Abstract
Climate change has been increasing the frequency and severity of drought periods. There is a need to understand how this water scarcity affects aquatic communities. The main aim of this work was to compare the macroinvertebrate communities and their bioindicator value (ecological status sensu the Water Framework Directive) in three Regions [northeast (N), central (C) and south (S)] of Portugal along a water availability and climate gradient, in two consecutive years that reflect hydrologically distinct scenarios (2018 versus 2019). The period prior to sampling (summer and autumn 2017) was extremely dry and hot, whereas climatic fluctuations in 2018–2019 (when the sampling occurred) were closer to the norm. A total of 28 sampling sites were surveyed in streams of Regions where water scarcity is differentially constitutive. The results showed a consistent environmental and ecological gradient in both years, despite the background differences. A coinciding mineralization, temperature, riparian cover and water availability gradient clearly separated Regions C (wettest) and S (driest), with Region N occupying an intermediate position. Region C had overall higher ecological quality (prevalence of sensitive organisms) in both years. Despite our a priori expectations that 2018 would represent a much more stressful condition, with larger differences across Regions (because of their constitutive background), the data suggest that macroinvertebrate communities may be resilient or adapted, to some degree, to hydrological fluctuations. Signs of more favourable conditions in 2019 were also observed (general improvement in ecological status). Studies across hydrological and climatic gradients (in time and space) are particularly important where water availability is becoming more challenging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate has been changing globally. Although it is expected that Earth suffers successive cycles of heating and cooling, the recent rate of heating has been much faster than expected if provoked by natural fluctuations. This has been proven by recent climate patterns, which are unparalleled for centuries (IPCC 2022). One of the most conspicuous signs of this reshaping is the change in precipitation patterns and ice melting rate, which have been causing alterations in hydrological systems and, consequently, in the availability of freshwater resources (EEA 2019; IPCC 2022). This jeopardizes freshwater as a resource for humans and as an ecosystem, adding to or interacting with the various other threats affecting freshwater biodiversity and ecosystem health (Woodward et al. 2010; Grizzetti et al. 2017; EEA 2019). In many regions of the world, water scarcity and prolonged droughts are becoming more frequent or are projected to increase their severity in the near future (EEA 2019; IPCC 2021). The consequences of such change may already be ongoing, and it is important to assess how resilient to such pressures aquatic communities are and whether the ecological status of riverine ecosystems is affected.
Drought may be defined as a temporary state of dryness, often resulting from low precipitation during an abnormally long period of time at a specific location (Mishra and Singh 2010). Drought periods are expected to happen naturally in dry seasons in regions with befitting climate types. However, the already complex sequence of intertwined events occurring in freshwater ecosystems during ordinary summer periods are complicated further by increasing drought extension and severity, and by its interaction with additional factors conditioning each ecosystem (e.g. land use). Indeed, such a scenario complicates the prediction of the net impacts of drought, especially in intermittent rivers (Chiu et al. 2017). Drought and flow intermittency may provoke fragmentation in habitats and biodiversity loss; indirectly, they may exacerbate the impacts of eutrophication and increase toxicant concentrations in the environment (Woodward et al. 2010; IPCC 2022), as well as induce changes in productivity, respiration rates and aeration of water (Mosley 2015). Also, the associated increase in water temperature interferes with oxygen solubility, thus affecting ecosystem structure and function (Kalff 2002). During drought periods, turbidity typically decreases due to lack of water input and sedimentation increases due to the lowered flow (Mosley 2015). Drought can also contribute to mineralization of waters and release of nutrients from organic matter (Hering et al. 2010; Gómez et al. 2017). Salinity increases during droughts due to evaporation and lower groundwater inflows (Cañedo-Argüelles et al. 2013; Gómez et al. 2017; Jones and van Vliet 2018). The consequences of drought lead to community structure shifts (Millennium Ecosystem Assessment 2005; IPBES 2019; Buffagni 2021; IPCC 2022) and ecosystems take time to recover from the effects of a period of drought after it ends (Heim Jr 2002), and sometimes those effects may even be irreversible (Djebou 2017).
Benthic macroinvertebrate communities are a good proxy for freshwater biodiversity and excellent bioindicators in riverine ecosystems. Benthic macroinvertebrates have adaptations and biological mechanisms (e.g. body armouring, adult flying ability, drift) that grant them resistance (ability to thrive albeit under unfavourable conditions) and/or resilience (ability to recover and recolonize after disturbance) to face adverse conditions, such as drought or river flow intermittence (Nimmo et al. 2015; Bogan et al. 2017; Stubbington et al. 2017). However, ecological alterations are occurring at such a pace that their ability to respond to setbacks may be compromised (Stubbington et al. 2017). Freshwater macroinvertebrate communities are negatively impacted by severe drought events, particularly through the decline of specific taxa, namely those that are very dependent of running water (e.g. Plecoptera larvae) (e.g. Bruno et al. 2014; Stubbington et al. 2017; Guareschi et al. 2018). These negative impacts are potentiated in streams that are expected to be perennial but face flow intermittency or marked flow alterations as a result of changing drought patterns (Smith et al. 2017; Buffagni et al. 2020; Arias Font et al. 2021; Buffagni 2021). The isolated effects of water scarcity and drought – especially under climate change – on the bioindicator value of the benthic macroinvertebrate community are difficult to ascertain (Buffagni et al. 2020; Munné et al. 2021; Santos et al. 2021). In Europe, such uncertainty undermines the assessment of the ecological status of waterbodies [sensu the Water Framework Directive (WFD)], thus compromising the conservation or restoration of aquatic ecosystems (in line with the WFD and European Green Deal). In countries with a Mediterranean climate (dry summers), macroinvertebrate-based metrics consider sensitive taxa that occur in intermittent or temporary rivers, which may indirectly tackle this uncertainty to some extent (Munné and Prat 2009; Feio et al. 2014b, a).
The main aim of the present work was to compare the macroinvertebrate communities and their bioindicator value (ecological status sensu WFD) across Regions differing in water availability and climate regimes (i.e. a gradient of Mediterranean influence) in two consecutive years, reflecting hydrologically distinct scenarios (high versus low). To do so, 28 sampling sites were surveyed in a total of 16 streams and six hydrographic basins, during the spring of 2018 and 2019, in three Regions representing distinct climatic features and water availability scenarios (Santos et al. 2023): Region N (northeast Portugal) is mountainous, generally experiencing rainy winters but hot and dry summers; Region S (south of Portugal) shares climate type with Region N, but with less rainy winters, being the most prone to drought; and Region C (central Portugal) is the less prone to drought, with a typical temperate climate expressed in rainy winters and dry, not very hot summers. The 2017–2019 period was highly variable in Portugal (publicly available data from the Portuguese Institute for Sea and Atmosphere – IPMA): 2017 was extremely dry and hot, whereas 2018 and 2019 were closer to the norm (1971–2000 period); the spring of 2018 was preceded by a long dry period (extreme drought through June to December 2017; IPMA 2017), which resulted in heterogeneous pressures and accumulated hydric stress in more vulnerable regions. A previous paper (Santos et al. 2023) analysed in detail the regional differences in the abiotic and biotic context of the sampled sites in 2019 and their interconnection to water availability. Here, we build upon this study by integrating data from spring 2018 and 2019. This sampling design and the opportunity provided by distinct hydrological years covered in the present study allowed us to hypothesize on whether macroinvertebrates show differential resilience (expressed through community recovery from 2018 to 2019), depending on the severity of ordinary and exceptional (2017) drought, across a climatic regime gradient provided by the different Regions sampled.
Methods
Study areas
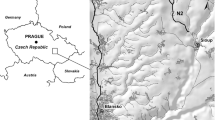
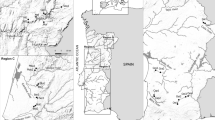
The work was developed in three Regions in Portugal (Regions C, N and S; Fig. 1), a country with a temperate climate type along a gradient of Mediterranean influence (Carvalho et al. 2011; IPCC 2022). Region C, in the centre of Portugal, presents temperate climate with rainy winters and dry and not very hot summers [type CSb, according to the Köppen–Geiger classification system (Beck et al. 2018; IPMA 2021)], displaying Cantabrian mixed forests in northern sampling sites and Southwest Iberian Mediterranean sclerophyllous (small, hard, thick leaves, adapted to dry climate) and mixed forests in southern sampling sites. This Region is the one with milder summers (i.e. lower temperature maxima and less dryness), therefore it is less expected to undergo stream intermittency. Region N, in northeastern Portugal, displays Iberian sclerophyllous and semi-deciduous forests, and a temperate climate with rainy winters and hot and dry summers (type Csa). This is a mountainous region, therefore most of the sampling sites lie at relatively high altitudes (250–600 m). Because of the slope, many portions of the riverine network tend to dry or become intermittent. Region S, in southern Portugal, also presents Csa climate type and Iberian sclerophyllous and semi-deciduous forests in the east but also Southwest Iberian Mediterranean sclerophyllous and mixed forests in the west. Moreover, most sampling sites of Region S are located at low altitudes (10–200 m), in contrast to Region N. It is the driest and hottest Region, and many of its small streams can experience intermittency.
Within each Region, small- to medium-size wadable streams were selected to be studied, as these water bodies are typically prone to undergo hydrological fluctuations such as a decrease or interruption of flow, with formation of non-flowing pools or complete drought, which is a situation that can last for long periods of time. The decision on the adequacy of the streams and sampling sites required a combination of previous knowledge of the terrain and observation of satellite photographic information, as well as a review on existing literature data (streams that had shown some degree of drought in the past were specifically included). After this task, a total of 28 sampling sites were selected (Fig. 1): seven sites (in five different streams) in Region N, ten sites (in four different streams) in Region S and eleven sites (in seven different streams) in Region C – additional information in Table S1 in Santos et al. (2023). Comparability among sites was ensured by selecting a common set of features, namely shallowness (< 1 m deep), narrowness (1–12 m wide) and riparian zone features (varying from absent to semi-continuous), and absence of visible anthropogenic impacts.
Sampling was performed in two consecutive years (2018 and 2019) in the spring, to match the orientations of nationally adopted WFD bioassessment protocols (INAG 2008). The first sampling campaign (in 2018) was preceded by a year when prolonged extreme drought events took place; by contrast, the second (in 2019) followed a year without such extreme climatic scenarios (see ‘Climate characterization’ for details on climatic data).
Climate characterization
Precipitation values in both May 2018 and 2019 (sampling months), as well as cumulative precipitation of the 3 and 6 months prior to sampling moments were provided by the Instituto Português do Mar e da Atmosfera – IPMA (www.ipma.pt). For the same time frames, the Palmer Drought Severity Index (PDSI) was retrieved, providing a clearer picture of the hydrological and climatic circumstances in each study region before sampling – PDSI considers the precipitation, air temperature and availability of ground water, classifying drought periods according to severity (Palmer 1965). This variable was categorized on an ordinal scale from 1 (no drought) to 5 (severe drought). Spatial resolution of the original database forced the sharing of climatological information for close sampling sites.
Water sampling and analysis
At each sampling site, water temperature (temp, °C), pH, dissolved oxygen (O2, mg/L and % saturation), conductivity (EC, µS/cm), and total dissolved solids (TDS, mg/L) were measured with a multi-parameter probe (Aquaread AP-2000). Water samples were collected and refrigerated until further analysis. In the laboratory, each water sample was mineralized with potassium persulphate (Ebina et al. 1983), followed by the measurement of total phosphorus content (TP, mg P/L) using the tin(II) chloride method (APHA 2005) and total nitrogen content (TN, mg N/L) with the cadmium reduction method (Lind 1979). To quantify total suspended solids (TSS, mg/L), each water sample was vacuum filtered through a GF/C filter (1.2 µm pore size) and the retained residue was then oven dried at 100 °C and weighed (APHA 2005). Coloured dissolved organic carbon (CDOC, as ε450, in m−1) was measured on the filtrate with absorption spectrophotometry (Williamson et al. 1999).
Hydromorphological characterization
Hydromorphological characterization of each sampling site comprised the measurement of channel width and depth, as well as flow velocity (flow meter; Global water, FP101), turbidity (visual estimation based on the visibility of the substrate) and continuity of riparian gallery in both banks and shading (% coverage). These variables were then categorized in an ordinal scale from 0 to 4 (flow, turbidity, riparian gallery) or 1 to 4 (shading, channel width and depth) (see Fig. S2 in Santos et al. (2023) for scaling details). Additionally, types of substrate in the river bed, logs and vegetation in the stream channel and signs of nearby human influence (e.g. infrastructure, agriculture, cattle passage) were recorded using a binary code for presence/absence (1/0). Elevation of each sampling site was obtained with Google Earth (https://earth.google.com/web/; assessed on 10/2022).
Macroinvertebrate sampling and identification
Benthic macroinvertebrate samples were collected at each sampling site with a standard kick net (0.30 m wide; 500 μm mesh size) by kick‐sampling six 1 m transects representing all microhabitats proportionally (Hering et al. 2003; INAG 2008). Samples were stored in plastic containers and immediately preserved with 70–80% v/v ethanol. In the laboratory, the organisms were sorted out and identified to the lowest practicable taxonomic level (generally the family and, whenever possible, genus or species) under a stereoscope (Olympus SZX10). Identification was assisted by specialized literature including general (Tachet et al. 2010) and taxon-specific taxonomical keys (Hynes 1993; Wallace et al. 2003; Edington and Hildrew 2005; Elliott and Humpesch 2010). The abundance of each macroinvertebrate taxon was recorded for each sample (full data are made available in Supporting Table S1).
Ecological status determination
Ecological status was determined using multi-metric indices derived from the macroinvertebrate community, by estimating the deviation from benchmarks corresponding to pristine reference conditions for each river typology. This is done by calculating ecological quality ratios (EQR) that are translated into ecological quality status classes (High, Good, Moderate, Poor, Bad). Following national bioassessment protocols (INAG 2009), multi-metric indices IPtIN or IPtIS (respectively, North and South Invertebrate Portuguese Index; INAG 2009; APA 2016)) were calculated according to their adequacy to the typology of each stream under evaluation (see Table S1), in conformity to the WFD scheme (APA 2016). These multi-metric indices assess general degradation impact on invertebrate fauna and are calculated as follows:
where S is the number of families (richness), EPT is the number of families from the orders Ephemeroptera, Plecoptera and Trichoptera (sensitive taxa), J′ is Pielou’s equitability index (evenness), IASPT is the average sensitivity score per taxon (derived from the Iberian adaptation of the BMWP index; Alba-Tercedor and Sánchez-Ortega 1988), and log (sel. ETD + 1) and log (sel. EPTCD + 1) stand for the logarithm of the sum of the abundances of selected sensitive taxa (INAG 2009; Munné and Prat 2009; van de Bund 2009; Feio et al. 2014b).
Before the calculation of the multi-metric indices, each macroinvertebrate metric was normalized to the corresponding reference values. A second normalization step included the division of the multi-metric index value by its type-specific reference value, thus obtaining the EQR and corresponding ecological status. For further details on river typology of each stream and the multi-metric index used in the calculation of the ecological status of each sampling site, see Table S1 in Santos et al. (2023).
Data analysis
Environmental variables were grouped in three datasets – climate, physicochemical and hydromorphology. Prior to any analysis, variables were standardized to accommodate differential units of measure and scales. Also, uninformative variables were discarded: variables coding for the presence of rocks and pebbles were not considered, as well as TN and water colour, which were overall homogeneous across samples. Principal component analysis (PCA) was used to analyse each environmental dataset separately, allowing to understand the main environmental gradients and sources of variation (regional versus annual).
The macroinvertebrate dataset was inspected and all taxa under (1) 5% occurrence or (2) under 10% occurrence and low total abundance (< 10 individuals) were excluded; also, partially unidentified specimens were excluded from the dataset. This species dataset was then analysed using principal coordinate analysis (unconstrained PCoA) applied to transformed abundance data (square root transformation followed by Wisconsin standardization), and computed on a distance matrix based on the square root of Bray–Curtis dissimilarity (see details in Santos et al. 2023). The PCoA allowed to infer the main sources of variation (regional versus annual), as well as understanding which taxa were associated with the most important ecological gradients. To explore the association between community structure and ecological status in 2018 versus 2019, PCoA sample (i.e. site) scores (from the first and second axis) were correlated with the corresponding site EQR (using Pearson correlation index, r) for both years (jointly and separately), and a heat map-like table was built with the EQRs and ecological status of each site in each of the sampling years.
To assess the contribution of explanatory environmental variables on the macroinvertebrate community, a distance-based redundancy analysis (db-RDA) based on Bray–Curtis dissimilarity was used, as a constrained form of PCoA (Legendre and Anderson 1999; Borcard et al. 2018). The steps prior to this analysis included, similarly to the unconstrained PCoA, the transformation of the macroinvertebrate abundance data (square root transformation followed by Wisconsin standardization) and of the Bray–Curtis dissimilarity distance matrix (square root). Standardized environmental variables were a priori selected for each environmental dataset (physicochemical, hydromorphology, climate), following a stepwise selection procedure coupled with the inspection of variance inflation factors (collinearity) and variable significance in the final model (Borcard et al. 2018). This combination of methods aimed at the construction of parsimonious models, avoiding the noise caused by redundant or less informative variables (Gauch Jr 1993).
The db-RDA allowed assessing the amount of variation explained by the environmental datasets (physicochemical, climate and hydromorphology), including their unique (‘pure’) and combined contribution (global model). To do so, the variation explained by each environmental dataset while partialling out the effect of the other datasets was computed using a variation partitioning technique (Borcard et al. 1992; Peres-Neto et al. 2006). A Venn diagram was built to illustrate the unique and joint (overlapping) contributions of the selected climate, hydromorphology and physicochemical variables. All models were tested for significance using a permutation test.
In all cases (PCA, unconstrained PCoA and db-RDA), we fitted two (additional) categorical predictors to the multi-variate models to statistically confirm interpretable differences among regions across years. The marginal effects of region (C, N and S), year (2018 and 2019) and their interaction were tested with a permutational multi-variate analysis of variance (PERMANOVA), using the function adonis2() from the ‘vegan’ package (Oksanen et al. 2022). In the presence of a significant Region × year interaction, we tested for significant differences among regions within each year.
Multi-variate analyses and graphics were rendered with R software (R Core Team 2023), using the IDE interface RStudio (RStudio Team 2021). Further details on required R packages and functions used to run PCA, PCoA and db-RDA, as well as their graphic representations, can be found in Santos et al. (2023). In all multi-variate ordinations (PCA, unconstrained PCoA and db-RDA), two dimensions were considered sufficient to represent the most important fraction of variance in the data (evaluated with the help of the evplot() function from Borcard et al. (2018)); the only exception was the PCA for hydromorphological dataset, where a third dimension could have been considered, but we kept the two-dimensional representation for the sake of coherence and simplification.
Results
Environmental gradients captured by the sampling design
Overall, the sampling years presented distinguishable climatic features, translated by a clear separation of the sites into two distinct groups (2018 versus 2019; Fig. 2). The spring of 2018 was wetter than the analogous period of 2019, hence the association between the precipitation in May with the samples from 2018 and the PDSI in March and May and the samples from 2019. In fact, the highest PDSI values in the spring 2018 (March, May) were close to the lowest ones recorded in 2019. However, the preceding PDSI values (6 months prior sampling) were positively associated with the 2018 samples, indicating the unusual dry period which occurred in 2017. This drought severity is confirmed by the PDSI measured 6 months prior to both sampling moments (December 2017 and 2018) which was very high in all Regions in 2017 (Fig. S1). In December 2018, PDSI presented values expected for the season. Distinct climatic features were recorded across Regions, mostly in terms of precipitation (Fig. 2). Region C was the wettest and S the driest, with N being intermediate; indeed, significant differences among Regions were statistically confirmed for 2018 and 2019 (Table 1). In the aftermath of the more severe drought conditions preceding the sampling period (second half of 2017), the climatic similarities between Regions N and S became more evident in 2018 (confirmed by the significant interaction between year and region; Table 1), with the exception of two S sites (Oei2 and Oei3 – the driest sites in 2018; Fig. S1) (Fig. 2).
PCA biplots for climatic (top), water physicochemical (middle) and hydromorphological (bottom) data. Symbol colours represent sampling sites according to geographical region (blue: Region C; yellow: Region N; red: Region S); symbol shapes represent sampling sites according to sampling year (circles: 2018; triangles: 2019; no annual variation in hydromorphology). Arrows represent environmental variables (corresponding labels shown close to tip). Ellipses (95% confidence) are shown in colour (filled areas) for each region (blue: Region C; yellow: Region N; red: Region S) and discontinuous lines for each year (dashed: 2018; dotted: 2019). The percent variation explained by each PC is given within parentheses. For simplification, labels of sampling sites are not shown, but can be found in Fig. S1 (Supporting Information). pdsi_dec and pdsi_mar stand for the PDSI in December and March, respectively, prior to sampling, and pdsi_may for the PDSI in May (sampling month); prec_6m and prec_3m stand for cumulative precipitation in the 6 and 3 months, respectively, prior to sampling, and prec_may for the cumulative precipitation in May. EC stands for electrical conductivity, temp for water temperature, TDS, total dissolved solids; TSS, total suspended solids; CDOC, coloured dissolved organic carbon; TP, total phosphorous; O2, dissolved oxygen; O2_sat, oxygen saturation of the water; macroph, presence of macrophytes in the channel; ranunc, presence of Ranunculus sp.; cattle and construc, presence of cattle and construction, respectively, in the vicinities of the site; sand, blocks and logs, presence of sand, blocks and logs, respectively, in the channel; width, channel width; turb, turbidity; herbac, herbaceous plants; depth, channel depth; agricul, the presence of agriculture in the banks; elevation, site elevation; ripar_r and ripar_l, riparian gallery in the right and left banks, respectively; flow, water flow; shadow, percentage of shading in the channel
The physicochemical context of the studied network was more homogeneous in 2018 than in 2019, i.e. local variation within Regions was more pronounced in 2019; this translated into a significant Region × year interaction (Table 1). Still, regions formed almost completely separated groups, indicating physicochemical differences among them in 2018, as well as in 2019 (Table 1). Regions N and S were locally heterogeneous (large dispersion in the PCA), while Region C showed high homogeneity. Xed and Vil1 (Region N; Fig. S1) and Oei1–Oei4 (Region S; Fig. S1) were the sites driving the heterogeneity found in the regions they belong to. Despite annual differences, separation across regions was mostly associated with water temperature and conductivity, suggesting coincident temperature and mineralization gradients (Region S > Region N > Region C). Dissolved oxygen was also important, especially in 2019, when low values were observed in some sites (most noticeably in Xed; Region N).
The general hydromorphological context did not change from 2018 to 2019 in any of the sites, despite the differential drought context. Regional differences in the hydromorphological profile were consistent in 2018 and 2019 (PERMANOVA: F2, 52 = 10.2, P < 0.001; Table 1). Sites from Region C were associated with higher flow, riparian cover and associated shadowing, features that were less intense in the other Regions (especially Region S). In parallel, almost all sites from Regions N and S showed strong presence of macrophytes (especially Ranunculus sp.) and terrestrial herbaceous plants in narrower river channels with sandy substrate. Further details on the hydromorphology of the sampling sites can be found as Supporting Information in Santos et al. (2023).
Macroinvertebrate community structure and ecological status
Overall, the segregation of sample scores by Region was consistent in both sampling years (Fig. 3, top panel), which was confirmed by PERMANOVA (no significant Region × year interaction and significantly marginal effect of Region; Table 1). Although the sampling in 2018 occurred in the aftermath of a severe drought period (summer and autumn 2017), the distribution of the samples in the PCoA pointed out the greater importance of region over drought severity (represented by the year) as a determinant of the recovery/structure of the macroinvertebrate communities. In both sampling years, Region C formed a homogeneous group of sites that was overall well separated from Regions S and N (along the first ordination axis). The separation of Regions N and S was less clear, because sites from Region N were more scattered. A few sites belonging to Regions N (Moi, Vil2) and S (Cap2) seemed more similar to the sites from Region C than to the regions they belong to in 2019, whereas in 2018 this only happened with Moi (Region N).
Unconstrained PCoA ordination of macroinvertebrate abundance data based on Bray–Curtis dissimilarity (top), and the relationship between ecological status [ecological quality ratios (EQR)] and PCoA site scores from the first axis (bottom). To aid in visualization, ordination plot is broken down by year (2018 versus 2019) for comparative purposes; correlation coefficient (Pearson’s r) and significance (P) are also shown per year. Sampling sites in each Region (blue: Region C; yellow: Region N; red: Region S) are shown as circles (2018) or triangles (2019), whereas highlighted areas represent 95% confidence ellipses for region. The per cent variation explained by each axis is given within parentheses
The first PCoA axis was correlated to ecological quality (measured as EQR; r = 0.53, P < 0.001), but not the second axis (r = − 0.079, P = 0.56). This was consistent when both years were separately analysed (Fig. 3, bottom panel), which was an indication of some correspondence between community composition and ecological quality in both years. This association was also underpinned by regional differences and High ecological status characterized the vast majority of the sites from Region C (Table 2 and Fig. 3). In fact, sites from this Region presented High or Good ecological status only, indicating that this region had a better ecological status in general. By contrast, Regions N and S presented a broader range of ecological statuses in their sites, varying from Poor to High. In general, Region N presented worse ecological statuses than the other regions, with sites having mostly Moderate ecological status. This was overall consistent across sampling year. A few changes were observed on the EQR and ecological status of the sites across years, with the overall tendency being the maintenance or improvement of classification from 2018 to 2019 (Table 2). Out of 28 sites, the ecological status class improved in eight (Oei1, Cal1, Cal2, Cat, Boi1, Boi2, Moi, Vil2), did not change in 17, and worsened only in three locations (Oei2, Oei3 and Oei4). Taxa exclusive to Region C were mostly sensitive (75% of taxa with an IBMWP score ≥ 7), whereas exclusive taxa from Regions N and S were mostly tolerant (82% and 87.5% had a score < 7, respectively).
Constrained analysis and variation partitioning
The final db-RDA models included the environmental variables that explained most variation (whilst avoiding redundancy) in the macroinvertebrate community, following the a priori selection procedure described in the methods section: (1) water temperature, dissolved oxygen (in mg/L) and EC (physicochemical dataset); (2) presence of Ranunculus sp., elevation, water flow, channel width and presence of riparian gallery on the right bank (hydromorphology dataset); and (3) precipitation in the 6 months prior to sampling and PDSI in December (climate dataset). The db-RDA with all explanatory environmental variables (global model; Fig. 4, top left) showed a clear separation of Region C from a shared area including sites from Regions N and S, as already displayed in the unconstrained analysis (see Fig. 3). Higher water flow, more cumulative precipitation in the 6 months prior to sampling and higher levels of dissolved oxygen in water were strongly associated with this separation. On the opposite side of these gradients, higher temperature, presence of Ranunculus sp. and other macrophytes in the channel and higher electrical conductivity of water seem to be associated with the sites from Regions N and S. The distribution of sites was similar in both sampling years, with very few exceptions (Fig. 4, bottom panel). Similarly to the unconstrained PCoA, the observed regional differences were consistent across years (Table 1).
Constrained PCoA ordination (db-RDA, based on Bray–Curtis dissimilarity) of macroinvertebrate abundance data (species represented by blue crosses, ×) in stream samples from distinct Regions (blue: Region C; yellow: Region N; red: Region S) in two consecutive years (sites are represented by circles for 2018 and triangles for 2019). Arrows represent selected physicochemical, hydromorphological and climatic explanatory variables, and highlighted areas represent 95% confidence ellipses for region (filled areas). To aid in visualization, the ordination plot is broken down by year (2018 versus 2019) for comparative purposes (bottom panel). The percent variation explained by each axis is given within parentheses on the top left triplot. For simplification, labels of sampling sites are not shown in the upper left ordination plot. Taxa abbreviations are decoded in Table S1 (Supporting Information). In the upper left panel: ranunc, presence of Ranunculus sp.; temp, water temperature; EC, electrical conductivity; pdsi_dec, the PDSI in December prior to sampling; width, channel width; elevation, site elevation; ripar_r, riparian gallery in the right bank; O2, dissolved oxygen in the water; prec_6m, cumulative precipitation in the 6 months prior to sampling; flow, water flow
Taxa associated with the separation of Region C in both years along the first axis of the PCoA (Fig. 4, top right) were Leuctridae (Leuct), Atherix sp. (Ather), Rhyacophila sp. (Rhya), Polycentropodidae (Polyce), Philopotamidae (Philop) and a Siphonoperla morphospecies (Siphon), due to their frequency and abundance in this region (and in the sites from other Regions that were associated with Region C: Moi, Vil2 and Cap2). All these taxa are sensitive to pollution (IBMWP ≥ 7). Similarly, Notonecta sp. (Noto), Dytiscidae sp. larvae and an adult morphospecies (Dytlar and Dyt2ad, respectively), Aquarius sp. (Aqua) and Oulimnius sp. larvae (Oullar) seem important to explain the position of sites from Region N and Cap1 (Region S) in the ordination. In parallel, Caenis sp. (Caen), some Chironomidae groups [Tanypodinae (Tanyp), Chironomini (Chini), Tanytarsini (Tanyt)], Atyaephyra sp. (Atyae), Hydracarina (Hidrac) and Simuliidae (Simul) were important to explain the position of sites from Region S and Vil1 (Region N). Overall, these taxa are the reason for the separation of these two regions along the second axis of the PCoA. The vast majority of these taxa are tolerant to pollution (IBMWP ≤ 4).
The global db-RDA model explained 18.7% of the variation in ecological distance, whereas the contribution of individual datasets varied from 7.7% (climate) to 12.9% (hydromorphology) (Fig. 5). Unique contributions of each of the three environmental datasets are substantially lower, ranging from 2.7% (climate) to 7.3% (hydromorphology). In fact, physicochemical, hydromorphological and climatic features were inter-correlated and the shared proportion of variance explained was higher (5.3%; Fig. 5). Interestingly, when this shared proportion of variation was removed (by partialling out the influence of other datasets), differences across regions became less apparent: in the ordination diagrams depicting ‘pure’ variation for each dataset (Supporting Figs. S2–S4), the ecological distance highlighted differences across specific sites, such as Oei2, Oei3 and Oei4 (associated with higher mineralization), or Cat and Can (associated with higher elevation). To some degree, there were annual differences (2018 versus 2019), which were most obvious for the climate dataset.
Partitioning of variation in macroinvertebrate abundance data based on Bray–Curtis dissimilarity explained by environmental variables, including unique (within circles) and shared (within parentheses) contributions of each dataset of explanatory variables (physicochemical, hydromorphology, climate). Areas depict an approximate representation of % explanation
Discussion
The main aim of the present work was to compare macroinvertebrate communities and their bioindicator value across Regions (differing in historical water availability) in two consecutive years differentially affected by drought. This context allowed us to explore whether communities respond similarly to different drought scenarios (2018 drier than 2019) in Regions where water scarcity is constitutive compared with Regions where this is not the case. The data showed a surprisingly consistent environmental and ecological gradient across Regions in both years, despite 2018 and 2019 representing distinct situations in terms of their climatic and water availability background. Indeed, 2017 was the second hottest and the third driest year since 1931, especially between April and December, leading to abnormally high levels of evapotranspiration and deficits in soil humidity throughout this period (IPMA 2017). Although all sites were flowing in spring 2018, temporary dryness of many of them in the preceding summer is likely, given the water availability scenario in 2017. This assumption is reinforced by the record of various dry study sites when we visited them in October–November 2018, with the most affected Region being Region N, with only two out of seven sites with running water (only occasional pools remained). Notwithstanding, 2018 had levels of temperature and precipitation within the expected when compared with the norm (1971–2000 period) (IPMA 2018), which confirms that the situation in the summer and autumn of 2017 (preceding the 2018 sampling) was much more drastic. Thus, the conditions preceding the 2018 and 2019 sampling campaigns were quite distinct. Given this distinct background, our expectations were that 2018 presented a much more stressed ecosystem, exacerbating differences across Regions, but this was not necessarily the case (see below).
A previous study with 2019 data (Santos et al. 2023) detailed the environmental and ecological (i.e., macroinvertebrate community composition) differences across Regions C, N and S. A coinciding mineralization, temperature, riparian cover and water availability gradient clearly separated Regions C (wettest) and S (driest), with Region N occupying an intermediate position. Sampling sites from Region C had more flow, more riparian cover, and had overall higher ecological quality and homogeneous macroinvertebrate communities, opposing Regions N and S. When comparing 2019 with 2018 (the present study), consistent differences across Regions during both years remained. The selected streams along this gradient must thus reflect cumulative pressures and the stress of intermittency and reduced flow over the years. In both years, physicochemical features of sampling sites uncovered regional differences, although variation within Regions was more pronounced in 2019. Some degree of overlapping between Regions, due to scatter of samples, indicated local variation within each Region. The importance of temperature and mineralization gradients as drivers of these differences emphasizes the importance of climate, together with local hydromorphogical characteristics, in the outcome of abiotic characteristics of a site.
Specific taxa associated to the regional gradient were mostly sensitive organisms, which were present or more abundant in Region C; thus, not only the water availability gradient reflected taxonomic differences across Regions, but it also partially reflected a gradient of ecological quality. Other studies have shown that reduced flow and intermittency can cause shifts that benefit tolerant taxa, although there is little information on how prolonged these effects are. A reduction of EPT taxa and increase of chironomid abundance was observed in Mediterranean intermittent rivers as consequence of drought periods, even 4 weeks after resumption of water flow (Sánchez-Montoya et al. 2018). Differences in macroinvertebrate communities from perennial to intermittent portions of rivers revealed a noteworthy shift in structure and composition from sensitive taxa to more tolerant taxa when intermittency occurred (Di Sabatino et al. 2022). Indeed, flow disturbances seem to benefit the prevalence of generalist and less specialized taxa (Leigh and Datry 2017; Di Sabatino et al. 2022). Long-term consequences of repeated drought periods include local diversity reduction and homogenization of biotic communities, favouring species that are better adapted to lentic conditions (Piano et al. 2019, 2020) and general differences in composition, structure and functional traits of macroinvertebrate communities (Di Sabatino et al. 2022). Various authors (e.g. Leigh and Datry 2017; Sánchez-Montoya et al. 2018) have pointed out the critical importance of studying the abiotic factors and regional characteristics influenced by climate and drought patterns in the shaping of the macroinvertebrate communities, namely in Mediterranean rivers and in the context of intermittent streams.
The significant correlation found between the ecological gradients and EQR values (with sites from Region C having higher ecological quality – see above) was consistent for both years, although various sites improved ecological status in 2019 (low drought stress) compared with 2018 (high drought stress). On the one hand, the consistent association between community composition gradients and ecological quality supports the view that there are historical pressures differentiating these Regions, which are due to intertwined (and indissociable) climate, hydromorphological and physicochemical influences (see Figs. 4, 5). On the other hand, the improvement of ecological quality from 2018 to 2019 may indicate recovery from the stressful conditions of the summer and autumn of 2017. Still, some contradictory results at the microscale are worth noting, such as the case of Oei2, Oei3 and Oei4 (Region S), where ecological status worsened in 2019 compared with 2018. In fact, these sites witnessed a decrease in richness, from 37 taxa present in 2018 to 20 taxa in 2019. This decrease in ecological status in Oeiras stream was clearly opposite to the overall maintenance or improvement of ecological quality in other sites. This may be related with accumulated effects of drought in this area, which are historically very prone to intermittency due to water shortage (Graça, 2018; Gama et al. 2020), as recorded during 2017, and summer, autumn and winter 2018–2019 (IPMA 2017, 2018, 2019). Relating ecological quality to community structure is important given the ongoing discussion (e.g. Pawlowski et al. 2018; Santos et al. 2021) on whether current WFD metrics for the evaluation of ecological status are sensitive to pressures other than organic pollution (or other well-known stressors, such as pesticides or metals). This is particularly important in the case of ecosystem health assessment in a Mediterranean context, due to the challenging features of intermittent rivers (Buffagni et al. 2020; Munné et al. 2021). Putative mismatches between structural changes and ecological status can be useful to assess how well is the WFD bioassessment scheme working under different frameworks (in this case, comparing Regions with different water availability in two hydrological distinct years).
Bearing in mind the different climatic background across years, it seemed reasonable to expect that the constitutive differences and ecophysiological profile of the communities would translate into community differences between 2018 (higher stress) and 2019 (lower stress). A possible outcome could be that communities from Region S would be less disturbed than the ones from Regions C and N in 2018 because they were historically adapted to low flow and intermittency, whereas communities of sites from Region C would be more affected by drought/intermittency. Alternatively, the impacts of the background condition in 2018 could be more intense in Region S due to the cumulative effect of more stressful conditions (lower water availability, higher temperatures) and a better buffering capacity and resilience in sites from Region C, given the better riparian cover and presence of sensitive macroinvertebrate taxa. The rationale of these two hypotheses for Region S extends to Region N, despite its higher variability, as it is closer to Region S in terms of climate and overall characteristics. Surprisingly, our data show approximately the same distribution patterns of samples in both years, with a distinct separation of Region C from Region S in both years, and Region N being intermediate and more variable. This indicates that regional characteristics together with climate features were present in both years, resulting in such marked differences. Therefore, macroinvertebrate communities may be resilient or adapted to some degree of drought, maintaining the ecological distance despite background differences in the hydrological state in the months prior to sampling. These constitutive differences do not guarantee, however, the absence of cumulative structure shifts due to hydrological pressures (especially droughts), such as the substitution of sensitive taxa for taxa tolerant to drought. These shifts that occur through repeated drought periods may imply long-term negative implications on the recovery capacity of the communities after severe drought events (Di Sabatino et al. 2022). On the other hand, the severity of the drought period of 2017, in intensity and duration, may still be affecting the systems in 2019. As Region C was the least affected by drought, its communities may have recovered faster, which would explain the more distinct separation of site scores in 2019 comparatively to 2018.
Although our results show consistent, perhaps constitutive, taxonomical differences between macroinvertebrate communities across Regions that are modulated by intertwined climatic, hydrological and hydromorphological drivers, other aspects need to be explored. It is likely that functional changes may accompany these taxonomical shifts, with unknown consequences for functional integrity of the ecosystem. In fact, there is evidence that increasing flow intermittence may cause irreversible functional alterations in river communities, even when drying is a predictable disturbance (Crabot et al. 2021), such as the case of the Mediterranean Region. Exploring this functional component may allow checking which traits are favoured in Region S that justify resilience to hydrological pressure (low flow and/or frequent droughts), or if there are specific traits that are lost in these harsher conditions.
There is still a degree of uncertainty on exactly how resilient these communities are and how reliable are the metrics used to estimate ecological status in temporary/intermittent streams. It is important to resolve these gaps of knowledge, so that sustainable management can be done properly. Although governance falls beyond the scope of this work, the results of the present study confirm Region specificities in the response of benthic communities to drought, thus supporting that drought management should be done considering global but also specific objectives and measures (preventive and reactive). Understanding which areas are more prone to drought and more sensitive to the effects of water scarcity provides important information on how to control response to water demands, helping the establishment of priority guidelines towards efficient conservation.
Studies such as this one are important to improve the knowledge on how resilient aquatic communities are to pressures associated with climate and water availability, and whether the ecological status of riverine ecosystems is affected by these variables. The overall improvement in the ecological status from 2018 to 2019 signals more favourable environmental conditions to sustain biological communities in 2019 (less dry). As hydromorphology did not change and physicochemical conditions are connected in some degree with climate conditions, normal conditions of temperature and precipitation (such as the ones registered in 2018–2019) seem to be paramount to the maintenance of healthy biological communities in freshwaters. Also, improvement in ecological status indicates a certain degree of resilience of the communities and, consequently, ecosystems. However, a longer time frame is required to accurately capture the cumulative impacts of extremely dry years (such as 2017) in aquatic ecosystems, and the recovery potential of biotic communities therein. Additionally, the overall connection among climate, physicochemical and hydromorphological characteristics, and their shared influences on macroinvertebrate community structure, was evidenced in this work. This is an indication of the importance of considering an array of environmental features when studying the effects of disturbances. The severity of the drought phenomena solely may not be enough to grasp the mechanisms driving changes in biodiversity patterns. Thus, an accurate study of the big picture is imperative. This is particularly important in Regions such as the Mediterranean Region, where water availability is becoming more and more challenging, with expected negative impacts in the ecological status of water bodies and freshwater biodiversity.
Data availability
Raw data are provided as supplementary materials.
References
Alba-Tercedor J, Sánchez-Ortega A (1988) Un método rápido y simple para evaluar la calidad biológica de las águas corrientes basado en el de Hellawell (1978). Limnética 4:51–56
APA (2016) Plano de Gestão de Bacia Hidrográfica, Parte 2—Caracterização e Diagnóstico
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association (APHA), American Water Works Association (AWWA) and the Water Environment Federation (WEF), Washington DC
Arias Font R, Khamis K, Milner AM, Sambrook Smith GH, Ledger ME (2021) Low flow and heatwaves alter ecosystem functioning in a stream mesocosm experiment. Sci Total Environ 777:146067. https://doi.org/10.1016/j.scitotenv.2021.146067
Beck HE, Zimmermann NE, McVicar TR, Vergopolan N, Berg A, Wood EF (2018) Present and future Köppen–Geiger climate classification maps at 1-km resolution. Sci Data 5:180214. https://doi.org/10.1038/sdata.2018.214
Bogan MT, Chester ET, Datry T, Murphy AL, Robson BJ, Ruhi A et al (2017) Resistance, resilience, and community recovery in intermittent rivers and ephemeral streams. Elsevier Inc, New York
Borcard D, Legendre P, Drapeau P (1992) Partialling out the spatial component of ecological variation. Ecology 73(3):1045–1055. https://doi.org/10.2307/1940179
Borcard D, Gillet F, Legendre P (2018) Numerical ecology with R, 2nd edn. Springer, New York
Bruno D, Belmar O, Sánchez-Fernández D, Guareschi S, Millán A, Velasco J (2014) Responses of Mediterranean aquatic and riparian communities to human pressures at different spatial scales. Ecol Ind 45:456–464. https://doi.org/10.1016/j.ecolind.2014.04.051
Buffagni A (2021) The lentic and lotic characteristics of habitats determine the distribution of benthic macroinvertebrates in Mediterranean rivers. Freshw Biol 66:13–34. https://doi.org/10.1111/fwb.13596
Buffagni A, Erba S, Cazzola M, Barca E, Belfiore C (2020) The ratio of lentic to lotic habitat features strongly affects macroinvertebrate metrics used in southern Europe for ecological status classification. Ecol Ind 117:106563. https://doi.org/10.1016/j.ecolind.2020.106563
Cañedo-Argüelles M, Kefford BJ, Piscart C, Prat N, Schäfer RB, Schulz C-J (2013) Salinisation of rivers: an urgent ecological issue. Environ Pollut 173:157–167. https://doi.org/10.1016/j.envpol.2012.10.011
Carvalho JC, Cardoso P, Crespo LC, Henriques S, Carvalho R, Gomes P (2011) Determinants of beta diversity of spiders in coastal dunes along a gradient of mediterraneity. Divers Distrib 17(2):225–234. https://doi.org/10.1111/j.1472-4642.2010.00731.x
Chiu M-C, Leigh C, Mazor R, Cid N, Resh V (2017) Anthropogenic threats to intermittent rivers and ephemeral streams. In: Intermittent Rivers and ephemeral streams: ecology and management. Elsevier Inc., New York, pp 433–454
Crabot J, Mondy CP, Usseglio-Polatera P, Fritz KM, Wood PJ, Greenwood MJ et al (2021) A global perspective on the functional responses of stream communities to flow intermittence. Ecography 44:1511–1523. https://doi.org/10.1111/ecog.05697
Di Sabatino A, Coscieme L, Cristiano G (2022) Effects of antecedent drying events on structure, composition and functional traits of invertebrate assemblages and leaf-litter breakdown in a former perennial river of Central Apennines (Aterno River, Abruzzo, Central Italy). Ecohydrology 15(5):e2358. https://doi.org/10.1002/eco.2358
Djebou DCS (2017) Bridging drought and climate aridity. J Arid Environ 144:170–180. https://doi.org/10.1016/j.jaridenv.2017.05.002
Ebina J, Tsutsui T, Shirai T (1983) Simultaneous determination of total nitrogen and total phosphorus in water using peroxodisulfate oxidation. Water Res 17(12):1721–1726. https://doi.org/10.1016/0043-1354(83)90192-6
Edington JM, Hildrew AG (2005) A revised key to the caseless caddis larvae of the British Isles. Freshwater Biological Association, London
EEA (2019) The European environment—state and outlook 2020. Knowledge for transition to a sustainable Europe. European Environment Agency, Copenhagen, Denmark. https://doi.org/10.2800/96749
Elliott JM, Humpesch UH (2010) Mayfly larvae (Ephemeroptera) of Britain and Ireland: keys and a review of their ecology. Freshwater Biological Association, London
Feio MJ, Aguiar FC, Almeida SFP, Ferreira J, Ferreira MT, Elias C et al (2014a) Least disturbed condition for European Mediterranean rivers. Sci Total Environ 476–477:745–756. https://doi.org/10.1016/j.scitotenv.2013.05.056
Feio MJ, Ferreira J, Buffagni A, Erba S, Dörflinger G, Ferréol M et al (2014b) Comparability of ecological quality boundaries in the Mediterranean basin using freshwater benthic invertebrates. Statistical options and implications. Sci Total Environ 476–477:777–784. https://doi.org/10.1016/j.scitotenv.2013.07.085
Gama M, Banha F, Moreira C, Gama H, Graça M, Anastácio P (2020) Patterns of distribution of Bivalve populations in a Mediterranean temporary river. Diversity 12(4):158. https://doi.org/10.3390/d12040158
Gauch HG Jr (1993) Prediction, parsimony and noise. Am Sci 81(5):468–478
Gómez R, Arce MI, Baldwin DS, Dahm CN (2017) Water physicochemistry in intermittent rivers and ephemeral streams. Intermittent rivers and ephemeral streams: ecology and management. Elsevier Inc., New York, pp 109–134. https://doi.org/10.1016/B978-0-12-803835-2.00005-X
Graça MAS (2018) Qualidade biológica das águas da Ribeira de Oeiras
Grizzetti B, Pistocchi A, Liquete C, Udias A, Bouraoui F, van de Bund W (2017) Human pressures and ecological status of European rivers. Sci Rep 7:205. https://doi.org/10.1038/s41598-017-00324-3
Guareschi S, Mellado-Díaz A, Puig MÁ, Sánchez-Fernández D (2018) On the Iberian endemism Eurylophella iberica Keffermuller and Da Terra 1978 (Ephemeroptera, Ephemerellidae): current and future potential distributions, and assessment of the effectiveness of the Natura 2000 network on its protection. J Insect Conserv 22:127–134. https://doi.org/10.1007/s10841-018-0044-1
Heim RR Jr (2002) A review of twentieth-century drought indices used in the United States. Am Meteorol Soc 83(8):1149–1165. https://doi.org/10.1175/1520-0477(2002)083%3c1149:AROTDI%3e2.3.CO;2
Hering D, Buffagni A, Moog O, Sandin L, Sommerhäuser M, Stubauer I et al (2003) The development of a system to assess the ecological quality of streams based on macroinvertebrates—design of the sampling programme within the AQEM project. Int Rev Hydrobiol 88(3–4):345–361. https://doi.org/10.1002/iroh.200390030
Hering D, Haidekker A, Schmidt-Kloiber A, Barker T, Buisson L, Graf W et al (2010) Monitoring the responses of freshwater ecosystems to climate change. In: Kernan M, Battarbee RW, Moss B (eds) Climate change impacts on freshwater ecosystems. Blackwell Publishing, pp 84–118
Hynes HBN (1993) A key to the adults and nymphs of the british stoneflies (Plecoptera): with notes on their ecology and distribution. Freshwater Biological Association, London
INAG (2008) Manual para a avaliação biológica da qualidade da água em sistemas fluviais segundo a Directiva Quadro da Água—Protocolo de amostragem e análise para os macroinvertebrados bentónicos. Ministério do Ambiente, Ordenamento do Território e do Desenvolvimento Regional, Portugal
INAG (2009) Critérios para a Classificação do Estado das Massas de Água Superficiais - Rios e Albufeiras. Ministério do Ambiente, Ordenamento do Território e do Desenvolvimento Regional, Portugal
IPBES (2019) Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. In: Díaz S, Settele J, Brondízio ES, Ngo HT, Guèze M, Agard J, Arneth A, Balvanera P, Brauman KA, Butchart SHM, Chan KMA, Garibaldi LA, Ichii K, Liu J, Subramanian SM, Midgley GF, Miloslavich P, Molnár Z, Obura D, Pfaff A, Polasky S, Purvis A, Razzaque R, Reyers B, Roy Chowdhury R, Shin YJ, Visseren-Hamakers IJ, Willis KJ, Zayas CN (eds) IPBES secretariat, Bonn, Germany, pp 56
IPCC (2021) Climate change 2021: the physical science basis—summary for policymakers. Contribution of Working Group I to the sixth assessment report of the intergovernmental panel on climate change, pp 31
IPCC (2022) Climate change 2022: impacts, adaptation and vulnerability. Contribution of Working Group II to the sixth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
IPMA (2017) Boletim Climático Anual, Portugal Continental. Lisboa, Portugal, 24 pp
IPMA (2018) Boletim Climático Anual, Portugal Continental. Lisboa, Portugal, 20 pp
IPMA (2019) Boletim Climático Anual, Portugal Continental. Lisboa, Portugal, 23 pp
IPMA (2021) Normais climatológicas. https://www.ipma.pt/pt/oclima/normais.clima/. Accessed Oct 2023
Jones E, van Vliet MTH (2018) Drought impacts on river salinity in the southern US: implications for water scarcity. Sci Total Environ 644:844–853. https://doi.org/10.1016/j.scitotenv.2018.06.373
Kalff J (2002) Limnology—Inland water ecosystems. Prentice Hall, Hoboken
Legendre P, Anderson MJ (1999) Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. Ecol Monogr 69(1):1–24. https://doi.org/10.1890/0012-9615(1999)069[0001:DBRATM]2.0.CO;2
Leigh C, Datry T (2017) Drying as a primary hydrological determinant of biodiversity in river systems: a broad-scale analysis. Ecography 40(4):487–499. https://doi.org/10.1111/ecog.02230
Lind OT (1979) Handbook of common methods in Limnology. Kendall Hunt Publishing, Dubuque
Millennium Ecosystem Assessment (2005) Ecosystems and human well-being: synthesis. Island Press, Washington
Mishra AK, Singh VP (2010) A review of drought concepts. J Hydrol 391(1–2):202–216. https://doi.org/10.1016/j.jhydrol.2010.07.012
Mosley LM (2015) Drought impacts on the water quality of freshwater systems; review and integration. Earth Sci Rev 140:203–214. https://doi.org/10.1016/j.earscirev.2014.11.010
Munné A, Prat N (2009) Use of macroinvertebrate-based multimetric indices for water quality evaluation in Spanish Mediterranean rivers: an intercalibration approach with the IBMWP index. Hydrobiologia 628(1):203–225. https://doi.org/10.1007/s10750-009-9757-1
Munné A, Bonada N, Cid N, Gallart F, Solà C, Bardina M et al (2021) A proposal to classify and assess ecological status in Mediterranean temporary rivers: research insights to solve management needs. Water 13(6):767. https://doi.org/10.3390/w13060767
Nimmo DG, Mac Nally R, Cunningham SC, Haslem A, Bennett AF (2015) Vive la résistance: reviving resistance for 21st century conservation. Trends Ecol Evol 30(9):516–523. https://doi.org/10.1016/j.tree.2015.07.008
Oksanen J, Simpson GL, Blanchet FG, Kindt R, Legendre P, Minchin PR et al (2022) Vegan: community ecology package
Palmer WC (1965) Meteorological drought. Research Paper No. 45. US Weather Bureau
Pawlowski J, Kelly-Quinn M, Altermatt F, Apothéloz-Perret-Gentil L, Beja P, Boggero A et al (2018) The future of biotic indices in the ecogenomic era: integrating (e)DNA metabarcoding in biological assessment of aquatic ecosystems. Sci Total Environ 637–638:1295–1310. https://doi.org/10.1016/j.scitotenv.2018.05.002
Peres-Neto PR, Legendre P, Dray S, Borcard D (2006) Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87(10):2614–2625. https://doi.org/10.1890/0012-9658(2006)87[2614:VPOSDM]2.0.CO;2
Piano E, Doretto A, Falasco E, Fenoglio S, Gruppuso L, Nizzoli D et al (2019) If Alpine streams run dry: the drought memory of benthic communities. Aquat Sci 81:32. https://doi.org/10.1007/s00027-019-0629-0
Piano E, Doretto A, Mammola S, Falasco E, Fenoglio S, Bona F (2020) Taxonomic and functional homogenisation of macroinvertebrate communities in recently intermittent Alpine watercourses. Freshw Biol 65(12):2096–2107. https://doi.org/10.1111/fwb.13605
R Core Team (2023) R: a language and environment for statistical computing
RStudio Team (2021) RStudio: integrated development environment for R
Sánchez-Montoya MM, von Schiller D, Barberá GG, Diaz AM, Arce MI, del Campo R et al (2018) Understanding the effects of predictability, duration, and spatial pattern of drying on benthic invertebrate assemblages in two contrasting intermittent streams. PloS One 13(3):e0193933. https://doi.org/10.1371/journal.pone.0193933
Santos JI, Vidal T, Gonçalves FJM, Castro BB, Pereira JL (2021) Challenges to water quality assessment in Europe—is there scope for improvement of the current Water Framework Directive bioassessment scheme in rivers? Ecol Ind 121:107030. https://doi.org/10.1016/j.ecolind.2020.107030
Santos JI, Silva C, Gonçalves FJM, Pereira JL, Castro BB (2023) Macroinvertebrate community structure and ecological status in Portuguese streams across climatic and water scarcity gradients. Hydrobiologia 850:967–984. https://doi.org/10.1007/s10750-023-05137-2
Smith CR, McCormick PV, Covich AP, Golladay SW (2017) Comparison of macroinvertebrate assemblages across a gradient of flow permanence in an agricultural watershed. River Res Appl 33(9):1428–1438. https://doi.org/10.1002/rra.3211
Stubbington R, Bogan MT, Bonada N, Boulton AJ, Datry T, Leigh C et al (2017) The biota of intermittent rivers and ephemeral streams: aquatic invertebrates. Elsevier Inc., New York. https://doi.org/10.1016/B978-0-12-803835-2.00007-3
Tachet H, Richoux P, Bournaud M, Usseglio-Polatera P (2010) Invertébrés d’eau douce: systématique, biologie, écologie. CNRS Editions
van de Bund W (2009) Water framework directive intercalibration technical report, part 1: rivers. https://doi.org/10.2788/23384
Wallace ID, Wallace B, Philipson GN (2003) Keys to the case-bearing caddis larvae of Britain and Ireland. Freshwater Biological Association, London
Williamson CE, Morris DP, Pace ML, Olson OG (1999) Dissolved organic carbon and nutrients as regulators of lake ecosystems: Resurrection of a more integrated paradigm. Limnol Oceanogr 44(3, part 2):795–803. https://doi.org/10.4319/lo.1999.44.3_part_2.0795
Woodward G, Perkins DM, Brown LE (2010) Climate change and freshwater ecosystems: impacts across multiple levels of organization. Philos Trans R Soc B 365(1549):2093–2106. https://doi.org/10.1098/rstb.2010.0055
Acknowledgements
This work was supported by national funds (through Fundação para a Ciência e Tecnologia, Portugal) by means of the institutional programmes UIDP/50017/2020, UIDB/50017/2020 and LA/P/0094/2020 (CESAM), as well as UID/BIA/04050/2019 and UIDB/04050/2020 (CBMA, https://doi.org/10.54499/UIDB/04050/2020). Joana Isabel Santos and Carlos Silva were supported by individual doctoral grants (SFRH/BD/121341/2016 and SFRH/BD/138389/2018) by FCT. Funding institutions played no role in study design, data collection and interpretation, nor in manuscript preparation and submission. The authors acknowledge the assistance of Sandra Nogueira, who produced Fig. 1 using GIS software (location of sites and regions).
Funding
Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Contributions
JIS: conceptualization, investigation, formal analysis, data curation, writing – original draft. CS: investigation, writing – review and editing. FJMG: conceptualization, writing – review and editing, supervision, funding acquisition. JLP: conceptualization, formal analysis, writing – review and editing, supervision, funding acquisition. BBC: conceptualization, formal analysis, writing – review and editing, supervision, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose. The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Santos, J.I., Silva, C., Gonçalves, F.J.M. et al. Macroinvertebrate communities reveal regional asymmetries in riverine ecosystems along a gradient of water availability in hydrologically distinct years. Aquat Sci 86, 21 (2024). https://doi.org/10.1007/s00027-023-01037-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-023-01037-8