Abstract
Periphyton is the dominant primary producer in mountain streams and sustains the higher trophic levels. While certain periphyton groups, particularly diatoms, have received extensive study, the comprehensive characterization of the entire community has been largely neglected. This study aims to investigate the temporal pattern of biofilm in mountain streams characterized by different water thermal regimes. A one-year quantitative campaign, involving monthly samplings, was conducted in five subalpine streams in Northern Italy’s Orobic Alps to collect epilithic biofilm from a wide surface area. The total biomass was quantified and the periphyton was analyzed both for composition (diatoms, green algae, cyanobacteria, and red algae) and for pigments. Disturbance, water temperature, physico-chemical conditions, nutrients, substrate diversity, and light availability were assessed concurrently with biofilm samplings. Results show sharp biofilm variations over months. In all sites, the disturbance was the primary factor reducing biomass and pigment content. Annually, all sites experienced similar turnover in periphyton composition mainly associated with light and water temperature. Overall, the study indicates that frequent quantitative investigations of biofilm help understand intra-annual variations and identify key drivers. Such information is useful to understand the ecosystem processes and the food web dynamics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biofilm is the assemblage of algae, bacteria, fungi, and meiofauna enclosed within a mucilaginous, polysaccharide matrix attached to wet surfaces (Hauer & Lamberti, 2017). The autotrophic component, called periphyton, encompasses a diverse range of organisms primarily from Bacillariophyta (diatoms), Chlorophyta (green algae), Cyanophyta (cyanobacteria), Rhodophyta (red algae), and Chrysophyta (gold algae) groups. Although primary producers are a minority in mountain lotic systems due to their constant instability, they play a vital role in supporting upper trophic levels and providing habitat for invertebrates, protists, and bacteria (Rott et al., 2006). In headwaters, periphyton can contribute up to 80% of autochthonous primary production (Hansson, 1992), serving as a crucial energy for the trophic chain (Hauer & Lamberti, 2017). In mountain streams, flow is the primary factor controlling biofilm growth. The current exerts shear stress on biofilm causing cell sloughing (Power & Stewart, 1987a; Biggs, 1988). Extreme flows can have a strong negative impact on benthic biota that, due to high velocity, overturning of stones and tumbling abrasion, flows away (Power & Stewart, 1987b; Robinson & Rushforth, 1987). Such effects primarily result in decreased taxonomic richness and abundances as highlighted in several studies (Biggs & Smith, 2002; Wellnitz & Rader, 2003; Luttenton & Baisden, 2006). However, the size and type of substrate (rough or smooth) as well as the riverbed typology (armored or not) can play a pivotal role (McAuliffe, 1984; Biggs & Smith, 2002). The pattern of recolonization is taxon specific, depends on hydrology and substrate type, and is generally faster especially just after a flood and in streams where disturbances are frequent (Biggs & Smith, 2002). On the other hand, drought events also strongly influence biofilm, particularly affecting diatoms productivity and altering the composition of the assemblages (Falasco et al., 2020). Besides hydrology and substrate, light and nutrients also control periphyton growth. In shaded streams, periphyton growth is limited by insufficient light, while in unshaded, high-altitude streams, it is constrained by both low nutrient concentration and UV exposure (Rosemond, 1993; Hill et al., 1995; Mosisch et al., 1999; Sudlow et al., 2023). Nutrient availability is controlled by inputs from riparian and terrestrial environments (Hedin et al., 1998; Bernal et al., 2015), with phosphorus (PO43−), nitrogen (NH4+, NO3−), and silica (SiO2) being the primary factors. However, in many streams, silica concentration often exceed the minimum request (Robinson et al., 2000). Overall, mountain running waters not subjected to pasture are very poor of phosphate, while nitrogen, available generally in the form of nitrate (NO3−), comes from the atmospheric deposition. Finally, the water temperature affects the biomass of periphyton (Morin et al., 1999), controlling the rate of primary production through its effects on photosynthetic physiology (Medlyn et al., 2002). Moreover, the periphyton composition depends on the thermal preference of each taxon, and temperature variations promote changes in the dominance of the main groups (green algae, cyanobacteria, diatoms, and red algae) throughout the seasons (Allan & Castillo, 2007) and along the watercourses (Li et al., 2022).
Multiple factors influence the growth and composition of biofilm. However, research focusing on the fine temporal patterns of stream biofilm and providing an overall characterization of the community is relatively scarce. Most of this research has been conducted in New Zealand (Quinn & Hickey, 1990; Biggs, 1995) and South America (Necchi et al., 1995; Branco & Necchi, 1996, 1998; Pizarro & Alemanni, 2005). In contrast, investigations conducted in the Alps have predominantly centered around diatoms and cyanobacteria in Trentino springs (Italy) (Cantonati et al., 2005, 2012b; Gerecke et al., 2011), algae assemblages of Swiss glacier streams (Uehlinger, 1991, 2006; Hieber et al., 2005), and of Austrian watercourses (Rott et al., 2006; Rott & Wehr, 2016).
Given the importance of studying biofilm to gain insights into ecosystem processes and food web dynamics, this study seeks to explore intra-annual changes in epilithic biofilm and the associated environmental variables. The research study was conducted in five stream sites of the Serio catchment (Orobic Alps, Northern Italy), characterized by distinct thermal regimes. At each stream site, biofilm was surveyed monthly, over a year and different approaches were combined to investigate changes in periphyton through a fast assessment (without a taxonomic identification). Specifically, the research aims to describe within-site monthly variations in biofilm biomass, pigments content, and the relative abundance of periphyton groups, to compare annual differences among sites, and to assess the relationship between environmental factors and biofilm structure. We supposed temporal variations in total biomass and pigments’ content would primarily result from floods, while monthly shifts in the relative abundance of periphyton groups would be influenced by variations in water temperature (i). As a result, we expected that sites experiencing high annual water temperature variability would display more pronounced temporal dissimilarity in periphyton composition, leading to spatial distinctions (ii). Furthermore, we hypothesize that differences in substrate, water quality, and light availability would explain variations in the biofilm structure among the sites (iii).
Methods
Monitoring plan and sites
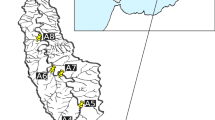
One-year campaign (June 2020–May 2021) with monthly samplings was carried out in five stream sites in the upper Serio catchment (Orobic Alps, Northern Italy). Streams are characterized by different water sources (snowmelt/stormwater and groundwater-fed streams) and anthropogenic pressures (presence of reservoirs) leading to different annual water temperature variabilities (annual daily maximum-annual daily minimum) ranging from ~ 1 to 16 °C. Sites S and G were situated in the Goglio catchment: S at 975 m a.s.l., on a tributary stream not subjected to flow regulation, and G at 1128 m a.s.l., on the Goglio stream, approximately 2-km downstream of reservoirs (volume of 5.4 Mm3 and dam height exceeding 20 m). Both streams are fed by snowmelt/storm waters. Due to flow regulation, G displays a lower water temperature variability (~ 16 vs ~ 9 °C). Sites U and D were situated upstream (970 m a.s.l.) and downstream (583 m a.s.l.) of a small reservoir (volume of 0.15 Mm3 and dam height not exceeding 12 m) in the Ogna catchment, along a stream fed by snowmelt/storm and groundwaters. In this case, the downstream site exhibits slightly lower variability compared to the upstream one (~ 7 °C vs ~ 8 °C). The N site was located in the Nossana catchment at 468 m a.s.l. on a groundwater-fed stream characterized by a constant temperature (~ 8 °C), situated about 200 m from the spring. All the monitored streams have similar morphology and substrates, with sizes ranging from gravel (0.2–2 cm) to large pebbles (> 40 cm). The study area, situated at altitudes ranging from 500 to 1200 m a.s.l, is covered by beeches and firs forests, while the riverbanks feature a variety of vegetation, including willows, alders, birches, hazels, maples, and shrubs (Fig. 1).
Biofilm sampling and quantification
Epilithic biofilm, i.e., that part of biofilm present on the stone surface in the river bed, was sampled following European and Italian methodology guidelines (APAT, 2007; CEN EN 14407, 2014). A dozen stones (~ 10–13), representing different microhabitats (akal/microlithal/mesolithal/macrolithal/megalithal) (Hering et al., 2004) were randomly selected from the riverbed to cover an area of about 0.5 m2. All stones from a specific site were then scrubbed, and the biomass was collected in 500 ml of stream water, resulting in a single composite biofilm sample for each sampling event (site × date). A 100-ml suspension of biomass was analyzed for dry weight (dried for 24 h at 105 °C) and ashes (burned for 4 h at 550 °C). The total biofilm mass was calculated as the ash-free dry mass (AFDM) normalized by the sampled surface (g/m2) following Hauer & Lamberti (2017). To calculate the sampling surface, we took a picture of the collected stones arranged on a squared sheet and measured the total area in QGIS. On the same day of the sampling, 10 ml of biofilm solution were frozen (− 20 °C) for the pigment extraction, while the rest was chilled to 4 °C and brought to the laboratory to be processed within 24 h to determine the composition of the algae community as described in the next paragraph.
Periphyton composition
The composition of the periphyton component of biofilm was determined analyzing the biofilm suspension by the Phyto-PAM II instrument (Heinz Walz GmbH, Effeltrich, Germany) that distinguishes the fluorescence signals emitted by four different groups of photosynthetic organisms: cyanobacteria, green algae, diatoms (and dinoflagellates), and red algae (organisms with phycoerythrin). The operating principle of PAM instrument is the same as BenthoTorch, commonly used for rapid assessment of phototrophic communities on river bottom substrates (Kahlert & McKie, 2014), but Phyto-PAM II can distinguish also red algae. The percentage of each algal group was calculated, and the composition diversity was estimated using the Shannon index. To assess the overall annual temporal community composition dissimilarity (Legendre, 2019) within a site, we calculated the multi-date β-diversity (i.e., also known as multi-site β-diversity (Baselga et al., 2022)) using the Sorensen dissimilarity (BSor) and its components (turnover and nestedness).
Pigment determination
Pigment content was determined as follows: 10 ml of biofilm solution was centrifuged at 10,000× g for 5 min; the supernatant was discarded and 10 ml of 99.9% methanol was added to the concentrated pellet, well mixed, and incubated at 45 °C for 24 h in dark. Pigment concentration was calculated according to the following equations as the mean of three replicates per sample (Lichtenthaler, 1987):
where Abs665, Abs652, and Abs470 are the absorbances measured by a spectrophotometer (DR600TM UV–VIS Spectrophotometer Hach Lange) at 665-, 652-, and 470-nm wavelength, respectively. The concentration of each pigment was then reported to the sampled surface and expressed as mg/m2.
Finally, the chlorophyll a concentration was used to calculate the autotrophic index (Weber, 1973) that estimated the ratio between heterotrophic organisms/organic detritus and autotrophic organisms.
Environmental variables
Stream microhabitat availability (HABITAT) was assessed using the standardized multi-habitat methodology used for macroinvertebrates sampling (Hering et al., 2004). When sampling biofilm, the relative proportion of each microhabitat covering more than 10% of the stream sampling site was measured and the Simpson index was calculated for each habitat. The light exposure (EXPOSURE) was estimated as a percentage of the open sky, based on photographic pictures of the surrounding trees (0% = full canopy, 100% = no canopy) (see Cantonati and Pipp, 2000). Each water sample was characterized for electric conductivity (CE), dissolved oxygen (DO), and oxygen saturation (O2) using a probe HACH-HQ40d (Loveland, USA). In addition, 0.5 l of water were collected and brought to the laboratory where pH was measured using a HANNA pH meter 211 (Woonsocket, USA), and nitrate nitrogen NO3–N (NNO3) and Chemical Oxygen Demand (COD) were determined using spectrophotometric test kits (Hach Lange, Düsseldorf, Germany, LCK 340, and LCK1414, respectively). Total phosphorus (Ptot) and ammoniacal nitrogen NH4–N (NNH4) concentrations were determined in the laboratory according to standard methods (APHA/AWWA/WEF, 2012). The water temperature was measured continuously (every 10 min) from Autumn 2019 to Autumn 2021 using iButton devices (DS1925L sensors: range − 40–80 °C, accuracy: ± 0.5 °C, resolution: ± 0.0625 °C) fixed in the riverbed of the five stream sites. The mean water temperature (TMean) of each sampling day was calculated as the mean of the 30 days before each sampling. Substrate movement associated with flood disturbance was measured using painted pebbles placed in the streambed according to Townsend et al. (1997). 15 locally sourced painted pebbles belonging to three size classes (50th, 75th, and 95th percentiles of the substrate size distribution) were placed in riffles, in random order in triplets (small, medium, and large), across the main flow of the stream at marked points on the stream bank. On each sampling day, the distance traveled by each stone was recorded and stones were placed back at their initial position (Death & Zimmermann, 2005). Flood disturbance (DISTURBANCE) was quantified for each sampling combining the displacement of the substrate with the moved mass by the following equation:
where m is the pebble mass and d is the displacement (1 = moved or 0 = not moved). Pebbles that could not be recovered were not considered since they could have been washed away or buried by sediments. In most cases (75%), all the pebbles were found. As several floods could occur within one month, the metric DISTURBANCE can be used as a proxy of the overall sediment movement between two consecutive sampling dates. Light availability (HLIGHT) in each site was calculated by the GRASS software. The “r.sun” function was used to obtain the hours of light at each site as a function of the day of the year and the topography (location and mountain shadowing). Each site was represented by an area of 250 m2 described by a digital terrain model with a resolution of 5 m. The light availability at each sampling day was measured as the mean of the daily light in the 30 days before. This indicator, coupled with the exposure, was considered more representative than the photosynthetically active radiation (PAR) that could have been measured monthly at each site: indeed, punctual PAR measures are very sensitive to weather conditions and to the specific riverbed position (Melbourne & Daniel, 2003) and, thus, are not representative of monthly changes.
Data analysis
To describe the temporal patterns of environmental variables monthly values were represented for each site apart for water temperature for which daily measures were used. Non-parametric Kruskal–Wallis and Dunn’s multiple comparison tests were performed to identify significant annual differences in the environmental variables among sites. The Bonferroni method was used to adjust p values for multiple comparisons. Analyses were performed using the “dunn.test” R package (Dinno, 2022). Similarly, the temporal variations of biofilm were represented by considering biomass/pigments and composition separately. First, the time series of biofilm biomass, periphyton pigments, and autotrophic index were plotted for each site. Then, the temporal change in community composition was represented by cumulative bar plots with the relative abundance of each algal group (cyanobacteria, green algae, diatoms, and red algae) for each site. To identify differences among temporal patterns of biomass/pigments in each site, Spearman correlations were used. The p-values were corrected for multiple inferences using Holm’s method using the rcorr.adjust function from the “RcmdrMisc” package (Fox et al., 2022). To visualize spatial variations, boxplots of biofilm metrics (biomass, pigments, periphyton groups, Shannon, and autotrophic indices) were reported for each site and Kruskal–Wallis and Dunn’s multiple comparison tests were performed to identify significant annual differences among sites.
To assess how environmental factors were associated with the biofilm structure, generalized linear mixed-effect models (GLMM) between biofilm metrics and environmental variables were developed and the relative contribution was determined. The analysis was performed using the “glm2” R package (Marschner & Donoghoe, 2022). Biomass, chlorophyll a, chlorophyll b, carotenoids, Shannon, and autotrophic indices were log + 1 transformed, while the relative abundance of each group was logit transformed (logit function from the “car” package (Fox et al., 2022) to normalize residuals and equalize variances. As predictors, the more explicative variables according to the PCA (Fig. 1A) were used; DO and pH were excluded as they were highly correlated with TMean (r Pearson > 0.7) and exhibited always optimal values as well as O2. HABITAT, COD, and NNH4 were also excluded as they did not show any temporal pattern and poorly contributed to the variance explained by the PCA. Thus, DISTURBANCE, CE, NNO3, TMean, and the interaction term between EXPOSURE and HLIGHT were included as fixed effects, while sites were included as random effects on the intercept. To account for temporal and spatial variations, the SEASON, identified as winter (December–January–February), spring (March–April–May), summer (June–July–August), and autumn (September–October–November), and the SITE (S, G, U, D, N), were included as fixed effects. The dredge function within the “MuMIn” package (Barton, 2022) was used to derive the optimal set of fixed effects tested within each GLM. This function fits different models comprising all the combinations of fixed effects and ranks them by the Akaike Information Criterion corrected for small sample size (AICc). The most parsimonious model within 2 AICc units of the best (the model exhibiting the lowest AICc value) was selected as the “optimal” model. The explanatory power of the statistical models was derived from marginal pseudo r-squared values (R2marginal, see Nakagawa et al., 2017), which quantify the variance explained by the fixed effects, obtained using the r.squaredGLMM function in “MuMIn.” The significance of each optimal model was obtained via likelihood ratio tests (see White et al., 2018); if SEASON or SITE was included in the optimal model, it was also included in the “null” model to test the joint effect of the other variables. Finally, to establish if differences in the water thermal regime could partially explain differences in periphyton composition among sites the annual water thermal variability was correlated with the annual temporal dissimilarity (BSor). All the analyses were performed in R-project software (R Core Team, 2020). Please note that the analyses are constrained by a small sample size (n = 60) and the temporal autocorrelation among within-site samples, which restrict the statistical power. Consequently, our emphasis is on the observed patterns and associations rather than statistical significance.
Results
Patterns of environmental variables
The daily mean temperature ranged from ~ 0 °C to ~ 16 °C in S, between ~ 3 and 12 °C in G; between ~ 3 and 11 °C in U, it was almost constant (~ 8 °C in N); nevertheless, according to the Dunn’s test, even with distinct thermal variability, all sites were characterized by a similar annual thermal average (P = 0.03, Fig. 2A). The water thermal variability was smaller in the sites with groundwater inputs (U and D) than in snowmelt/stormwater sites (S and G), while the stream fed exclusively by groundwater (NN) had a stable thermal profile. DO exhibited a temporal pattern opposite to the thermal one (P = 0.05, Fig. 2B), while pH followed the thermal behavior with slightly differences among S, N, and the other sites (P = 0.08, Fig. 2C). DISTURBANCE displayed strong temporal fluctuations, synchronous in all sites, with abrupt peaks in June, September, October, and May and maximum values (70–100%) in autumn without annual differences among sites (P = 0.95, Fig. 2D). HLIGHT strongly varied through the year reaching 8–12 h in summer and 0–3 h in winter (P = 0.6, Fig. 2E); of course, EXPOSURE had higher values in winter, in the absence of leaves on the trees, especially in Ogna sites (U and D) that were different from the Goglio sites (S and G) and the N site (P = 0, Fig. 2F). The other environmental variables did not show any seasonal pattern but displayed some annual differences among sites according to the Dunn’s test. First, CE was below 85 µS/cm in sites dominated by snowmelt/stormwater (S and G), while it was above 200 µS/cm in sites influenced by groundwater inflows (U, D, N) and especially in the D site where it reached 480 µS/cm as annual average. It was also slightly higher in regulated sites (D and G) than in the unregulated ones (U and S) (P = 0, Fig. 2H). Second, NNO3 was lower (< 0.6 mg/l) in Goglio sites (S and G) than in the others and in D was higher (~ 1.3 mg/l annual mean) (P = 0, Fig. 2I). COD was below 10 mg/l in all sites, indicating a low concentration of oxidable compounds (P = 0.34, Fig. 2L), including ammoniacal nitrogen (NNH4 < 0.005 mg/l) (P = 0.27, Fig. 2M) and the concentration of total phosphorus (Ptot) was always below the methodological sensitivity (0.001 mg/l). The streambed HABITAT availability was consistent both among and within the sites (P = 0.01, Fig. 2N) (Table 1).
Biofilm temporal patterns
Photosynthetic pigments and total biomass showed similar temporal patterns in each site, as indicated by their strong positive Spearman correlation (ƿ always greater than 0.5, Table 1A). The highest values were observed in winter, while lower values occurred in summer and after the floods (Fig. 3A). By contrast, the autotrophic index was negatively correlated with biomass and photosynthetic pigments in all sites (ƿ always lower than − 0.32, Table 1A) and had the highest values in summer and autumn (Fig. 3A). Winter promoted green algae, while summer cyanobacteria and red algae; diatoms were the dominant group in all sites (Fig. 3B). In N, an exceptional algal bloom occurred in November when the total biomass reached 90 g/m2, while Chlorophyll a and b were 81 mg/m2, and 73 mg/m2, respectively. Annually, the sites exhibited similar changes in periphyton composition as the temporal dissimilarity was 0.63, 0.61, 0.57, 0.49, and 0.58 in S, G, U, D, and N, respectively. The total β-diversity (BSor) corresponded to the turnover component (the ratio between turnover and Sorensen indices was 0.97) outlining that the temporal changes in community composition were driven almost only by the replacement and not by the loss/gain of groups.
Temporal pattern of A biofilm biomass, periphyton pigments, and autotrophic index and B periphyton community composition. Black arrows represent the sampling dates following a flood (with the highest occurring in September and October). Missing data in community composition were due to Phyto-PAM malfunction or loss of samples
Biofilm spatial patterns
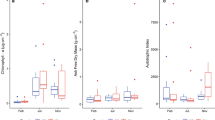
The overall epilithic biofilm biomass was 9.4 ± 12.7 g/m2 on average (Table 2). According to the Dunn’s test sites, D and U sites showed marked differences from the others, with lower and higher annual averages, respectively (the annual mean is 4.4 ± 2.5 g/m2 and 12.9 ± 8.94 g/m2, respectively, P = 0.01, Fig. 4A). Sites below dams (G and D) had higher annual biomass compared to their unregulated counterparts (S and U). The sites exhibited similar annual pigment content (P = 0.63, 0.24, and 0.11 for carotenoids, chlorophyll a, and b, respectively, Fig. 4B). However, site U displayed the lowest content of chlorophyll a, carotenoids, and chlorophyll b (2.9 ± 3.2, 1.0 ± 1.4, and 1.3 ± 1.3 mg/m2, respectively), while site D had the highest (11.1 ± 14.5, 2.6 ± 4.7, and 8.7 ± 12.6 mg/m2, respectively). Diatoms were the most represented group (58.6%), followed by cyanobacteria (18.6%), red algae (17.1%), and green algae (14.1%), with high variability among samplings (Table 2). For example, green algae were slightly more abundant in G and S (15.7% and 11.4%, respectively) and absent in N. Red algae represented 22.7% of the community in N and U. Annually, sites displayed similar abundances of diatoms, green algae, and red algae (P = 0.19, P = 0.12, and P = 021, respectively, Fig. 4C) but not of cyanobacteria, which dominated in N and were scarce in S (P = 0.02, Fig. 4C). The Shannon and autotrophic indices were similar in all sites (~ 2800–3700) (P = 0.92 and 0.97, Fig. 4D and E, respectively).
Boxplots comparing the annual variability of A biofilm biomass, B pigment’s component, C the percentage of the main groups, D Shannon index, and E Autotrophic index in the five stream sites. The p value of Kruskal–Wallis test is reported above each plot and lowercase letters indicate which comparisons are significant (Dunn’s multiple comparison test, alpha = 0.05). Outliers were excluded for a better visualization
Environmental factors and biofilm
In general, the selected environmental variables explained 0.11–0.51 of the biofilm metrics’ variance except for the Shannon diversity index, which remained unexplained (Table 3). Biomass, Chlorophyll a, Chlorophyll b, Diatoms, and Carotenoids displayed strong negative association with DISTURBANCE (P < 0.01) in contrast to the Autotrophic index and the relative abundance of Red algae that were positively associated (P < 0.001 and 0.05 ≤ P < 0.1, respectively). Light availability, expressed as the interaction between EXPOSURE and HLIGHT, was weakly negatively correlated with biomass and the relative abundance of diatoms (0.01 ≤ P < 0.05), while it was strongly positively related to the relative abundance of cyanobacteria (P < 0.001). NNO3 exhibited a weak positive association with chlorophyll b (0.05 ≤ P < 0.1) and was strongly correlated with cyanobacteria abundance (P < 0.001). TMean poorly explained the relative abundance of green algae and red algae (0.01 ≤ P < 0.05 and 0.05 ≤ P < 0.1, respectively). The time (SEASON) was pivotal to explain the amount of Chlorophyll a and carotenoids, which exhibited different behaviors during winter compared to the other months. Space (SITE) was important in explaining differences in biofilm biomass (Online Table 2A). Annual water thermal variability and annual temporal diversity (BSor) were poorly correlated (R2 = 0.18, p = 0.48).
Discussion
Temporal patterns
One-year biofilm samplings revealed a pronounced seasonal pattern in total biomass and pigment concentration common to all sites. Autumn floods drastically reduced biofilm, altering the balance between autotrophic and heterotrophic organisms/organic detritus. Subsequently, in November, the autotrophic index peaked, followed by a biofilm recovery during winter, with increased pigment content persisting into spring. The results indicate that the biofilm biomass is primarily influenced by the disturbance, consistent with other studies conducted in mountain streams. For instance, Power and Stewart (1987a, b) observed a 55% decrease in algal coverage following severe floods in a Oklahoma stream (New Zealand). Similarly, Biggs (1995) reported a negative correlation between chlorophyll a and flood disturbance frequency in various New Zealand streams. According to our field observations, the abrasion was the primary mechanism of biofilm removal because of the substrate overturning and tumbling. Although the pebble displacement method had already proven to be valid for the assessment of the ecological effect of floods (Power & Stewart, 1987a; Biggs, 1995), flow data, unfortunately unavailable here, would have contributed to explain biomass variations during free flood periods and similarly, the concentration of suspended solids. Indeed, biofilm biomass responds also to changes in current velocity and sediment scouring (Horner & Welch, 1981; Horner et al., 1990; Biggs, 1996; Francoeur & Biggs, 2006; Niedrist et al., 2018).
In winter, a sparce canopy can enhance biofilm growth due to the limited light in small streams under dense tree cover (DeNicola, 1996; McConnell & Singler, 1999; Larned & Santos, 2000). Consequently, periphyton tends to peak just before canopy development, declining through the summer, as observed in our surveyed streams, especially those with lower exposure (S, D, U and D) (Hill & Dimick, 2002). However, in this case, the time (SEASON) better explained the monthly changes in chlorophyll a and carotenoids than light availability (EXPOSURE: LIGHT), indicating similar temporal variations across all sites. Light availability impacts photosynthesis, which in turn affects carbon fixation and elemental ratios (Sterner et al., 2007). Consequently, the sparse winter canopy may boost primary production and elevate autochthonous carbon content to the detriment of inorganic nutrients, with effects on the diet of consumers (Martyniuk et al., 2016). The opposite pattern observed between the autotrophic index and the pigment concentrations suggests changes in the benthic metabolism. Thus, floods shifted stream metabolism toward heterotrophy as primary production is more affected than ecosystem respiration (Uehlinger and Naegeli, 1998). Moreover, with this shift, heterotrophic bacteria and fungi associated with periphyton may emerge as significant resource for consumers, diminishing the relative importance of algal inputs (Hillebrand et al., 2002).
Following the autumn floods, the N site experienced an exceptional algal bloom, likely attributable to Hydrurus foetidus (Villars) Trevisan, which covered the entire riverbed. This fast colonization has been observed in glacier streams and karstic springs too (Uehlinger et al., 1998; Hieber et al., 2001; Cantonati et al., 2006; Rott et al., 2006). Reduced summer light availability was associated with cyanobacteria abundance, whereas higher temperatures, as indicated by Hieber et al. (2001) and Allan and Castillo (2007), were related to red algae dominance in spring and summer. In contrast, diatoms remained the dominant group for most months, with higher abundances in winter, when light availability and disturbance were low (Table 3). Green algae were notably present in winter, contrary to the expectation that they thrive in higher temperatures (Allan & Castillo, 2007). However, Hill et al., (1995) suggested that green algae also require high light intensity, often lacking in summer due to the canopy. Therefore, limited light availability may outweigh temperature as the key factor in these stream sites. However, given the documented variations in periphyton composition assessment using fluorimetric methods (Kahlert & McKie, 2014), we advise caution when interpretating community composition responses.
Spatial pattern
The annual biofilm biomass varied between 4.4 and 15.0 g/m2, aligning with findings from studies in diverse environments, such as glacial streams (Joos, 2003; Robinson et al., 2016; Peszek et al., 2022), meadows streams (Elsaholi, 2011), and forested streams (Biggs, 1988; Fernandes & Esteves, 2003). Annually, sites regulated by reservoirs (G and D) had higher biomass (9.0 and 12.9 g/m2) compared to unregulated sites (S and U, with 5.7 and 4.4 g/m2, respectively). This confirmed the positive impact of upstream regulation on the biofilm, attributed to enhanced flow stability, consistent with findings in the Soca River (Slovenia) by Smolar-Žvanut & Mikoš (2014). However, due to the absence of gauging sites, the assessment of small flow variations was not possible, and disturbance, estimated by pebble movement, revealed similar annual magnitude across all sites (Fig. 3D). Biofilm growth was not associated with nutrient concentration which is always very low (nitrate and phosphate concentrations were less than 1.4 mg/l and < 0.001 mg/l, respectively) as low-light availability and disturbance probably overrode nutrient limitations (Larned & Santos, 2000; Bernhardt & Likens, 2004).
Apart from biomass, the other biofilm metrics annually were similar among sites. For example, chlorophyll a content averaged 2.9 to 10.2 mg/m2, in line with forest stream studies (Rier & Stevenson, 2002; Bernhardt & Likens, 2004; Pizarro & Alemanni, 2005; Francoeur & Biggs, 2006) but considerably lower than the 20–80 mg/m2 observed in several unshaded streams (Biggs, 1988; Quinn et al., 1996; Cattaneo et al., 1997; Robinson et al., 2016). This discrepancy was attributed to the combined influence of disturbance and canopy cover, typically found in mountain-shaded streams. However, light utilization efficiency increases under low-light conditions leading to elevated cellular nutrition content which potentially benefit consumers through enhanced trophic energy transfer (Martyniuk et al., 2019). Additionally, the autotrophic index annually averaged around 3000, exceeding values typically found in mountain streams, despite known high seasonal variability (this study, Biggs, 1988; Joos, 2003). These high values suggest that primary producers have a diminished functional role within the biofilm matrix of the surveyed streams.
Contrary to our assumptions, periphyton groups were not associated with site-specific environmental conditions but primarily with factors, like light availability, water temperature, disturbance, and nitrate concentration, which exhibited marked temporal variations (Table 3). However, the N site showed a high percentage of cyanobacteria in summer (> 50%) and red algae in late winter–spring (20%), consistent with observations in other groundwater streams (Hieber et al., 2001; Uehlinger, 2006). On the other hand, the absence of green algae may be attributed to the short distance from the source (~ 200 m), which limits the colonization by green algae from terrestrial environments. In contrast, the high abundance of green algae in G (15%) could be promoted by the colonization of Chlorophyceae and Conjugatophyceae from high-altitude reservoirs. These lakes host several species of green algae, including genera such as Staurastrum, Staurodesmus, Crucigeniella, Planctosphaeria, and Sphaerocystis (Gentili et al., 2001). Cyanobacteria were particularly abundant in N (36%), positively associated with light availability and nitrate concentration. This contrasts with prior studies where nitrate enrichment did not promote Cyanobacteria density, likely due to their nitrogen-fixing capacity (Allan & Castillo, 2007). Unlike our assumptions, all the investigated sites displayed similar annual turnover in periphyton composition, poorly related to the annual water thermal variability. However, we believe that a detailed taxonomic investigation is necessary to assess how periphyton taxa’s occurrence and abundance respond to changes in water temperature. A species-based investigation may also be useful to detect differences in community composition related to lithology (limestone vs silica), hydrology, and shading as reported in several studies (Gesierich & Kofler, 2010; Larned, 2010; Cantonati et al., 2012a; Kamberovic et al., 2019). Overall, the biofilm variations observed within and among the surveyed streams suggest that, at small catchment scale, the seasonal variations outweigh the spatial ones. However, the limited number of sites (n = 5) hindered a rigorous evaluation of biofilm patterns and the factors influencing them; therefore, further research including several sites is needed. Similarly, conducting investigations at the mesohabitat scale may provide a better understanding of the role of within stream reach variability in biofilm patterns at larger scale (across stream sites and samplings).
Conclusions
In a year-long survey with monthly samplings, marked temporal variability in epilithic biofilm was observed across all sites, mainly driven by disturbance and seasonality. Floods dropped the biofilm biomass, leading to a shift in the benthic metabolism toward higher heterotrophy. While various environmental factors affected periphyton community, water temperature, and light availability were the most influential in shaping its composition. A succession of the main groups was encountered through the seasons with green algae dominating in autumn, followed by cyanobacteria and red algae in spring–summer and summer–autumn, respectively. Spatially, higher biofilm biomass values were measured in regulated sites, and no green algae were found in the groundwater stream. Despite varying annual temperature variability at the sites, they displayed similar annual turnover in composition. Overall, the study highlights the importance of conducting a quantitative and frequent (monthly) investigation of biofilm to understand temporal changes. It also indicates that Phyto-PAM deconvolution is an effective tool to detect major changes in periphyton composition. Nevertheless, a deeper investigation involving a larger number of sites would be valuable for a comprehensive community characterization and a more thorough assessment of the role of each environmental driver.
Data availability
Data are available at: https://doi.org/10.6084/m9.figshare.24431287
References
Allan, J. D. & M. M. Castillo, 2007. Stream ecology, Springer, Dordrecht:
APAT, 2007. Annuario dei dati ambientali Edizione 2007. .
Barton, K., 2022. Package ‘MuMIn’ Version 1.46.0. R Package.
Baselga, A., D. Orme, S. Villeger, J. De Bortoli, L. Fabien, M. Logez, R. Henriques-Silva, S. Mart, R. Mart, C. Guez, & R. Crujeiras, 2022. Betapart.
Bernal, S., A. Lupon, M. Ribot, F. Sabater & E. Martí, 2015. Riparian and in-stream controls on nutrient concentrations and fluxes in a headwater forested stream. Biogeosciences 12: 1941–1954.
Bernhardt, E. S. & G. E. Likens, 2004. Controls on periphyton biomass in heterotrophic streams. Freshwater Biology 49: 14–27.
Biggs, B. J. F., 1988. Artificial substrate exposure times for periphyton biomass estimates in rivers. New Zealand Journal of Marine and Freshwater Research 22: 507–515.
Biggs, B. J. F., 1995. The contribution of flood disturbance, catchment geology and land use to the habitat template of periphyton in stream ecosystems. Freshwater Biology 33: 419–438.
Biggs, B. J. F., 1996. Hydraulic habitat of plants in streams. Regulated Rivers: Research and Management 12: 131–144.
Biggs, B. J. F. & R. A. Smith, 2002. Taxonomic richness of stream benthic algae: effects of flood disturbance and nutrients. Limnology and Oceanography 47: 1175–1186.
Branco, C. C. & O. Necchi, 1996. Survey of stream macroalgae of eastern atlantic rainforest of São Paulo state, Southeastern Brazil. Hydrobiologia 333: 139–150.
Branco, H. L. & O. Necchi, 1998. Distribution of stream macroalgae in three tropical drainage basins of southeastern Brazil. Archiv Fur Hydrobiologie 142: 241–256.
Cantonati, M. & E. Pipp, 2000. Longitudinal and seasonal differentiation of epilithic diatom communities in the uppermost sections of two mountain spring-fed streams. Vereinigung Für Theoretische Und Angewandte Limnologie 27: 1591–1595.
Cantonati, M., E. Bertuzzi, R. Gerecke, K. Ortler & D. Spitale, 2005. Long-term ecological research in springs of the Italian Alps: six years of standardised sampling. Internationale Vereinigung Für Theoretische Und Angewandte Limnologie: Verhandlungen 29: 907–911.
Cantonati, M., R. Gerecke & E. Bertuzzi, 2006. Springs of the Alps - Sensitive ecosystems to environmental change: from biodiversity assessments to long-term studies. Hydrobiologia 562: 59–96.
Cantonati, M., N. Angeli, E. Bertuzzi, D. Spitale & H. Lange-Bertalot, 2012a. Diatoms in springs of the Alps: spring types, environmental determinants, and substratum. Freshwater Science 31: 499–524.
Cantonati, M., E. Rott, D. Spitale, N. Angeli & J. Komárek, 2012b. Are benthic algae related to spring types? Freshwater Science 31: 481–498.
Cattaneo, A., T. Kerimian, M. Roberge & J. Marty, 1997. Periphyton distribution and abundance on substrata of different size along a gradient of stream trophy. Hydrobiologia 354: 101–110.
European Committee for standardizations (CEN), 2014. Water quality - Guidance for the identification and enumeration of benthic diatom samples from rivers and lakes.
Death, R. G. & E. M. Zimmermann, 2005. Interaction between disturbance and primary productivity in determining stream invertebrate diversity. Oikos 111: 392–402.
DeNicola, D. M., 1996. Periphyton responses to temperature at different ecological levels. Algal Ecology. Elsevier: 149–181.
Dinno, A., 2022. Package ‘dunn.test.’, 1–7.
Elsaholi, M., 2011. Nutrient and light limitation of algal biomass in selected streams in Ireland. Inland Waters 1: 74–80.
Falasco, E., A. Doretto, S. Fenoglio, E. Piano & F. Bona, 2020. Supraseasonal drought in an Alpine river: effects on benthic primary production and diatom community. Journal of Limnology 79: 97–110.
Fernandes, V. O. & F. A. Esteves, 2003. The use of indices for evaluating the periphytic community in two kinds of substrate in Imboassica lagoon, Rio de Janeiro, Brazil. Brazilian Journal of Biology 63: 233–243.
Fox, M. J., R. Muenchen, & D. Putler, 2022. Package ‘RcmdrMisc.’
Francoeur, S. N. & B. J. F. Biggs, 2006. Short-term effects of elevated velocity and sediment abrasion on benthic algal communities. Hydrobiologia 561: 59–69.
Gentili, G., A. Romano, A. Bucchini & M. Bardazzi, 2001. Studio sull’ecologia dei laghi alpini della Provincia di Bergamo, Provincia di Bergamo, Bergamo:
Gerecke, R., M. Cantonati, D. Spitale, E. Stur & S. Wiedenbrug, 2011. The challenges of long-term ecological research in springs in the northern and southern Alps: indicator groups, habitat diversity, and medium-term change. Journal of Limnology 70: 168–187.
Gesierich, D. & W. Kofler, 2010. Are algal communities from near-natural rheocrene springs in the Eastern Alps (Vorarlberg, Austria) useful ecological indicators? Algological Studies 133: 1–28.
Hansson, L. A., 1992. Factors regulating periphytic algal biomass. Limnology and Oceanography 37: 322–328.
Hauer, F. R. & G. A. Lamberti, 2017. Methods in stream ecology, Elsevier, San Diego:
Hedin, L. O., J. C. Von Fischer, N. E. Ostrom, B. P. Kennedy, M. G. Brown & G. Philip Robertson, 1998. Thermodynamic constraints on nitrogen transformations and other biogeochemical processes at soil-stream interfaces. Ecology 79: 684–703.
Hering, D., O. Moog, L. Sandin, & P. F. M. Verdonschot, 2004. Integrated assessment of running waters in Europe. Integrated Assessment of Running Waters in Europe 1–20.
Hieber, M., C. T. Robinson, S. R. Rushforth & U. Uehlinger, 2001. Algal communities associated with different alpine stream types. Arctic, Antarctic, and Alpine Research 33: 447–456.
Hieber, M., C. T. Robinson, U. Uehlinger & J. V. Ward, 2005. A comparison of benthic macroinvertebrate assemblages among different types of alpine streams. Freshwater Biology 50: 2087–2100.
Hill, W. R. & S. M. Dimick, 2002. Effects of riparian leaf dynamics on periphyton photosynthesis and light utilisation efficiency. Freshwater Biology 47: 1245–1256.
Hill, W. R., M. G. Ryon & E. M. Schilling, 1995. Light limitation in a stream ecosystem: responses by primary producers and consumers. Ecology 76: 1297–1309.
Hillebrand, H., M. Kahlert, A. L. Haglund, U. G. Berninger, S. Nagel & S. Wickham, 2002. Control of microbenthic communities by grazing and nutrient supply. Ecology 83: 2205–2219.
Horner, R. R. & E. B. Welch, 1981. Stream periphyton development in relation to current velocity and nutrients. Canadian Journal of Fisheries and Aquatic Sciences 38: 449–457.
Horner, R. R., E. B. Welch, M. R. Seeley & R. R. Homer, 1990. Responses of periphyton to changes in current velocity, suspended sediment and phosphorus concentration. Freshwater Biology 24: 215–232.
Joos, N., 2003. Spatial-temporal distribution of periphyton in the lower parts of the River Thur: the influence of morphology, hydraulics and hydrology.
Kahlert, M. & B. G. McKie, 2014. Comparing new and conventional methods to estimate benthic algal biomass and composition in freshwaters. Environmental Science: Processes and Impacts Royal Society of Chemistry 16: 2627–2634. https://doi.org/10.1039/C4EM00326H.
Kamberovic, J., A. Plenković-Moraj, K. K. Borojevic, M. G. Udovic, P. Žutinic, D. Hafner & M. Cantonati, 2019. Algal assemblages in springs of different lithologies (ophiolites vs. limestone) of the Konjuh Mountain (Bosnia and Herzegovina). Acta Botanica Croatica 78: 66–81.
Larned, S. T., 2010. A prospectus for periphyton: recent and future ecological research. Journal of the North American Benthological Society 29: 182–206.
Larned, S. T. & S. R. Santos, 2000. Light and nutrient-limited periphyton in low order streams of Oahu Hawaii. Hydrobiologia 432: 101–111.
Legendre, P., 2019. A temporal beta-diversity index to identify sites that have changed in exceptional ways in space–time surveys. Ecology and Evolution 9: 3500–3514.
Li, N., Y. Hao, H. Sun, Q. Wu, Y. Tian, J. Mo, F. Yang, J. Song & J. Guo, 2022. Distribution and photosynthetic potential of epilithic periphyton along an altitudinal gradient in Jue River. Freshwater Biology 67: 1761–1773.
Lichtenthaler, H. K., 1987. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods in Enzymology 148: 350–382.
Luttenton, M. R. & C. Baisden, 2006. The relationships among disturbance, substratum size and periphyton community structure. Hydrobiologia 561: 111–117.
Marschner, I., & M. W. Donoghoe, 2022. Package ‘glm2’.
Martyniuk, N., B. Modenutti & E. Balseiro, 2016. Forest structure affects the stoichiometry of periphyton primary producers in mountain streams of Northern Patagonia. Ecosystems 19: 1225–1239.
Martyniuk, N., B. Modenutti & E. G. Balseiro, 2019. Light intensity regulates stoichiometry of benthic grazers through changes in the quality of stream periphyton. Freshwater Science 38: 391–405.
McAuliffe, J. R., 1984. Resource depression by a stream herbivore: effects on distributions and abundances of other grazers. Oikos 42: 327.
McConnell, W. J. & W. F. Singler, 1999. Chlorophyll and productivity in a mountain river. Limnology and Oceanography 4(3): 335–351.
Medlyn, B. E., E. Dreyer, D. Ellsworth, M. Forstreuter, P. C. Harley, M. U. F. Kirschbaum & X. L. E. Roux, 2002. Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant, Cell and Environments 25: 1167–1179.
Melbourne, B. A. & P. J. Daniel, 2003. A low-cost sensor for measuring spatiotemporal variation of light intensity on the streambed. Journal of the North American Benthological Society 22: 143–151.
Morin, A., W. Lamoureux & J. Busnarda, 1999. Empirical models predicting primary productivity from chlorophyll a and water temperature for stream periphyton and lake and ocean phytoplankton. Journal of the North American Benthological Society 18: 299–307.
Mosisch, T. D., S. E. Bunn, P. M. Davies & C. J. Marshall, 1999. Effects of shade and nutrient manipulation on periphyton growth in a subtropical stream. Aquatic Botany 64: 167–177.
Nakagawa, S., P. C. D. Johnson & H. Schielzeth, 2017. The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. Journal of the Royal Society Interface 14(134): 20170213.
Necchi, O., C. C. Z. Branco, R. C. G. Simão & L. H. Z. Branco, 1995. Distribution of stream macroalgae in the northwest region of São Paulo State, southeastern Brazil. Hydrobiologia 299: 219–230.
Niedrist, G. H., M. Cantonati & L. Füreder, 2018. Environmental harshness mediates the quality of periphyton and chironomid body mass in alpine streams. Freshwater Science 37: 519–533.
Peszek, Ł, B. Kawecka & C. T. Robinson, 2022. Long-term response of diatoms in high-elevation streams influenced by rock glaciers. Ecological Indicators 144: 109515.
Pizarro, H. & M. E. Alemanni, 2005. Variables físico-químicas del agua y su influencia en la biomasa del perifiton en un tramo inferior del Río Luján (Provincia de Buenos Aires). Ecología Austral 15: 73–88.
Power, M. E. & A. J. Stewart, 1987a. Disturbance and recovery of an algal assemblage following flooding in an Oklahoma stream. The American Midland Naturalist 117: 333–345.
Power, M. E. & A. J. Stewart, 1987b. Disturbance and recovery of an algal assemblage following flooding in an Oklahoma stream. American Midland Naturalist 117: 333–345.
Quinn, J. M. & C. W. Hickey, 1990. Characterisation and classification of benthic invertebrate communities in 88 New Zealand rivers in relation to environmental factors. New Zealand Journal of Marine and Freshwater Research 24: 387–409.
Quinn, J. M., C. W. Hickey & W. Linklater, 1996. Hydraulic influences on periphyton and benthic macroinvertebrates: simulating the effects of upstream bed roughness. Freshwater Biology 35: 301–309.
R Core Team, 2020. R: A Language and Environment for Statistical Computing. http://www.R-project.org.
Rier, S. T. & R. J. Stevenson, 2002. Effects of light, dissolved organic carbon, and inorganic nutrients on the relationship between algae and heterotrophic bacteria in stream periphyton. Hydrobiologia 489: 179–184.
Robinson, C. T. & S. R. Rushforth, 1987. Effects of physical disturbance and canopy cover on attached diatom community structure in an Idaho stream. Hydrobiologia 154: 49–59.
Robinson, C. T., D. Tonolla, B. Imhof, R. Vukelic & U. Uehlinger, 2016. Flow intermittency, physico-chemistry and function of headwater streams in an Alpine glacial catchment. Aquatic Sciences Springer Basel 78: 327–341.
Robinson, C. T., U. Uehilinger, & M. O. Gessner, 2000. Nutrient limitation In Ward, J. V., & U. Uehlingher (eds), Ecology of a Glacial Flood Plain. Springer: 231–241.
Rosemond, A. D., 1993. Interactions among irradiance, nutrients, and herbivores constrain a stream algal community. Oecologia 94: 585–594.
Rott, E., M. Cantonati, L. Füreder & P. Pfister, 2006. Benthic algae in high altitude streams of the Alps - A neglected component of the aquatic biota. Hydrobiologia 562: 195–216.
Rott, E., & J. D. Wehr, 2016. The spatio-temporal development of macroalgae in rivers In Necchi, O. (ed), River Algae. Springer: 1–279.
Smolar-Žvanut, N. & M. Mikoš, 2014. Impact de la régularisation du débit causée par les barrages hydroélectriques sur la communauté périphytique de la rivière Soca, Slovénie. Hydrological Sciences Journal Taylor & Francis 59: 1032–1045. https://doi.org/10.1080/02626667.2013.834339.
Sterner, R. W., J. J. Elser, E. J. Fee, S. J. Guildford & T. H. Chrzanowski, 2007. The light:nutrient ratio in lakes: the balance of energy and materials affects ecosystem structure and process. American Naturalist 150: 663–684.
Sudlow, K., S. S. Tremblay & R. D. Vinebrooke, 2023. Glacial stream ecosystems and epilithic algal communities under a warming climate. Environmental Reviews 00: 1–13.
Townsend, C. R., M. R. Scarsbrook, S. Doledec & S. Dolédec, 1997. Quantifying disturbance in streams: alternative measures of disturbance in relation to macroinvertebrate species traits and species richness. Journal of the North American Benthological Society 16: 531–544.
Uehlinger, U., 1991. Spatial and temporal variability of the periphyton biomass in a prealpine river (Necker, Switzerland). Archiv Für Hydrobiologie 123: 219–237.
Uehlinger, U., 2006. Annual cycle and inter-annual variability of gross primary production and ecosystem respiration in a floodprone river during a 15-year period. Freshwater Biology 51: 938–950.
Uehlinger, U. & M. W. Naegeli, 1998. Ecosystem metabolism, disturbance, and stability in a prealpine gravel bed river. Journal of the North American Benthological Society 17: 165–178.
Uehlinger, U., R. Zah, & H. Bürgi, 1998. The Val Roseg project: temporal and spatial patterns of benthic algae in an Alpine stream ecosystem influenced by glacier runoff. IAHS publication. : 419–424.
Weber, C., 1973. Biological field and laboratory methods for measuring the quality of surface waters and effluents. National environmental research center office of research and development. .
Wellnitz, T. & R. B. Rader, 2003. Mechanisms influencing community composition and succession in mountain stream periphyton: Interactions between scouring history, grazing, and irradiance. Journal of the North American Benthological Society 22: 528–541.
White, J. C., A. House, N. Punchard, D. M. Hannah, N. A. Wilding, & P. J. Wood, 2018. Macroinvertebrate community responses to hydrological controls and groundwater abstraction effects across intermittent and perennial headwater streams. Science of the Total Environment Elsevier B.V. 610–611: 1514–1526, https://doi.org/10.1016/j.scitotenv.2017.06.081.
Acknowledgements
We sincerely thank Marco Mantovani, Pietro Rigoldi, and Alberto Bonacina for the assistance with sampling and field measurements. Pietro Rigoldi helped us to analyze periphyton and extract pigments. Additionally, we extend our gratitude to an anonymous reviewer who contributed to enhancing the manuscript.
Funding
Open access funding provided by Università degli Studi di Milano - Bicocca within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
Conceptualisation, developing methods, and conducting the field survey: LB, RF, and FM. Data analysis, interpretation, and writing of the manuscript: LB. Funding: VM.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Handling editor: Judit Padisák
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bonacina, L., Fornaroli, R., Mezzanotte, V. et al. Temporal patterns of stream biofilm in a mountain catchment: one-year monthly samplings across streams of the Orobic Alps (Northern Italy). Hydrobiologia 851, 2081–2097 (2024). https://doi.org/10.1007/s10750-023-05399-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05399-w