Abstract
Freshwater salinization increasingly threatens river ecosystems in arid regions. In situ studies on effects of salinity on freshwater communities are still scarce, especially in largely understudied areas of Africa. To compare macroinvertebrate communities in differing salinity levels, we conducted a confluence-based study in the Draa River basin in Morocco by focusing on two tributaries and their joint downstream sections, in the immediate vicinity of three confluences. Our study revealed that α-diversity differed only minimal. Although only around five taxa comprised over 90% of specimens per section, the more saline sections exhibited proportionally more salt-tolerant generalist species. There was lower β-diversity between the downstream section and each tributary compared to between tributaries, indicating a mixed community after the confluence. The trait profile of the saline El Mellah displayed more resistance and resilience traits to disturbances than the less saline Iriri. Furthermore, low water flow reduced the abundance of sensitive taxa. Overall, we observed minimal differences in macroinvertebrate community composition, due to low γ-diversity in the basin. However, the confluence-based study design remains valuable for investigating effects of specific stressors on ecosystems by excluding large-scale geographic patterns, as compared sites are close and therefore share the same climate, geology, and altitude.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite increasing scientific interest in the issue of freshwater salinization in recent years, there are still significant knowledge gaps on the effects of salinization on ecosystems, particularly in less studied regions such as Africa (Cunillera-Montcusí et al., 2022). Saline rivers are common in semi-arid and arid regions with endorheic basins, and result from the so-called primary salinization; the release of salts from rocks and soils into the water, followed by solar evaporation (Williams, 1999; Warner et al., 2013). Increasingly arid conditions induced by climate change (Beck et al., 2018) and anthropogenic activities (i.e., secondary salinization), such as the use of saline freshwater for irrigation, cause further salinization of freshwater ecosystems (Williams, 1999; Cañedo-Argüelles et al., 2013; Herbert et al., 2015; Hssaisoune et al., 2020). This poses a growing threat to biotic communities and the functions and services they provide, which also threatens human well-being (Cañedo-Argüelles et al., 2016; Berger et al., 2019; Cunillera-Montcusí et al., 2022; Kaczmarek et al., 2023). Increasing water salinity diminishes the quality and usability of water and thus affects aquatic communities through a loss of saline-sensitive species or a change in the community composition (Cañedo-Argüelles et al., 2013; Herbert et al., 2015).
Macroinvertebrates play a crucial role in river ecosystems (Wallace & Webster, 1996), yet high salinity can negatively impact them at individual, population, community, and ecosystem levels (Cañedo-Argüelles et al., 2013). High salinity levels limit macroinvertebrate development, growth, and fertility by increasing stress and the energy required for osmoregulation (Cañedo-Argüelles et al., 2013; Kefford et al., 2016). Saline and intermittent river ecosystems are mainly home to highly adapted organisms that can withstand harsh environmental conditions and extreme changes (Millán et al., 2011). Variations in species' responses to elevated salinity levels alter the composition of the entire community (Cañedo-Argüelles et al., 2013), resulting in a decline in abundance, or local extinction, of sensitive taxa and an advantage for salt-tolerant species that may benefit from reduced competition and predation (Velasco et al., 2006; Kefford et al., 2016). Resistance and resilience traits for adaptation to high salinities, such as small body size, multi-voltinism, and ovoviviparity, promote survival in saline conditions (Piscart et al., 2006; Díaz et al., 2008; Kaczmarek et al., 2021). Additionally, a lower abundance of scrapers and piercers and higher abundance of filter and deposit feeders have been associated with higher salinity levels (Piscart et al., 2006).
Tributaries with lower salinity levels in saline river basins serve as important refuges for salt-sensitive species (Robson et al., 2013; Benson et al., 2019), and often exhibit distinct macroinvertebrate community compositions compared to their saline counterparts (Kefford, 1998). They also play a role in reducing downstream salinity through dilution. However, water withdrawals for agricultural use and the return of salinized water (Thorslund et al., 2021) can compromise their ability to dilute and to serve as refuge. On the other hand, naturally saline ecosystems harbor unique species adapted to high salinities that can colonize habitats affected by secondary salinization (Velasco et al., 2006; Kefford et al., 2016). Therefore, it is essential for the conservation of macroinvertebrate communities to consider protecting both fresh and saline rivers (Cañedo-Argüelles et al., 2013, 2016) and the connectivity of entire stream networks of a basin.
The composition of macroinvertebrate communities is shaped by multiple environmental conditions, such as differences in climate, the presence or absence of different mineral and organic substrates, and anthropogenic impacts such as pollution. Such environmental conditions change naturally, especially on a large geographic scale (i.e., river basin wide changes in habitat characteristics; Kefford, 1998; Heino et al., 2004). Laboratory or mesocosm experiments can be used to study the effects of specific stressors on biotic communities in isolation of confounding factors. However, they can only reflect the natural environmental conditions to a certain extent and results are often influenced by the selection of the initial community composition (Kefford et al., 2021). Therefore, results do not necessarily predict effects in natural ecosystems (Kefford et al., 2023). Kefford (1998) implemented a paired difference design to study the effect of salinity on the dissimilarity between macroinvertebrate communities in situ at closely spaced pairs of saline and non-saline tributaries. In his study design, macroinvertebrate communities were not influenced by large-scale geographic patterns. Therefore, the design proved to be useful for investigating dissimilarities in community structure associated with salinity (Kefford, 1998).
Following the study design of Kefford (1998), we conducted an in situ study in the Draa river basin in southern Morocco with the aim of providing further insights into the effects of salinity on the composition of macroinvertebrate communities and their trait profiles in the immediate vicinity of confluences, while excluding large-scale geographic patterns (i.e., investigation of large differences in salinity on a small spatial scale of a few hundred meters to exclude the effects of climate, geology, and altitude). To do so, we compared the macroinvertebrate communities at two confluences with tributary pairs of similar size but differing salinity levels and one confluence with tributaries of similar salinity levels. In contrast to Kefford (1998), we also included the joint downstream section of each confluence to receive additional insights into community responses after the two tributaries merge, as intermediate salinity levels, dispersal-related processes (e.g., dispersal from source sites by macroinvertebrate drift) could result in a combination of communities (Cellot, 1996; Heino, 2013).
We hypothesize (1) that the tributaries with higher electrical conductivity show lower macroinvertebrate taxon richness compared to the less saline tributaries, due to the exclusion of salt-sensitive taxa that are not able to survive or complete their life cycles in saline environments (Kefford et al., 2016). We further hypothesize (2) that beta-diversity between upstream macroinvertebrate communities is higher than between up- and downstream communities, based on the coexistence of salt-tolerant and salt-sensitive taxa, dispersal-related processes, and source-sink dynamics (Cellot, 1996; Velasco et al., 2006; Heino, 2013). We expect community composition consisting of more and a higher abundance of generalist taxa (e.g., Diptera, Coleoptera, Hemiptera) adapted to saline conditions in the more saline tributaries, while we expect to find more sensitive taxa (e.g., sensitive taxa of Ephemeroptera and Trichoptera) in the less saline tributaries (Kefford et al., 2016). Therefore, we hypothesize (3) that we find a larger proportion of traits associated with resilience and resistance to disturbances (e.g., smaller size and multivoltine species) in the trait profiles of the macroinvertebrate communities of the more saline tributary, compared to the less saline one, as these traits are needed to guarantee survival in saline environments (Díaz et al., 2008).
Materials and methods
Study area
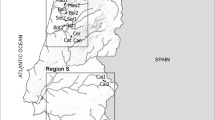
The Draa River flows through a semi-arid to arid basin in southern Morocco, situated south of the High Atlas Mountains and originates in the Barrage El Mansour Eddahbi (BEME) reservoir (Fig. 1). Tributaries to the BEME reservoir in the more mountainous Upper Draa basin, such as the rivers Dades, M'Goun, and Iriri typically have low levels of salinity. An exception is the saline El Mellah river, which can reach electrical conductivity (EC) levels of up to 20,000 µS/cm through primary salinization from the release of ions from rocks and soils into the water (Warner et al., 2013; own measurements). Tributaries to the Draa River downstream of the BEME reservoir, such as Tissint river, generally have higher salinity levels due to an increasingly arid climate (i.e., higher evaporation) in the lower reaches of the basin close to the Saharan desert (Warner et al., 2013). While secondary salinization due to irrigation practices is a growing problem in rivers worldwide (Cañedo-Argüelles, 2013) and increasingly in the Middle Draa (Warner et al., 2013; Moumane et al., 2021), the salinity levels in the confluences studied can most likely mainly be attributed to natural causes (Warner et al., 2013). The macroinvertebrate fauna of the region comprises mostly genera that are commonly found in Europe, with a limited number of additional Afrotropical species (Beauchard et al., 2003).
Site selection
Two confluences in the Draa river basin were selected, with tributaries per confluence characterized by similar flow rate and differing EC levels (Fig. 1). In the first confluence (A, altitude = 583 m), the mean EC levels were 5610 ± 20 µS/cm (A1) and 10,180 ± 1150 µS/cm (A2). In the second confluence (B, 1191 m), the mean EC levels were 810 ± 60 µS/cm (B1) and 2440 ± 280 µS/cm (B2). A third confluence (C, 1357 m) was selected, characterized by similar EC levels (1160 ± 200 µS/cm (C1) to 1280 ± 20 µS/cm (C2)) for the tributaries, but the flow rate varied by 13.4 times (0.014 to 0.188 m3/s). The three confluences differed by large-scale geographic patterns such as altitude and climate. Due to changes of habitat characteristics within small distances per confluence, we selected three 50-m-long sites within each tributary (A1, B1, and C1 with lower and A2, B2, and C2 with higher salinity; Fig. 1) and downstream section (A3, B3, C3). Distances between sites ranged between 50 and 300 m (Fig. 1). Sites were selected to be comparable to the sites selected in the other sections of each confluence (e.g., comparable low and high flow sites in A1, A2, and A3) and pooled afterward to account for a larger variety of site conditions per section. Sampling took place in June 2022 and September/October 2022 to capture potential seasonal variation of the macroinvertebrate communities over seasons. All rivers were considered permanent based on literature (Cappy, 2007) and own observations in recent years, with some variation in water flow depending on seasons and rain events (Cappy, 2007). However, a prolonged dry period prevented sampling in September/October at the confluence C due to the complete drying up of the rivers Dades and M’Goun.
Environmental parameters
Physical and chemical parameters and stream bed characteristics were measured to describe the river sites and to detect differences in habitat characteristics. A multi-parameter meter (WTW MultiLine® Multi 3510 IDS) was used to measure water temperature, pH, and EC. River width and depth were measured using a tape measure. Flow velocity was measured using a hydrological impeller (SEBA Hydrometrie) and subsequently combined with the area of the cross profile to calculate flow rate. We used a MACHEREY–NAGEL VISOCOLOR reagent case with the photometer PF-12Plus and VISOCOLOR Eco colorimetric test kits to measure ion composition in field, measuring chloride (Cl−), sulfate (SO 2−4 ), nitrate (NO −3 ), nitrite (NO −2 ), ammonium (NH +4 ), orthophosphate (PO 3−4 ), potassium (K+), total hardness (TH), and carbonate hardness (CH). Concentrations of nitrate, nitrite, ammonium, orthophosphate, and potassium lower than 4, 0.02, 0.1, 0.6, and 2 mg/l, respectively, were below detection level. If a value was below detection level, we used half of the detection level (see Clarke, 1998). Ion composition was measured in the midstream sites of each section, only. Stream bed characteristics were assessed at all sites by estimating percentages of coverage for mineral (megalithal (> 40 cm), macrolithal (> 20–40 cm), mesolithal (> 6–20 cm), microlithal (> 2–6 cm), akal (> 2 mm–2 cm), psammal/psammopelal (> 6 µm–2 mm)), and organic (algae, macrophytes) substrates (Hering et al., 2004). Additionally, benthic algal concentrations of green algae, cyanobacteria, and diatoms were measured during the second field campaign using a handheld spectrofluorometer (bbe BenthoTorch), except for confluence C due to the drought. Furthermore, flow measurements are missing for two sites and nitrate and sulfate values for September/October due to technical issues.
Macroinvertebrates
Macroinvertebrates were sampled during both sampling campaigns (see “Site selection” section) using a 25 × 25 cm Surber sampler (mesh size 500 µm). Surber samplers were reported to catch a slightly higher species richness and more rare taxa compared to other methods (Storey et al., 1991; Torralba-Burrial & Ocharan, 2007), even though highly mobile taxa might be missed more easily (Ghani et al., 2016). Additionally, they provide an approximation of the number and abundance of taxa in a specific stretch of river which allows to analyze community composition more precisely (Torralba-Burrial & Ocharan, 2007). Quantitative samples were taken at 10 spots per site which were selected to cover all microhabitats based on the proportion of microhabitats in a 50-m reach. The samples were conserved in 95% ethanol. Taxa were identified to species level, except for Diptera (family or subfamily), Odonata (family or genus), some Crustacea (order), some Mollusca (genus), Annelida (sub-class), and Tricladida (class). For each taxon, the number of specimens was counted.
We calculated α- (taxon richness for each section), β- (number of taxa of two sections that are unique to only one of them), and γ-diversity (taxon richness at confluence) for the three confluences for each season and all seasons summarized. Additionally, we calculated the ratio of the generally more sensitive Ephemeroptera and Trichoptera to the generally more tolerant Diptera, Coleoptera, Odonata, and Hemiptera taxa (Kefford et al., 2016).
We calculated the IBMWP (Iberian biological monitoring working party; Jáimez-Cuéllar et al., 2002) and IBGN (Indice Biologique Global Normalisé; Archaimbault & Dumont, 2010) to indicate differences in the presence of sensitive taxa. These multimetric biotic indices describe the biological quality of rivers by using indicator organisms. Sampling methods differed from the methods described in the protocols of each index.
We performed non-metric multidimensional scaling analysis (NMDS; two dimensions, 50 runs) to check for similarities of macroinvertebrate communities between confluences and seasons using log-transformed (log(x + 1)) abundance data and Bray–Curtis distance. Additionally, we performed a nested Analysis of Similarity (ANOSIM) using Bray–Curtis distance with the confluences (seasons separated) as first and the section (i.e., more saline, less saline, downstream) as second level. For each confluence, we checked for typical taxa using an Indicator Species Analysis. We used R v.4.0.4 (R Core Team, 2021) and RStudio (version 1.2.5019) with the packages “ggplot2” (Wickham, 2011), “indicspecies” (De Cáceres et al., 2016), and “vegan” (Oksanen et al., 2010).
Trait profiles
To compare adaptations in biological traits associated with resilience and resistance to disturbances of the macroinvertebrate communities (Bonada et al., 2007), we used the trait database of Tachet et al. (2010) to obtain information for the macroinvertebrate taxa, including 11 biological traits with 62 modalities (Supplementary Table 1). For each of these biological traits, different trait modalities are defined and the affinity of the taxa to each trait modality is represented by fuzzy coded scores (0 = no affinity, 1 = low affinity, 2 = moderate affinity, 3 = high affinity). These codes were transformed to relative frequencies for a given taxon. For each section, we calculated a macroinvertebrate community trait profile based on the relative frequencies of trait modalities for each taxon and the log-transformed (log(x + 1)) abundance data (to reduce the weight of abundant taxa) at a given section, by taking the sum for each trait modality from all present taxa and transforming these sums into relative frequencies for each trait (similar to Mondy et al, 2016). If traits were defined for a lower taxonomic level than the level to which we identified the taxon, we assigned the traits of the lower levels using mean values for all available taxa (Kunz et al., 2022). Trait information of Melanopsis cariosa (Linnaeus, 1767) was added based on Bonada & Dolédec (2011). Trait information was missing for four out of 38 taxa. These taxa were not considered for the calculation of the macroinvertebrate community trait profiles.
Results
River section characteristics
High EC in tributaries was associated with high concentrations of major ions (chloride, sulfate, and potassium), while intermediate concentrations were observed after the confluences (Table 1; Supplementary Tables 2 and 3, and Supplementary Fig. 1). Other ion values were mostly close to or under detection level. The high concentration of the anion chloride and the comparatively low concentration of potassium point toward a non-measured cation that balances the anion. In Dades and M'Goun, this cation was likely calcium, whereas in El Mellah, a high concentration of sodium has been reported, followed by calcium (Cappy, 2007). In the south (toward Tissint), this cation is also likely sodium (Szczucińska et al., 2019). Water temperature and pH were similar among sites at each confluence. The percentage of coverage of mineral and organic substrate, width, depth, and flow velocity differed between sites within a section, with habitat changing within a few hundred meters. Therefore, site samples per section were pooled (see “Site selection” section). Habitat characteristics such as altitude, water temperature, and substrate (e.g., higher presence of emergent macrophytes in confluence A) differed largely between confluences (Supplementary Table 2). The concentration of cyanobacteria was higher in the most saline confluence A than in B, with the highest value in A1. The diatom concentration differed only slightly, while the green algae concentration was generally low, except for section A3 (Supplementary Tables 2 and 3; Supplementary Fig. 2).
Macroinvertebrate community composition
We found 38 taxa (γ-diversity overall), with the highest number of taxa (γ-diversity per confluence) at the least saline confluence C (Dades / M’Goun) and lowest in the most saline confluence A (Tissint; Fig. 2). In contrast, the most saline confluence A had the highest total abundance, while the least saline confluence C had the lowest. Taxon richness (α-diversity) was only slightly higher in the less saline tributaries than in the more saline ones and the joint downstream sections for the confluences A and B (Iriri / El Mellah). α-diversity was only slightly higher in the lower flow tributary C1 than in C2. Communities showed only slightly more unique species (β-diversity) between the two tributaries than between each tributary and the joint downstream section. The ratio of Ephemeroptera and Trichoptera to Diptera, Coleoptera, Odonata, and Hemiptera taxa was lower in the more saline than in the less saline tributaries of the confluences A and B, with the joint downstream sections showing higher or intermediate values (Table 2). All these trends varied sometimes within the two sampling campaigns (Fig. 2) and taxon richness varied between the up-, mid-, and downstream sites per section (Supplementary Tables 2 and 3). The ANOSIM (R = − 0.0003, P = 0.1577) indicated an even distribution of taxa, with no significant differences between the communities in the individual sections, while there are significant differences between confluences (R = 0.3735, P = 0.0001). Indicator species were found only for the first field campaign for the less saline tributary of confluence A (Micronecta sp., P = 0.04), for Iriri (Orthocladiinae, Nebrioporus clarkia (Wollaston, 1862), Tricladida, P = 0.04 for each of the three taxa), for the joint downstream section of Iriri and El Mellah (Hydroptila vectis Curtis, 1834, P = 0.04), for Dades (Caenis luctuosa (Burmeister, 1839), P = 0.03), and for M’Goun and the joint downstream section (Baetis pavidus Grandi, 1951, P = 0.03).
α-, β-, and γ-diversity at the three confluences. α- and γ-diversity (per section; γ-diversity overall = 38) describe taxon richness, β-diversity describes the number of taxa for two sections, which are unique to only one of them. Numbers in brackets represent diversity values for the first/second sampling (no sampling in September/October at the confluence C). Numbers at river lines indicate electrical conductivity in µS/cm
Non-metric multidimensional scaling analysis (NMDS) revealed a distinct macroinvertebrate community for confluence A, while C showed similarities with B (Fig. 3). A shift of communities can be seen from June to September/October, which was smaller for the confluence A (Fig. 3).
At each section, 91.2 ± 2.2% of the total number of specimens made up only 5 ± 1.1 taxa, while only 8.8 ± 2.2% of the community contained 13 ± 1.3 taxa (Fig. 4). While the freshwater snail Melanopsis cariosa and the Chironomidae subfamily Orthocladiinaea made up a large proportion of specimens of the less saline tributary (A1; 5613 µS/cm) of confluence A, the more saline (A2; 10,177 µS/cm) and downstream sections (A3) were characterized by a higher abundance of the Ephemeropterans Caenis luctuosa and Baetis pavidus. These two Ephemeropterans also made up large parts of the communities in the confluences B and C. Simulium sp. and Tricladida were present in the less saline B1 (807 µS/cm), whereas Oligochaeta were present in the more saline B2 (2442 µS/cm). C1 (1160 µS/cm) showed three abundant species, of which Caenis luctuosa represented 78% (Fig. 4). Total abundance was similar in the two tributary sections for the confluences A and C, but lower in the joint downstream section. In the confluence B, total abundance was lower in the more saline tributary (B2), while it was similar in the other two sections (Fig. 4).
The highest IBGN scores were ten out of 20, mostly with no consistent differences between sections (Table 2). The ecological status by IBWMP was moderate to good. Only small differences were observed for the confluence A, while the less saline section B1 attained a higher score than the more saline B2 and their joint downstream section (B3; Table 2).
Trait profiles
Differences in trait profiles per confluence were generally small (Fig. 5; Supplementary Fig. 3). The trait profile of B1 indicated macroinvertebrate communities with taxa of larger sizes, longer life cycle duration, less cycles per year, no respiration through plastron, and a higher presence of taxa that use living micro- and macroinvertebrates as a source of food compared to B2 and B3 (Fig. 5). Differences tended to be smaller for the other traits and at the other confluences (Fig. 5; Supplementary Table 1 and Supplementary Fig. 3).
Discussion
We observed only a slightly lower α-diversity in the more saline tributaries. However, the differences ranged only from one to three taxa and varied over both seasons. Therefore, the hypothesized lower taxon richness in the more saline tributaries could not be confirmed. The generally low α-diversity per section in the entire study area, which aligns well with the results of other studies conducted in arid regions of Northwest Africa (Kaczmarek et al., 2021), is likely the reason for relatively minor differences between sites. The observed low taxon richness can largely be attributed to the mostly high aridity levels in the basin, which increase toward the Saharan desert (Beauchard et al., 2003; Warner et al., 2013; Kaczmarek et al., 2023). The absence of permanent rivers that would provide a refuge for sensitive taxa during droughts as well as source populations reduces taxon richness dramatically (Heino, 2013; Doretto et al., 2018). The minimal differences in macroinvertebrate community composition between sections in the most saline and least diverse Tissint confluence indicate a drought- and salinity-adapted regional species pool (Pallarés et al., 2017), while the greater differences in the less saline and more diverse tributaries point to a larger species pool. The harsh habitat conditions of naturally saline rivers in arid regions are also indicated by generally low scores of the multimetric biotic indices IBMWP and IBGN (Kaczmarek et al., 2023). Inconsistent differences between less and more saline tributaries can be explained by the absence of sensitive taxa (e.g., sensitive Plecoptera, Ephemeroptera, and Trichoptera) that are typically associated with high index scores (Jáimez-Cuéllar et al., 2002; Archaimbault & Dumont, 2010). This indicates a macroinvertebrate community that largely consists of salt-tolerant generalist taxa (e.g., Oligochaeta), which can withstand elevated salinity levels (Wolf et al., 2008). This is also reflected by a lower ratio of generally more salt-sensitive Ephemeroptera and Trichoptera to more salt-tolerant Diptera, Coleoptera, Odonata, and Hemiptera taxa (Millán et al., 2011; Kefford et al., 2016), which we observed for the more saline tributaries. However, low scores in the indices do not necessarily indicate poor environmental conditions due to anthropogenic activities, as aridity and salinity are largely induced by natural processes in the region (Warner et al., 2013) and, hence, largely describe the natural state of the river ecosystem (Velasco et al., 2006). To detect anthropogenic impacts (e.g., secondary salinization and pollution) in saline and intermittent rivers, specific indices should be developed, and indicator organisms should be identified (Gutiérrez-Cánovas et al., 2019; Arias-Real et al., 2022).
Only around five dominant taxa per section made up more than 90% of the total number of specimens of the whole community, mainly composed of generalist and dominant species that can withstand high salinity levels, such as the salt-tolerant ephemeropterans Caenis luctuosa and Baetis pavidus, the trichoptera Hydropsyche sp., or the Oligochaete (Kefford, 1998; Berezina, 2003; Wolf et al., 2008; Pond, 2012; Arribas et al., 2019; Samraoui et al., 2021; Benlasri et al., 2023). Saline ecosystems favor generalist and salinity specialist species that can survive in conditions of high salinity as resources are freed up by the loss of salt-sensitive species (Velasco et al., 2006; Kefford et al., 2016). Piscart et al (2005) reported no influence of salinity on total macroinvertebrate abundance. We found a lower total abundance in the saline El Mellah compared to the other sections at this confluence. However, total abundance was lower in the downstream section of the other two confluences. The fact that we found highest abundances at the most saline confluence might be explained by differences in habitat characteristics (e.g., amount of emergent macrophytes) or a high autotrophic primary production of desert streams such as Tissint (Harms et al., 2008). Here, we found, compared to the confluence of Iriri and El Mellah, higher concentrations of cyanobacteria in sections A1 and A2 and the highest concentration of green algae in section A3. Therefore, the increased abundance of herbivores at the most saline confluence, where we also found the highest percentage of scrapers, may be attributed to the presence of autotrophic food sources. Therefore, we cannot argue that salinity was the main driver of total abundance. In the saline tributaries, we observed proportionally more specimens of the abovementioned taxa, while salt-sensitive taxa [e.g., Cloeon simile Eaton, 1870, Tricladida, Melanopsis cariosa (Velasco et al., 2006; Piscart et al., 2011; Benlasri et al., 2023)] were absent or decreased proportionally. We also found a significantly lower abundance of the water beetle Nebrioporus clarkii (Wollaston, 1862) in El Mellah compared to Iriri, which is known to be adapted to saline environments. However, its upper salinity range (Pallarés et al., 2015) is not exceeded directly at the confluence, but further upstream in El Mellah, where the electrical conductivity reaches nearly 20,000 µS/cm. These salt-sensitive taxa are primarily at risk of extinction in aggravating salinizing habitats (Kaczmarek et al., 2021). However, it is also important to consider protecting naturally saline ecosystems that harbor unique species adapted to high salinities (Velasco et al., 2006; Gutiérrez-Cánovas et al., 2019). These species’ abilities to colonize impaired habitats (Kefford et al., 2016) can help to maintain ecosystem functions (e.g., decomposition; Wallace & Webster, 1996) and the health of salinized ecosystems in the future. Therefore, protecting saline ecosystems should be considered in future policies and action plans (Kaczmarek et al., 2023).
We found, in accordance with our second hypothesis, that the dissimilarities between macroinvertebrate communities (β-diversity) were lower between each tributary and the downstream section (i.e., sections 1 and 3 or 2 and 3) than between the two tributaries (i.e., sections 1 and 2), indicating a combined species pool downstream of the confluence. However, again, differences were only small, not always congruent over seasons, and should, therefore, be treated with caution. The intermediate levels of salinity in the joint downstream section may have allowed for the coexistence of salt-tolerant and salt-sensitive species, as explained by Velasco et al. (2006). Another explanation for the lower β-diversity between the downstream section and each tributary is the immigration of species by macroinvertebrate drift, which could have led to a higher number of taxa being brought downstream with the current due to high salinity levels upstream (Cellot, 1996; Beermann et al., 2018). Given the higher β-diversity between the two tributaries, an immigration of taxa by drift from the tributaries would likely result in highest α -diversity after the confluence, which we did not observe. Biotic species interactions could be an important factor excluding species at the downstream sites (García-Girón et al., 2020), which could prevent the permanent colonization of species that have drifted here. However, the study area was limited to just a few hundred meters around the confluence. Expanding the area up- and downstream could provide a better understanding of the transition and turn-over of macroinvertebrate communities around non-saline–saline confluences and allow for conclusions about the presence of macroinvertebrates due to drift or source-sink dynamics (Heino, 2013). Therefore, we expect that a confluence-based study design, with the extension of the study area, could provide further potential to study the effects of salinity and other stressors on macroinvertebrate communities, especially in terms of metacommunity concepts (Heino, 2013; Cunillera-Montcusí et al., 2022). Difficulties, however, include the identification of confluences that suit the study design (i.e., the stressor of interest) and differences in habitat conditions (e.g., flow and substrate) between the tributaries and between sites in each tributary.
Adaptations for resistance and resilience to disturbances were observed for macroinvertebrate communities in the more saline El Mellah and the joint downstream section, compared to the less saline Iriri, which is in accordance with our third hypothesis. These adaptations included smaller body size and shorter life cycles, which are typical of r-strategists and promote survival in harsh environments (Díaz et al. 2008; Kaczmarek et al. 2021), as well as the ability for air breathing. The expected higher abundance of filter and deposit feeders in the more saline sections was only minimal in El Mellah compared to Iriri (Piscart et al., 2006). However, contrary to our hypothesis, differences in trait profiles were generally small, especially in the Tissint river sections. As EC here exceeds 5600 µS/cm in the less saline tributary, the community of all sections seems to show adaptations to salinity such as small life cycle duration, ovoviviparity, and air breathing via plastron (Kaczmarek et al., 2021). The macroinvertebrate community of Dades showed more resistance and resilience adaptations compared to that of M’Goun, which might be related to low flow conditions at Dades. Low flow conditions also favor a higher number of cycles per year and air breathing, which support survival in arid conditions (Stubbington et al. 2017; Kaczmarek et al. 2021). However, differences in trait profiles were low, likely caused by the high abundance of few dominant taxa. Furthermore, for the creation of the community trait profiles, we had to aggregate traits for a large part of the taxa, as trait data for some taxa were not available in the database of Tachet et al. (2010). The availability of trait data for the species present in our arid study area could have resulted in larger differences of trait profiles between saline and non-saline tributaries. This highlights the need to complete databases on macroinvertebrate salinity tolerances and traits, especially in understudied regions (Schäfer et al. 2011; Cunillera-Montcusí et al. 2022).
Our study shows that the macroinvertebrate communities underwent seasonal changes during the hot and dry summer period, as it was observed in various studies (Dallas, 2004; Eriksen et al., 2021). It should be noted that not all observations described above were consistent across seasons. Seasonal dissimilarities can be explained by varying life history strategies such as the timing of hatching and emergence, which could lead to the presence (e.g., Ostracoda in Tissint) or absence (e.g., Hydracarina in Iriri / El Mellah) of seasonal species in the second sampling in September/October (Ferguson, 1944; Meyer, 1994). We observed a significant increase in the abundance of Simulium sp. (Iriri) and Caenis luctuosa (Tissint) during the dry summer period. These findings highlight the importance of considering seasonal variability in studies and biomonitoring of macroinvertebrate communities (Johnson et al., 2012).
We observed only small trends in α- and β-diversity in response to salinity. This might be due to the overall low diversity of the community, and the difficulty of excluding confounding factors and not necessarily to the absence of effects (e.g., differences in flow, ion composition, and habitat). Although we pooled sites per section to mitigate the impact of habitat conditions, it is difficult to completely exclude the influence of other environmental parameters in in situ studies, as described by Heino et al (2004) and Kefford (1998). However, we observed a large difference in community composition between Dades and M’Goun, which were similar in salinity levels, but differed in flow rate and velocity, caused by a prolonged drought in recent years and water abstractions upstream of the Dades site. Low flow conditions influence the macroinvertebrate community and cause higher drift propensities (Heino et al., 2004; Beermann et al., 2018). Although Dades showed the highest α-diversity due to generally good water quality in the region (Kaczmarek et al., 2023), its community exhibited higher dissimilarity compared to M’Goun and the joint downstream section, and nearly four-fifths of the whole specimens belonged to the generalist species Caenis luctuosa. The low abundance and the presence of other taxa might be caused by an increasing pressure of drought conditions in recent years. A shift from permanent to intermittent flow can strongly change the macroinvertebrate community, with a loss of non-adapted species (Piano et al., 2020). Both normally permanent rivers Dades and M’Goun (Cappy, 2007) dried up completely in August due to a drought. We also had to omit a fourth confluence with large differences in EC (3890–19,330 µS/cm) from our study, as one of the tributaries was falling dry due to upstream water abstractions. These events caused issues for our study and highlight the increasing impact of climate change in the region, which will increase the pressure on water resources and compromise ecosystem health and human well-being (Kaczmarek et al., 2023).
With increasingly arid climate and ongoing primary and secondary salinization harsh habitat conditions will increase in Northwest Africa (Beck et al., 2018; Kaczmarek et al., 2021). This will lead to a loss of salt- and drought-sensitive species (Kaczmarek et al., 2021), highlighting the importance of limiting global warming and of protecting aquatic ecosystems from the consequences of climate change and salinization (Cañedo-Argüelles et al., 2013; Schuler et al., 2019). However, as described above, we found only limited effects of salinity on the community composition around the confluences, affecting mainly rare taxa. Macroinvertebrate communities in the Draa River basin are generally low in diversity and dominated by a few highly abundant salt- and drought-tolerant taxa. Species’ cross-tolerance to salinity and desiccation could provide important physiological advantages to survive drying streams of desert environments (Pallarés et al., 2017). With a high abundance of tolerant species, the community is likely to be relatively resilient and resistant to climate change (Pallarés et al., 2017), with the ability to withstand phases of droughts while being able to return to a functioning community in the wet phase (Colloff & Baldwin, 2010). Nevertheless, the increasing drying of rivers due to climate change and anthropogenic water abstractions may lead to a loss of resilience (Colloff & Baldwin, 2010). It is important to ensure connectivity of permanent rivers by preventing long periods of intermittency to allow for recolonization from source populations and to ensure river ecosystem functioning (Hughes, 2007; Colloff & Baldwin, 2010). It is therefore crucial to understand the importance of metapopulation and metacommunity processes (e.g., colonization–extinction and source-sink dynamics) for the functioning of arid ecosystems (Cunillera-Montcusí et al., 2022).
Conclusion
Our study found only small differences between macroinvertebrate communities and their trait profiles from more and less saline tributaries, if any at all. However, larger differences were found between confluences on a large scale (e.g., Dades/M’Goun in higher altitude and Tissint toward the desert). Despite this, we believe that a confluence-based study design remains valuable for investigating the effects of specific stressors on macroinvertebrate community composition, as it enables the comparison of community responses while excluding these large-scale geographic patterns. The impact of salinity is likely more pronounced in river basins with more diverse communities that do not show any pre-adaptation to salinity and aridity. To build upon our findings, we suggest extending the study area up- and downstream to explore metacommunity concepts such as macroinvertebrate drift and source-sink dynamics. Identifying taxa to a lower taxonomic level could potentially reveal further effects of salinity. However, finding comparable rivers with similar habitat and flow characteristics may prove challenging. Setting up mesocosm experiments in the field using river water could help to gain further insights into the combined effects of salinity and drought. It is crucial to gain a better understanding of macroinvertebrate salinity tolerances and their resistance and resilience traits through the extension of global databases. This knowledge, coupled with efforts to limit future secondary salinization, will help to understand and efficiently protect river ecosystems worldwide.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Archaimbault, V. & B. Dumont, 2010. L’indice biologique global normalisé (IBGN): principes et évolution dans le cadre de la directive cadre européenne sur l’eau. Sciences Eaux & Territoires. https://doi.org/10.3917/set.001.0036.
Arias-Real, R., C. Gutiérrez-Cánovas, M. Menéndez & I. Muñoz, 2022. Drying niches of aquatic macroinvertebrates identify potential biomonitoring indicators in intermittent and ephemeral streams. Ecological Indicators 142: 109263. https://doi.org/10.1016/j.ecolind.2022.109263.
Arribas, P., C. Gutiérrez-Cánovas, M. Botella-Cruz, M. Cañedo-Argüelles, J. Antonio Carbonell, A. Millán, S. Pallarés, J. Velasco & D. Sánchez-Fernández, 2019. Insect communities in saline waters consist of realized but not fundamental niche specialists. Philosophical Transactions of the Royal Society b: Biological Sciences Royal Society 374: 20180008. https://doi.org/10.1098/rstb.2018.0008.
Beauchard, O., J. Gagneur & S. Brosse, 2003. Macroinvertebrate richness patterns in North African streams. Journal of Biogeography 30: 1821–1833. https://doi.org/10.1111/j.1365-2699.2003.00954.x.
Beck, H. E., N. E. Zimmermann, T. R. McVicar, N. Vergopolan, A. Berg & E. F. Wood, 2018. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Scientific Data Nature Publishing Group 5: 1–12. https://doi.org/10.1038/sdata.2018.214.
Beermann, A. J., V. Elbrecht, S. Karnatz, L. Ma, C. D. Matthaei, J. J. Piggott & F. Leese, 2018. Multiple-stressor effects on stream macroinvertebrate communities: A mesocosm experiment manipulating salinity, fine sediment and flow velocity. Science of the Total Environment 610–611: 961–971. https://doi.org/10.1016/j.scitotenv.2017.08.084.
Benlasri, M., N. Kaczmarek, M. El Alami, M. Ghamizi & E. Berger, 2023. Inventory and pattern of distribution of mayflies (Insecta, Ephemeroptera) in the Draa river basin, southern Morocco. Alpine Entomology 7: 13–20. https://doi.org/10.3897/alpento.7.96436.
Benson, J. A., P. G. Close, B. A. Stewart & A. J. Lymbery, 2019. Freshwater tributaries provide refuge and recolonization opportunities for mussels following salinity reversal. Science of the Total Environment Elsevier 683: 231–239. https://doi.org/10.1016/j.scitotenv.2019.05.286.
Berezina, N. A., 2003. Tolerance of freshwater invertebrates to changes in water salinity. Russian Journal of Ecology Springer 34: 261–266. https://doi.org/10.1023/A:1024597832095.
Berger, E., O. Frör & R. B. Schäfer, 2019. Salinity impacts on river ecosystem processes: a critical mini-review. Philosophical Transactions of the Royal Society b: Biological Sciences Royal Society 374: 20180010. https://doi.org/10.1098/rstb.2018.0010.
Bonada, N., & S. Dolédec, 2011. Do mediterranean genera not included in Tachet et al. 2002 have mediterranean trait characteristics? Limnetica 30: 0129–0142. https://doi.org/10.23818/limn.30.11
Bonada, N., S. Dolédec & B. Statzner, 2007. Taxonomic and biological trait differences of stream macroinvertebrate communities between mediterranean and temperate regions: implications for future climatic scenarios. Global Change Biology Wiley Online Library 13: 1658–1671.
Cañedo-Argüelles, M., B. J. Kefford, C. Piscart, N. Prat, R. B. Schäfer & C.-J. Schulz, 2013. Salinisation of rivers: An urgent ecological issue. Environmental Pollution 173: 157–167. https://doi.org/10.1016/j.envpol.2012.10.011.
Cañedo-Argüelles, M., C. P. Hawkins, B. J. Kefford, R. B. Schäfer, B. J. Dyack, S. Brucet, D. Buchwalter, J. Dunlop, O. Frör & J. Lazorchak, 2016. Saving freshwater from salts. Science American Association for the Advancement of Science 351: 914–916. https://doi.org/10.1126/science.aad3488.
Cappy, S., 2007. Hydrogeological characterization of the Upper Drâa catchment: Morocco. (Doctoral dissertation) Universitäts und Landesbibliothek Bonn.
Cellot, B., 1996. Influence of side-arms on aquatic macroinvertebrate drift in the main channel of a large river. Freshwater Biology Wiley Online Library 35: 149–164.
Clarke, J. U., 1998. Evaluation of censored data methods to allow statistical comparisons among very small samples with below detection limit observations. Environmental Science & Technology 32: 177–183. https://doi.org/10.1021/es970521v.
Colloff, M. J. & D. S. Baldwin, 2010. Resilience of floodplain ecosystems in a semi-arid environment. The Rangeland Journal 32(3): 305–314. https://doi.org/10.1071/RJ10015.
Cunillera-Montcusí, D., M. Beklioğlu, M. Cañedo-Argüelles, E. Jeppesen, R. Ptacnik, C. A. Amorim, S. E. Arnott, S. A. Berger, S. Brucet & H. A. Dugan, 2022. Freshwater salinisation: a research agenda for a saltier world. Trends in Ecology & Evolution Elsevier. https://doi.org/10.1016/j.tree.2021.12.005.
Dallas, H. F., 2004. Seasonal variability of macroinvertebrate assemblages in two regions of South Africa: implications for aquatic bioassessment. African Journal of Aquatic Science Taylor & Francis 29: 173–184. https://doi.org/10.2989/16085910409503808.
De Caceres, M., F. Jansen, & M. M. De Caceres, 2016. Package ‘indicspecies’. indicators 8(1).
Díaz, A. M., M. L. S. Alonso & M.R.V.-A. Gutiérrez, 2008. Biological traits of stream macroinvertebrates from a semi-arid catchment: patterns along complex environmental gradients. Freshwater Biology 53: 1–21. https://doi.org/10.1111/j.1365-2427.2007.01854.x.
Doretto, A., E. Piano, E. Falasco, S. Fenoglio, M. C. Bruno & F. Bona, 2018. Investigating the role of refuges and drift on the resilience of macroinvertebrate communities to drying conditions: an experiment in artificial streams. River Research and Applications 34(7): 777–785.
Eriksen, T. E., J. E. Brittain, G. Søli, D. Jacobsen, P. Goethals & N. Friberg, 2021. A global perspective on the application of riverine macroinvertebrates as biological indicators in Africa, South-Central America, Mexico and Southern Asia. Ecological Indicators Elsevier 126: 107609. https://doi.org/10.1016/j.ecolind.2021.107609.
Ferguson, E., Jr., 1944. Studies on the seasonal life history of three species of freshwater Ostracoda. American Midland Naturalist JSTOR. https://doi.org/10.1016/j.scitotenv.2018.12.253.
García-Girón, J., J. Heino, F. García-Criado, C. Fernández-Aláez & J. Alahuhta, 2020. Biotic interactions hold the key to understanding metacommunity organisation. Ecography 43(8): 1180–1190. https://doi.org/10.1111/ecog.05032.
Ghani, W. M. H. W. A., C. S. M. Rawi, S. Abd Hamid & S. A. Al-Shami, 2016. Efficiency of different sampling tools for aquatic macroinvertebrate collections in Malaysian streams. Tropical Life Sciences Research 27(1): 115.
Gutiérrez-Cánovas, C., D. Sánchez-Fernández, M. Canedo-Argüelles, A. Millán, J. Velasco, R. Acosta, P. Fortuño, N. Otero, A. Soler & N. Bonada, 2019. Do all roads lead to Rome? Exploring community trajectories in response to anthropogenic salinization and dilution of rivers. Philosophical Transactions of the Royal Society B 374(1764): 20180009. https://doi.org/10.1098/rstb.2018.0009.
Harms, T. K., R. A. Sponseller & N. Grimm, 2008. Desert streams. In Encyclopedia of Ecology, Five-Volume Set (pp. 871–879). Elsevier Inc. https://doi.org/10.1016/B978-008045405-4.00325-6
Heino, J., 2013. The importance of metacommunity ecology for environmental assessment research in the freshwater realm. Biological Reviews Wiley Online Library 88: 166–178. https://doi.org/10.1111/j.1469-185X.2012.00244.x.
Heino, J., P. Louhi & T. Muotka, 2004. Identifying the scales of variability in stream macroinvertebrate abundance, functional composition and assemblage structure. Freshwater Biology 49: 1230–1239. https://doi.org/10.1111/j.1365-2427.2004.01259.x.
Herbert, E. R., P. Boon, A. J. Burgin, S. C. Neubauer, R. B. Franklin, M. Ardón, K. N. Hopfensperger, L. P. Lamers & P. Gell, 2015. A global perspective on wetland salinization: ecological consequences of a growing threat to freshwater wetlands. Ecosphere Wiley Online Library 6: 1–43. https://doi.org/10.1890/ES14-00534.1.
Hering, D., O. Moog, L. Sandin & P. F. Verdonschot, 2004. Overview and application of the AQEM assessment system. Hydrobiologia 516: 1–20. https://doi.org/10.1023/B:HYDR.0000025255.70009.a5.
Hssaisoune, M., L. Bouchaou, A. Sifeddine, I. Bouimetarhan & A. Chehbouni, 2020. Moroccan groundwater resources and evolution with global climate changes. Geosciences Multidisciplinary Digital Publishing Institute 10: 81. https://doi.org/10.3390/geosciences10020081.
Hughes, J. M., 2007. Constraints on recovery: using molecular methods to study connectivity of aquatic biota in rivers and streams. Freshwater Biology 52(4): 616–631. https://doi.org/10.1111/j.1365-2427.2006.01722.x.
Jáimez-Cuéllar, P., S. Vivas, N. Bonada, S. Robles, A. Mellado, M. Álvarez, J. Avilés, J. Casas, M. Ortega & I. Pardo, 2002. Protocolo GUADALMED (prece). Limnetica 21: 187–204.
Johnson, R. C., M. M. Carreiro, H.-S. Jin & J. D. Jack, 2012. Within-year temporal variation and life-cycle seasonality affect stream macroinvertebrate community structure and biotic metrics. Ecological Indicators Elsevier 13: 206–214. https://doi.org/10.1016/j.ecolind.2011.06.004.
Kaczmarek, N., R. B. Schaefer & E. Berger, 2021. Environmental change threatens freshwater insect communities in Northwest Africa: a meta-analysis. Frontiers in Environmental Science Frontiers Media SA 9: 671715. https://doi.org/10.3389/fenvs.2021.671715.
Kaczmarek, N., I. Mahjoubi, M. Benlasri, M. Nothof, R. B. Schäfer, O. Frör & E. Berger, 2023. Water quality, biological quality, and human well-being: water salinity and scarcity in the Draa River basin. Morocco. Ecological Indicators Elsevier 148: 110050. https://doi.org/10.1016/j.ecolind.2023.110050.
Kefford, B. J., 1998. The relationship between electrical conductivity and selected macroinvertebrate communities in four river systems of south-west Victoria, Australia. International Journal of Salt Lake Research Springer 7: 153–170. https://doi.org/10.1007/BF02441884.
Kefford, B. J., D. Buchwalter, M. Cañedo-Argüelles, J. Davis, R. P. Duncan, A. Hoffmann & R. Thompson, 2016. Salinized rivers: degraded systems or new habitats for salt-tolerant faunas? Biology Letters Royal Society 12: 20151072. https://doi.org/10.1098/rsbl.2015.1072.
Kefford, B., J. Bray, S. Nichols, J. Fraser, R. Mac Nally, A. O’Reilly-Nugent & G. Kon Kam King, & R. Thompson, 2021. Understanding salt-tolerance and biota–stressor interactions in freshwater invertebrate communities. Marine and Freshwater Research 73: 140. https://doi.org/10.1071/MF21164.
Kefford, B. J., R. V. Hyne, A. J. Brooks, J. P. Bray, M. Shenton, K. Hills & S. J. Nichols, 2023. Single-species acute lethal toxicity tests are not predictive of relative population and community effects of two salinity types. Limnology and Oceanography Letters 8: 181–189. https://doi.org/10.1002/lol2.10208.
Kunz, S., B. J. Kefford, A. Schmidt-Kloiber, C. D. Matthaei, P. Usseglio-Polatera, W. Graf, N. L. Poff, L. Metzeling, L. Twardochleb & C. P. Hawkins, 2022. Tackling inconsistencies among freshwater invertebrate trait databases: harmonising across continents and aggregating taxonomic resolution. Freshwater Biology Wiley Online Library 67: 275–291. https://doi.org/10.1111/fwb.13840.
Meyer, E. I., 1994. Species composition and seasonal dynamics of water mites (Hydracarina) in a mountain stream (Steina, Black Forest, southern Germany). Hydrobiologia Springer 288: 107–117. https://doi.org/10.1007/BF00007130.
Millán, A., J. Velasco, C. Gutiérrez-Cánovas, P. Arribas, F. Picazo, D. Sánchez-Fernández & P. Abellán, 2011. Mediterranean saline streams in southeast Spain: what do we know? Journal of Arid Environments 75: 1352–1359. https://doi.org/10.1016/j.jaridenv.2010.12.010.
Mondy, C. P., I. Muñoz & S. Dolédec, 2016. Life-history strategies constrain invertebrate community tolerance to multiple stressors: a case study in the Ebro basin. Science of the Total Environment Elsevier 572: 196–206. https://doi.org/10.1016/j.scitotenv.2016.07.227.
Moumane, A., F. E. El Ghazali, J. Al Karkouri, J. Delorme, M. Batchi, D. Chafiki & A. Karmaoui, 2021. Monitoring spatiotemporal variation of groundwater level and salinity under land use change using integrated field measurements, GIS, geostatistical, and remote-sensing approach: case study of the Feija aquifer, Middle Draa watershed, Moroccan Sahara. Environmental Monitoring and Assessment 193: 1–21. https://doi.org/10.1007/s10661-021-09581-2.
Oksanen, J., 2010. Vegan: community ecology package. http://CRAN.R-project.org/package= vegan.
Pallarés, S., P. Arribas, D. T. Bilton, A. Millan & J. Velasco, 2015. The comparative osmoregulatory ability of two water beetle genera whose species span the fresh-hypersaline gradient in inland waters (Coleoptera: Dytiscidae, Hydrophilidae). PLoS One 10(4): e0124299. https://doi.org/10.1371/journal.pone.0124299.
Pallarés, S., M. Botella-Cruz, P. Arribas, A. Millán & J. Velasco, 2017. Aquatic insects in a multistress environment: cross-tolerance to salinity and desiccation. Journal of Experimental Biology 220(7): 1277–1286. https://doi.org/10.1242/jeb.152108.
Piano, E., A. Doretto, S. Mammola, E. Falasco, S. Fenoglio & F. Bona, 2020. Taxonomic and functional homogenisation of macroinvertebrate communities in recently intermittent Alpine watercourses. Freshwater Biology 65(12): 2096–2107. https://doi.org/10.1111/fwb.13605.
Piscart, C., J. C. Moreteau & J. N. Beisel, 2005. Biodiversity and structure of macroinvertebrate communities along a small permanent salinity gradient (Meurthe River, France). Hydrobiologia 551: 227–236. https://doi.org/10.1007/s10750-005-4463-0.
Piscart, C., P. Usseglio-Polatera & J.-C.B. Moreteau, 2006. The role of salinity in the selection of biological traits of freshwater invertebrates. Archiv Für Hydrobiologie Schweizerbart’sche Verlagsbuchhandlung. https://doi.org/10.1127/0003-9136/2006/0166-0185.
Piscart, C., B. J. Kefford & J.-N. Beisel, 2011. Are salinity tolerances of non-native macroinvertebrates in France an indicator of potential for their translocation in a new area? Limnologica 41: 107–112. https://doi.org/10.1016/j.limno.2010.09.002.
Pond, G. J., 2012. Biodiversity loss in Appalachian headwater streams (Kentucky, USA): Plecoptera and Trichoptera communities. Hydrobiologia 679: 97–117. https://doi.org/10.1007/s10750-011-0858-2.
R Core Team, 2021. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Robson, B. J., E. T. Chester, B. D. Mitchell & T. G. Matthews, 2013. Disturbance and the role of refuges in mediterranean climate streams. Hydrobiologia 719: 77–91. https://doi.org/10.1007/s10750-012-1371-y.
Samraoui, B., Z. Bouhala, K. Chakri, J. Márquez-Rodríguez, M. Ferreras-Romero, H. A. El-Serehy, F. Samraoui, M. Sartori & J.-L. Gattolliat, 2021. Environmental determinants of mayfly assemblages in the Seybouse River, north-eastern Algeria (Insecta: Ephemeroptera). Biologia Springer 76: 2277–2289. https://doi.org/10.1007/s11756-021-00726-9.
Schäfer, R. B., B. J. Kefford, L. Metzeling, M. Liess, S. Burgert, R. Marchant, V. Pettigrove, P. Goonan & D. Nugegoda, 2011. A trait database of stream invertebrates for the ecological risk assessment of single and combined effects of salinity and pesticides in South-East Australia. Science of the Total Environment 409: 2055–2063. https://doi.org/10.1016/j.scitotenv.2011.01.053.
Schuler, M. S., M. Canedo-Argüelles, W. D. Hintz, B. Dyack, S. Birk & R. A. Relyea, 2019. Regulations are needed to protect freshwater ecosystems from salinization. Philosophical Transactions of the Royal Society B the Royal Society 374: 20180019. https://doi.org/10.1098/rstb.2018.0019.
Storey, A. W., D. H. D. Edward & P. Gazey, 1991. Surber and kick sampling: a comparison for the assessment of macroinvertebrate community structure in streams of south-western Australia. Hydrobiologia 211: 111–121. https://doi.org/10.1007/BF00037367.
Stubbington, R., M. T. Bogan, N. Bonada, A. J. Boulton, T. Datry, C. Leigh & R. Vander Vorste, 2017. Chapter 43—the biota of intermittent rivers and ephemeral streams: aquatic invertebrates. In Datry, T., N. Bonada & A. Boulton (eds), Intermittent rivers and ephemeral streams Academic Press, Cambridge: 217–243.
Szczucińska, A., M. Dłużewski, R. Kozłowski & P. Niedzielski, 2019. Hydrochemical diversity of a large alluvial aquifer in an arid zone (Draa river, S Morocco). Ecological Chemistry and Engineering S 26(1): 81–100. https://doi.org/10.1515/eces-2019-0007.
Tachet, H., P. Richoux, M. Bournaud, & P. Usseglio-Polatera, 2010. Invertébrés d’eau douce: systématique, biologie, écologie. CNRS éditions Paris.
Thorslund, J., M. F. P. Bierkens, G. H. P. Oude Essink, E. H. Sutanudjaja & M. T. H. van Vliet, 2021. Common irrigation drivers of freshwater salinisation in river basins worldwide. Nature Communications Nature Publishing Group 12: 4232. https://doi.org/10.1038/s41467-021-24281-8.
Torralba-Burrial, A. & F. J. Ocharan, 2007. Comparación del muestreo de macroinvertebrados bentónicos fluviales con muestreador surber y con red manual en ríos de Aragón (NE Península Ibérica). Limnetica 26(1): 013–024. https://doi.org/10.23818/limn.26.02.
Velasco, J., A. Millán, J. Hernández, C. Gutiérrez, P. Abellán, D. Sánchez & M. Ruiz, 2006. Response of biotic communities to salinity changes in a Mediterranean hypersaline stream. Saline Systems 2: 12. https://doi.org/10.1186/1746-1448-2-12.
Wallace, J. B., & J. R. Webster, (1996). The Role of Macroinvertebrates in Stream Ecosystem Function. https://doi.org/10.1146/annurev.en.41.010196.000555
Warner, N., Z. Lgourna, L. Bouchaou, S. Boutaleb, T. Tagma, M. Hsaissoune & A. Vengosh, 2013. Integration of geochemical and isotopic tracers for elucidating water sources and salinization of shallow aquifers in the sub-Saharan Drâa Basin, Morocco. Applied Geochemistry 34: 140–151. https://doi.org/10.1016/j.apgeochem.2013.03.005.
Wickham, H., 2011. ggplot2. Wiley Interdisciplinary Reviews: Computational Statistics Wiley Online Library 3: 180–185. https://doi.org/10.1046/j.1440-1770.1999.00089.x.
Williams, W. D., 1999. Salinisation: a major threat to water resources in the arid and semi-arid regions of the world. Lakes & Reservoirs: Research & Management Wiley Online Library 4: 85–91.
Wolf, B., E. Kiel, A. Hagge, H.-J. Krieg & C. Feld, 2008. Using the salinity preferences of benthic macroinvertebrates to classify running waters in brackish marshes in Germany. Ecological Indicators. https://doi.org/10.1016/j.ecolind.2008.10.005.
Acknowledgements
We would like to thank our colleagues, especially all members of the SaliDraa-juj project, for all their help during the fieldwork, and for all helpful discussions during the project. We kindly thank M. Huszarik for language editing. We thank the German Ministry of Education and Research (BMBF) for the financial support (SALIDRAAjuj-01UU1906).
Funding
Open Access funding enabled and organized by Projekt DEAL. Funding was provided by Bundesministerium für Bildung und Forschung (Grant Number 01UU1906).
Author information
Authors and Affiliations
Contributions
NK contributed toward conceptualization, methodology, validation, formal analysis, investigation, writing—original draft, and visualization. MB contributed toward methodology, investigation, formal analysis. RBS contributed toward conceptualization, methodology, validation, formal analysis, writing—original draft, supervision, and funding acquisition. AA contributed toward conceptualization, and investigation. MN: contributed toward methodology, validation, and investigation. MG contributed toward resources and supervision. KL contributed toward investigation. EB contributed toward conceptualization, methodology, validation, formal analysis, resources, and writing—original draft, supervision, project administration, and funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Consent to participate
The research leading to the results presented in this article did not involve animal experiments or humans.
Ethical approval
The authors have no ethical conflict to state.
Additional information
Handling editor: Sally A. Entrekin
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaczmarek, N., Benlasri, M., Schäfer, R.B. et al. Macroinvertebrate community responses to salinity around non-saline–saline confluences in the Draa River basin, Morocco. Hydrobiologia 851, 2189–2204 (2024). https://doi.org/10.1007/s10750-023-05445-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05445-7