Abstract
The biochemical composition and bioavailability of particulate organic matter (POM) were studied in relation to abiotic (water column stratification, macronutrient concentrations) and biotic factors (phytoplankton biomass and functional groups) in the open Ross Sea and coastal area of Terra Nova Bay, Antarctica, during the austral summer 2017. The presence of different sub-systems was highlighted by spatial and temporal variations of all the considered variables, characterised by higher concentrations of particulate organic carbon and nitrogen in Terra Nova Bay, where diatoms dominated, while in the open Ross Sea was higher the contribution of haptophytes (Phaeocystis antarctica Karsten, 1905). The southernmost Ross Sea was characterised by significantly higher concentrations of carbohydrates in the entire water column. High lipid contribution and low photosynthetic activity indicated aged POM, suggesting that POM was composed mainly by the remnants of previous haptophytes production. The higher lability of the carbohydrates indicated that an increase of haptophytes in the area could alter the biogeochemical C cycle, reducing the storage of refractory carbon in the depths, and favouring the transfer and respiration of C in the trophic web, with a decrease of export rate in the Ross Sea.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The organic matter (OM) in the seawater is a complex pool of substances that, operationally, are divided into a particulate pool (POM, larger than 0.2–0.7 µm) and a dissolved one (DOM, smaller than 0.2–0.7 µm) (Lønborg et al., 2017). The POM pool contains detrital as well as living fractions, including viruses, bacteria, phytoplankton, heterotrophic protozoans, and small metazoans (Delmont et al., 2014; Hyun et al., 2016; Williams et al., 2016). The POM fraction has a pivotal role in the trophic webs (Jo et al., 2021; Kharbush et al., 2020), showing variable susceptibility to consumption (Benner & Amon, 2015) and, therefore, persisting for different time lags in the environment before being respired and converted again to CO2 and inorganic nutrients (Marsay et al., 2015). In the Southern Ocean, the production of POM in the water column is regulated by phytoplanktonic organisms (Smith & Asper, 2001, Kim et al., 2018). The phytoplanktonic-derived POM is consumed by heterotrophic organisms and can be transformed or recycled within the water column as well as sink to the bottom, feeding deep environments via Antarctic Bottom Water formation. The balance between these processes has given the Southern Ocean the role of carbon-sink area (Arrigo et al., 2008; Gruber et al., 2009), especially the highly productive continental shelf waters of the Ross Sea. The Ross Sea, in fact, is one of the most productive areas of the entire Southern Ocean (Smith et al., 2000; Schine et al., 2015), characterised by high spatial-related variability with a mosaic of different subsystems, due to the variable environmental conditions driven by, for instance, marine-ice presence, occurrence of continental-ice melting and hydrodynamism (Povero et al., 2006; Smith et al., 2011a; Kim et al., 2018; Bolinesi et al., 2020a; Rivaro et al., 2020). All these conditions can emphasise differences between coastal and offshore areas (Povero et al., 2006). The phytoplankton blooms in the Ross Sea are dominated by diatoms and the haptophyte Phaeocystis antarctica Karsten, 1905 (Smith et al., 2014) showing different temporal and spatial distribution, bypassing in such a way competition and exclusion (Kropuenske et al., 2009; Smith et al., 2011a, b; Heiden et al., 2016). The role of the two groups in the food webs and biogeochemical processes is generally different, as they show different elemental composition (Dunbar et al., 2003) and trophic fate (Smith et al., 2006). P. antarctica is known to aggregate in mucilaginous colonies usually reaching 2–3 mm in diameter (Peperzak et al., 2000) that are mainly composed by carbohydrates (Mathot et al., 2000). When massive blooms of P. antarctica occur, they can supply the water column with huge amounts of molecules with different chemical characteristics and palatability to consumers (Aldekamp et al., 2007). P. antarctica has extremely low losses (Dunbar et al., 1998; Caron et al., 2000; Smith & Asper, 2001; Tagliabue and Arrigo, 2003), while diatoms support the grazing food chain representing the main trophic source for krill (Haberman et al., 2003; Smith et al., 2007). In the last decades, some studies have shown that the timing and spatial distribution of phytoplankton blooms changed, with potential alteration of the common pathways of POM distribution, quantity and quality (Mangoni et al., 2017, 2019; Misic et al., 2017; Bolinesi et al., 2020a, b). Such anomalies could influence the allocation of the POM-derived materials in the food webs and determine changes of its use and recycling. This study focuses on the biochemical composition of POM in the Ross Sea during the summer 2017, analysing the quantitative and qualitative properties of the main biomolecules (proteins, carbohydrates and lipids) at different stations located in coastal and open sea areas in relation to phytoplankton community structure, to understand whether different forcings influence the bioavailability of POM and its fate in the water column.
Materials and methods
An oceanographic cruise was carried out in the Ross Sea during the austral summer 2017 (from 13 January to 11 February) in the framework of the Italian National Antarctic Research Programme (PNRA) research projects CELEBeR (CDW effects on glacial melting and on bulk of Fe in the Western Ross Sea) and P-ROSE (Plankton biodiversity and functioning of the Ross Sea ecosystems in a changing Southern Ocean).
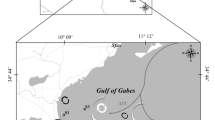
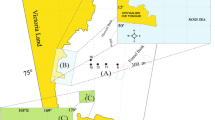
Sampling activities were carried out at 19 stations located in two distinct areas of the Ross Sea (Table 1, Fig. 1): the coastal area of Terra Nova Bay, and the offshore waters of the Ross Sea. In the Ross Sea, stations S53, S55 and S59 were in the northern sector (RS-North), and stations from S46 to S50 were in the southern sector (RS-South). The western sector (RS-West) included stations S43 and S57. Stations S15 (in Terra Nova Bay) and S57 (in RS-West) were repeatedly sampled in different days (Table 1).
Water column characteristics
A Sea Bird Electronics SBE-911plus CTD profiler was used to define temperature and salinity profiles along the water column. Water samples were collected with 10 l Niskin bottles at discrete depths, chosen according to the fluorescence profile and the physical structure of the water column: surface, subsurface depth between 15 and 35 m, intermediate depth from 45 to 60 m, 100 m, 200 m, 300–500 m, and 10 m from the bottom.
The upper mixed layer (UML) depth was determined as the depth at which the in-situ density (σt) changed by 0.05 kg m−3 over a 5 m depth interval (Rivaro et al., 2017). The deeper layer (DL) has been defined as the layer below UML down to 500–600 m. In the Terra Nova Bay area, several stations showed bottom depth higher than 600 m. The samplings at these depths were called “bottom” depths (Table 1).
Inorganic nutrient, phytoplankton biomass and main functional groups
Water samples for the determination of inorganic nutrient (nitrates, ammonia, orthophosphates, silicates) concentrations were collected in 20-ml vials and immediately stored at − 20°C until analysis. The analyses were performed using a FlowSys Systea autoanalyzer (Hansen & Grasshoff, 1983).
For the determination of total phytoplankton biomass, 0.5 l of seawater was drawn from the Niskin bottle for each sampled depth (0–100 m depth), filtered on GF/F Whatman (25-mm diameter) for the analyses of total chlorophyll-a, proxy of phytoplankton biomass, and stored in liquid nitrogen until the analyses in laboratory. A Shimatzu spectrofluorometer was calibrated and checked daily with a chlorophyll-a standard solution (Anacystis nidulans, Sigma) according to Holm-Hansen et al. (1965).
The analyses of phytoplankton functional groups were carried out by chemotaxonomic method in the water layer from surface to 100 m. To this end, 3 l of seawater was drawn from the Niskin bottle and filtered on GF/F Whatman filters (47-mm diameter). Filters were stored in liquid nitrogen until the analysis of pigments by a Hewlett Packard (mod. 1100 Series) HPLC (Vidussi et al., 1996). The system was equipped with an HP 1050 photodiode array detector and a HP 1046A fluorescence detector for the determination of chlorophyll degradation products. Instrument calibration was carried out with external standard pigments provided by the International Agency for 14C determination-VKI Water Quality Institute. The concentrations of pigments were used to estimate the contributions of the main functional groups to the total chlorophyll-a using a matrix factorization software CHEMTAX 1.95.
Maximum quantum efficiency
The maximum PSII photochemical efficiency (Fv/Fm) was determined using a Phyto_PAM II compact unit (Walz) in the layer from surface to 50 m. All samples were acclimated at the dark for 30 min before analysis to minimise the non-photochemical dissipation of excitation, and measurements were blank corrected filtering the sample through 0.2 μm filter (Cullen & Davis, 2003). As regard Fv/Fm, (Fv = momentary fluorescence level of an illuminated sample measured shortly before application of a Saturation Pulse; Fm = fluorescence maximum after a saturation pulse), samples were illuminated with a saturating pulse as reported in Maxwell & Johnson (2000).
Particulate organic matter (POM)
For the determination of POM, 0.5–1.5 l of seawater, depending on the depth, was filtered through pre-combusted Whatman GF/F glass-fibre filters (25-mm diameter, nominal pore size 0.45 µm) in duplicate for each variable. The filters were immediately frozen until analysis in laboratory. Particulate organic carbon (POC) and particulate nitrogen (PN) were analysed following Hedges and Stern (1984), using a Carlo Erba Mod. 1110 CHN Elemental Analyzer. Samples were acidified with HCl fumes to remove inorganic carbon, and cyclohexanone 2–4-dinitrophenyl hydrazone was used as a reference standard. The relative standard deviations were 0.13 and 0.11 for PN and POC, respectively. Particulate protein, carbohydrate, and lipid concentrations were determined following Hartree (1972), Dubois et al. (1956), Bligh and Dyer (1959) and Marsh and Weinstein (1966). Briefly, the protein concentrations were estimated with the colorimetric method based on the binding of copper (provided by a copper sulphate solution) to the aminoacids in an alkaline medium and a further treatment with the Folin-Ciocalteu reagent. The method for carbohydrates, mainly structural polymers such as cellulose, was based on the reaction of the carbohydrates to phenol (5% in water solution) in an acid medium (sulphuric acid). Lipids (including all the non-polar substances soluble in organic solvents) were extracted with a solution of chloroform and water and measured following carbonization with sulphuric acid. Albumin, glucose, and tripalmitin solutions were used to calibrate a Jasco V530 spectrophotometer. The specific standard deviations were 0.15, 0.13 and 0.17 for proteins, carbohydrates, and lipids, respectively. Proteins, carbohydrates and lipids were transformed in C equivalents with the coefficients of 0.49 µg C µg protein−1, 0.40 µg C µg carbohydrate−1 and 0.70 µg C µg lipid−1 (Fabiano & Pusceddu, 1998). The enzyme-hydrolysable fraction of particulate carbohydrates was determined following the protocols of Gordon (1970), Mayer et al. (1995), and Dell’Anno et al. (2000). The sample filters were placed in plastic containers with a solution (100 mg l−1 in 0.1 M Na-phosphate buffer) of β-glucosidase purchased from Sigma-Aldrich. This enzyme is extracted from plants but have hydrolytic activities like natural marine organisms. Incubation lasted 2 h at the optimal temperatures and pH for the enzyme. After hydrolysis, each filter was carefully removed from its container, placed in a filter-holder and rinsed with the solution remaining in the dish and 5 ml of deionised water, to return any particles that may have floated off the filter (Gordon et al., 1970). Then, the filters were processed for the determinations of carbohydrate concentrations as above. Other filters for each station and depth were treated only with the buffer solution, to evaluate the mechanical removal of molecules due to the flushing of the buffer. The concentrations detected after hydrolysis, corrected for the eventual error just mentioned (never higher than 20% of the total carbohydrate concentrations), were subtracted from the total concentrations to obtain the hydrolysable carbohydrates. The relative standard deviation was 0.22.
Statistical analyses
Univariate statistical analysis was performed with STATISTICA software. One-way ANOVA was performed (F and P values are provided in the tables and text, when not indicated the differences are statistically not significant), to highlight whether the variables showed different values in the selected sub-areas and depth.
Then, the relationship between POM variables and the phytoplanktonic variables were tested in the 0–100 m water layer, using Pearson correlation.
To have a wider and comprehensive information of the characteristics of the stations, Principal Component Analysis (PCA) and Analysis of Variance (ANOSIM) (PRIMER software, Clarke & Warwick, 2001) were performed on the average data for all POM variables, nutrient concentrations, and phytoplankton in the 0–100 m layer of each station, after normalisation. The ANOSIM is a resampling technique that uses permutation/randomisation methods on Euclidean distance matrices to identify the differences among groups of samples. Large values of the test statistic (R) indicate complete separation of groups, and small values point to high intra-group variability, denoting a high degree of heterogeneity within groups. Analysis of Redundancy (RDA) Brodgar software (Brodgar 2.5.6 package, 2011, Highland Statistics Ltd.) was applied (Zuur et al., 2007) on the normalised data of POM, nutrient concentrations, and phytoplankton in the 0–100 m water layer. RDA investigates the influence exerted by the variation of one set of variables (explanatory variables: physical characteristics, nutrient and phytoplanktonic variables) on the variation of another set of variables (response variables: POC, PN, proteins, lipids, hydrolysable and not-hydrolysable carbohydrates). An automated forward selection model was applied to test the order of importance of the explanatory variables. In particular, the “conditional effects” that show the increase in total sum of eigenvalues after including a new variable during the forward selection were calculated. Finally, a permutation test was applied (number of permutations: 499) to test the null hypothesis that the explained variation is larger than a random contribution.
Results
Physical setting
The physical setting of the investigated areas has been described in Bolinesi et al. (2020a) and Zaccone et al. (2020) regarding the first 100-m of the water column. The UML depth (Table 1) was significantly shallower in the coastal area of Terra Nova Bay (26 ± 13 m) than in the open Ross Sea (46 ± 26 m) (one-way ANOVA F(1,16)= 7.5, P = 0.02), with a deepening in RS-North (49 ± 10 m) probably due to the bad wind-wave conditions occurred before the sampling (Bolinesi et al., 2020a). In two cases, no UML was detected (S21 and S46), indicating a feeble stratification of the water column. Nevertheless, the distribution of the other variables with depth (see following paragraphs) suggested previous stratification of these stations similar to those observed for the neighbouring stations. Therefore, we considered the first three sampling depths of S46 in the UML and the 100 m depth as the first of DL. For S21, instead, the UML was limited to the first two sampling depths. The S43 showed a double stratification, with a shallower UML placed at 13 m and a weaker one between 50 and 60 m, that was considered as UML due to the POM profiles.
In the coastal Terra Nova Bay, the UML was characterized by the presence of Antarctic Surface Water (salinity lower than 34.60, Orsi & Wiederwohl, 2009) and Dense Shelf Water (Bolinesi et al., 2020a). The wide range of salinity observed in the UML of Terra Nova Bay (Table 2) depended on the proximity of some stations (such as S18 and S23) to the ice tongue of the Drygalski glacier.
The salinity of some of the bottom depths was consistent with a signal of mixing with High Salinity Shelf Water (Castagno et al., 2019), reaching values of 34.82 at stations S13, S18, S19, S21 and S23 and 34.81 at S20 (Table 2).
In the open Ross Sea, the RS-South UML was generally warmer and saltier than the other offshore areas. In the deeper Ross Sea, temperature and salinity suggested the influence of Modified Circumpolar Deep Water (Rivaro et al., 2015; Bolinesi et al., 2020a), while mixing with denser water (salinity > 34.76) was found only next to the bottom at stations S59, S46 and S43.
Nutrients
Nutrients were collected and analyzed only down to 100 m. In stations S57r1 (Ross Sea), S5 and 15r2 (Terra Nova Bay), nutrient concentrations are not available. In all the areas, mean values of nitrates and orthophosphates were significantly lower in UML than in the depths below down to 100 m (Table 3) (one-way ANOVA nitrates: F(1,63) = 85.45, P < 0.0001; orthophosphates: F(1,63) = 51.27, P < 0.0001). In RS-West, RS-South and Terra Nova Bay, a different trend was observed for silicates, showing quite constant concentrations. In RS-North silicates were significantly higher in UML (one-way ANOVA: F(1,11) = 5.95, P = 0.035) (Table 3). Ammonia showed similar values in the water column of all the sub-areas.
Phytoplankton biomass and main functional groups
In the UML, chlorophyll-a concentrations (Table 3, Fig. 2) were significantly higher in Terra Nova Bay (on average 2.0 ± 0.4 µg l−1) than Ross Sea (1.3 ± 0.7 µg l−1) (P < 0.05, Table 4), although the concentrations at S15 (Terra Nova Bay) strongly decreased with time (lowest value on 11th February, S15r2). In the Ross Sea, the lowest values were found in RS-South and RS-West, the former characterised also by a lower photosynthetic activity (Fv/Fmax 0.19 ± 0.13 in S46, Fig. 2). In the layer below UML down to 100 m, RS-South showed chlorophyll-a values significantly higher than in Terra Nova Bay and RS-West (P < 0.05 and P < 0.01, respectively, Table 5).
In the UML, differences between Terra Nova Bay and Ross Sea areas were significant also in terms of Fv/Fm (P < 0.05, Table 4), with the lowest activity in RS-West (mean values of 0.25 ± 0.09), followed by RS-South (0.26 ± 0.14) and the highest in Terra Nova Bay (0.34 ± 0.11). In RS-North, the ratio was 0.30 ± 0.09.
The functional groups showed a low contribution of chlorophytes, cryptophytes, cyanophytes, dinophytes and prasinophytes (Fig. 3) accounting totally up to 10% and 21% of total phytoplankton community within and below UML, respectively. In the UML, significant differences were observed between Terra Nova Bay and Ross Sea for diatoms and haptophytes (P < 0.01, Table 4). In Terra Nova Bay and RS-West, a higher diatom contribution was recorded in the UML (93.6 ± 4.1% and 94.6 ± 4.2%, respectively) than in the other sub-areas (63.6 ± 6.6% for RS-North and 65.1 ± 7.3 % for RS-South, Table 3, one-way ANOVA F(1,43) = 81.84, P = 0.00000). On the contrary the contribution of haptophytes was higher in the eastern part of Ross Sea (31.4 ± 4.7% in RS-North and 31.2 ± 6.9% in RS-South) than in Terra Nova Bay (4.7 ± 4.1%) and RS-West (5.1 ± 4.0%) (Table 3, one-way ANOVA F(1,43) = 114.46, P = 0.00000). In the layer below the UML, haptophytes showed contribution higher than 80% only in RS-South (S49 and S50) (Fig. 3). The repeated station S15 in Terra Nova Bay showed increases of the contribution of haptophytes in the UML, in the layer below the contribution was irregular.
Contribution of the phytoplanktonic functional groups in the upper mixed layer (UML) and deep layer (DL, down to 100 m depth) of the stations sampled in Ross Sea (RS, upper panel) and Terra Nova Bay (TNB, lower panel) (others include: Chlorophyceae, Cryptophyceae, Cyanophyceae, Dinophyceae and Prasinophyceae)
POM
Differences between the sampling areas were recorded also in terms of POM (Table 3). In UML, Terra Nova Bay showed an accumulation of the entire POM pool (namely POC and PN, Fig. 4), while the biochemical components of POM (proteins, lipids and carbohydrates) mainly accumulated in the Ross Sea (Figs. 6, 7).
In UML, Terra Nova Bay showed significantly higher values for POC (281.4 ± 54.8 µg l−1) and PN (45.9 ± 10.9 µg l−1) than Ross Sea (on average 172.6 ± 43.9 and 25.6 ± 6.8 µg l−1, respectively) (P < 0.0001, Fig. 4, Tables 3, 4). In Ross Sea, no significant differences were found between the areas. In DL, values were quite variable.
In UML, the contribution of the different biochemical components of POM (Fig. 5) showed the general dominance of proteins, without significant differences between the areas. On average, protein contribution was 63 ± 5% in Terra Nova Bay and 59 ± 9% in Ross Sea, where RS-West showed the highest contribution (65 ± 7%) and RS-South the lowest (53 ± 10%). Nevertheless, RS-South showed protein concentrations (186.0 ± 60.2 µg l−1) significantly lower than Terra Nova Bay (226.9 ± 51.2 µg l−1) and RS-North (227.7 ± 35.5 µg l−1) (P < 0.05, Table 5).
Contribution of the different biochemical classes to the POM in the upper mixed layer (UML). White: proteins, black: lipids, dark grey: not-hydrolysable carbohydrates, light grey: hydrolysable carbohydrates. The pie chart of the repeated stations 15 and 57 is related to the data of the first samplings
In the UML of Ross Sea, the values were significantly higher than in Terra Nova Bay for hydrolysable carbohydrates (P < 0.05, on average 39.7 ± 29.6 and 24.5 ± 19.9 µg l−1, respectively), lipids (P < 0.05, on average 55.4 ± 26.4 and 39.3 ± 7.2 µg l−1, respectively) and the contribution of lipids to POC (P < 0.0001, on average 23.0 ± 11.0 and 10.2 ± 2.6%, respectively) (Table 4).
The vertical distribution of the different POM variables is reported in Figs. 6 and 7. In all the areas and sub-areas (Table 3), particulate organic matter in the UML showed significantly higher values than in the respective DL (P < 0.01) except for RS-South, where carbohydrate (not-hydrolysable and hydrolysable) and lipid concentrations did not change significantly with depth.
Vertical profiles of the POM variables in the different sub-areas: particulate nitrogen (PN), particulate organic carbon (POC), proteins and total carbohydrates. Open Ross Sea (RS-North, RS-South and RS-West) data are reported in the upper panels, and Terra Nova Bay (TNB) data are reported in the lower ones. Lines denote the average value for each depth in the sub-area
Vertical profiles of the POM variables in the different sub-areas: non-hydrolysable carbohydrates (nh-carbohydrates), hydrolysable carbohydrates (h-carbohydrates), and lipids. Open Ross Sea (RS-North, RS-South and RS-West) data are reported in the upper panels, and Terra Nova Bay (TNB) data are reported in the lower ones. Lines denote the average value for each depth in the sub-area
In the DL, proteins were distributed quite homogeneously in the entire study area, without any significant differences.
Total carbohydrates, instead, showed significantly higher values in RS-South (72.5 ± 45.0 µg l−1) than Terra Nova Bay (25.7 ± 20.7 µg l−1), RS-North (36.5 ± 22.7 µg l−1) and RS-West (15.3 ± 3.8 µg l−1) (P < 0.001, P < 0.05 and P < 0.01, respectively, Table 5).
Lipid concentrations and the contribution of lipids to POC showed large variations (Table 3). The two variables showed significantly higher values in Ross Sea (on average 27.4 ± 14.4 µg l−1 and 37.6 ± 15.3%, respectively) than Terra Nova Bay (12.8 ± 7.1 µg l−1 and 27.7 ± 12.4 %, respectively) (P < 0.05 and P < 0.01, respectively, Table 4), as previously observed for the UML. The RS-South values associated with these variables (lipids: 37.3 ± 21.5 µg l−1, lipids/POC: 38.7 ± 16.1%) were higher than those of the other areas, showing significant differences with Terra Nova Bay (lipids: 12.8 ± 7.1 µg l−1, lipids/POC: 27.7 ± 12.4%, P < 0.001) and RS-West (lipids only: 20.7 ± 10.6 µg l−1, P < 0.01) (Table 5).
The protein + total carbohydrate contribution to POC was higher in the open Ross Sea (ca. 90%, 87 ± 14 and 97 ± 25% for UML and DL, respectively) than in the coastal Terra Nova Bay (ca. 60%, 57 ± 12 and 62 ± 12% for UML and DL, respectively) (P < 0.0001, Table 4). In the Ross Sea, slightly higher mean values were observed in the DL (from 93 ± 10 to 97 ± 14% for RS-South and RS-West, respectively), while in the UML RS-South showed the lowest contribution (80 ± 15%, one-way ANOVA F(1,25) = 5.00, P = 0.035).
A higher detail is given in Fig. 8 for the carbohydrate fractions in the two water layers. Generally, the refractory (not-hydrolysable) fraction (UML: Terra Nova Bay 76 ± 14%, Ross Sea 69 ± 14%; DL: Terra Nova Bay 76 ± 19%, Ross Sea 76 ± 22%) of carbohydrates was dominant over the labile (hydrolysable) one. The histograms related to RS-South hydrolysable carbohydrates highlighted higher values (UML: 46.2 ± 32.8 µg l−1, DL: 22.8 ± 20.0 µg l−1) than the other areas, significantly higher than Terra Nova Bay UML (24.5 ± 19.9 µg l−1) and DL (7.5 ± 8.3 µg l−1) (P < 0.05, Table 5). In the UML, the not-hydrolysable carbohydrates were lower in RS-West (15.9 ± 5.3 µg l−1) than in the other areas (on average 72.9 ± 16.0 µg l−1), one-way ANOVA F(1,16) = 27.55, P < 0.001), while, in the DL, RS-South (50.2 ± 34.9 µg l−1) showed again significantly higher values (P < 0.05, Table 5).
Data related to depths higher than 600 m (bottom layer) for POM are available only in Terra Nova Bay. The values (Table 3) were lower, despite not significantly, than the DL ones, being the bottom average values from 27% (PN) to 36% (proteins) of the DL ones. The hydrolysable carbohydrates were an exception, being the values of the bottom layer 62% of those of the DL.
Relationships between POM and physical–chemical–biological characteristics
PCA applied on the 0-100 m-related values for each station (Fig. 9, PC1 explaining 38.0 %, PC2 23.2%) confirmed the previous differences between Terra Nova Bay stations and the Ross Sea ones at a multivariate level. ANOSIM (Table 6) highlighted the highest difference between Terra Nova Bay and RS-South, followed by Terra Nova Bay vs RS-North.
Applying the RDA, we tried to find out which variables, between physical characteristics (temperature and salinity), nutrients and phytoplankton functional groups, influenced POM variations in the first 100 m. Table 7 reports the results for the main areas, namely Ross Sea and Terra Nova Bay. In Ross Sea, the variations of POM were explained by a quite high number of chemical and biological explanatory variables (nutrients as well as phytoplanktonic groups such as diatoms and haptophytes). In Terra Nova Bay, instead, physical characteristics and diatoms determined the POM variations.
Correlation analysis was carried out to investigate the relationships between the POM variables and the phytoplankton-related ones (Table 8). In Terra Nova Bay (no. of observations 65 for POM variables, 39 for phytoplankton-related ones), POM variables showed always highly significant correlations among them. Chlorophyll-a and diatoms were significantly positively correlated with POM, while haptophytes showed a negative correlation with proteins. The photochemical efficiency (Fv/Fm, no. of observations 20) showed significant negative correlations with POM components (hydrolysable carbohydrates, proteins and lipids).
In Ross Sea the correlations depended on the sub-area (no. of observations for POM variables: 18, for phytoplankton: 12). RS-North was the area with the highest number of significant correlations, despite hydrolysable carbohydrates and lipids showed trends that were often different. RS-South and RS-West showed the lowest number of significant correlations. Hydrolysable carbohydrates and lipids, again, showed trends that were seldom correlated to the other variables and often the correlations were weaker. The absence of correlation with haptophytes was generally observed. In all the areas, the few significant correlations found for Fv/Fm (due also to the very low no. of observations, Table 8) highlighted that the higher the photochemical efficiency, the lower the POM concentrations.
Discussion
During the austral summer 2017, the Ross Sea showed high spatial and temporal variability in terms of physical (e.g. UML depth changes, temperature and salinity variations, peculiar water mass presence), chemical (e.g. nutrient concentration variations) and biological characteristics (e.g. primary production and activity fluctuations, dominance of different phytoplanktonic functional groups) of the water column in different areas. All these variations drove the distribution of living and detrital POM, as well as its biochemistry and bioavailability.
It is well known that DOM and POM are mainly composed by biochemical components that can persist in the environment, showing a strong refractivity to degradation, and molecules that are subjected to biological consumption and chemical degradation (Hansell, 2013; Lønborg et al., 2017). Among these, proteins and carbohydrates are generally highly represented (Fraga, 2001; Benner, 2002). We found a very large fraction of POC (considered as bulk POM) built by proteins and carbohydrates in the offshore Ross Sea (on average 92 ± 21%) and in the coastal Terra Nova Bay (on average 62 ± 15%). Since the typology of the analyses for the determination of the concentrations is quite different, as reported in the “Materials and methods” section, these percentages should be interpreted with caution. Nevertheless, the data highlighted that the contribution of these kind of molecules was relevant, especially in the offshore area. The other biochemical components of POC (such as refractory compounds, fulvic and humic acids, for instance), particularly abundant in Terra Nova Bay compared to Ross Sea, indicated the accumulation in the coastal site of other substances deriving from the ageing and transformation of previously produced POM.
Proteins dominated in all the areas, with fluctuations in RS-South. The seawater particulate proteins are generally labile and palatable for the consumers (microbes and metazoans), while the organic compound rich in carbon (such as carbohydrates) is considered less bioavailable due to the preferential degradation of N and P rich compounds (Álvarez-Salgado et al., 2006; Lønborg & Álvarez-Salgado, 2012). Nevertheless, changes of the bioavailability can modify the actual value of the protein pool to consumers. For instance, Fabiano & Pusceddu (1998) found that in a shallow coastal station in Terra Nova Bay, 50% of the proteins was hydrolysable, while Misic et al. (2017) observed in Ross Sea that the hydrolysable contribution to the total protein concentration was only 35 %. However, the high protein concentrations we found during the austral summer 2017 indicate a large and diffused reservoir of high-trophic quality POM (Etcheber et al., 1999) that can meet the nutritional demand of the heterotrophic organisms (Pjontek et al., 2014; Zaccone et al., 2020).
Some help to determine the origin of these proteins was given by the multivariate analysis. We used RDA to highlight whether the explanatory variables had a role in the variations of the POM characteristics and, in case of significant role, which of them exerted the main effect. The RDA results showed that in the coastal Terra Nova Bay as well as the offshore Ross Sea, the POM was regulated by diatoms. The pivotal role of diatoms in POM characterisation was shown by previous studies, as diatoms were responsible of POC production during late summer, since the iron-limitation increases the C content of cells (Smith & Kaufman, 2018). It is well known that proteins contribute significantly to the diatom biomass (Young et al., 2015) especially during the exponential growth phase (Jo et al., 2021). This can explain the strong correlation between chlorophyll-a and the particulate proteins reported in the Ross Sea by Misic et al., 2017 and in the present study, and indicate diatoms as one of the main responsible of protein production. However, at station S43 (RS-West) a high protein concentration was associated with the presence of large choanoflagellate blooms (Escalera et al., 2019), in accordance with the protein composition of the lorica (Agatha & Simon, 2012).
The slightly higher protein concentrations in DL of RS-South may be due to the transfer of proteins to the sediments by faecal pellets (Isla et al., 2006). These materials represent a food resource for microbes, whose activities further increase the protein concentrations due to the production of protein-rich biomass (Povero et al., 2003). Microbial heterotrophic reworking of detrital POM increases the N content also of the autotrophic colonies (Carlson et al., 1998), especially during summer (Misic et al., 2017). In RS-North, together with the possible contribution of faecal pellets, the deepening of the UML due to bad wind-wave conditions could have favoured the downward transfer of freshly produced proteins from the upper part of the water layer to the depth.
Another result of the multivariate RDA was that haptophytes have had a role in the variability of the POM characteristics in the open Ross Sea, and the presence of P. antarctica is a common feature especially of RS-South during spring (Hong et al., 1997; Arrigo & Van Dijken, 2004). In RS-South and RS-West haptophytes did not correlate significantly with the POM fractions, because of the quite low biomass (lower values of chlorophyll-a than in RS-North, for instance) and activity. Nevertheless, the qualitative features of POM could depend also on an indirect influence of haptophytes, based on the biology of this phytoplanktonic group. In general, phytoplankton produce and release significant quantities of carbohydrates, in relation to growth phase and events of nutrient limitation (Alderkamp et al., 2007). Phaeocystis genus is known to exudate huge amounts of mucopolysaccharides during colonial stage (Alderkamp et al., 2007) mostly during the stationary growth phase (contribution of carbohydrate to the entire colony up to 60%). Kim et al. (2018) observed high carbohydrate concentrations in the Amundsen Sea in the presence of P. antarctica blooms. Therefore, we believe that the higher carbohydrate concentrations in RS-South depends on decaying haptophytes blooms, as indicated by the low Fv/Fm values suggesting a senescent phytoplanktonic biomass.
Arrieta and Herndl (2002) showed that during the collapse of large Phaeocystis blooms in the North Sea the β-glucosidase activity was significantly higher, indicating that the OM of degraded colonies was hydrolysable by this kind of enzyme. Complex carbohydrates such as cellulose constitute the structure of the cell wall and generally induce the activity of β-glucosidase that is the hydrolytic enzyme that has been used to hydrolyse the carbohydrates in the present study. β-glucosidase is known to hydrolyse also other substrates, such as β-d-galactosides, α-l-arabinosides, β-d-xylosides and β-d-fucosides (Piontek et al., 2014). Therefore, our hydrolysable carbohydrates can be a variety of molecules and not only the sole cellulose, increasing the value of this kind of variable in ecological studies related to trophodynamic.
In the RS-South, hydrolysable carbohydrates correlated with lipids. It has been reported that older OM have a higher lipid content (Rios et al., 1998; Volkman & Tanoue, 2002). Therefore, a higher concentration of lipids, and a higher contribution of lipid C to POC, was a signal of aged POM in RS-South. This agrees with the seasonal biological processes, starting in spring along the Ross Ice Shelf and developing northward following the opening of the Ross Sea summer polynya (Smith et al., 2007). Therefore, the correlation between lipids and hydrolysable carbohydrates found in RS-South supported the hypothesis that this kind of carbohydrates was the remnant of a previous production activity. Following this hypothesis, a significant correlation of hydrolysable carbohydrates with the living P. antarctica was not expected. This hypothesis fits also with what we found at S43 (RS-West), where a quite large hydrolysable carbohydrate concentration was found (like RS-South especially in the UML), despite the contribution of Haptophytes was very low (below 2% in the UML). S43 showed physical and biological properties similar to the eastern station of Terra Nova Bay, but the presence of high hydrolysable carbohydrate concentration in UML and DL can be interpreted as a signal of previous biological development similar to that of the offshore RS-South.
A notable activity of microbial β-glucosidase was recorded by Zaccone et al. (2020) during the austral summer of 2017 in the Ross Sea. Since the expression of this kind of activity is energy expensive because it requires a synergic action of different enzymatic systems (Celussi et al., 2015), the environmental conditions should have been very favourable. POM composition could have had a role. The high protein concentrations available to microbes, in fact, could have supported the enhanced glycolytic activities. In addition, a higher lability/palatability of carbohydrates could have given a higher than usual gain to consumers. In fact, in the present study the contribution of hydrolysable carbohydrates to the total carbohydrates was 25.7 ± 18.3% in Terra Nova Bay and 31.8 ± 19.5% in Ross Sea, vs 13.1 ± 10.8% in a similar area during summer 2014 (Misic et al., 2017).
Conclusions
The Ross Sea has been described as a mosaic of different sub-systems following alternative pathways of primary production (Smith et al., 2014; Bolinesi et al., 2020a, b). This observation fits with our results, as the characteristics of POM reflect the extent of phytoplankton blooms and their composition in terms of dominant groups. In this study, we have observed that a higher contribution of haptophytes in the southern sector of the Ross Sea (RS-South), during spring and during summer, influenced the POM characteristics, increasing the availability of POM to exploitation and consumption especially by bacterial community in the surface water masses. One of the main questions dealing with the Southern Ocean is the degree of variation of biogeochemical processes related to climatic changes. These changes are known to directly affect the phytoplankton community in terms of biomass and functional groups (Mangoni et al., 2019; Park et al., 2019). Some studies suggested the possible success of P. antarctica in outcompeting diatoms that are more sensible to changes of light and Fe availability (Trimborn et al., 2019). Whether this scenario is going to occur in the next years is still under debate. However, if this will be true, the potential more hydrolysable POM resulting from a P. antarctica dominance will favour the transfer of C towards upper trophic level and decrease its storage in the deep layers, limiting the sink efficiency of the Southern Ocean.
Data availability
All data generated or analysed during this study are included in this published article.
References
Agatha, S. & P. Simon, 2012. On the Nature of Tintinnid Loricae (Ciliophora: Spirotricha: Tintinnina): a histochemical, enzymatic, EDX, and high-resolution TEM study. Acta Protozoologica 51: 1–19.
Alderkamp, A. C., A. G. J. Buma & M. Van Rijssel, 2007. The carbohydrates of Phaeocystis and their degradation in the microbial food web. Biogeochemistry 83: 99–118. https://doi.org/10.1007/s10533-007-9078-2.
Álvarez-Salgado, X. A., M. Nieto-Cid, J. Gago, S. Brea, C. G. Castro, M. D. Doval & F. F. Pérez, 2006. Stoichiometry of the degradation of dissolved and particulate biogenic organic matter in the NW Iberian upwelling. Journal of Geophysical Research 111: C07017. https://doi.org/10.1029/2004JC002473.
Arrieta, J. M. & G. J. Herndl, 2002. Changes in bacterial beta-glucosidase diversity during a coastal phytoplankton bloom. Limnology and Oceanography 47: 594–599. https://doi.org/10.4319/lo.2002.47.2.0594.
Arrigo, K. R. & G. L. van Djiken, 2004. Annual changes in sea-ice, chlorophyll a, and primary production in the Ross Sea, Antarctica. Deep-Sea Research I 51: 117–138. https://doi.org/10.1016/j.dsr2.2003.04.003.
Arrigo, K. R., G. L. Van Dijken & M. Long, 2008. Coastal Southern Ocean: a strong anthropogenic CO2 sink. Geophysical Research Letters 35: L21602. https://doi.org/10.1029/2008GL035624.
Benner, R., 2002. Chemical composition and reactivity. In Hansell, D. A. & C. A. Carlson (eds), Biogeochemistry of Marine Dissolved Organic Matter Academic Press, San Diego: 90.
Benner, R. & R. M. Amon, 2015. The size-reactivity continuum of major bioelements in the Ocean. Annual Review of Marine Science 7: 185–205. https://doi.org/10.1146/annurev-marine-010213-135126.
Bligh, E. G. & W. J. Dyer, 1959. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology 37: 911–917.
Bolinesi, F., M. Saggiomo, F. Ardini, P. Castagno, A. Cordone, G. Fusco, P. Rivaro, V. Saggiomo & O. Mangoni, 2020. Spatial-related community structure and dynamics in phytoplankton of the Ross Sea. Antarctica. Frontiers in Marine Science 7: 1092. https://doi.org/10.3389/fmars.2020.574963.
Bolinesi, F., M. Saggiomo, S. Aceto, A. Cordone, E. Serino, M. C. Valoroso & O. Mangoni, 2020. On the relationship between a novel Prorocentrum sp. and Colonial Phaeocystis antarctica under iron and vitamin B12 limitation: ecological implications for Antarctic waters. Applied Sciences 10(19): 6965. https://doi.org/10.3390/app10196965.
Carlson, C. A., H. W. Ducklow, D. A. Hansell & W. O. Smith, 1998. Organic carbon partitioning during spring phytoplankton blooms in the Ross Sea polynya and the Sargasso Sea. Limnology and Oceanography 43: 275–386. https://doi.org/10.4319/lo.1998.43.3.0375.
Caron, D. A., M. R. Dennett, D. J. Lonsdale, D. M. Moran & L. Shalapyonok, 2000. Micro-zooplankton herbivory in microzooplankton herbivory in the Ross Sea, Antarctica. Deep-Sea Research II 47: 15–16. https://doi.org/10.1016/S0967-0645(00)00067-9.
Castagno, P., V. Capozzi, G. R. DiTullio, P. Falco, G. Fusco, S. R. Rintoul, G. Spezie & G. Budillon, 2019. Rebound of shelf water salinity in the Ross Sea. Nature Communications 10: 5441. https://doi.org/10.1038/s41467-019-13083-8.
Celussi, M., C. Fabbro, M. Bastianini, R. Urbani & P. Del Negro, 2015. Polysaccharide degradation and utilisation during a spring phytoplankton bloom in the northwestern Adriatic Sea. Hydrobiologia 757: 209–222. https://doi.org/10.1007/s10750-015-2253-x.
Clarke, K. R. & R. M. Warwick, 2001. Changes in Marine Communities: An Approach to Statistical Analysis and Interpretation,. Second PRIMER-E, Plymouth:
Cullen, J. J. & R. F. Davis, 2003. The blank can make a big difference in oceanographic measurements. Limnology and Oceanography Bulletin 12: 29–35. https://doi.org/10.1002/lob.200312229.
Dell’Anno, A., M. Fabiano, M. L. Mei & R. Danovaro, 2000. Enzymatically hydrolysed protein and carbohydrate pools in deep-sea sediments estimates of the potentially bioavailable fraction and methodological considerations. Marine Ecology-Progress Series 196: 15–23. https://doi.org/10.3354/meps196015.
Delmont, T. O., K. M. Hammar, H. W. Ducklow, P. L. Yager & A. F. Post, 2014. Phaeocystis antarctica blooms strongly influence bacterial community structures in the Amundsen Sea polynya. Frontiers in Microbiology 5: 646. https://doi.org/10.3389/fmicb.2014.00646.
Dubois, M., K. A. Gilles, J. K. Hamilton, P. A. Rebers & F. Smith, 1956. Colorimetric method for determination of sugars and related substances. Analytical Chemistry 28: 350–356.
Dunbar, R. B., A. R. Leventer & D. A. Mucciarone, 1998. Water column sediment fluxes in the Ross Sea, Antarctica: atmospheric and sea ice forcing. Journal of Geophysical Research 103: 741–759. https://doi.org/10.1029/1998JC900001.
Dunbar, R.B., K.R. Arrigo, M. Lutz, G.R. DiTullio, A.R. Leventer, M.P. Lizotte, M.P. Van Woert & D.H. Robinson, 2003. In: DiTullio, G.R. & R.B. Dunbar (eds). Non-Redfield production and export of marine organic matter: a recurrent part of the annual cycle in the Ross Sea, Antarctica. Biochemistry of the Ross Sea, Antarctic Research Series, vol. 78, pp. 179–195.
Escalera, L., O. Mangoni, F. Bolinesi & M. Saggiomo, 2019. Austral summer bloom of loricate Choanoflagellates in the Central Ross Sea Polynya. Journal of Eukaryotic Microbiology 66: 849–852. https://doi.org/10.1111/jeu.12720.
Etcheber, H., J.-C. Relexans, M. Beliard, O. Weber, R. Buscail & S. Heussner, 1999. Distribution and quality of sedimentary organic matter on the Aquitanian margin (Bay of Biscay). Deep-Sea Res. 46: 2249–2288.
Fabiano, M. & A. Pusceddu, 1998. Total and hydrolizable particulate organic matter (carbohydrates, proteins and lipids) at a coastal station in Terra Nova Bay (Ross Sea, Antarctica). Polar Biology 19: 125–132. https://doi.org/10.1007/s003000050223.
Fraga, F., 2001. Phytoplankton biomass synthesis: application to deviations from Redfield stoichiometry. Scientia Marina 65: 153–169.
Gordon, D. C., Jr., 1970. Some studies on the distribution and composition of particulate organic carbon in the North Atlantic Ocean. Deep-Sea Research 17: 233–243. https://doi.org/10.1016/0011-7471(70)90017-3.
Gruber, N., M. Gloor, F. S. E. Mikaloff, S. C. Doney, S. Dutkiewicz, M. J. Follows, M. Gerber, A. R. Jacobson, F. Joos, K. Lindsay, D. Menemenlis, A. Mouchet, S. A. Müller, J. L. Sarmiento & T. Takahashi, 2009. Oceanic sources, sinks, and transport of atmospheric CO2. Global Biogeochemical Cycles 23: 1–21. https://doi.org/10.1029/2008GB003349.
Haberman, K. L., R. M. Ross & L. B. Quetin, 2003. Diet of the Antarctic krill (Euphausia superba Dana): II. Selective grazing in mixed phytoplankton assemblages. Journal of Experimental Marine Biology and Ecology 283: 97–113. https://doi.org/10.1016/S0022-0981(02)00467-7.
Hansell, D. A., 2013. Recalcitrant dissolved organic carbon fractions. Annual Review of Marine Science 5: 421–445. https://doi.org/10.1146/annurev-marine-120710-100757.
Hansen, H. P. & K. Grasshoff, 1983. Automated chemical analysis. In Grasshoff, K., M. Ehrardt & K. Kremlin (eds), Methods of Seawater Analysis Chemie, Weinheim: 347–379.
Hartree, E. F., 1972. Determination of proteins: a modification of the Lowry method that gives a linear photometric response. Analytical Biochemistry 48: 422–427. https://doi.org/10.1016/0003-2697(72)90094-2.
Hedges, J. I. & J. H. Stern, 1984. Carbon and nitrogen determination of carbonate-containing solids. Limnology and Oceanography 29: 657–663. https://doi.org/10.4319/lo.1984.29.3.0657.
Heiden, J. P., K. Bischof & S. Trimborn, 2016. Light intensity modulates the response of two Antarctic diatom species to ocean acidification. Frontiers in Marine Science 3: 260. https://doi.org/10.3389/fmars.2016.00260.
Holm-Hansen, O., C. J. Lorenzen, R. W. Holmes & J. D. H. Strickland, 1965. Fluorometric determination of chlorophyll. ICES Journal of Marine Science 30: 3–15. https://doi.org/10.1093/icesjms/30.1.3.
Hong, Y., W. O. Smith & A. M. White, 1997. Studies on transparent exopolymer particles (TEP) produced in the Ross Sea (Antarctica) and by Phaeocystis antarctica (Prymnesiophyceae). Journal of Phycology 33: 368–376. https://doi.org/10.1111/j.0022-3646.1997.00368.x.
Hyun, J.-H., S.-H. Kim, E. J. Yang, A. Choi & S. H. Lee, 2016. Biomass, production, and control of heterotrophic bacterioplankton during a late phytoplankton bloom in the Amundsen Sea polynya, Antarctica. Deep-Sea Research Part II 123: 102–112. https://doi.org/10.1016/j.dsr2.2015.10.001.
Isla, E., S. Rossi, A. Palanques, J.-M. Gili, D. Gerdes & W. Arntz, 2006. Biochemical composition of marine sediment from the eastern Weddell Sea (Antarctica): high nutritive value in a high benthic-biomass environment. Journal of Marine Systems 60: 255–267. https://doi.org/10.1016/j.jmarsys.2006.01.006.
Jo, N., H. S. La, J.-H. Kim, K. Kim, B. K. Kim, M. J. Kim, W. Son & S. H. Lee, 2021. Different biochemical compositions of particulate organic matter driven by major phytoplankton communities in the northwestern Ross Sea. Frontiers in Microbiology 12: 623600. https://doi.org/10.3389/fmicb.2021.623600.
Kharbush, J. J., H. G. Close, B. A. S. Van Mooy, C. Arnosti, R. H. Smittenberg, F. A. C. Le Moigne, G. Mollenhauer, B. Scholz-Böttcher, I. Obreht, B. P. Koch, K. W. Becker, M. H. Iversen & W. Mohr, 2020. Particulate organic carbon deconstructed: molecular and chemical composition of particulate organic carbon in the ocean. Frontiers in Marine Science 7: 518. https://doi.org/10.3389/fmars.2020.00518.
Kim, B. K., S. H. Lee, S.-Y. Ha, J. Jung, T. W. Kim, E. J. Yang, N. Jo, J. Park & S. H. Lee, 2018. Vertical distributions of macromolecular composition of particulate organic matter in the water column of the Amundsen Sea Polynya during the summer in 2014. Journal of Geophysical Research: Oceans 123: 1393–1405. https://doi.org/10.1002/2017JC013457.
Kropuenske, L. R., M. M. Mills, G. L. van Dijken, S. Bailey, D. H. Robinson, N. A. Welschmeyer & K. R. Arrigo, 2009. Photophysiology in two major Southern Ocean phytoplankton taxa: photoprotection in Phaeocystis antarctica and Fragilariopsis cylindrus. Limnology and Oceanography 54: 1176–1196. https://doi.org/10.4319/lo.2009.54.4.1176.
Lønborg, C. & X. A. Álvarez-Salgado, 2012. Recycling versus export of bioavailable dissolved organic matter in the coastal ocean and efficiency of the continental shelf pump. Global Biogeochemical Cycles. https://doi.org/10.1029/2012GB004353.
Lønborg, C., J. Doyl, M. Furnasa, P. Menendez, J. A. Benthuysen & C. Carreira, 2017. Seasonal organic matter dynamics in the Great Barrier Reef lagoon: contribution of carbohydrates and proteins. Continental and Shelf Research 138: 95–105. https://doi.org/10.1016/j.csr.2017.01.010.
Mangoni, O., V. Saggiomo, F. Bolinesi, F. Margiotta, G. Budillon, Y. Cotroneo, C. Misic, P. Rivaro & M. Saggiomo, 2017. Phytoplankton blooms during austral summer in the Ross Sea, Antarctica: driving factors and trophic implications. PLoS ONE 12(4): e0176033. https://doi.org/10.1371/journal.pone.0176033.
Mangoni, O., M. Saggiomo, F. Bolinesi, M. Castellano, P. Povero, V. Saggiomo & G. R. DiTullio, 2019. Phaeocystis antarctica unusual summer bloom in stratified Antarctic coastal waters (Terra Nova Bay, Ross Sea). Marine Environmental Research 151: 104733. https://doi.org/10.1016/j.marenvres.2019.05.012.
Marsay, C. M., R. J. Sanders, S. A. Henson, K. Pabortsava, E. P. Achterberg & R. S. Lampitt, 2015. Attenuation of sinking particulate organic carbon flux through the mesopelagic ocean. Proceedings of the National Academy of Sciences USA 112: 1089–1094. https://doi.org/10.1073/pnas.1415311112.
Marsh, J. B. & D. B. Weinstein, 1966. A simple charring method for determination of lipids. Journal of Lipid Research 7: 574–576.
Mathot, S., Jr., W. O. Smith, C. A. Carlson & D. L. Garrison, 2000. Estimate of Phaeocystis sp. carbon biomass: methodological problems related to the mucilaginous nature of the colonial matrix. Journal of Phycology 36: 1049–1056.
Maxwell, K. & G. N. Johnson, 2000. Chlorophyll fluorescence—a practical guide. Journal of Experimental Botany 51: 659–668. https://doi.org/10.1093/jexbot/51.345.659.
Mayer, L. M., L. L. Schick, T. Sawyer, C. J. Plante, P. A. Jumars & R. L. Self, 1995. Bioavailable amino acids in sediments: a biomimetic, kinetics, based approach. Limnology and Oceanography 40: 511–520. https://doi.org/10.4319/lo.1995.40.3.0511.
Misic, C., A. Covazzi Harriague, O. Mangoni, Y. Cotroneo, G. Aulicino & P. Castagno, 2017. Effects of physical constraints on the lability of POM during summer in the Ross Sea. Journal of Marine Systems 166: 132–143. https://doi.org/10.1016/j.jmarsys.2016.06.012.
Orsi, A. H. & C. L. Wiederwohl, 2009. A recount of Ross Sea waters. Deep Sea Research Part II 56: 778–795. https://doi.org/10.1016/j.dsr2.2008.10.033.
Park, J., J.-H. Kim, H.-C. Kim, J. Hwang, Y.-H. Jo & S. H. Lee, 2019. Environmental forcings on the remotely sensed phytoplankton bloom phenology in the central Ross Sea Polynya. Journal of Geophysical Research: Oceans 124: 5400–5417. https://doi.org/10.1029/2019JC015222.
Peperzak, L., F. Colijn, E. G. Vrieling, W. W. C. Gieskes & J. C. H. Peeters, 2000. Observations of flagellates in colonies of Phaeocystis globosa (Prymnesiophyceae); a hypothesis for their position in the life cycle. Journal of Plankton Research 22: 2181–2203. https://doi.org/10.1093/plankt/22.12.2181.
Piontek, J., M. Sperling, E. M. Nothing & A. Engel, 2014. Regulation of bacterioplankton activity in Fram Strait (Arctic Ocean) during early summer: the role of organic matter supply and temperature. Journal of Marine Systems 132: 83–94. https://doi.org/10.1016/j.jmarsys.2014.01.003.
Povero, P., C. Misic, C. Ossola, M. Castellano & M. Fabiano, 2003. The trophic role and ecological implications of oval faecal pellets in Terra Nova Bay (Ross Sea). Polar Biology 26: 302–310. https://doi.org/10.1007/s00300-003-0485-0.
Povero, P., M. Castellano, N. Ruggieri, L. S. Monticelli, V. Saggiomo, M. Chiantore & R. Cattaneo-Vietti, 2006. Water column features and their relationship with sediments and benthic communities along the Victoria Land coast, Ross Sea, summer 2004. Antarctic Science 18: 603–613. https://doi.org/10.1017/S0954102006000642.
Rios, A. F., F. Fraga, F. F. Perez & F. G. Figueiras, 1998. Chemical composition of phytoplankton and particulate organic matter in the Ria de Vigo. Scientia Marina 62: 257–271. https://doi.org/10.3989/scimar.1998.62n3257.
Rivaro, P., C. Ianni, E. Magi, S. Massolo, G. Budillon & W. M. Jr Smethie, 2015. Distribution and ventilation of water masses in the western Ross Sea inferred from CFC measurements. Deep-Sea Research Part I 97: 19–28. https://doi.org/10.1016/j.dsr.2014.11.009.
Rivaro, P., C. Ianni, L. Langone, C. Ori, G. Aulicino, Y. Cotroneo, M. Saggiomo & O. Mangoni, 2017. Physical and biological forcing on the mesoscale variability of the carbonate system in the Ross Sea (Antarctica) during the summer season 2014. Journal of Marine Systems 166: 144–158. https://doi.org/10.1016/j.jmarsys.2015.11.002.
Rivaro, P., F. Ardini, D. Vivado, R. Cabella, P. Castagno, O. Mangoni & P. Falco, 2020. Potential sources of particulate iron in surface and deep waters of the Terra Nova Bay (Ross Sea, Antarctica). Water 12: 3517. https://doi.org/10.3390/w12123517.
Schine, C. M. S., G. L. van Dijken & K. R. Arrigo, 2015. Spatial analysis of trends in primary production and relationship with large-scale climate variability in the Ross Sea, Antarctica (1997–2013). Journal of Geophysical Research 121: 368–386. https://doi.org/10.1002/2015JC011014.
Smith, W. O., Jr. & V. L. Asper, 2001. The influence of phytoplankton assemblage composition on biogeochemical characteristics and cycles in the southern Ross Sea, Antarctica. Deep-Sea Research Part I 48: 137–161. https://doi.org/10.1016/S0967-0637(00)00045-5.
Smith, W. O., Jr. & D. E. Kaufman, 2018. Climatological temporal and spatial distributions of nutrients and particulate matter in the Ross Sea. Progress in Oceanography 168: 192–185. https://doi.org/10.1016/j.pocean.2018.10.003.
Smith, W. O., Jr., J. Marra, M. R. Hiscock & R. T. Barber, 2000. The seasonal cycle of phytoplankton biomass and primary productivity in the Ross Sea, Antarctica. Deep-Sea Research Part II 47: 3119–3140. https://doi.org/10.1016/S0967-0645(00)00061-8.
Smith, W. O., Jr., A. R. Shields, J. A. Peloquin, G. Catalano, S. Tozzi & M. S. Dinniman, 2006. Interannual variations in nutrients, net community production, and biogeochemical cycles in the Ross Sea. Deep-Sea Research Part II 53: 815–833. https://doi.org/10.1016/j.dsr2.2006.02.014.
Smith, W. O., Jr., D. G. Ainley & R. Cattaneo Vietti, 2007. Trophic interactions within the Ross Sea continental shelf ecosystem. Philosophical Transactions of the Royal Society B 362: 95–111. https://doi.org/10.1098/rstb.2006.1956.
Smith, W. O., Jr., V. Asper, S. Tozzi, X. Liu & S. E. Stammerjohn, 2011. Surface layer variability in the Ross Sea, Antarctica as assessed by in situ fluorescence measurements. Progress in Oceanography 88: 28–45. https://doi.org/10.1016/j.pocean.2010.08.002.
Smith, W. O., Jr., A. R. Shields & J. Dreyer, 2011. Interannual variability in vertical export in the Ross Sea: magnitude, composition, and environmental correlates. Deep-Sea Research Part I 58: 147–159. https://doi.org/10.1016/j.dsr.2010.11.007.
Smith, W. O., Jr., D. G. Ainley, K. R. Arrigo & M. S. Dinniman, 2014. The oceanography and ecology of the Ross Sea. Annual Review in Marine Science 6: 469–87. https://doi.org/10.1146/annurev-marine-010213-135114.
Tagliabue, A. & K. R. Arrigo, 2003. Anomalously low zooplankton abundance in the Ross Sea: an alternative explanation. Limnology and Oceanography 48: 686–699. https://doi.org/10.4319/lo.2003.48.2.0686.
Trimborn, S., S. Thoms, K. Bischof & S. Beszteri, 2019. Susceptibility of two Southern Ocean phytoplankton key species to iron limitation and high light. Frontiers in Marine Science 6: 167. https://doi.org/10.3389/fmars.2019.00167.
Vidussi, F., H. Claustre, J. Bustillos-Guzman, C. Cailliau & J. C. Marty, 1996. Determination of chlorophylls and carotenoids of marine phytoplankton: separation of chlorophyll a from divinylchlorophyll a and zeaxanthin from lutein. Journal of Plankton Research 18: 2377–2382. https://doi.org/10.1093/plankt/18.12.2377.
Volkman, J. K. & E. Tanoue, 2002. Chemical and biological studies of particulate organic matter in the ocean. Journal of Oceanography 58: 265–279. https://doi.org/10.1023/A:1015809708632.
Williams, C. M., A. M. Dupont, J. Loevenich, A. F. Post, J. Dinasquet & P. L. Yager, 2016. Pelagic microbial heterotrophy in response to a highly productive bloom of Phaeocystis antarctica in the Amundsen Sea Polynya, Antartica. Elementa: Science of Anthropocene 4: 000102. https://doi.org/10.12952/journal.elementa.000102.
Young, J. N., J. A. L. Goldman, S. A. Kranz, P. D. Tortell & F. M. M. Morel, 2015. Slow carboxylation of Rubisco constrains the rate of carbon fixation during Antarctic phytoplankton blooms. New Phytologist 205: 172–181. https://doi.org/10.1111/nph.13021.
Zaccone, R., C. Misic, F. Azzaro, M. Azzaro, G. Maimone, O. Mangoni, G. Fusco, A. C. Rappazzo & R. La Ferla, 2020. Regulation of microbial activity rates by organic matter in the Ross Sea during the Austral Summer 2017. Microorganisms 8: 1273. https://doi.org/10.3390/microorganisms8091273.
Zuur, A., E.N. Ieno & G.M. Smith, 2007. Statistics for Biology and Health. Gail, M. & J. M. Samet, (eds) Springer, Berlin.
Acknowledgements
This study was funded by the Italian National Antarctic Program (PNRA), in the framework of the projects P-ROSE (PNRA16_00239) and CELEBeR (PNRA16_00207). We thank the officers, crew, and technical personnel on board the R/V Italica.
Funding
Open access funding provided by Università degli Studi di Genova within the CRUI-CARE Agreement. Funding was provided by Progetto Nazionale Ricerca in Antartide (Italy) (Grant Nos. PNRA16_00239, PNRA16_00207).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling editor: Diego Fontaneto
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Misic, C., Bolinesi, F., Castellano, M. et al. Factors driving the bioavailability of particulate organic matter in the Ross Sea (Antarctica) during summer. Hydrobiologia 851, 2657–2679 (2024). https://doi.org/10.1007/s10750-024-05482-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-024-05482-w