Abstract
The Younger Dryas-Holocene transition represents a period of significant thermal change, comparable in magnitude to modern warming, yet in a colder context and without the effect of anthropogenic disturbance. This is useful as a reference to tackle how biodiversity is affected by temperature in natural conditions. Here, we addressed the thermal change during this period in a southern Baltic coastal lake (Konarzewo Lake, Poland), as inferred by chironomid remains. We evaluated changes in chironomid communities and used Hill numbers to explore how commonness and rarity underlie biodiversity changes attributable to warming. We found evidence of warming at Konarzewo Lake during the Younger Dryas-Holocene transition, with inferred temperatures in the Younger Dryas period supporting the NW–SE gradient in Younger Dryas summer temperatures across Europe. Chironomid communities drastically changed during the thermal transition. However, Hill numbers showed no response to temperature when rare morphotypes were emphasized (order q = 0) or a weak response when they were balanced with common morphotypes (order q = 1). Hill number of order q = 2, emphasizing the most common morphotypes, consistently increased with temperature across different sample sizes or coverages. This illustrates how common morphotypes, rather than the rare ones, may boost biodiversity facing warming.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exploring past effects of temperature on biodiversity might be insightful for understanding how biodiversity responds to current climate warming (Fordham et al., 2020). The Younger Dryas-Holocene transition provides a context in which thermal conditions were colder compared with current temperatures. The rate of temperature increase was lower than in modern days (Osman et al., 2021), but it still constitutes the most recent sharp warming event we have available to compare with recent climate change. This provides an opportunity to explore the effects of warming on biodiversity in the past, during a time period void of anthropogenic disturbance on ecosystem functioning. In this sense, it can be regarded as a reference scenario representing natural conditions.
Attempting to quantify the effect of temperature on biodiversity back in time several thousands of years ago requires the usage of sedimentary sequences where the environmental history is appropriately registered. Both past temperatures and biodiversity can be inferred from organismal remains pertaining to specimens that were once inhabiting the area under study. Chironomids (Diptera: Chironomidae) can be very helpful in this regard. They are a highly diverse group of insects with an aquatic larval stage (Armitage et al., 1995) that present narrow thermal tolerances and an excellent preservation of their cephalic capsules in lake sediments (Eggermont & Heiri, 2012). Hence, previous studies have successfully proven their usefulness to infer past air temperatures in lacustrine environments (e.g., Levesque et al., 1997; Heiri et al., 2003; Porinchu et al., 2009; Massaferro & Larocque-Tobler, 2013; Zhang et al., 2017; Tarrats et al., 2018; Tóth et al., 2022). Their high species richness makes chironomids also an ideal group to study changes in biodiversity in relation to temperature (Williams et al., 2016, 2019a, b; Engels et al., 2020; Mayfield et al., 2021; Belle et al., 2022).
Concerning biodiversity metrics, Hill numbers represent a general framework in which specific diversity measures can be fit (Hill, 1973; Chao et al., 2014a, b). This provides a tool to address the relative contribution of rare and common species in biodiversity changes, as shown by previous ecological studies. For example, Thorn et al. (2020) used Hill numbers to show that after wind-storm disturbance, community dissimilarities between salvage logged forest plots and unlogged forest plots in southeastern Germany were generally driven by rare species, in a wide variety of organismal groups. Tarifa et al. (2021) showed that the effect of agricultural intensification on herb assemblages in a Mediterranean landscape was stronger on rare species than on common or dominant species, using Hill numbers. Tsafack et al. (2021) reported a reduced alpha diversity in exotic forests compared to native forests, in a series of arthopod groups in the Azores. In this case, Hill numbers evidenced that the effect of exotic forests on rare and common species varied between arthropod groups. Maturo & Di Battista (2018) used Hill numbers to develop a functional multivariate approach for biomonitoring programs which considers both species richness and evenness. In the modeling area, Zhang et al. (2019) evaluated the consistency of different models predicting fish biodiversity in the North Yellow Sea (China), by using Hill numbers to progressively downweight the influence of rare species. Despite the wide variety of possibilities that the simultaneous use of different Hill numbers can offer to ecological studies, this approach has not yet been widely applied in the field of paleoecology (but see, e.g., Pla-Rabés et al., 2011; Yasuhara et al., 2016; Pla-Rabés & Catalan, 2018; Figueroa-Rangel et al., 2020; Negash et al., 2022).
Any given Hill number qD is mathematically defined as a function of S, pi, and q, where S is the number of species in the entire community assemblage, pi is the relative abundance of the ith species, and q is the order of the diversity number (q ≥ 0, q ≠ 1), following Chao et al. (2014b):
When q = 1, the above equation is undefined, but the limit of qD as q approaches 1 is the exponential of the Shannon index. Hill numbers of order q = 0, q = 1, and q = 2 exactly match species richness (0D), the exponential of the Shannon index (1D), and the Gini-Simpson index (2D). While 0D exaggerates the importance of rare species (i.e., all species contribute equally to biodiversity), 1D and 2D progressively shift the focus onto more abundant species (Jost, 2006; Chao et al., 2014a, b). Hill numbers can compare samples with unequal number of individuals (Colwell et al., 2012) or different sample coverage (Chao & Jost, 2012). The latter is defined as the proportion of individuals of the entire community that belong to the subset of species collected in the sample. In both variations, the standardized value of choice can be below or above the observed value in the collected sample. In the former case, interpolation (rarefaction) applies; in the latter, extrapolation is used considering the theoretical complete community.
Here, we aimed to achieve two goals. First, we attempted to reconstruct past temperatures during the Younger Dryas-Holocene transition at a southern Baltic coastal lake (Konarzewo Lake, NW Poland), using chironomid remains. The lithostratigraphic and geochemical profiles of the sediment core were also analyzed to better understand the main environmental changes at that time. We expected to find a marked temperature increase, as found in other sedimentary sequences across Europe and Greenland (Schwander et al., 2000; Brooks & Birks, 2001; Cacho et al., 2001; Johnsen et al., 2001; Heiri et al., 2007; Pawłowski et al., 2016; Müller et al., 2021). Secondly, we aimed to evaluate Hill numbers for standardized values of sample size and sample coverage, in order to tackle the relative contribution of commonness and rarity to the biodiversity–temperature relationship. We expected a general increase in chironomid richness with increasing temperature, in the colder-than-today context of this thermal warming, following the previous results by Engels et al. (2020). The specific expectancy concerning the relative contribution of rare and common chironomids to biodiversity changes was unclear. Previous paleolimnological studies have used the Hill’s N2 effective number of occurrences (e.g., Luoto et al., 2020; Šeirienė et al., 2021), which is analogous to 2D (ter Braak, 1990, 2019; ter Braak & Verdonschot, 1995). However, combining different Hill numbers to assess the relative relevance of rare and common species on biodiversity changes has not yet been widely addressed in paleolimnological studies (but see, e.g., Pla-Rabés et al., 2011; Pla-Rabés & Catalan, 2018).
Materials and methods
Study site
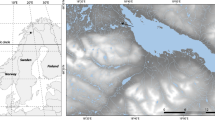
Konarzewo Lake (in Polish, Konarzewskie Bagno or Bagno Pogorzelickie, 54.09259° N, 15.13817° E), is a coastal lake located in the southern Baltic Sea coast 3 km southeast of the village of Niechorze (Fig. 1, Supplementary Information Fig. S1). Currently, this lake covers an area of 71.6 ha, with a maximum depth of 2.5 m and a level of 2.6 m a.s.l. The lake was formed in a kettle hole on a morainic upland build of till of the Vistulian (Weichselian) Glaciation. In the Late Glacial, the kettle hole was filled with dead ice, which started melting in the Oldest Dryas, triggering the development of the lake. During the Holocene, the northern part of the morainic upland was covered by aeolian sand, which nowadays limits the lake from the west, north, and east (Dobracka, 2009, 2021; Sydor, 2021). Existing data (Sydor & Uścinowicz, 2023) suggest that the lake was not affected by the sea.
a Map of Europe showing the location of the P1 core used in this study and five more cores used to compare results on inferred mean July air temperature: 1, Pawłowa Lake (central Poland); 2, Kråkenes Lake (Norway); 3, Lake Hijkermeer (The Netherlands); 4, MD95-2043 (Alboran Sea); 5, Gerzensee (Switzerland). The GISP2 ice core from central Greenland was also used for comparisons (not shown in the map). b Map showing the location of Konarzewo Lake (NW Poland), studied here as well as the precise point where the P1 core was retrieved
Sediment coring
In the summer of 2016, a sediment core (P1) of 7 m in length was retrieved from the center of the lake, using an Eijkelkamp Instorf sampler (Supplementary Information Fig. S1). This is a steel peat sampler which must be pressed into the sediment by hand to collect the core, with outside diameter of 60 mm, core diameter of 52 mm, work length of 50 cm, and capacity of 0.5 L (half section). The sediment sampler was used on a naturally developed floating island located above the maximum depth of the lake (Supplementary Information Fig. S1). The geographical location of Konarzewo Lake and the P1 core placement are presented in Fig. 1. The P1 core was analyzed from 5.7 to 7.0 m depth (which corresponded to the earliest period of the lake's existence). Laboratory analysis comprised radiocarbon dating, grain size analysis, geochemistry analysis, and the analysis (sorting and identification) of chironomid head capsules (Fig. 2).
General lithostratigraphic characteristics of the P1 core of Konarzewo Lake. Dating points, as well as the samples collected for chironomid, geochemical, and grain size analyses are indicated across the depth profile. Short-dash horizontal lines indicate the Younger Dryas boundaries of Müller et al. (2021), which could be established within the range of depths of the dating points (see text for details)
Radiocarbon dating
Radiocarbon dating analysis was performed at the Kraków Radiocarbon Laboratory for five samples from depths 5.92, 6.10, 6.21, 6.33, and 6.63 m (Fig. 2, Supplementary Information Table S1) using Accelerator Mass Spectrometry (AMS, 4 samples) and Liquid Scintillation Counting (LSC, 1 sample). All radiocarbon dates were calibrated in OxCal v4.4 software (Bronk Ramsey, 2009) using the IntCal20 calibration curve (Reimer et al., 2020). The dated material was gyttja (bulk sample). Due to the high fragmentation of the material and thus the risk that the sample size for dating was insufficient, we decided to date a bulk sample over plant remains and shells. Because of the presence of carbonates in lake sediments, radiocarbon age may be exaggerated (reservoir effect). However, there were no data on the reservoir effect for the Konarzewo Lake (nowadays and in the past) so there was no possibility to take this into account during calibration. One of the samples analyzed (code MKL-3717A, depth 6.10 m) was excluded when building the age-depth model (Supplementary Information Fig. S2), since it appeared to be contaminated by older redeposited material, a common situation in organic deposits such as gyttja.
Grain size analysis
The lower part of the P1 core (6.37–7.00 m) was void of plant remains, and it was used to perform grain size analysis for eight samples (Fig. 2). All samples were dried and sieved on a Fritsch Vibratory Sieve Shaker Analysette 3 PRO. The mesh interval of the sieves was 0.25 phi. For each sample, textural parameters were calculated following Folk & Ward (1957) and using GRADISTAT software (Blott & Pye, 2001). Basic fractions were determined using the classification of Wentworth (1922), modified by Krumbein (1934) and Urbaniak-Biernacka (1975). To determine the type of sediment transport, the frequency and cumulative curves as well as the C/M diagram (Passega & Byramjee, 1969) were drawn. The analysis of the cumulative curves was performed using the classification of Sindowski (1957) and the method proposed by Moss (1962, 1963) and Visher (1969), modified by Viard & Breyer (1979).
Geochemistry
For geochemical analysis, twelve samples were used, covering from ca. 5.7 to 6.5 m (Fig. 2). The analysis was performed in the Chemical Laboratory of the Polish Geological Institute—National Research Institute following the protocol described by Bojakowska & Tołkanowicz (2015). The geochemical variables in the sediments were measured as soluble concentrations (65% HNO3 + 37–38% HCl + 70% HClO4 + 38% HF; 1 g sample in 50 ml digestion). The contents of Ti were obtained using the ICP-MS method (Perkin Elmer ELAN DRC II spectrometer); Cu, Fe, Mn, Zn, Pb, and Ca were obtained using the ICP-OES method (Thermo Scientific iCAP 6500 spectrometer); N, S, and P were obtained using a flow injection analyzer (FIA Compact, MLE GmbH factory); and TOC was obtained using the coulometric method (Ströhlein Coulomat 702/LI). Additionally, combined Al + K + Na + Mg measurements were obtained (ICP-OES method), and the ratio TOC/N was computed. The proportions of the analyzed compounds are often the basis for the reconstruction of environmental changes in lakes and mires and their catchments (Meyers & Teranes, 2001; Apolinarska et al., 2012; Petera-Zganiacz et al., 2022).
Chironomid sorting and identification
Thirty-four 1-cm-thick samples were retrieved from the P1 core at different depths, spanning from 5.96 to 6.62 m, to analyze the composition of chironomid remains (Fig. 2). Wet sediment samples were first sieved through 355 μm and 100 μm meshes and residues treated (if needed) in an ultrasonic bath for three seconds to break up the clay aggregates that may adhere to the chironomid remains, making their identification more difficult. Then, a chemical treatment followed, according to the standard laboratory procedure of Brooks et al. (2007), essentially consisting of sample deflocculation in 10% KOH for 10 min at 70 °C. Samples were then transferred into a Bogorov counting tray and subfossil chironomid head capsules were sorted and counted under a stereo microscope, at ×25 magnification. In the eventual encounter of head capsules of Ceratopogonidae (Diptera), these were also counted, yet not further identified. Separated head capsules were dehydrated in 96% ethanol and mounted in Euparal on microscope slides for their identification with an optical microscope (ZEISS Axio Lab.A1). Chironomid remains were identified following Cranston (1982), Wiederholm (1983), Schmid (1993), Klink & Moller Pillot (2003), Brooks et al. (2007), and Andersen et al. (2013). The whole collection was deposited and made available at the Polish Geological Institute—National Research Institute Pomeranian Branch in Szczecin (Poland).
Temperature reconstruction and comparison
Chironomids were used to reconstruct past mean July air temperatures at Konarzewo Lake by estimating first the thermal optimum of each morphotype with a modern Swiss-Norwegian-Polish training set (Kotrys et al., 2020), which includes 357 lakes, 134 taxa, and a temperature range of 3.5–20.0 °C. The training set’s root mean square error of prediction (RMSEP) and coefficient of determination (R2jack) were 1.39 °C and 0.91, respectively (i.e., WA-PLS model in Kotrys et al., 2020). The modern analogues were assessed through the minimum dissimilarity between core and training set samples (minDC), using the squared chi-square dissimilarity as the distance, as in Luoto et al. (2020). Then, a Detrended Correspondence Analysis (DCA) was performed to estimate the gradient length in chironomid turnover, through detrending by segments and downweighting rare taxa, with the package ‘vegan’ (Oksanen et al., 2020) of R software (R Core Team, 2020). This step is convenient to check the extent to which there exists taxa replacement in chironomid communities through time, which reduces the risk of finding spurious chironomid–temperature relationships. The correlation between temperature and DCA values (axis 1) was eventually tested, after checking for normality of variables with a Shapiro–Wilk test. Finally, because few indeterminate chironomids occurred after taxonomic sample processing, the DCA was considered first after proportionally merging indeterminate taxa into their determined counterparts and then repeated after removing first the indeterminate taxa.
Reconstructed temperatures were compared to that of other sites in Europe, with specific attention to chironomid-inferred temperatures during the Younger Dryas-Holocene transition in Lake Gościąż, central Poland (Müller et al., 2021; Płóciennik et al., 2022), Lake Hijkermeer, The Netherlands (Heiri et al., 2007), and Kråkenes Lake, Norway (Brooks & Birks, 2000, 2001). The Lieporiai paleolake, northern Lithuania (Šeirienė et al., 2021), was used only as a complementary source, since very few samples in that study can be assigned to the Younger Dryas-Holocene transition. Inferred temperatures using cladocerans in central Poland were also used in comparisons (Pawłowski et al., 2016), as well as general reference standards such as alkenones in the core MD95-2043, Alboran Sea (Cacho et al., 2001), and oxygen stable isotopes in both Gerzensee, Switzerland (Schwander et al., 2000), and the GISP2 core, central Greenland (Johnsen et al., 2001).
Chironomid stratigraphic zonation
For each chironomid taxon, percentage data were used to create vertical area plots using SigmaPlot version 10.0 (Systat Software, San Jose CA, USA). Individual plots were eventually merged into stratigraphic diagrams with the selected chironomids (i.e., common or rare chironomids). In each stratigraphic diagram, chironomids were arranged (from left to right) from higher to lower average depth of the samples in which they occurred, weighted by relative abundance. Based on the chironomid composition of each sample, statistically significant assemblage zones were determined using constrained clustering based on a similarity distance matrix between samples. We specifically used constrained incremental sum of squares (CONISS) analysis, in combination with a broken stick null model, to determine the number of significant zones in the stratigraphic diagram (MacArthur, 1957; Grimm, 1987; Bennett, 1996). To compute the similarity distance matrix between samples, percentage data were previously divided by 100 to obtain abundance values bounded between 0 and 1 for each chironomid, and thereafter square-root transformed so as to, in practice, use Euclidean distances operating as Hellinger distances (Legendre & Gallagher, 2001). This is convenient as it drastically reduces the risk of spurious similarity between two given samples, resulting from double zeros. After data transformation, CONISS analysis was first executed with all chironomid taxa involved and then repeated with increasing thresholds limiting chironomid inclusion: first, the condition that the chironomids involved needed to be present in at least three samples was required; then, a minimum relative abundance in at least one of the samples was additionally required. This abundance minimum was progressively increased from 2% to 3% to 5%. All CONISS analyses and the associated significance tests (broken stick model) were performed with the package ‘rioja’ (Juggins, 2020) in R software (R Core Team, 2020). As in the DCA analyzing chironomid community changes, the statistical analysis of the stratigraphic zonation was considered first after merging indeterminate taxa into their determined counterparts and then repeated after removing first the indeterminate taxa.
Biodiversity–temperature relationship
In order to tackle biodiversity–temperature relationships, biodiversity was first defined by Hill numbers of order q = 0, q = 1, and q = 2 (0D, 1D, and 2D, respectively), at different values of sample size and coverage, using the R package ‘iNEXT’ (Chao et al., 2014a; Hsieh et al., 2020). Sample size values of choice started at 10 individuals and were increased at constant intervals of 10 individuals up to an upper limit defined as twice as much the amount of the maximum number of individuals observed in any of the samples. This is because extrapolating beyond twice the observed value in the sample likely results in a large prediction bias for 0D (Chao et al., 2014a). For sample coverage, values of choice started at 0.500 and were increased at 0.025 intervals, up to 0.975. Thereafter, 0.995, 0.999, 0.9995, 0.99995, and 0.999995 values were used. As the value of 1 was approached, the calculations were computationally more intense, and the specific value of 1 could not be reached because too long a vector was generated. As a complementary comparative exercise between samples, the relationship between Hill numbers and the number of individuals was compared at a specific sample coverage value of 0.900, indicating whether interpolation or extrapolation from the observed value was needed to reach this value, in each sample. The relationship of biodiversity with temperature at all values of size and coverage considered was established by Pearson correlation coefficient tests. Normality of variables was tested with a Shapiro–Wilk test at all times, and Spearman correlation tests were additionally performed to complement Pearson correlation tests, in case departure from normality was observed in a specific variable. Finally, a specific sample size of 200 individuals, and a specific sample coverage of 0.975, were chosen to visually plot the linear relationship between Hill numbers (0D, 1D, and 2D), and temperature. As in the DCA and stratigraphic zonation analyses, biodiversity–temperature relationships were considered first after merging indeterminate taxa into their determined counterparts, and then repeated after removing first indeterminate taxa. For these specific values of sample size and coverage, we also plotted the variation of the Hill numbers together with temperature along the stratigraphic sequence.
Results
Sediment core macroscopical description
The P1 core comprised some lithological variation that can be described macroscopically (Fig. 2). The deepest part of the core (6.37–7.00 m) was gray in color and dominated by very fine sand with carbonates, with no plant remains or shells. Then (depth 6.20–6.37 m) plant remains were recorded, with the sediment still gray in color but now more dominated by sandy silt. At depth 6.20, the color changed to light brown, and the sediment was dominated by carbonate gyttja, with shells (6.10–6.20 m). The upper part of the P1 core at depth 5.70–6.10 m was characterized by fine detritus gyttja with Chara remains and a dark brown color.
Chronology and Younger Dryas boundaries
Our age-depth chronology model was based on four samples, as one of the initial five samples was eventually discarded because it appeared contaminated by older redeposited material, resulting in considerable deviation in the estimated age, with respect to the other four samples. Overall, the uncertainty in calibrated ages was relatively moderate (Supplementary Information Table S1, Fig. S2). The resulting model established that the main lithostratigraphic changes observed at 6.10, 6.20, and 6.37 m depth (Fig. 2) occurred at 10.60, 11.31, and 12.27 cal ka BP, respectively. On the other hand, the Allerød-Younger Dryas and Younger Dryas-Holocene boundaries could be assumed at ca. 12.85 and 11.65 cal ka BP, respectively, following a standard definition (Rasmussen et al., 2006). However, Müller et al. (2021) defined these boundaries at 12.62 and 11.47 cal ka BP, respectively, in the sedimentary sequence analysis of Lake Gościąż, central Poland, based on observed shifts on 18O content in bulk carbonate, and concomitant vegetation changes. We adopted here the boundaries defined by Müller et al. (2021) given the geographical proximity of their study with respect to ours. In accordance with this, we established the Allerød-Younger Dryas boundary at 6.539 m depth, and the Younger Dryas-Holocene boundary at 6.234 m depth in the P1 core of Konarzewo Lake, following our age-depth model (Supplementary Information Tables S1, S2, Fig. S2). No lithostratigraphic change was observed at the Allerød-Younger Dryas boundary, while the Younger Dryas-Holocene boundary was established near the transition from sandy silt to carbonate gyttja (Fig. 2). The general standard definition of these boundaries (Rasmussen et al., 2006) could be established below our deepest sample at 6.62 m and at 6.257 m depth, respectively, which is valuable information for comparison purposes.
Grain size analysis
Grain size analysis was performed for the lower part of P1 core at depth 7.00–6.37 m (Supplementary Information Fig. S3). The analysis revealed that this part of the core mostly contained very fine sand (46–56%) but with a significant share of very coarse silt (16–30%) and fine sand (10–30%). In general, the deposit was moderately sorted, i.e., with a low variance in sediment sizes (sI = 0.71–0.96). There was, however, some vertical variability in grain size parameters, with larger and more variable grain sizes in the upper part of the analyzed section. Overall, the grain size distribution was slightly negatively skewed but nearly symmetrical (SkI from − 0.31 to − 0.03) with moderate values of kurtosis (KG = 1.02–1.21). A more detailed analysis revealed five modes in the frequency curves: 2.0 phi (0.25 mm), 3.25 phi (0.1 mm), 3.75 phi (0.071 mm), 4.25 phi (0.05 mm), and 5.0 phi (0.032 mm). Cumulative curves were moderately inclined and only showed a coarse truncation point in three of the samples, at 0.25 phi (0.8 mm, one sample) and 0.5 phi (0.71 mm, two samples). In contrast, fine truncation points were evident in all the samples at 4.75 phi (0.036 mm). Overall, the dominant type of transport was saltation (96%), with relatively low shares of suspension (3.6%) and traction (0.08%). By using a C/M diagram, which is a representation to assess particle transport mechanisms where C represents the one percentile and M the median of the grain size distribution (Passega, 1957), all samples were located in the fields VII and V in the R-Q segment, which describe deposits transported in saltation and formed from suspension in conditions of reduced environmental dynamic activity (Passega & Byramjee, 1969; Racinowski et al., 2001).
Geochemistry
The P1 core geochemical analyses showed the dominance of mineral sediments during the Younger Dryas period, with very low TOC (below 2%) and N (below 0.5%) content (Supplementary Information Fig. S4). At the beginning of the Holocene (11.47 cal ka BP), the concentration of Ti (above 200 mg/kg) started to decrease slightly to ca. 150 mg/kg, and thereafter (ca. 10.95 cal ka BP), a sharp decrease was observed (below 50 mg/kg). The strong reduction in Ti was coincident with an increase of Ca (initially from ca. 5 to 10% at ca. 11.25 cal ka BP, and then to above 20% at ca. 10.95 cal ka BP) and Mn (from initial values of ca. 50–100 mg/kg during the Younger Dryas and the Younger Dryas-Holocene transition, to ca. 600 mg/kg at ca. 10.95 cal ka BP). Ca and Mn decreased later at ca. 10.05 cal ka BP, back again to their previous values observed during the Younger Dryas. At 10.05 cal ka BP, TOC, N, and S displayed a considerable increase (above twice as much their previous values, to end up at 30%, 3%, and 2%, respectively). The concentration of lithogenic elements (i.e., Al + K + Na + Mg) decreased sharply (from ca. 1.6 to 0.8%) at the Younger Dryas-Holocene transition (11.47 cal ka BP), and then, the decrease continued to 0.4% at ca. 10.95 cal ka BP when they eventually stabilized.
Chironomid identification
A total amount of 2677 chironomid head capsules were found, representing 85 different morphotypes, of which 35 were present in at least three samples and at the same time showed a relative abundance above 3% in at least one of the samples (Supplementary Information Fig. S5). The rest of the taxa were represented by 50 morphotypes (Supplementary Information Fig. S6a). The percentage of indeterminate chironomids was relatively low, mostly referred to Paratanytarsus, with a very low percentage of indeterminate morphotypes resolved at the tribe level. The number of chironomids counted was low in two of the 34 samples (25 and 19 individuals, at 6.34 and 6.63 m, respectively), and these two samples were merged with adjacent samples (at 6.35 and 6.60 m, respectively). After merging, 32 samples were available for statistical analysis, with a number of chironomids counted exceeding 50 individuals in all samples, except two, at 5.96 and 6.44 m, with 41 and 33 individuals, respectively (Supplementary Information Fig. S6b). Also, 66 Ceratopogonidae head capsules were found overall, mostly in the upper part of the sediment core (Supplementary Information Fig. S6b). The most common chironomids, present in at least 3 samples and with a relative abundance of 15% or higher in at least one of the samples, were represented by 10 morphotypes (Fig. 3). The chironomid Microtendipes pedellus-type was clearly dominant during the Younger Dryas, while dominant taxa were mainly Ablabesmyia, Dicrotendipes nervosus-type, and Chironomus plumosus-type during the early Holocene (Fig. 3).
Stratigraphy of the 10 most common chironomid taxa (i.e., with a relative abundance of at least 15% in at least 1 sample). Chironomids are ordered from higher to lower average depth of the samples in which they occurred, weighed up by relative abundance. The Younger Dryas boundaries are delimited by short-dash horizontal lines
Chironomid-inferred temperatures
The reconstructed temperature showed the thermal transition between the Younger Dryas and the onset of the Holocene (Fig. 4, Supplementary Information Table S2). The mean July air temperature reconstruction values ranged from 13.9 °C (i.e., in our deepest sample at 6.62 m core depth, 12.76 cal ka BP, already in the Allerød period) to 20.6 °C (6.05 m, 10.13 cal ka BP, early Holocene). Temperature values for the Younger Dryas section varied from 14.1 °C (6.50 m, 12.54 cal ka BP) to 19.2 °C (6.44 m, 12.41 cal ka BP), and for the early Holocene section from 15.6 °C (6.16 m, 11.12 cal ka BP) to 20.6 °C (6.05 m, 10.13 cal ka BP). The reconstruction gave, on average, 2.4 °C lower temperatures during the Younger Dryas (average 15.9 °C) in comparison with the early Holocene section (average 18.3 °C). For the Younger Dryas period, a warmer phase at the beginning (6.52–6.33 m, 12.59–12.20 cal ka BP), followed by a cooler phase (6.30–6.24 m, 12.05–11.51 cal ka BP), could be observed. During the latter, one exceptionally high (outlying) temperature of 19.1 °C occurred at 6.25 m (11.59 cal ka BP).
Chironomid-inferred mean July air temperature reconstructed for Konarzewo Lake, compared to the mean July air temperature inferred with chironomids at Lake Gościąż (central Poland), with Cladocera at Pawłowa Lake (central Poland), with chironomids in Lake Hijkerneer (The Netherlands) and Kråkenes Lake (Norway), and with surrogates of temperature change following the models MD95-2043 (Alboran Sea), Gerzensee (Switzerland), and GISP2 (central Greenland). The Younger Dryas boundaries are delimited by gray dash-dotted horizontal lines following a standard definition (Rasmussen et al., 2006) and with black short-dash horizontal lines for the Polish sites following Müller et al. (2021). Modern mean July air temperature at Konarzewo Lake is indicated by a red short-dash vertical line. The blue y-axis indicates sample depth (for Konarzewo Lake only)
The minimum dissimilarity (squared chi-square distance) observed between core and training set chironomid samples (minDC) was lower than the 5th percentile of the dissimilarity values in the training set in four of the samples, and lower than the 10th percentile in 11 of the samples. This suggests that many of the samples in the sediment core have acceptable modern analogues. The DCA analysis revealed a strong association between the variation of the first DCA axis sample scores (as a surrogate of chironomid community changes) and chironomid-inferred temperatures. Correlations were computed as Spearman correlations, since DCA values (axis 1) departed from normality following a Shapiro–Wilk test, either when merging indeterminate taxa (P = 0.0030) or when excluding them (P = 0.0104). Spearman correlations were strong either when merging indeterminate taxa into their determined counterparts (Spearman rho = − 0.717, P < 0.0001), or after removing first the indeterminate taxa (Spearman rho = − 0.664, P < 0.0001). The gradient length revealed by the DCA was moderately long in both cases (i.e., 2.37 and 2.35 standard deviation units, respectively).
Chironomid stratigraphic zonation
CONISS analysis in combination with the broken stick model (to establish the number of significant zones in the chironomid stratigraphy) determined the presence of two clusters (two zones), which were delimited by the Younger Dryas-Holocene boundary (Fig. 5), following the definition by Müller et al. (2021) of this boundary at 11.47 cal ka BP in central Poland. This result was quite robust to changes in the threshold imposed for chironomid inclusion in the analysis (in terms of incidence and relative abundance) and to the decision regarding whether or not the few indeterminate chironomids found should be included by merging them into their corresponding determined counterparts (Fig. 5, Supplementary Information Figs. S7, S8). The exact place where these two stratigraphic clusters were divided was subjected to slight variation after modifying these criteria, but this division certainly occurred around the Younger Dryas-Holocene boundary.
Constrained incremental sum of squares (CONISS) analysis of chironomid stratigraphy. The analysis is run with all morphotypes (left panel) and with the most common morphotypes only (i.e., present in at least three samples, and with a maximum relative abundance across samples, max. ab., of at least 5%; right panel). The number of taxa involved in each analysis is shown in brackets. The Younger Dryas boundaries are delimited by short-dash horizontal lines, following Müller et al. (2021). Blue and red colors indicate the existence of two different clusters following the broken stick model
Biodiversity–temperature relationships
Temperature values slightly departed from normality (P = 0.0323, Shapiro–Wilk test), while the vast majority of biodiversity variants were normally distributed. As the latter is our dependent variable, this is what matters most with regard to the normality assumption, in the context of linear regression (Zar, 1999). Exceptions concentrated in the analysis of 0D under a large sample coverage, where extrapolation exceeded twice as much the reference sample size (Supplementary Information Appendix S1), which may lead to large prediction bias in the estimation of 0D (Chao et al., 2014a). This situation was avoided in advance when the analysis was referred to a specific sample size, but could not be anticipated for sample coverage values since the number of individuals necessary to reach a certain coverage value is unknown prior to the execution of the analysis. As to the relationship between biodiversity and temperature, the results obtained did not fundamentally vary when the analyses were repeated using Spearman instead of Pearson correlation tests, which supports the idea that linear regression models are robust against departures from normality (Schmidt & Finan, 2018).
Hill numbers were obtained either with interpolation or extrapolation from the observed value in the sample, which implied different numbers of individuals across samples (Supplementary Information Fig. S9). Hill numbers showed different patterns in relation to temperature: while 0D showed no correlation except for cases with limited size or coverage, 1D showed a rather weak correlation that did not hold for high values of coverage, and 2D was consistently and positively correlated with temperature independently of size and coverage (Fig. 6). The pattern was also independent on the decision about how to treat the few indeterminate chironomids found (Supplementary Information Figs. S10, S11), yet in the case of sample coverage with indeterminates being removed prior to statistical analysis, the correlation between 2D and temperature was mostly marginally significant (i.e., P < 0.10) when based on the Spearman correlation coefficient (Supplementary Information Fig. S11). The relationship between biodiversity and temperature was inspected in more detail for a sample size of 200 individuals and a sample coverage of 0.975. This relationship was positive in the case of sample size, for 1D (rather weak) and 2D (stronger), and only significant for 2D in the case of sample coverage (Fig. 7). When indeterminate chironomids were first removed, the positive relationship was observed again for 2D (Supplementary Information Fig. S12). The variation of the different Hill numbers across the stratigraphic sequence also showed that common chironomids were more closely linked to temperature changes than rare chironomids (Fig. 8, Supplementary Information Fig. S13).
Pearson correlation (top row) and associated P-value (bottom row) of the relationship between biodiversity and temperature. Biodiversity is computed as alpha diversity in three different manners, following Hill numbers of order q = 0, q = 1, and q = 2; marked in purple (circles), brown (squares), and green (diamonds), respectively. The analysis is repeated for different values of sample size (left column) and sample coverage (right column). Sample size values ranged from 10 to 230, in increments of 10, while sample coverage values ranged from 0.500 to 0.975 in increments of 0.025, with the addition of 0.995, 0.999, 0.9995, 0.99995, and 0.999995 values. Sample size and sample coverage values were obtained either through interpolation or extrapolation, depending on the desired value. For the analysis of P-values (bottom row), the short-dash horizontal line indicates P = 0.05
Chironomid biodiversity–temperature relationships during the Younger Dryas-Holocene transition, considering Hill numbers of order q = 0, q = 1, and q = 2. The analysis is performed for a sample size of 200 individuals (top row) and a sample coverage of 0.975 (bottom row). A red line is plotted when there is a significant linear relationship (P < 0.05, Pearson correlation test), with the grayed area representing its 95 confidence interval envelope. The level of significance is indicated in parentheses: ns, not significant; *P < 0.05; **P < 0.01
Stratigraphic profile of Hill numbers of order q = 0, q = 1, and q = 2 for sample size 200 and sample coverage 0.975, with indeterminate taxa merged into their determined counterparts (see also Supplementary Information Fig. S13, where indeterminate taxa were excluded). Temperature and Hill numbers are represented as standardized z-scores for comparability
Discussion
Temperatures were inferred during the Younger Dryas-Holocene transition by using subfossil chironomid remains in Konarzewo Lake, and then, we established a correlation between biodiversity and temperature, emphasizing the role of common and rare chironomid morphotypes in contributing to such pattern. We found that biodiversity tended to increase with temperature during the Younger Dryas-Holocene transition, in accordance with the idea that biodiversity is boosted by warming in colder-than-today past periods (Engels et al., 2020). When comparing the contribution of common and rare morphotypes to this pattern, a priori, we did not have any expectancy of what the result would be, and we eventually found that common morphotypes, not the rare ones, were mostly responsible for maintaining the positive relationship observed between biodiversity and temperature during the Younger Dryas-Holocene transition.
Depositional environment at the Younger Dryas-Holocene boundary
The sediment core analyzed was dominated by very fine sand or sandy silt during the Younger Dryas, mostly deposited through saltation, indicating generally poorly dynamic environmental activity (Fig. 2, Supplementary Information Fig. S3). In the early Holocene, the sediment changed in color to light brown and became more dominated by carbonate gyttja, with the presence of shells. Later, at 6.10 m depth (10.6 cal ka BP, Supplementary Information Table S2), the sediment turned dark brown and Chara became dominant in replacement of shells, indicating main changes in the freshwater communities inhabiting the lake. The Younger Dryas was also richer in mineral materials, and poorer in organic carbon and nitrogen, compared with the early Holocene (Supplementary Information Fig. S4). Overall, this supports the idea of warmer, perhaps more productive, environmental conditions at Konarzewo Lake during the early Holocene, compared with the Younger Dryas period.
Reconstructed temperatures during the Younger Dryas-Holocene transition
The Konarzewo Lake mean July air temperature reconstruction results were clearly different between the Younger Dryas and the early Holocene periods. The temperature rise was coherent with sedimentological and geochemical proxies, indicating the onset of the Holocene period (Fig. 4, Supplementary Information Figs. S3, S4). When reconstructed temperatures at Konarzewo Lake were compared to other Late Glacial and early Holocene reconstructions in Europe, they were consistent with a NW–SE incremental gradient in summer temperatures, as previously described by Brooks & Langdon (2014) and Heiri et al. (2014). Previous studies indicate that sites located in the peri-Atlantic region reveal very clear summer temperature drops during the Younger Dryas compared with earlier Interglacial periods and the beginning of the Holocene (Brooks & Birks, 2000, 2001; Cacho et al., 2001; Johnsen et al. 2001). Interestingly, Schenk et al. (2018, 2020) argue that summers in central-eastern Europe were warmer than in western Europe, with Younger Dryas cooling mostly happening in the form of severe winters.
The signal of the Younger Dryas cooling, however, also shows variability within western and central-eastern Europe. For example, in the Netherlands (Heiri et al., 2007), the summer temperature decrease is less pronounced than in the Alps (Schwander et al., 2000). In central-eastern Europe, the temperature changes during the transitions from Allerød to Younger Dryas and from the latter to the Holocene have been estimated to be negligible at Pawłowa Lake, central Poland, as reconstructed from cladoceran remains (Pawłowski et al., 2016). This lake is located ca. 400 km southeast of Konarzewo Lake. In contrast, the Younger Dryas cooling period was clearly observed, with the onset of the Holocene clearly warmer, when temperatures were reconstructed from chironomid remains at Lake Gościąż (Müller et al., 2021; Płóciennik et al., 2022), which is only 120 km north of Pawłowa.
In general, the Younger Dryas and early Holocene reconstructed temperatures at Konarzewo Lake were similar to the temperatures reconstructed at Lake Gościąż (Müller et al., 2021; Płóciennik et al., 2022). Temperatures were generally higher than those reconstructed at the Lieporiai Palaeolake in Lithuania (Šeirienė et al., 2021), which is located 560 km northeast of Konarzewo Lake, although in this case only a few samples were covering the Younger Dryas-Holocene transition, which prevents comparisons from being reliable. Arguably, western Baltic Ice Lake coast could have been less exposed to northern air mass oscillations from the Scandinavian Ice Sheet at that time, in comparison with the area of modern Lithuanian coast (Muschitiello, 2016). There exists evidence that the area of modern Pomeranian Bay, at the shore of which Konarzewo Lake is located, was already free of ice in the Oldest Dryas (Uścinowicz, 2014).
The higher reconstructed temperature values from Konarzewo Lake and other recent reconstructions from Poland (Kotrys et al., 2020; Müller et al., 2021; Płóciennik et al., 2021, 2022) compared to other chironomid-based reconstructions in Europe (Brooks & Birks, 2000, 2001; Heiri et al., 2007; Kaufman et al. 2020) may also be indicative of the relevance of training sets in the final output. An extended training set (e.g., Kotrys et al., 2020; Müller et al., 2021; Płóciennik et al., 2022) may result in higher temperatures. Specifically, adding 102 Polish sites to the Swiss-Norwegian training set (Heiri et al., 2011; Kotrys et al., 2020) increases the chances of finding modern analogues representing higher temperatures, and thus, modeled species optima are also likely to be higher.
Biodiversity–temperature relationships
Our results supported the use of different Hill numbers to assess the relative importance of rare and common species on biodiversity changes following temperature variation. In the paleolimnological context of our study, we found a sufficiently large thermal gradient at the Younger Dryas-Holocene transition to explore this question. We found 85 chironomid morphotypes among the 32 samples analyzed, covering the period between ca. 12.76 and 9.39 cal ka BP. Species richness per sample (standardized for 200 individuals) varied considerably across samples, from 7.0 to 50.8 during the Younger Dryas, and from 18.9 to 36.2 during the early Holocene (Supplementary Information Table S2). Despite the large variability, chironomid morphotypes were not evenly distributed across the period under study. During the Younger Dryas, the dominant morphotype was Microtendipes pedellus-type, which was suddenly replaced at the transition to the early Holocene by a series of chironomids that rapidly became relatively abundant, particularly Chironomus plumosus-type, Dicrotendipes nervosus-type, and Ablabesmyia (Fig. 3, Supplementary Information Fig. S5). In contrast, rare morphotypes followed a rather smooth replacement pattern (Supplementary Information Fig. S6). At the community level, we observed a sharp change at the transition, which was mainly driven by changes in common morphotypes, independently of the decision as to how to handle the few indeterminate chironomids found (Fig. 5, Supplementary Information Figs. S7, S8). Again common morphotypes, rather than the rare ones, largely influenced the biodiversity–temperature relationship observed (Figs. 6, 7, Supplementary Information Figs. S10, S11, S12). Common chironomids also appeared more tightly associated to temperature changes than rare chironomids along the stratigraphic sequence (Fig. 8, Supplementary Information Fig. S13).
Previous ecological studies suggest that the importance of rare and common species on biodiversity changes is context-dependent (Thorn et al., 2020; Tarifa et al. 2021; Tsafack et al., 2021). There is also some confusion as to what is “common” and “rare” in studies using Hill numbers. While order q = 1 can be regarded as a balance between the influence of common and rare species (Jost, 2006), many studies describe q = 1 as the order in which the influence of common or “typical” species is favored, and reserve the order q = 2 for “very common” or “dominant” species (Chao et al., 2014a, b; Thorn et al., 2020; Tarifa et al., 2021; Tsafack et al., 2021). We find the former definition, where q = 1 represents an equilibrium, more in line with the classical concept of commonness and rarity at the extremes (Preston, 1962) which is still widely used (e.g., Enquist et al., 2019). Following this simpler commonness-rarity gradient, we can argue that common wide-ranging species are also the most numerous at a local scale in terms of number of individuals (Gaston, 1996), and they have been shown to determine biodiversity patterns in the context of spatial constraints in previous literature (e.g., Jetz & Rahbek, 2002). However, rare species account for the vast majority of species richness inventories (Magurran & Henderson, 2003) and, therefore, they could lead patterns of biodiversity change as well. In this study, we found that (most) common morphotypes (i.e., order q = 2 and, to a much lesser extent, order q = 1), but not the rarest ones (i.e., order q = 0), are responsible for driving biodiversity changes under shifting thermal conditions, during the Younger Dryas-Holocene transition, at Konarzewo Lake.
Many aquatic ecosystems (like Konarzewo Lake) can be regarded as islands of favorable environmental conditions (for aquatic organisms) on a land matrix of unfavorable conditions. In this sense, one potential explanation for our findings may rely on metacommunity theory, whereby community assembly is governed by both environmental and spatial factors. This is because ecological communities in each of these fragmented habitats are connected through dispersal, and shaped by environmental factors as well. Essentially, four main different processes may be at play in a non-mutually exclusive way: patch dynamics, source-sink dynamics (mass effects), species-sorting, and neutrality (Leibold et al., 2004; Logue et al., 2011; Heino et al., 2015; de Mendoza et al., 2018). When dispersal is limited by spatial constraints and the ability of species to disperse becomes of utmost importance for colonization, it may happen that common wide-ranging species have an advantage to colonize new habitats faster than, and eventually outcompete, more rare narrow-ranging species (Xu et al., 2023). Also, common species are usually more tolerant of environmental changes than rare species are, which facilitates the colonization process, since the number of suitable patches to colonize can rapidly increase with environmental tolerance. This may result in a higher relevance of environmental variables for rare narrow-ranging specialists and of spatial variables for common wide-ranging generalists (Pandit et al., 2009; Heino & de Mendoza, 2016; de Mendoza et al., 2018). In general, the biodiversity shift observed in Konarzewo Lake during the Younger Dryas-Holocene transition, which is mainly driven by common morphotypes, supports the idea that common chironomids are able to respond faster than rare chironomids to temperature changes because they might be in an advantageous position during the colonization process from nearby habitats, in comparison with rare species.
In the context of establishing reference models of biodiversity changes in the past to apply to biological conservation strategies in the present, our results provide support to the idea that common species, and not only the rare ones, are responsible for biodiversity changes following environmental variation. This has implications for biodiversity conservation actions, where common species are often overlooked in favor of rare species.
Future directions
One potential criticism to our findings concerning biodiversity–temperature relationships relies on the fact that subfossil chironomid remains are usually not determined to species level, and several species per morphotype may exist (Langton, 1991; Brooks et al., 2007; Andersen et al., 2013). It is possible that more than one species is present within a given chironomid morphotype in our study. If this were the case, our observations would have been only possible if all (or most) species within a given chironomid morphotype responding to temperature were responding in the same direction, or at least without considerable interference. Since we observed clear stratigraphic changes among the morphotypes analyzed, we think that many of these morphotypes probably belong to one species. It is true that apparently common morphotypes could consist of more than one species which might not be so common when analyzed individually. Following the same rationale above, several not-so-common species within a common morphotype, all responding in a similar way to temperature changes, seems an unlikely scenario. These uncertainties are not to be resolved in the future by using morphological characters for chironomid identification. Genetic tools for species recognition are needed. Sedimentary DNA is a promising tool for investigating past biodiversity (Domaizon et al., 2017), and chironomid species coded in public barcode databases are increasing, yet they still represent a small fraction of the total number of chironomid species known (Han et al., 2023).
On the other hand, we acknowledge that when it comes to the analysis of biodiversity–temperature relationships, both variables should ideally be obtained through independent proxies, and this is certainly not the case in this study and in other studies (e.g., Engels et al., 2020). However, our results generally indicated no correlation between chironomid richness and temperature. Only when chironomid abundances were taken into account, particularly when these were overweighted through the specific usage of 2D, the relationship between biodiversity and temperature became evident. Also, in Engels et al. (2020), the association between biodiversity and temperature was considerably weakened above 14 °C, providing further evidence that species richness (i.e., 0D) does not necessarily drive the biodiversity–temperature relationship under all circumstances. Future directions for improvement in this regard should consider inferred temperature and biodiversity data from different proxies. This is relatively easy to achieve taking into account the different types of aquatic organism remains (e.g., chironomids, cladocerans, diatoms, chrysophytes, etc.) that are often encountered in lake sediments.
Finally, the use of Hill numbers can also be applied to functional and phylogenetic biodiversity (Chao et al., 2010, 2014b; Chiu & Chao, 2014; Thorn et al., 2020; Tarifa et al., 2021). In the case of paleolimnological studies using chironomids, functional diversity in particular seems to overcome the problem of the uncertainty in taxonomic resolution, since functional traits can often be defined at the genus level (e.g., Serra et al., 2016; Antczak-Orlewska et al., 2021). Comparing taxonomic and functional diversity across sedimentary sequences with chironomids sheds light onto the ecosystem processes that are subjected to change following the environmental variation in the past (Antczak-Orlewska et al., 2021; Belle et al., 2022).
Conclusion
Inferred past temperatures in Konarzewo Lake provided clear evidence of a temperature rise during the Younger Dryas-Holocene transition. Temperature values were consistent with a northwest-southeast incremental gradient in Europe, and in line with the temperatures inferred in Lake Gosciaż in central Poland. Our results also showed that Hill numbers proved successful in evaluating the differential contribution of rare and common morphotypes on biodiversity patterns following temperature changes across paleolimnological sequences. Common chironomid morphotypes were more relevant than the rare ones in shaping the biodiversity changes during the Younger Dryas-Holocene transition at Konarzewo Lake. This is consistent with the idea that common species may easily outcompete rare species when colonizing new sites from other sources, in a spatial context of fragmented suitable habitats.
Studying community changes in the past is a fundamental step to establish models toward biological conservation strategies under current environmental change. Common species deserve more attention in biodiversity conservation actions, which are often focused mainly on rare species. Chironomid remains can play an important role in this regard. In this study, they proved successful in supporting the idea that common species, not only the rare ones, might be essential in shaping biodiversity patterns across environmental gradients. Overall, our results showed the potential of paleolimnological research to incorporate ideas from metacommunity theory, with potential implications for conservation actions.
Data availability
Data will be made available upon reasonable request.
References
Andersen, T., P. S. Cranston & J. H. Epler, 2013. Chironomidae of the Holarctic region. Keys and diagnoses—larvae. Insect Systematics & Evolution, Supplement 66: 1–573.
Antczak-Orlewska, O., M. Płóciennik, R. Sobczyk, D. Okupny, R. Stachowicz-Rybka, M. Rzodkiewicz, J. Siciński, A. Mroczkowska, M. Krąpiec, M. Słowiński & P. Kittel, 2021. Chironomidae morphological types and functional feeding groups as a habitat complexity vestige. Frontiers in Ecology and Evolution 8: 583831. https://doi.org/10.3389/fevo.2020.583831.
Apolinarska, K., M. Woszczyk & M. Obremska, 2012. Late Weichselian and Holocene palaeoenvironmental changes in northern Poland based on the Lake Skrzynka record. Boreas 41: 292–307. https://doi.org/10.1111/j.1502-3885.2011.00235.x.
Armitage, P. D., L. C. Pinder & P. S. Cranston, 1995. The Chironomidae: Biology and Ecology of Non-biting Midges, Chapman & Hall, London: 572.
Belle, S., F. Klaus, M. A. González Sagrario, T. Vrede & W. Goedkoop, 2022. Unravelling chironomid biodiversity response to climate change in subarctic lakes across temporal and spatial scales. Hydrobiologia 849: 2621–2633. https://doi.org/10.1007/s10750-022-04890-0.
Bennett, K. D., 1996. Determination of the number of zones in a biostratigraphical sequence. New Phytologist 132: 155–170. https://doi.org/10.1111/j.1469-8137.1996.tb04521.x.
Blott, S. J. & K. Pye, 2001. GRADISTAT: a grain size distribution and statistics package for the analysis of unconsolidated sediments. Earth Surface Processes and Landforms 26: 1237–1248. https://doi.org/10.1002/esp.261.
Bojakowska, I. & E. Tołkanowicz, 2015. Zróżnicowanie zawartości pierwiastków śladowych w osadach torfowisk Otalżyno, Huczwa i Stoczek. Biuletyn Państwowego Instytutu Geologicznego 464: 5–16.
Bronk Ramsey, C., 2009. Bayesian analysis of radiocarbon dates. Radiocarbon 51: 337–360. https://doi.org/10.1017/S0033822200033865.
Brooks, S. J. & H. J. B. Birks, 2000. Chironomid-inferred late-glacial and early-Holocene mean July air temperatures for Kråkenes Lake, western Norway. Journal of Paleolimnology 23: 77–89. https://doi.org/10.1023/A:1008044211484.
Brooks, S. J. & H. J. B. Birks, 2001. Chironomid-inferred air temperatures from Lateglacial and Holocene sites in north-west Europe: progress and problems. Quaternary Science Reviews 20: 1723–1741. https://doi.org/10.1016/S0277-3791(01)00038-5.
Brooks, S. J. & P. G. Langdon, 2014. Summer temperature gradients in northwest Europe during the Lateglacial to early Holocene transition (15–8 ka BP) inferred from chironomid assemblages. Quaternary International 341: 80–90. https://doi.org/10.1016/j.quaint.2014.01.034.
Brooks S. J., P. G. Langdon & O. Heiri, 2007. The identification and use of palaearctic chironomidae larvae in palaeoecology. Technical Guide No. 10. Quaternary Research Association. London, UK: 276.
Cacho, I., J. O. Grimalt, M. Canals, L. Sbaffi, N. J. Shackleton, J. Schönfeld & R. Zahn, 2001. Variability of the western Mediterranean Sea surface temperature during the last 25,000 years and its connection with the Northern Hemisphere climatic changes. Paleoceanography and Paleoclimatology 16: 40–52. https://doi.org/10.1029/2000PA000502.
Chao, A. & L. Jost, 2012. Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than size. Ecology 93: 2533–2547. https://doi.org/10.1890/11-1952.1.
Chao, A., C.-H. Chiu & L. Jost, 2010. Phylogenetic diversity measures based on Hill numbers. Philosophical Transactions of the Royal Society B 365: 3599–3609. https://doi.org/10.1098/rstb.2010.0272.
Chao, A., N. J. Gotelli, T. C. Hsieh, E. L. Sander, K. H. Ma, R. K. Colwell & A. M. Ellison, 2014a. Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecological Monographs 84: 45–67. https://doi.org/10.1890/13-0133.1.
Chao, A., C.-H. Chiu & L. Jost, 2014b. Unifying species diversity, phylogenetic diversity, functional diversity, and related similarity/differentiation measures through Hill numbers. Annual Review of Ecology, Evolution, and Systematics 45: 297–324. https://doi.org/10.1146/annurev-ecolsys-120213-091540.
Chiu, C.-H. & A. Chao, 2014. Distance-based functional diversity measures and their decomposition: a framework based on Hill numbers. PLoS ONE 9: e100014. https://doi.org/10.1371/journal.pone.0100014.
Colwell, R. K., A. Chao, N. J. Gotelli, S.-Y. Lin, C. X. Mao, R. L. Chazdon & J. T. Longino, 2012. Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. Journal of Plant Ecology 5: 3–21. https://doi.org/10.1093/jpe/rtr044.
Cranston, P. S., 1982. A Key to the Larvae of the British Orthocladiinae (Chironomidae). Scientific Publication No. 45. Freshwater Biological Association. Cumbria, UK: 152.
de Mendoza, G., R. Kaivosoja, M. Grönroos, J. Hjort, J. Ilmonen, O.-M. Kärnä, L. Paasivirta, L. Tokola & J. Heino, 2018. Highly variable species distribution models in a subarctic stream metacommunity: patterns, mechanisms and implications. Freshwater Biology 63: 33–47. https://doi.org/10.1111/fwb.12993.
Dobracka, E., 2009. Szczególowa mapa geologiczna Polskie w skali 1: 50 000, arkusz Niechorze (77)—reambulacja. Państwowy Instytut Geologiczny—Państwowy Instytut Badawczy, Warszawa.
Dobracka, E., 2021. Objaśnienia do Szczegółowej mapy geologicznej Polski w skali 1: 50 000, arkusz Niechorze (77)—reambulacja. Państwowy Instytut Geologiczny—Państwowy Instytut Badawczy, Warszawa.
Domaizon, I., A. Winegardner, E. Capo, J. Gauthier & I. Gregory-Eaves, 2017. DNA-based methods in paleolimnology: new opportunities for investigating long-term dynamics of lacustrine biodiversity. Journal of Paleolimnology 58: 1–21. https://doi.org/10.1007/s10933-017-9958-y.
Eggermont, H. & O. Heiri, 2012. The chironomid-temperature relationship: expression in nature and palaeoenvironmental implications. Biological Reviews 87: 430–456. https://doi.org/10.1111/j.1469-185X.2011.00206.x.
Engels, S., A. S. Medeiros, Y. Axford, S. J. Brooks, O. Heiri, T. P. Luoto, L. Nazarova, D. F. Porinchu, R. Quinlan & A. E. Self, 2020. Temperature change as a driver of spatial patterns and long-term trends in chironomid (Insecta: Diptera) diversity. Global Change Biology 26: 1155–1169. https://doi.org/10.1111/gcb.14862.
Enquist, B. J., X. Feng, B. Boyle, B. Maitner, E. A. Newman, P. M. Jørgensen, P. R. Roehrdanz, B. M. Thiers, J. R. Burger, R. T. Corlett, T. L. P. Couvreur, G. Dauby, J. C. Donoghue, W. Foden, J. C. Lovett, P. A. Marquet, C. Merow, G. Midgley, N. Morueta-Holme, D. M. Neves, A. T. Oliveira-Filho, N. J. B. Kraft, D. S. Park, R. K. Peet, M. Pillet, J. M. Serra-Diaz, B. Sandel, M. Schildhauer, I. Šímová, C. Violle, J. J. Wieringa, S. K. Wiser, L. Hannah, J.-C. Svenning & B. J. McGill, 2019. The commonness of rarity: global and future distribution of rarity across land plants. Science Advances 5: eaaz0414. https://doi.org/10.1126/sciadv.aaz0414.
Figueroa-Rangel, B. L., M. Olvera-Vargas, S. Lozano-García, G. Islebe, N. Torrescano, S. Sosa-Najera & A. P. Del Castillo-Batista, 2020. Forests diversity in the Mexican Neotropics: a paleoecological view. In Rull, V. & A. Carnaval (eds), Neotropical Diversification: Patterns and Processes. Fascinating Life Sciences Springer, Cham: 449–473. https://doi.org/10.1007/978-3-030-31167-4_17.
Folk, R. L. & W. C. Ward, 1957. Brazos river bar, a study of significance of grain size parameters. Journal of Sedimentary Petrology 27: 3–26. https://doi.org/10.1306/74D70646-2B21-11D7-8648000102C1865D.
Fordham, D. A., S. T. Jackson, S. C. Brown, B. Huntley, B. W. Brook, D. Dahl-Jensen, M. T. P. Gilbert, B. L. Otto-Bliesner, A. Svensson, S. Theodoridis, J. M. Wilmshurst, J. C. Buettel, E. Canteri, M. McDowell, L. Orlando, J. Pilowsky, C. Rahbek & D. Nogues-Bravo, 2020. Using paleo-archives to safeguard biodiversity under climate change. Science 369: eabc5654. https://doi.org/10.1126/science.abc5654.
Gaston, K. J., 1996. The multiple forms of the interspecific abundance–distribution relationship. Oikos 76: 211–220. https://doi.org/10.2307/3546192.
Grimm, E. C., 1987. CONISS: a FORTRAN 77 program for stratigraphically constrained cluster analysis by the methods of incremental sum of squares. Computers & Geoscience 13: 13–35. https://doi.org/10.1016/0098-3004(87)90022-7.
Han, W., H. Tang, L. Wei & E. Zhang, 2023. The first DNA barcode library of Chironomidae from the Tibetan Plateau with an evaluation of the status of the public databases. Ecology and Evolution 13: e9849. https://doi.org/10.1002/ece3.9849.
Heino, J. & G. de Mendoza, 2016. Predictability of stream insect distributions is dependent on niche position, but not on biological traits or taxonomic relatedness of species. Ecography 39: 1216–1226. https://doi.org/10.1111/ecog.02034.
Heino, J., A. S. Melo, T. Siqueira, J. Soininen, S. Valanko & L. M. Bini, 2015. Metacommunity organisation, spatial extent and dispersal in aquatic systems. Patterns, processes and prospects. Freshwater Biology 60: 845–869. https://doi.org/10.1111/fwb.12533.
Heiri, O., A. F. Lotter, S. Hausmann & F. Kienast, 2003. A chironomid-based Holocene summer air temperature reconstruction from the Swiss Alps. The Holocene 13: 477–484. https://doi.org/10.1191/0959683603hl640ft.
Heiri, O., H. Cremer, S. Engels, W. Z. Hoek, W. Peeters & A. F. Lotter, 2007. Lateglacial summer temperatures in the Northwest European lowlands: a chironomid record from Hijkermeer, the Netherlands. Quaternary Science Reviews 26: 2420–2437. https://doi.org/10.1016/j.quascirev.2007.06.017.
Heiri, O., S. J. Brooks, H. J. B. Birks & A. F. Lotter, 2011. A 274-lake calibration data-set and inference model for chironomid-based summer air temperature reconstruction in Europe. Quaternary Science Reviews 30: 3445–3456. https://doi.org/10.1016/j.quascirev.2011.09.006.
Heiri, O., S. J. Brooks, H. Renssen, A. Bedford, M. Hazekamp, B. Ilyashuk, E. S. Jeffers, B. Lang, E. Kirilova, S. Kuiper, L. Millet, S. Samartin, M. Toth, F. Verbruggen, J. E. Watson, N. van Asch, E. Lammertsma, L. Amon, H. H. Birks, H. J. B. Birks, M. F. Mortensen, W. Z. Hoek, E. Magyari, C. Muñoz Sobrino, H. Seppä, W. Tinner, S. Tonkov, S. Veski & A. F. Lotter, 2014. Validation of climate model-inferred regional temperature change for late-glacial Europe. Nature Communications 5: 4914. https://doi.org/10.1038/ncomms5914.
Hill, M., 1973. Diversity and evenness: a unifying notation and its consequences. Ecology 54: 427–432. https://doi.org/10.2307/1934352.
Hsieh, T. C., K. H. Ma & A. Chao, 2020. iNEXT: iNterpolation and EXTrapolation for species diversity. R package version 2.0.20. http://chao.stat.nthu.edu.tw/wordpress/software-download/.
Jetz, W. & C. Rahbek, 2002. Geographic range size and determinants of avian species richness. Science 297: 1548–1551. https://doi.org/10.1126/science.1072779.
Johnsen, S. J., D. Dahl-Jensen, N. Gundestrup, J. P. Steffensen, H. B. Clausen, H. Miller, V. Masson-Delmotte, A. E. Sveinbjörnsdottir & J. White, 2001. Oxygen isotope and palaeotemperature records from six Greenland ice-core stations: Camp Century, Dye-3, GRIP, GISP2, Renland and NorthGRIP. Journal of Quaternary Science 16: 299–307. https://doi.org/10.1002/jqs.622.
Jost, L., 2006. Entropy and diversity. Oikos 113: 363–375. https://doi.org/10.1111/j.2006.0030-1299.14714.x.
Juggins, S., 2020. Rioja: Analysis of Quaternary Science Data, R package version 0.9-26. https://cran.r-project.org/package=rioja.
Kaufman, D., N. McKay, C. Routson, M. Erb, B. Davis, O. Heiri, S. Jaccard, J. Tierney, C. Dätwyler, Y. Axford, T. Brussel, O. Cartapanis, B. Chase, A. Dawson, A. de Vernal, S. Engels, L. Jonkers, J. Marsicek, P. Mofa-Sánchez, C. Morrill, A. Orsi, K. Rehfeld, K. Saunders, P. S. Sommer, E. Thomas, M. Tonello, M. Tóth, R. Vachula, A. Andreev, S. Bertrand, B. Biskaborn, M. Bringué, S. Brooks, M. Caniupán, M. Chevalier, L. Cwynar, J. Emile-Geay, J. Fegyveresi, A. Feurdean, W. Finsinger, M.-C. Fortin, L. Foster, M. Fox, K. Gajewski, M. Grosjean, S. Hausmann, M. Heinrichs, N. Holmes, B. Ilyashuk, E. Ilyashuk, S. Juggins, D. Khider, K. Koinig, P. Langdon, I. Larocque-Tobler, J. Li, A. Lotter, T. Luoto, A. Mackay, E. Magyari, S. Malevich, B. Mark, J. Massaferro, V. Montade, L. Nazarova, E. Novenko, P. Pařil, E. Pearson, M. Peros, R. Pienitz, M. Płóciennik, D. Porinchu, A. Potito, A. Rees, S. Reinemann, S. Roberts, N. Rolland, S. Salonen, A. Self, H. Seppä, S. Shala, J.-M. St-Jacques, B. Stenni, L. Syrykh, P. Tarrats, K. Taylor, V. van den Bos, G. Velle, E. Wahl, I. Walker, J. Wilmshurst, E. Zhang & S. Zhilich, 2020. A global database of Holocene paleotemperature records. Scientific Data 7: 115. https://doi.org/10.1038/s41597-020-0445-3.
Klink, A. G. & H. K. M. Moller Pillot, 2003. Chironomidae Larvae. Key to Higher Taxa and Species of the Lowlands of Northwestern Europe. CD-ROM, Expert Center for Taxonomic Information, University of Amsterdam. Amsterdam, The Netherlands.
Kotrys, B., M. Płóciennik, P. Sydor & S. J. Brooks, 2020. Expanding the Swiss-Norwegian chironomid training set with Polish data. Boreas 49: 89–107. https://doi.org/10.1111/bor.12406.
Krumbein, W. C., 1934. Size frequency distribution of sediments. Journal of Sedimentary Petrology 4: 65–77. https://doi.org/10.1306/D4268EB9-2B26-11D7-8648000102C1865D.
Langton, P. H., 1991. A Key to Pupal Exuviae of West Palearctic Chironomidae. Privately Published by P. H. Langton, 3 St Felix Road, Ramsey Forty Foot, Huntingdon. Cambridgeshire, UK: 324.
Legendre, P. & E. D. Gallagher, 2001. Ecologically meaningful transformations for ordination of species data. Oecologia 129: 271–280. https://doi.org/10.1007/s004420100716.
Leibold, M. A., M. Holyoak, N. Mouquet, P. Amarasekare, J. M. Chase, M. F. Hoopes, R. D. Holt, J. B. Shurin, R. Law, D. Tilman, M. Loreau & A. Gonzalez, 2004. The metacommunity concept: a framework for multi-scale community ecology. Ecology Letters 7: 601–613. https://doi.org/10.1111/j.1461-0248.2004.00608.x.
Levesque, A. J., L. C. Cwynar & I. R. Walker, 1997. Exceptionally steep north–south gradients in lake temperatures during the last deglaciation. Nature 385: 423–426. https://doi.org/10.1038/385423a0.
Logue, J. B., N. Mouquet, H. Peter & H. Hillebrand, 2011. Empirical approaches to metacommunities: a review and comparison with theory. Trends in Ecology & Evolution 26: 482–491. https://doi.org/10.1016/j.tree.2011.04.009.
Luoto, T. P., E. H. Kivilä, B. Kotrys, M. Płóciennik, M. V. Rantala & L. Nevelainen, 2020. Air temperature and water level inferences from northeastern Lapland (69°N) since the Little Ice Age. Polish Polar Research 41: 23–40. https://doi.org/10.24425/ppr.2020.132568.
MacArthur, R. H., 1957. On the relative abundance of bird species. Proceedings of the National Academy of Sciences of the United States of America 43: 293–295. https://doi.org/10.1073/pnas.43.3.293.
Magurran, A. E. & P. A. Henderson, 2003. Explaining the excess of rare species in natural species abundance distributions. Nature 422: 714–716. https://doi.org/10.1038/nature01547.
Massaferro, J. & I. Larocque-Tobler, 2013. Using a newly developed chironomid transfer function for reconstructing mean annual air temperature at Lake Potrok Aike, Patagonia, Argentina. Ecological Indicators 24: 201–210. https://doi.org/10.1016/j.ecolind.2012.06.017.
Maturo, F. & T. Di Battista, 2018. A functional approach to Hill’s numbers for assessing changes in species variety of ecological communities over time. Ecological Indicators 84: 70–81. https://doi.org/10.1016/j.ecolind.2017.08.016.
Mayfield, R. J., P. G. Langdon, C. P. Doncaster, J. A. Dearing, R. Wang, G. Velle, K. L. Davies & S. J. Brooks, 2021. Late Quaternary chironomid community structure shaped by rate and magnitude of climate change. Journal of Quaternary Science 36: 360–376. https://doi.org/10.1002/jqs.3301.
Meyers, P. A. & J. L. Teranes, 2001. Sediment organic matter. In Last, W. M. & J. P. Smol (eds), Tracking Environmental Change Using Lake Sediments. Physical and Geochemical Methods, Vol. 2. Kluwer Academic Publishers, Dordrecht: 239–269.
Moss, A. J., 1962. The physical nature of common sandy and pebbly deposits. Part I. American Journal of Science 260: 337–373. https://doi.org/10.2475/ajs.260.5.337.
Moss, A. J., 1963. The physical nature of common sandy and pebbly deposits. Part II. American Journal of Science 261: 297–343. https://doi.org/10.2475/ajs.261.4.297.
Müller, D., R. Tjallingii, M. Płóciennik, T. P. Luoto, B. Kotrys, B. Plessen, A. Ramisch, M. J. Schwab, M. Błaszkiewicz, M. Słowinski & A. Brauer, 2021. New insights into lake responses to rapid climate change: the Younger Dryas in Lake Gosciaż, central Poland. Boreas 50: 535–555. https://doi.org/10.1111/bor.12499.
Muschitiello, F., 2016. Deglacial impact of the Scandinavian Ice Sheet on the North Atlantic climate system. PhD Thesis. Stockholm University.
Negash, E. W., R. Bobe, Z. Alemseged & J. Wynn, 2022. Mammalian diversity patterns and paleoecology in the Lower Omo Valley, Ethiopia. In Reynolds, S. & R. Bobe (eds), African Paleoecology and Human Evolution Cambridge University Press, Cambridge: 289–297. https://doi.org/10.1017/9781139696470.024.
Oksanen, J., F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O'Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, E. Szoecs & H. Wagner, 2020. Vegan: Community Ecology Package. R package version 2.5–7. https://CRAN.R-project.org/package=vegan.
Osman, M. B., J. E. Tierney, J. Zhu, R. Tardif, G. J. Hakim, J. King & C. J. Poulsen, 2021. Globally resolved surface temperatures since the Last Glacial Maximum. Nature 599: 239–244. https://doi.org/10.1038/s41586-021-03984-4.
Pandit, S. N., J. Kolasa & K. Cottenie, 2009. Contrasts between habitat generalists and specialists: an empirical extension to the basic metacommunity framework. Ecology 90: 2253–2262. https://doi.org/10.1890/08-0851.1.
Passega, R., 1957. Texture as characteristic of clastic deposition. Bulletin of the American Association of Petroleum Geologists 41: 1952–1984. https://doi.org/10.1306/0BDA594E-16BD-11D7-8645000102C1865D.
Passega, R. & R. Byramjee, 1969. Grain-size image of clastic deposits. Sedimentology 13: 233–252. https://doi.org/10.1111/j.1365-3091.1969.tb00171.x.
Pawłowski, D., R. K. Borówka, G. A. Kowalewski, T. P. Luoto, K. Milecka, L. Nevalainen, D. Okupny, J. Tomkowiak & T. Zieliński, 2016. Late Weichselian and Holocene record of the paleoenvironmental changes in a small river valley in Central Poland. Quaternary Science Reviews 135: 24–40. https://doi.org/10.1016/j.quascirev.2016.01.005.
Petera-Zganiacz, J., D. Dzieduszyńska, K. Milecka, D. Okupny, M. Słowiński, D. J. Michczyńska, J. Forysiak & J. Twardy, 2022. Climate and abiotic landscape controls of Younger Dryas environmental variability based on a terrestrial archive (the Żabieniec mire, Central Poland). Catena 219: 106611. https://doi.org/10.1016/j.catena.2022.106611.
Pla-Rabés, S. & J. Catalan, 2018. Diatom species variation between lake habitats: implications for interpretation of paleolimnological records. Journal of Paleolimnology 60: 169–187. https://doi.org/10.1007/s10933-018-0017-0.
Pla-Rabés, S., R. J. Flower, E. M. Shilland & A. M. Kreiser, 2011. Assessing microbial diversity using recent lake sediments and estimations of spatio-temporal diversity. Journal of Biogeography 38: 2033–2040. https://doi.org/10.1111/j.1365-2699.2011.02530.x.
Płóciennik, M., A. Jakiel, J. Forysiak, P. Kittel, D. K. Płaza, D. Okupny, D. Pawłowski, M. Obremska, S. J. Brooks, B. Kotrys & T. P. Luoto, 2021. Multi-proxy inferred hydroclimatic conditions at Bęczkowice Fen (central Poland); the influence of fluvial processes and human activity in the Stone Age. Acta Geographica Lodziensia 111: 135–157. https://doi.org/10.26485/AGL/2021/111/10.
Płóciennik, M., I. Zawiska, M. Rzodkiewicz, A. M. Noryśkiewicz, M. Słowinski, D. Müller, A. Brauer, O. Antczak-Orlewska, M. Kramkowski, O. Peyron, L. Nevalainen, T. P. Luoto, B. Kotrys, H. Seppä, J. Camuera Bidaurreta, M. Rudna, M. Mielczarek, E. Zawisza, E. Janowska & M. Błaszkiewicz, 2022. Climatic and hydrological variability as a driver of the Lake Gościąż biota during the Younger Dryas. Catena 212: 106049. https://doi.org/10.1016/j.catena.2022.106049.
Porinchu, D., N. Rolland & K. Moser, 2009. Development of a chironomid-based air temperature inference model for the central Canadian Arctic. Journal of Paleolimnology 41: 349–368. https://doi.org/10.1007/s10933-008-9233-3.
Preston, F. W., 1962. The canonical distribution of commonness and rarity: Part I. Ecology 43: 185–215. https://doi.org/10.2307/1931976.
R Core Team, 2020. R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. https://www.R-project.org/.
Racinowski, R., T. Szczypek & J. Wach, 2001. Prezentacja i interpretacja wyników badań uziarnienia osadów czwartorzędowych, Wydawnictwo Uniwersytetu Śląskiego, Katowice: 146.
Rasmussen, S. O., K. K. Andersen, A. M. Svensson, J. P. Steffensen, B. M. Vinther, H. B. Clausen, M.-L. Siggaard-Andersen, S. J. Johnsen, L. B. Larsen, D. Dahl-Jensen, M. Bigler, R. Röthlisberger, H. Fischer, K. Goto-Azuma, M. E. Hansson & U. Ruth, 2006. A new Greenland ice core chronology for the last glacial termination. Journal of Geophysical Research 111: D06102. https://doi.org/10.1029/2005JD006079.
Reimer, P. J., W. E. N. Austin, E. Bard, A. Bayliss, P. G. Blackwell, C. Bronk Ramsey, M. Butzin, H. Cheng, R. L. Edwards, M. Friedrich, P. M. Grootes, T. P. Guilderson, I. Hajdas, T. J. Heaton, A. G. Hogg, K. A. Hughen, B. Kromer, S. W. Manning, R. Muscheler, J. G. Palmer, C. Pearson, J. van der Plicht, R. W. Reimer, D. A. Richards, E. M. Scott, J. R. Southon, C. S. M. Turney, L. Wacker, F. Adolphi, U. Büntgen, M. Capano, S. M. Fahrni, A. Fogtmann-Schulz, R. Friedrich, P. Köhler, S. Kudsk, F. Miyake, J. Olsen, F. Reinig, M. Sakamoto, A. Sookdeo & S. Talam, 2020. The IntCal20 northern hemisphere radiocarbon age calibration curve (0–55 cal kBP). Radiocarbon 62: 725–757. https://doi.org/10.1017/RDC.2020.41.
Schenk, F., M. Väliranta, F. Muschitiello, L. Tarasov, M. Heikkilä, S. Björck, J. Brandefelt, A. V. Johansson, J.-O. Näslund & B. Wohlfarth, 2018. Warm summers during the Younger Dryas cold reversal. Nature Communications 9: 1634. https://doi.org/10.1038/s41467-018-04071-5.
Schenk, F., O. Bennike, M. Väliranta, R. Avery, S. Björck & B. Wohlfarth, 2020. Floral evidence for high summer temperatures in southern Scandinavia during 15–11 cal ka BP. Quaternary Science Reviews 233: 106243. https://doi.org/10.1016/j.quascirev.2020.106243.
Schmid, P. E., 1993. A Key to the Larval Chironomidae and their Instars from Austrian Danube Region Streams and Rivers with Particular Reference to a Numerical Taxonomic Approach. Part I. Diamesinae, Prodiamesinae and Orthocladiinae. Federal Institute of Water Quality of the Ministry of Agriculture and Forestry, Wien, Austria: 514.
Schmidt, A. F. & C. Finan, 2018. Linear regression and the normality assumption. Journal of Clinical Epidemiology 98: 146–151. https://doi.org/10.1016/j.jclinepi.2017.12.006.
Schwander, J., U. Eicher & B. Ammann, 2000. Oxygen isotopes of lake marl at Gerzensee and Leysin (Switzerland), covering the Younger Dryas and two minor oscillations, and their correlation to the GRIP ice core. Palaeogeography, Palaeoclimatology, Palaeoecology 159: 203–214. https://doi.org/10.1016/S0031-0182(00)00085-7.
Šeirienė, V., N. Gastevičienė, T. P. Luoto, L. Gedminienė & M. Stančikaitė, 2021. The Lateglacial and early Holocene climate variability and vegetation dynamics derived from chironomid and pollen records of Lieporiai palaeolake. North Lithuania. Quaternary International 605–606: 55–64. https://doi.org/10.1016/j.quaint.2020.12.017.
Serra, S. R. Q., F. Cobo, M. A. S. Graça, S. Dolédec & M. J. Feio, 2016. Synthesising the trait information of European Chironomidae (Insecta: Diptera): Towards a new database. Ecological Indicators 61: 282–292. https://doi.org/10.1016/j.ecolind.2015.09.028.
Sindowski, K.-H., 1957. Die synoptische Methode des Kornkurven-Vergleiches zur Ausdeutung fossiler Sedimentationsräume. Geologisches Jahrbuch 73: 235–275.
Sydor, P., 2021. Rozwój wybrzeża mierzejowego we wschodniej części Zatoki Pomorskiej. Przegląd Geologiczny 69: 21–32. https://doi.org/10.7306/2021.2.
Sydor, P. & S. Uścinowicz, 2023. Driving forces and determinants of barrier coast evolution in the Holocene observed on the southern coast of the Baltic Sea. The Holocene 33: 759–780. https://doi.org/10.1177/09596836231163507.
Tarifa, R., C. Martínez-Núñez, F. Valera, J. P. González-Varo, T. Salido & P. J. Rey, 2021. Agricultural intensification erodes taxonomic and functional diversity in Mediterranean olive groves by filtering out rare species. Journal of Applied Ecology 58: 2266–2276. https://doi.org/10.1111/1365-2664.13970.
Tarrats, P., O. Heiri, B. Valero-Garcés, M. Cañedo-Argüelles, N. Prat, M. Rieradevall & P. González-Sampériz, 2018. Chironomid-inferred Holocene temperature reconstruction in Basa de la Mora Lake (Central Pyrenees). The Holocene 28: 1685–1696. https://doi.org/10.1177/0959683618788662.
ter Braak, C. J. F., 1990. Update notes: CANOCO version 3.1, Agricultural Mathematics Group, Wageningen, The Netherlands.
ter Braak, C. J. F., 2019. New robust weighted averaging- and model-based methods for assessing trait–environment relationships. Methods in Ecology and Evolution 10: 1962–1971. https://doi.org/10.1111/2041-210X.13278.
ter Braak, C. J. F. & P. F. M. Verdonschot, 1995. Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquatic Sciences 57: 255–289. https://doi.org/10.1007/BF00877430.
Thorn, S., A. Chao, M. Bernhardt-Römermann, Y.-H. Chen, K. B. Georgiev, C. Heibl, J. Müller, H. Schäffer & C. Bässler, 2020. Rare species, functional groups, and evolutionary lineages drive successional trajectories in disturbed forests. Ecology 101: e02949. https://doi.org/10.1002/ecy.2949.
Tóth, M., O. Heiri, I. Vincze, M. Braun, Z. Szabó & E. K. Magyari, 2022. Limnological changes and chironomid-inferred summer air temperature from the Late Pleniglacial to the Early Holocene in the East Carpathians. Quaternary Research 105: 151–165. https://doi.org/10.1017/qua.2021.36.
Tsafack, N., S. Fattorini, M. Boieiro, F. Rigal, A. Ros-Prieto, M. T. Ferreira & P. A. V. Borges, 2021. The role of small lowland patches of exotic forests as refuges of rare endemic Azorean arthropods. Diversity 13: 443. https://doi.org/10.3390/d13090443.
Urbaniak-Biernacka, U., 1975. Nowoczesna klasyfikacja osadów klastycznych według wielkości ziarn. Przegląd Geologiczny 3: 137–139.
Uścinowicz, S., 2014. The Baltic Sea continental shelf. In Chiocci, F. L. & A. R. Chivas (eds), Continental Shelves of the World: Their Evolution During the Last Glacio-Eustatic Cycle. Geological Society, London, Memoirs, Vol. 41. The Geological Society of London, London: 69–89. https://doi.org/10.1144/M41.7.
Viard, J. P. & J. A. Breyer, 1979. Description and hydraulic interpretation of grain size cumulative curves from the Platte River system. Sedimentology 26: 427–439. https://doi.org/10.1111/j.1365-3091.1979.tb00918.x.
Visher, G. S., 1969. Grain-size distributions and depositional processes. Journal of Sedimentary Petrology 39: 1074–1106. https://doi.org/10.1306/74D71D9D-2B21-11D7-8648000102C1865D.
Wentworth, C. K., 1922. A scale of grade and class terms of clastic sediments. The Journal of Geology 30: 377–392. https://doi.org/10.1086/622910.
Wiederholm, T., 1983. Chironomidae of the Holarctic region. Keys and diagnoses. Part 1—Larvae. Entomologica Scandinavica, Supplement 19: 1–457.
Williams, N., M. Rieradevall, D. Añón Suárez, A. Rizzo, R. Daga, S. Ribeiro Guevara & M. A. Arribére, 2016. Chironomids as indicators of natural and human impacts in a 700-year record from the northern Patagonian Andes. Quaternary Research 86: 120–132. https://doi.org/10.1016/j.yqres.2016.07.002.
Williams, N., D. Añón Suárez, M. Rieradevall, A. Rizzo, R. Daga, M. A. Arribére & S. Ribeiro Guevara, 2019a. Response of Chironomidae to environmental disturbances in a high mountain lake in Patagonia during the last millennium. Quaternary Research 92: 273–287. https://doi.org/10.1017/qua.2019.5.
Williams, N., D. Añón Suárez, M. Rieradevall, A. Rizzo, R. Daga, M. A. Arribére & S. Ribeiro Guevara, 2019b. Response of Chironomidae to environmental disturbances in a high mountain lake in Patagonia during the last millennium – Corrigendum. Quaternary Research 92: 605. https://doi.org/10.1017/qua.2019.28.
Xu, W.-B., S. A. Blowes, V. Brambilla, C. F. Y. Chow, A. Fontrodona-Eslava, I. S. Martins, D. McGlinn, F. Moyes, A. Sagouis, H. Shimadzu, R. van Klink, A. E. Magurran, N. J. Gotelli, B. J. McGill, M. Dornelas & J. M. Chase, 2023. Regional occupancy increases for widespread species but decreases for narrowly distributed species in metacommunity time series. Nature Communications 14: 1463. https://doi.org/10.1038/s41467-023-37127-2.
Yasuhara, M., H. Doi, C.-L. Wei, R. Danovaro & S. E. Myhre, 2016. Biodiversity–ecosystem functioning relationships in long-term time series and palaeoecological records: deep sea as a test bed. Philosophical Transactions of the Royal Society B 371: 20150282. https://doi.org/10.1098/rstb.2015.0282.
Zar, J. H., 1999. Biostatistical Analysis, 4th ed. Pearson Prentice Hall, Upper Saddle River: 663.
Zhang, E., J. Chang, Y. Cao, H. Tang, P. Langdon, J. Shulmeister, R. Wang, X. Yang & J. Shen, 2017. A chironomid-based mean July temperature inference model from the southeast margin of the Tibetan Plateau, China. Climate of the Past 13: 185–199. https://doi.org/10.5194/cp-13-185-2017.
Zhang, C., Y. Chen, B. Xu, Y. Xue & Y. Ren, 2019. How to predict biodiversity in space? An evaluation of modelling approaches in marine ecosystems. Diversity and Distributions 25: 1697–1708. https://doi.org/10.1111/ddi.12970.
Acknowledgements
We greatly appreciate the comments provided by two anonymous reviewers, which were very useful guidelines to improve considerably this manuscript.
Funding
This study was funded by the statutory research grant of the Polish Geological Institute—National Research Institute (Project No. 61.2608.1601.00.0), and the Polish National Agency for Academic Exchange (NAWA, Agreement No. PPN/ULM/2020/1/00092/U/00001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: Jasmine Saros
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Mendoza, G., Kotrys, B., Płóciennik, M. et al. Common chironomids drive the biodiversity–temperature relationship during the Younger Dryas-Holocene transition in a southern Baltic coastal lake. Hydrobiologia 851, 503–525 (2024). https://doi.org/10.1007/s10750-023-05337-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05337-w