGD discharge could have detrimental effects at many levels. Climate change is likely to promote P. micans blooms. Peperzak, (2003) in a study of the North Sea concluded that increased temperature and strengthened haloclines would be detrimental to certain phytoplankton species but for a species like P. micans would double its growth rate. Furthermore with increased summer-time precipitation comes increased land run off loaded with nutrients, promoting blooms. This makes a clear case for more research into mitigation and sustainable development of SGD fed bays. Even though P.micans is not considered toxic, it does create a very unsightly environment and by sheer numbers of a red tide bloom, can cause fish and shellfish kills due to oxygen depletion.
Conclusion
The data presented clearly show the effects of nutrient enrichment and salinity variation on primary production in a bay fed by SGD compared to one that has no SGD or fluvial input. It demonstrates that large variations may exist between adjacent waters even at the scale of a few kilometres. High nutrient SGD input in summer increases phytoplankton abundance while only marginally affecting species diversity. While the increased abundance helps to sustain shellfish aquaculture and draw down CO2, it can also lead to blooms of harmful algae. The submarine estuary may be controlling the input of phosphate, leading to phosphate limitation, and SGD enrichment experiments would be useful to analyse the effects of increasing phosphate inputs. Lab-based experimental work controlling factors such as nutrients, salinity and temperature provide good insights when compared with stable environments, but can only have limited application in areas like the indented Irish west coast, which experiences highly variable climate controls, even on a micro scale of 24 h.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available as they are in the process of being submitted to the Environmental Protection Agency (Ireland) where they will become part of the wider dataset on shellfish waters maintained by this Agency. They are available from the corresponding author on reasonable request.
References
Boycott, T., Bruce, C., Cronin, P., Drew, D., Farrant, A., Hobbs, S., Mullan, G., Self, C., Simms, M., Wilson, L., Members of University of Bristol Spelaeological Society, 2003. Caves of County Clare and South Galway, University of Bristol Spelaeological Society, Bristol:
Boyd, T. J., et al., 2010. Variation in ultrafiltered and LMW organic matter fluorescence properties under simulated estuarine mixing transects: 2. Mixing with photoexposure. Journal of Geophysical Research 115(3): 1–14. https://doi.org/10.1029/2009JG000994.
Brzezinski, M. A., 1985. The Si:C: N of marine diatoms: Interspecific variability and the effect of some environmental variables. Journal of Phycology 21: 347–357.
Burnett, W. C., et al., 2003. Groundwater and pore water inpunts to the coastal zone. Biogeochemistry 66: 3–33. https://doi.org/10.1023/B:BIOG.0000006066.21240.53.
Cave, R. R. & T. Henry, 2011. Intertidal and submarine groundwater discharge on the west coast of Ireland. Estuarine, Coastal and Shelf Science. https://doi.org/10.1016/j.ecss.2011.01.019.
Drew, D. P. & D. Daly, 1993. Groundwater and karstification in mid-Galway, south Mayo and north Clare. Geological Survey of Ireland (No. 93).
Edler, L. & M. Elbrächter, 2010. The Utermöhl method for quantitative phytoplankton analysis. Microscopic and Molecular Methods for Quantitative Phytoplankton Analysis. https://doi.org/10.1016/j.resp.2011.02.009.
Einsiedl, F., 2012. Sea-water/groundwater interactions along a small catchment of the European Atlantic coast. Applied Geochemistry 27(1): 73–80. https://doi.org/10.1016/j.apgeochem.2011.09.004.
Fichot, C. G., et al., 2016. Predicting dissolved lignin phenol concentrations in the coastal ocean from chromophoric dissolved organic matter (CDOM) absorption coefficients. Frontiers in Marine Science 3: 1–15. https://doi.org/10.3389/fmars.2016.00007.
Fleury, P., M. Bakalowicz & G. de Marsily, 2007. Submarine springs and coastal karst aquifers: a review. Journal of Hydrology 339(1–2): 79–92. https://doi.org/10.1016/j.jhydrol.2007.03.009.
Gameiro, C., J. Zwolinkski & V. Brotas, 2011. Light control on phytoplankton production in a shallow and turbid estuary. Hydrobiologia 669: 249–263. https://doi.org/10.1007/s10750-011-0695-3.
Garcés, E., G. Basterretxea & A. Tovar-Sánchez, 2011. Changes in microbial communities in response to submarine groundwater input. Marine Ecology Progress Series 438: 47–58. https://doi.org/10.3354/meps09311.
Gill, L. W., et al., 2013. Characterisation of hydrogeological connections in a lowland karst network using time series analysis of water levels in ephemeral groundwater-fed lakes (turloughs). Journal of Hydrology 499: 289–302. https://doi.org/10.1016/j.jhydrol.2013.07.002.
Gobler, C. J., 2020. Climate change and harmful algal blooms: insights and perspective. Harmful Algae 91: 101731. https://doi.org/10.1016/j.hal.2019.101731.
Goddjin, L. M. & M. White, 2006. Using a digital camera for water quality measurements in Galway Bay. Estuarine, Coastal and Shelf Science 66: 429–436.
Gregory, C., et al., 2020. The interaction of retention areas and submarine ground water (SGD) in small bays: implications for phytoplankton growth. Estuarine, Coastal and Shelf Science. https://doi.org/10.1016/j.ecss.2020.106681.
Honda, H., R. Sugimoto & S. Kobayashi, 2018. Submarine groundwater discharge and its influence on primary production in Japanese coasts: case study in Obama Bay, The Water-Energy-Food Nexus Springer, London: 101–115.
Horstman, D.A. 1981. Reported red-water outbreaks and their effects on fauna of the West and South Coasts of South Africa, 1959-1980. Fish Bull S Afr 15:71-88
Hydes, D. J. et al., 2010. High precision and inter-comparability using gas-segmented. Atlantic, pp. 1–87.
Kieber, R. J., X. Zhou & K. Mopper, 1990. Formation of carbonly compounds from UV-induced photodegradation of humic substances in natural waters: fate of riverine carbon in the sea. Limnology and Oceanography 35: 1503–1515. https://doi.org/10.4319/lo.1990.35.7.1503.
Knee, K. L. & A. Paytan, 2012. Submarine Groundwater Discharge: A Source of Nutrients, Metals, and Pollutants to the Coastal Ocean, Treatise on Estuarine and Coastal Science, Elsevier Inc., Amsterdam: https://doi.org/10.1016/B978-0-12-374711-2.00410-1.
Kratzer, S. & P. Tett, 2009. Using bio-optics to investigate the extent of coastal waters: a Swedish study. Hydrobiologia 629: 169–186. https://doi.org/10.1007/s10750-009-9769-x.
Lecher, A.L. & K.R.M. Mackey. 2018. Synthesizing the effects of submarine groundwater discharge on marine biota. Hydrology 5(4): 60. https://doi.org/10.3390/hydrology5040060.
Liu, J., et al., 2018. Nutrient input through submarine groundwater discharge in two major Chinese estuaries: the Pearl River Estuary and the Changjiang River Estuary. Estuarine, Coastal and Shelf Science 203: 17–28. https://doi.org/10.1016/j.ecss.2018.02.005.
Livingston, R. J., 2002. Eutrophication precesses in coastal systems: origin and succession of plankton blooms and effects on secondary production in Gulf Coast Estuaries. Copeia 2002: 248–249. https://doi.org/10.1023/A:1020124525263.
Maie, N., J. N. Boyer, C. Yang & R. Jaffe, 2006. Spatial, geomorphological, and seasonal variability of CDOM in estuaries of the Florida Coastal Everglades. Hydrobiologia 569: 135–150. https://doi.org/10.1007/s10750-006-0128-x.
McGrath, T., E. McGovern, C. Gregory & R. R. Cave, 2019. Local drivers of the seasonal carbonate cycle across four contrasting coastal systems. Regional Studies in Marine Science. https://doi.org/10.1016/j.rsma.2019.100733.
McGrath, T., M. Cronin, E. Kerrigan, D. Wallace, C. Gregory, C. Normandeau & E. McGovern, 2019. A rare intercomparison of nutrient analysis at sea: lessons learned and recommendations to enhance comparability of open-ocean nutrient data. Earth System Science Data. https://doi.org/10.5194/essd-11-355-2019.
Van Meerssche, E. & J. L. Pinckney, 2019. Nutrient loading impacts on estuarine phytoplankton size and community composition: community-based indicators of eutrophication. Estuaries and Coasts 42(2): 504–512. https://doi.org/10.1007/s12237-018-0470-z.
Moore, W. S., 1999. The subterranean estuary: a reaction zone of ground water and sea water. Marine Chemistry 65(1–2): 111–125. https://doi.org/10.1016/S0304-4203(99)00014-6.
Moore, W. S., 2010. The effect of submarine groundwater discharge on the ocean. Annual Review of Marine Science 2(1): 59–88. https://doi.org/10.1146/annurev-marine-120308-081019.
Naselli-Flores, L. & J. Padisák, 2022. Ecosystem services provided by marine and freshwater phytoplankton. Hydrobiologia. https://doi.org/10.1007/s10750-022-04795-y.
Nolan, G., M. Gillooly, & K. Whelan. (eds.). 2009. Irish ocean climate and ecosystem status report 2009. Marine Institute of Ireland. http://hdl.handle.net/10793/81
Paerl, H. W., 1997. Coastal eutrophication and harmful algal blooms: Importance of atmospheric deposition and groundwater as “new” nitrogen and other nutrient sources. Limnology and Oceanography 42(5): 1154–1165. https://doi.org/10.4319/lo.1997.42.5_part_2.1154.
O'Boyle, S., Trodd, W., Bradley, C., Tierney, D., Wilkes, R., Ni Longphuirt, S., Smith, J., Stephens, A., Barry, J., Maher, P., McGinn, R., Mockler, E., Deal=kin, J., Craig, M. & M. Gurrie, 2019. Water quality in Ireland 2013–2018. Environmental Protection Agency. 102 pp. Available from https://www.epa.ie/publications/monitoring--assessment/freshwater--marine/water-quality-in-ireland-2013-2018.php.
Pain, A. J., et al., 2020. Carbon and phosphorus processing in a carbonate karst aquifer and delivery to the coastal ocean. Geochimica et Cosmochimica Acta 269: 484–495. https://doi.org/10.1016/j.gca.2019.10.040.
Park, J., H. J. Jeong, Y. D. Yoo & E. Y. Yoon, 2013. Mixotrophic dinoflagellate red tides in Korean waters: distribution and ecophysiology. Harmful Algae 30S: S28–S40.
Peperzak, L., 2003. Climate change and harmful algal blooms in the North Sea. Acta Oecologica 24: S139-144.
Pinto, J.D.S., & E.D.S. Silva. 1956. The toxicity of Cardium edule L. and its possible relation to the dinoflagellate Prorocentrum micans. Notas e estudos di Instituto do Biologia Marítima 12: 1–20.
Pybus, C., 1990. Blooms of prorocentrum micans (dinophyta) in the galway bay area. Journal of the Marine Biological Association of the United Kingdom 70(4): 697–705. https://doi.org/10.1017/S0025315400058987.
Rocha, C., J. Wilson, J. Scholten, et al., 2015. Retention and fate of groundwater-borne nitrogen in a coastal bay (Kinvara Bay, Western Ireland) during summer. Biogeochemistry 125: 275–299. https://doi.org/10.1007/s10533-015-0116-1.
Rodellas, V., et al., 2014. Submarine groundwater discharge as a source of nutrients and trace metals in a Mediterranean bay (Palma Beach, Balearic Islands). Marine Chemistry 160: 56–66. https://doi.org/10.1016/j.marchem.2014.01.007.
Shannon, C. E., 1948. A mathematical theory of communication. Bell System Technical Journal 27(4): 623–656. https://doi.org/10.1002/j.1538-7305.1948.tb00917.x.
Simpson, E. H., 1949. Measurement of diversity. Nature 163: 688. https://doi.org/10.1038/163688a0.
Slomp, C. P. & P. Van Cappellen, 2004. Nutrient inputs to the coastal ocean through submarine groundwater discharge: controls and potential impact. Journal of Hydrology 295(1–4): 64–86. https://doi.org/10.1016/j.jhydrol.2004.02.018.
Smayda, T., 1997. Harmful algal blooms: their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnology and Oceanography 42: 1137–1153.
Smith, A. M. & R. R. Cave, 2012. Influence of fresh water, nutrients and DOC in two submarine-groundwater-fed estuaries on the west of Ireland. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2012.07.094.
Smith, V. H., G. D. Tilman & J. C. Nekola, 1999. Eutrophication: impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environmental Pollution 100(1–3): 179–196. https://doi.org/10.1016/S0269-7491(99)00091-3.
Statham, P. J., 2012. Nutrients in estuaries - an overview and the potential impacts of climate change. Science of the Total Environment 434: 213–227. https://doi.org/10.1016/j.scitotenv.2011.09.088.
Sφrensen, T. 1948. A method of establishing groups of equal amplitude in plant sociologybased on similarity of species content and its application to analyses of the vegetation on Danish commons’, Biol. Skr., K. danske Vidensk, 5, pp. 1–34. Available at: http://ci.nii.ac.jp/naid/10010257467/en/ (Accessed: 15 April 2021).
Tett, P., M. G. Kelly & G. M. Hornberger, 1977. Estimation of chlorophyll a and pheophytin a in methanol1. Limnology and Oceanography 22(3): 579–580. https://doi.org/10.4319/lo.1977.22.3.0579.
Utermöhl, H., 1958. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. SIL Communications 9(1): 1–38. https://doi.org/10.1080/05384680.1958.11904091.
Welschmeyer, N. A., 1994. Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and pheopigments. Limnology and Oceanography 39(8): 1985–1992. https://doi.org/10.4319/lo.1994.39.8.1985.
Acknowledgements
The authors thank Robert Wilkes at the Irish Environmental Protection Agency (EPA) for assistance with some of the sampling and Triona McGrath at the Marine Institute for assistance with nutrient analysis.
Funding
Open Access funding provided by the IReL Consortium. This work was funded by the Irish Research Council and the Irish Environmental Protection Agency (EPA) under the Enterprise Partnership Scheme (Postgraduate), grant no. EPSPG/2015/128, and was hosted by the University of Galway, Ireland.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by CG as part of his Ph.D. research, under the supervision of RRC, RR and GM. The first draft of the manuscript was written by CG and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling Editor: Jonne Kotta
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gregory, C., Cave, R.R., Raine, R. et al. Phytoplankton abundance and biodiversity in adjacent estuaries: the importance of submarine groundwater discharge. Hydrobiologia 851, 3323–3339 (2024). https://doi.org/10.1007/s10750-024-05497-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-024-05497-3