Abstract
Population growth and increasing production demands threaten the highly diverse Andean freshwater ecosystems. Biological indicators constitute a valuable tool for evaluating the ecological quality of freshwater ecosystems under different pressures. Diatom and macroinvertebrate assemblages are the most used bioindicators to assess water pollution, whereby these biotic groups occupy the first and second trophic levels and respond to habitat pollution. Several studies have explored the response of these communities to water pollution in other regions, but no studies have examined their performance in Andean rivers. In this context, this research aimed to evaluate the responses and relationships of both groups of bioindicators in the Upper Guayllabamba basin. We collected macroinvertebrate and diatom samples from nine sites in this basin during the dry and wet seasons, calculated trophic indices for both groups, and related them to environmental characteristics. The results indicated that both bioindicators were sensitive to changes in land use and nutrients. Epilithic diatoms were more sensitive to changes in water chemistry and macroinvertebrates to changes in fluvial habitat and land use. The index based on macroinvertebrates better-detected changes in quality classes between sites and seasons. Therefore, both indices gave complementary information, and their joint use seems suitable in Andean streams.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Tropical Andes provide significant freshwater resources to South America, supplying water to the Amazon, Pacific, and tropical Atlantic basins (Buytaert et al., 2006; Vergara, 2007). These high-altitude ecosystems are characterized by large diel temperature variations and high ultraviolet radiation and solar energy inputs (Luteyn, 1999). These environmental conditions and the area’s diverse geology and geomorphology promote high speciation rates and endemism (Buytaert et al., 2006; Madriñán et al., 2013). Also, Andean mountain streams are heterogeneous, with changes in physical, chemical, and hydromorphological characteristics along an altitudinal gradient (Villamarin et al., 2014). Human populations in the Andes rely heavily on paramo and mountain river systems for multiple ecosystem services, including tourism, hydropower, and water supply for urban, industrial, agricultural, and livestock activities (Buytaert et al., 2006). The paramo directly provides water resources to about 40 million people in the Andes (Josse et al., 2009), providing 85% of the water supply to Quito, the capital of Ecuador (Buytaert et al., 2006).
Increasing human pressures in the Andes have produced high afforestation rates, land use changes, water pollution, and flow reduction (Anderson & Maldonado‐Ocampo, 2011), influencing the physical and chemical and biological attributes in Andean streams. Urban, industrial, and agricultural activities contribute to the input of nutrients (Carpenter et al., 1998; Smith et al., 2006; Ríos-Touma et al., 2022), pesticides (Stone et al., 2014), and sediments (Trimble, 1997) into streams. In addition, many Andean cities discharge untreated wastewater directly into these freshwater systems (Walteros & Ramírez, 2020). These activities contribute to many disturbances, including the eutrophication of surface waters, consequently producing harmful algal blooms and oxygen depletion in these environments (Carpenter et al., 1998; Smith et al., 2006). Stream pollution can affect the ecological integrity of freshwater habitats and have adverse public health effects on Andean and downstream populations in Ecuador, making water quality monitoring and assessment imperative (Acosta et al., 2009; Guerrero-Latorre et al., 2018; Vinueza et al., 2021; Ríos-Touma et al., 2022).
Developing monitoring tools and programs to set management plans and restoration goals remain important for Andean streams. Many biotic indices have been developed for various regions and included in technical standards for monitoring water quality, such as the European Water Framework Directive (2000/60/EC) (European Commission, 2000). Bioindicators, such as macroinvertebrates and diatoms, remain a widely used tool (Hodkinson & Jackson, 2005; Carter et al., 2006; Ríos-Touma et al., 2014; Lobo et al., 2016). These organisms are considered sensitive to habitat changes, in which different taxa have different responses to pollution, and are widely distributed in freshwater habitats (Boothroyd & Stark, 2000; Hodkinson & Jackson, 2005; Chang et al., 2014; Lobo et al., 2015). They show water quality conditions through changes in the proportion of sensitive taxa and community composition and structure shifts (Kaesler et al., 1978; Lobo et al., 2016). In Latin America, the use of diatoms in water quality assessment started in the 1990s. Based on studies by Frenguelli (1947), Argentina developed the Pampean Diatom Index (Gómez et al., 2001). In Brazil, Wetzel et al. (2002) published the first description of a Brazilian saprobic system, characterizing three differential diatom groups: highly pollution-tolerant species, pollution-tolerant species, and less pollution-tolerant species. In Ecuador, Castillejo et al. (2018) tested this index for Ecuadorian Andean rivers, concluding that species’ tolerances are not always a suitable way of diagnosing water quality. The use of macroinvertebrates as bioindicators in Latin America started in the 1970s with the first adaptations in Colombia (Roldan et al., 1973), followed by adaptations of the BMWP (Biological Monitoring Working Party, Armitage et al., 1983) from the United Kingdom during the early 2000s with considerable success in the application (e.g., Domínguez & Fernández, 1998; Figueroa et al., 2003). Moreover, this index uses family-level identifications, which have shown consistent tolerance to pollution values worldwide (Chang et al, 2014) in streams located at over 2,000 m.a.s.l.; the Andean Biotic Index is widely used in Peruvian, Ecuadorian, and Colombian streams.
However, the relationship between the responses of both bioindicators to human pressures on Andean streams has yet to be evaluated. Diatom assemblages are typically closely associated with chemical variables (Hering et al., 2006), and macroinvertebrates are more sensitive to organic pollution (Wang et al., 2007) and alterations in physical habitat structure (Voss et al., 2012). Differences in responses to stressors of multiple assemblages make them complementary in standard ecological assessments or freshwater monitoring programs. Thus, changes in the composition and abundance of macroinvertebrate and diatom assemblages are expected in the face of all the human pressures placed upon aquatic ecosystems (Silva et al., 2017; González-Paz et al., 2020). Due to the importance of the biodiversity and ecosystem services that Andean streams provide (Encalada et al., 2019) and the increasing human pressures on these ecosystems, we aimed to evaluate how Andean diatoms and macroinvertebrate assemblages respond to the multiple stressors present in high-altitude Andean streams. Finally, we assessed the benefits of using diatoms and macroinvertebrates to determine the ecological status of the Upper Guayllabamba River basin.
Materials and methods
Study area
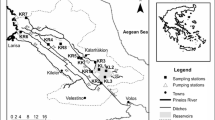
The Upper Guayllabamba River basin is in the southern part of Pichincha Province, serving Quito (Fig. 1). Nine sampling sites were selected along the basin (Table 1), including the Pita and Santa Clara sub-basins. The headwaters of Pita begin between Sincholagua (4899 m.a.s.l.) and Cotopaxi (5890 m.a.s.l) volcanoes and form a tributary of the San Pedro River (2415 m.a.s.l) (MAE, 2012). The Pita River flows in a south-north direction, covering 1 km2 (Castillejo et al., 2018). The river basin supplies 2200 L/s of drinking water to the Metropolitan District of Quito (FONAG, 2012) and is mainly impacted by livestock, agriculture, industrial activities, and urbanization (FONAG, 2012). Two sites were located within the Santa Clara River basin (Table 1; Fig. 1). The headwaters of this basin begin at an altitude of 4110 m.a.s.l in Rumiñahui Canton in southeast Pichincha and cover an area of 0.4 km2, flowing into the San Pedro River at 2440 m.a.s.l (Masabanda et al., 2016). The main land uses in the Santa Clara River basin include livestock, agricultural, industrial, and urban activities (GAD Cantón Rumiñahui, 2015).
Our sampling design aimed to capture the variation between the different reaches of the basin that are strongly influenced by an elevational and anthropogenic gradient. Sampling sites 1.1PI, 1.3PI, and 1.4PI are surrounded by paramo vegetation. Meanwhile, 2.1PI, 3.2PI, and 3.4PI are in pastureland but with well-preserved riparian areas. SC1, SC2, and SP3 are in urban areas.
Sampling
Macroinvertebrate, epilithic diatom, and water samples were collected at each site (Table 1). Sampling was performed in August 2020 and May 2021 to include dry and wet conditions. Riparian and stream habitats were assessed using the Andean Riparian Habitat Quality Index (QBR-And) (Acosta et al., 2009) and the Fluvial Habitat Index (IHF) (Pardo et al., 2002; Acosta et al., 2009). The QBR-And index assesses vegetation cover, structure, quality, and channel alteration, while the IHF index evaluates physical channel quality, focusing on heterogeneity factors related to benthic community diversity (Pardo et al., 2002). A QBR-And score of 96 or higher suggests undisturbed riparian habitat, and a score of 50 or below indicates a highly impacted habitat (Acosta et al., 2009). An IHF score of 40 or less indicates poor habitat quality for aquatic invertebrates, while a score of 75 or more suggests optimal habitat quality (Pardo et al., 2002).
We measured in situ environmental parameters—pH, specific conductance, total dissolved solids (TDS), temperature, barometric pressure, and dissolved oxygen saturation and concentration—using the YSI Pro1030 and ProODO instruments (YSI, USA). We measured water velocity using the FP111 Global Water Flow Probe (Xylem, USA), as well as depth and length along a transect to calculate channel discharge. Additionally, we collected water samples for laboratory analysis. These samples were stored at 4 ºC until their arrival at the laboratory in less than 5 h.
Water samples
In the laboratory, we determined color, turbidity, alkalinity, concentrations of chloride, sulfate, nitrite, nitrate, ammonium, and phosphate, Biochemical Oxygen Demand (BOD), Chemical Oxygen Demand (COD), and oil and fats (Table 2). Furthermore, we contrasted our measured environmental values with the parameter limits set by the Ecuadorian Environment Ministry’s legislation on water quality for aquatic life preservation or agricultural and livestock use (Ministerio del Ambiente de Ecuador, MAE, 2015).
Diatom sampling
Epilithic diatoms were collected at each site. The samples were scrubbed from the upper surface of three stones 10–20 cm in diameter using a toothbrush following the method described by Kobayasi & Mayama (1982). The samples were cleaned with sulfuric and hydrochloric acids and mounted on permanent slides with Naphrax®. To calculate the relative abundances of the species, all organisms found along random transects on the prepared permanent slides were identified and counted, with a minimum of 400 valves, using a Leica DM750 light microscope. Species with above-average numbers of individuals (number of species divided by the total number of individuals) were considered abundant, following the criterion proposed by Lobo & Leighton (1986). For species identification, the following taxonomic references were used: Lobo et al. (2014; 2016b), Metzeltin & Lange-Bertalot (1998; 2007), Rumrich et al. (2000), Metzeltin & García-Rodríguez (2003), and Metzeltin et al. (2005).
Scanning Electron Microscope (SEM) images were taken using an ultra-high-resolution Tescan MIRA3 analytical field emission scanning electron microscope operated at 10 kV to differentiate Nitzschia soratensis E.Morales and M.L.Vis from Nitzschia inconspicua Grunow. As suggested by Lobo et al. (1990), the percentage of N. soratensis vs. N. inconspicua found under the SEM was extrapolated in order to be counted under a light microscope.
Macroinvertebrate sampling
At each site, we collected benthic macroinvertebrates with a 250 µ D-net (Ríos-Touma et al., 2014) in all habitat types for 3 min (Acosta et al., 2009). We also collected three 0.09 m2 Surber samples from riffles in each sampling transect to obtain quantitative samples for biodiversity analysis (Ríos-Touma et al., 2022). We preserved the samples in 96% alcohol and sorted them with an Olympus SZ61 stereomicroscope (Olympus, Japan). Then, we identified macroinvertebrates to order and family levels to calculate the Andean Biotic Index (Ríos-Touma et al., 2014) and to genus or morphospecies levels related to abundances for biodiversity analysis. We used Dominguez & Fernandez (2009) and Hamada et al. (2019) to identify macroinvertebrates.
Data analysis
We used the Trophic Water Quality Index (TWQI) for subtropical and temperate Brazilian lotic systems developed by Lobo et al. (2014, 2015) to diagnose water quality using the composition of epilithic diatoms. This method assigned trophic values (TV) of 1, 2.5, and 4 to species, corresponding to low, medium, and high tolerance to eutrophication, respectively. The trophic values (tv) assigned to each species were taken from the OMNIDIA software (www.omnidia.fr).
We calculated the Andean Biotic Index (ABI, Rios-Touma, et al., 2014), which is an adaptation of the BMWP Index (Armitage et al., 1983). This index uses macroinvertebrate tolerance values from 1 (more tolerant) to 10 (more sensitive). This adaption provides tolerance values for families of macroinvertebrates present in lotic environments above 2,000 m.a.s.l in Ecuador and Peru. For this basin, if the sum of the tolerance values of the families present is higher than 96, it means excellent quality. If the sum is between 59 and 96, the site’s quality is marked as very good; between 35 and 58 means fair; between 14 and 34 means poor, and below 14 denotes very poor quality (Rios-Touma et al., 2014).
The environmental similarity between sites and seasons was evaluated with normalized variables using Euclidean distances with an average cluster. Then, the site’s environmental data were analyzed with a Principal Component Analysis (PCA) of mean values for both seasons for each site. We initially performed Spearman’s Rank Correlation on the environmental variables to choose a subset of variables to reduce collinearity in the analysis. Then, the variables were normalized before the PCA analysis. Both quality indices, TWQI and ABI, were related to Axis 1 of the PCA that best explained the pollution gradient, using JAMOVI statistical software (Jamovi, 2021).
Diatom and macroinvertebrate assemblages were evaluated using cluster and Non-Metric Multidimensional Scaling (NMDS) analyses. The taxon density (macroinvertebrates) or proportion (diatoms) were converted to square root prior to the analysis. First, we performed an average cluster analysis using Bray–Curtis species similarity (macroinvertebrates and diatoms independently) among the sites. We assessed the significance of the clustering using the Similarity Profile routine (SIMPROF) that tests the data against the null hypothesis of no structure (Clarke et al., 2008). Second, we performed an NMDS with the average densities/proportions of the two sampling seasons. We performed a RELATE procedure in the NMDS in which the previously selected environmental variables were related to the composition of taxa to assess the main environmental variables that determine species composition (> 0.65 Spearman correlations). All these ordination analyses were performed using PRIMER 6 statistical software (Clarke & Gorley, 2006). We calculated richness, abundance, and Shannon diversity (measured as the effective number of species) for each group (diatoms and macroinvertebrates at each site). For this analysis, we used the package iNEXT in R statistical software (Hsieh et al., 2016). We assessed differences in these metrics between sites using a Kruskall-Wallis analysis of variance and between seasons using Jamovi statistical software (Jamovi, 2021).
Results
Environmental gradients
According to the Ecuadorian legislation of quality for aquatic life preservation or agricultural and livestock use (MAE, 2015), all sampling sites except for 1.3PI, 2.1PI, and 3.4PI during the dry season and 2.1PI during the wet season exceed the chloride limit. Oxygen saturation at all sites during the dry season does not meet the indicated limit. SP3 during the wet season is the only site whose ammonium concentration is over the legal limit (Table 3).
Sites SC1, SC2, and SP3 showed higher turbidity, COD, temperature, discharge, and levels of ammonium, nitrites, nitrates, oil, and fats. In contrast, sites 1.1PI, 1.3PI, 1.4PI, 2.1PI, 3.2PI, and 3.4PI had higher DO concentration, specific conductance, sulfate, chloride, and QBR values. All sites for both seasons had IHF scores within or above the threshold for suitable habitats (> 60). The less polluted sites (1.1PI, 1.3PI, 1.4PI, 2.1PI, 3.2PI, and 3.4PI) in both seasons have QBR-And scores of “good” or “excellent.” In contrast, the most polluted sites in both seasons have QBR-And scores of “poor.”
The average cluster analysis with environmental variables (Fig. 2a) found five significant groups. The first was composed of SP3 in both seasons and SC2 in the wet season. The second group contained 1.1PI and 1.4PI during the wet season, and the third group SC1 and SC2 in the dry season. The fourth group included 2.1PI, 3.2PI, 3.4PI, and 1.3PI during both seasons and SC1 during the wet season. The fifth group consisted of 1.1PI and 1.4PI during the dry season. According to the SIMPROF cluster analysis test, only SC1, SC2, 1.1PI, and 1.4PI showed significant seasonal differences in environmental characteristics.
a Cluster analysis (Group Average) based on physical and chemical data from nine sampling sites during both seasons in the Upper Guayllabamba basin, Ecuador. b PCA analysis biplot including PC1 and PC2 (PC1: 35.2%, EV = 3.17; PC2: 21.9%, EV = 1.97) according to nine abiotic variables. Amm: ammonia; Phos: phosphate; spc_Cond: conductivity; QBR: Riparian Quality Index; DO_Sat: oxygen saturation; IHF: Fluvial Habitat Index; Discharge; and &_Agro: percentage of agricultural use in a drainage area in nine sampling stations (1.3PI, 1.3PI, 1.4PI, 2.1PI, 3.2PI, 3.4PI, SC1, SC2, and SP3) in the Upper Guayllabamba basin, Ecuador
The PCA with nine previously selected variables (Fig. 2b) discriminated between our nine sampling sites. The first two components explain a cumulative variance of 57.1% (PC1: 35.2% EV = 3.17; PC2: 21.9% EV = 1.97). The first component (PC1) differentiates sites based on ammonium and phosphate concentrations, contrasting more polluted sites (SC1, SC2, and SP3) with those containing healthier concentrations. Furthermore, less polluted sites (1.1PI, 3.2PI, 1.4PI, 2.1PI, and 1.3PI) were distinguished by better riparian areas (QBR-And) and higher oxygen saturation (DO_sat). On the other hand, PC2 separates sites 3.4PI, SC1, and SC2 by having higher discharge and percentage of agricultural land use than the other sites, and separates SP3, 2.1PI, and 1.4PI for their higher pH values.
Bioindicator assemblages and environmental variables
We identified 85 diatom species and 29 genera. Twenty-seven taxa were considered abundant species (Appendix 1— Supplementary Material.), with Nitzschia Hassall and Gomphonema Ehrenberg being the most representative genera with 17 (20%) and 11 (12.9%) species, respectively. In both seasons, Achnanthidium minutissimum (Kützing) Czarnecki and Planothidium frequentissimum (Lange-Bertalot) Lange-Bertalot were abundant exclusively in non-urban sites (1.1PI, 1.3PI, 1.4PI, 2.1PI, 3.2PI, and 3.4PI). Nitzschia soratensis was abundant in every sample except for the upper stations: 1.1PI and 1.3PI. Abundant species in the most eutrophic sample (SP3) were described in the literature as meso-tolerant or tolerant to eutrophication. Tolerant species include Nitzschia palea (Kützing) W.Smith, Nitzschia clausii Hantzsch, Sellaphora saugerresii (Desm.) C.E. Wetzel et D.G. Mann, Navicula gregaria Donkin, Navicula lanceolata Ehrenberg, and Eolimna minima, and meso-tolerant species include Sellaphora seminulum (Grunow) D.G.Mann, Gomphonema pumilum (Grunow) Reichardt & Lange-Bertalot, and Navicula cryptotenella Lange-Bertalot. In particular, Eolimna minima was previously classified as a pollution-tolerant species in an Ecuadorian study. The sites in middle reaches (2.1PI, 3.2PI, and 3.4PI) contained abundant species with oligotrophic and mesotrophic preferences. Reimeria sinuata (W.Gregory) Kociolek & Stoermer, an abundant species in our samples, has an oligo-mesotrophic preference (Carayon et al., 2019).
According to our TWQI, the only site with an oligotrophic pollution level for both seasons was 1.1PI. There was a positive pollution gradient from 1.3PI to 3.4PI, with all sites showing mean trophic values within the β-mesotrophic range. The most polluted sites (SC1, SC2, and SP3) showed mean tv values within the α-mesotrophic range (Table 4). The cluster formed with diatom assemblages (Fig. 3a) had smaller discernible differences compared to the environmental cluster (Fig. 2a). In general, sites clustered together (dry and wet seasons). The first two clusters are upstream sites with better water quality (1.1PI and 1.3PI). Then, one set is composed of a cluster of SP3 (both seasons) and SC1 (dry season), both sites with strong environmental impacts, and another set of SC2 and 3.4PI (in both seasons) along with 3.2PI during the wet season, which could be explained by considering a mix of strong to medium environmental affected streams. Finally, we have a cluster of 2.1PI and 1.4PI (during both seasons) together with 3.2PI during the dry season, sites with fewer environmental impacts than the previous group.
a Average cluster analysis (with SIMPROF test) based on diatom species abundance from the nine sampling sites during two seasons (May: wet; and August: dry) in the Upper Guayllabamba basin. b Average Cluster analysis (with SIMPROF test) based on macroinvertebrate taxa abundance from nine sampling sites during two seasons (May = wet and August = dry) in the upper Guayllabamba basin, Ecuador
We found 64 macroinvertebrate taxa at all sites during both samplings. Oligochaeta and Chironomidae were present in all sites (Appendix 2— Supplementary Material.). Hydracarina, Ceratopogonidae, Simuliidae, Baetidae, Hydroptilidae, and Hyalellidae were also present in most sites (90–100%). Austrelmis, Paltostoma, Mortoniella, Atopsyche, Anomalocosmoecus Tanitarisini (Chironomidae), and Tanypodinae (Chironomidae) were absent from most polluted sites (SC1, SC2, and SP3). The group average cluster (Fig. 3b) showed 4 significant groups. The first cluster contained two branches: one composed of paramo sites (1.1PI and 1.4PI in both seasons), and the other included 1.3PI during both seasons. The second cluster comprised all of the low and middle elevation sites (2.1PI and 3.2PI in both seasons along with 3.4PI in the dry season). The third cluster was composed of the most polluted sites (SC1, SC2, and SP3 in both seasons) and 3.4PI in the wet season.
The Andean Biotic Index (ABI, Table 4) showed sites ranging from “excellent” to “bad” water quality. Overall, biological quality was better in the dry season. On average, only 3.2PI had “excellent” biological quality, while four upstream and middle sites had “very good” quality (1.1PI, 1.3PI, 2.1PI, and 3.4PI). Three highly polluted sites (SC1, SC2, and SP3) belonged to the “bad” biological quality category. Only SP3 had “very poor” quality during the wet season.
Both indices relate to PC1 (35% of environmental variation explained, Fig. 2b), which discriminates more polluted from less polluted sites. TWQI had a direct relationship with this PC, and the ABI had an inverse relationship (Fig. 4). ABI and TWQI indices showed a significant negative correlation (Spearman rho = − 0.71; P < 0.5) as TWQI values increase with pollution and ABI decrease. TWQI correlated with land uses (%Agro = 0.81, P < 0.01; %Urban = 0.91, P < 0.01) and nutrient concentration (Ammonia = 0.91, P < 0.01; Nitrite = 0.91, P < 0.01; Nitrate = 0.88, P < 0.01). ABI correlated positively with oxygen and negatively with chemical oxygen demand (DO = 0.75, P < 0.05; COD = − 0.83, P < 0.01), and both indices correlated with QBR (ABI = 0.81, P < 0.01; TWQI = − 0.73, P < 0.05). (Appendix 3). The non-parametric Kruskal–Wallis test did not show significant differences in diversity measurements between sites (Shannon diversity and Richness, Table 4).
Community composition and environmental variables
Our NMDS with diatom assemblages were clearly related to environmental variables (Fig. 5a). Ammonium (Spearman rho: − 0.93 with NMDS 1), agricultural land use (Spearman rho: -0.77 with NMDS 1), and discharge (Spearman rho: − 0.67 with NMDS 1) were strongly related to polluted sites (SP3, SC1, and SC2). At the same time, better riparian quality (QBR-And Spearman correlation: 0.71 with NMDS 1) and oxygen saturation (DO_sat, Spearman rho: 0.62 with NMDS2) were related to sites 1.1PI, 1.3PI, 1.4PI, 2.1PI, and 3.2PI.
a Non-Metric Multidimensional Scaling of diatom assemblages with related environmental factors (> 0.65 Spearman correlation) with NMDS axes in nine sampling stations (1.3PI, 1.3PI, 1.4PI, 2.1PI, 3.2PI, 3.4PI, SC1, SC2, SP3). b Non-Metric Multidimensional Scaling of macroinvertebrate assemblages with related environmental factors (> 0.65 Spearman correlation) with NMDS axes in nine sampling stations (1.3PI, 1.3PI, 1.4PI, 2.1PI, 3.2PI, 3.4PI, SC1, SC2, SP3)
Macroinvertebrate assemblages showed paramo streams (1.1PI, 1.3PI, and 1.4PI) to be clearly separate from the rest of the sites (Fig. 5b) on the negative side of MDS1. The percentage of agricultural land use (Spearman rho: 0.67 with MDS1) discriminated the paramo sites (with less agricultural or non-agricultural land use) from the rest of the sites. Phosphates (Spearman rho: 0.83 with MDS2), discharge, and ammonium (both with Spearman rho: 0.62 with MDS2) were related to the polluted sites– SP3, SC1, and SC2. At the same time, 1.3PI, 2.1PI, and 3.2PI showed lower concentrations of these pollutants and lower discharge.
Discussion
Ecuadorian environmental laws do not establish the use of biological indicators for the analysis of water resources, despite the abundance of research on the subject in the country (e.g., Acosta et al., 2009; Ríos-Touma et al., 2014; Castillejo et al., 2018; Rosero‐López et al., 2020). In this study, we compared the performance of two different bioindicators to detect the impacts of anthropogenic pressures. Our results showed that the biological quality indices of the two biological communities used (diatoms and macroinvertebrates) were significantly correlated (Spearman rho = − 0.71; P < 0.05), and, at the same time, were also correlated to changes in the environmental and anthropogenic gradient studied.
The vast majority of our sampling sites are affected by mixed sources of pollution, e.g., wastewater effluents, agricultural land uses, and nutrient concentrations (e.g., ammonia), which are highly correlated (Spearman rho = > 0.65) with diatom and macroinvertebrate assemblages. This shows that both assemblages respond to these environmental pressures (Fig. 5a). Moreover, the TWQI and ABI were significantly correlated with stressor variation among the sites (PC1), and between them, showing that the indices were also sensitive to the degradation of environmental quality in these streams (Fig. 2b). However, this is not always the case: other biological quality indices based on diatoms and macroinvertebrates were reported to be uncorrelated in South Africa (De la Rey et al., 2004) and highly correlated in Mediterranean countries (Torrisi et al., 2010). In Portugal, both indices showed a similarity of 76% in their responses to water quality assessments but with complementary responses to environmental pressures (Feio et al., 2007). Neither in our work did the TWQI and ABI responses to anthropic pressures completely overlap. We showed that diatoms responded to variations of water quality, such as nutrient concentrations and riparian forest quality, while macroinvertebrates responded to decreased oxygen saturation, increased chemical oxygen demand, and land use change, as reported in the literature (e.g.,Soininen & Könönen, 2004; Hering et al., 2006; Blanco et al., 2007; Karaouzas et al., 2018). Compared to macroinvertebrates, diatoms have rapid growth rates that allow them to react faster to chemical changes, especially nutrient enrichment (e.g., Morin et al, 2016), detecting thus the first steps of degradation (Schneider et al., 2012), as opposed to macroinvertebrates (Karaouzas et al., 2018). Hence, the latter are more sensitive to changes affecting structural parameters, e.g., microhabitat composition, flow regime, etc. (Soininen and Könönen, 2004; Hering et al., 2006; Blanco et al., 2007). Our results suggest that in paramo streams, where cattle and agriculture are significant pressures, epilithic diatoms show more specificity for pristine and less impacted sites. In contrast, macroinvertebrates responded and discriminated better in sites in the lower inter-Andean reaches, where multiple stressors occur, such as increasing urbanization, agriculture, and intensive cattle farming.
Both indices showed worse water quality in the wet season in urban sites. Nonetheless, previous studies have suggested the relevance of seasonality in macroinvertebrates but not in diatoms (Almeida et al., 2014), giving diatoms an advantage when assessing water quality over a wide time frame. This inconsistency can be explained by the decrease in water quality in Ecuadorian Andean rivers in the wet season due to increased runoff from agriculture and urban areas, especially in places with a lack of adequate riparian vegetation cover and of wastewater treatment (Jacobsen, 1998; Ríos-Touma et al., 2022). Thus, the TWQI varies depending on chemical variables that affect water quality, as described for other diatom-based quality indices (Schneider et al., 2012; Morin et al., 2016). Our work shows that both biological groups are complementary, and their use is suitable for Andean freshwater ecosystems.
We found no differences in the classic biodiversity metrics between the sites and the environmental gradient. Biological indicators and community composition were more informative regarding the degradation of environmental conditions. Moreover, the study of the community through the NMDS and its relationship with the environmental variables contributed to a better understanding of how assemblages change and respond to changes in nutrients, land use, and oxygen availability. This is a powerful tool for comprehending ecological drivers of change and better adapting trophic scores of tolerance values for bioindicators. We used general trophic values for diatoms (OMNIDIA) and adapted tolerance values for Andean streams (Ríos-Touma et al., 2014). However, our data showed that some taxonomic groups might respond differently to the multiple stressors of these streams, as observed in macroinvertebrates in urban areas in this same basin (e.g., Psychodidae in Ríos-Touma et al., 2022).
Feio et al. (2021) indicate the importance of selecting appropriate bioindicators for monitoring programs to assess the ecological status of rivers. The expertise of local technicians is essential to correctly identify the taxa present in the samples. New molecular techniques, such as the genetic barcode approach (Ballesteros et al., 2020), can help overcome limitations and increase the quality of stream assessments. Both indices, employed in temperate areas and adapted to the location, responded to human pressures in streams. Nevertheless, our dataset is limited to two seasons in the Upper Guayllabamba basin. Strong monitoring data relies on long-term studies that are still lacking in most Andean countries. Therefore, filling the knowledge gap on how these streams and their communities respond is essential in order to improve the monitoring tools and the information they provide.
Data availability
Data used for this analysis is included in the supplementary material.
References
Acosta, R., B. Ríos, M. Rieradevall & N. Prat, 2009. Propuesta de un protocolo de evaluación de la calidad ecológica de ríos andinos (CERA) y su aplicación a dos cuencas en Ecuador y Perú. Limnetica 28: 35–64. https://doi.org/10.23818/limn.28.04.
Almeida, S. F. P., C. Elias, J. Ferreira, E. Tornés, C. Puccinelli, F. Delmas, G. Dörflinger, G. Urbanič, S. Marcheggiani & J. Rosebery, 2014. Water quality assessment of rivers using diatom metrics across Mediterranean Europe: a methods intercalibration exercise. Science of the Total Environment 476: 768–776. https://doi.org/10.1016/j.scitotenv.2013.11.144.
Anderson, E. P. & J. A. Maldonado-Ocampo, 2011. A regional perspective on the diversity and conservation of tropical Andean fishes. Conservation Biology 25: 30–39. https://doi.org/10.1111/j.1523-1739.2010.01568.x.
Armitage, P. D., D. Moss, J. F. Wright & M. T. Furse, 1983. The performance of a new biological water quality score system based on macroinvertebrates over a wide range of unpolluted running-water sites. Water Research 17: 333–347. https://doi.org/10.1016/0043-1354(83)90188-4.
Ballesteros, I., P. Castillejo, A. P. Haro, C. C. Montes, C. Heinrich & E. A. Lobo, 2020. Genetic barcoding of Ecuadorian epilithic diatom species suitable as water quality bioindicators. Comptes Rendus. Biologies 343: 41–52. https://doi.org/10.5802/crbiol.2.
Blanco, S., E. Bécares, H.-M. Cauchie, L. Hoffmann & L. Ector, 2007. Comparison of biotic indices for water quality diagnosis in the Duero Basin (Spain). Large Rivers 3: 267–286. https://doi.org/10.1127/lr/17/2007/267.
Boothroyd, I. & J. Stark, 2000. Use of invertebrates in monitoring. New Zealand stream invertebrates: ecology and implications for management. New Zealand Limnological Society 20: 344–373.
Buytaert, W., R. Célleri, B. De Bièvre, F. Cisneros, G. Wyseure, J. Deckers & R. Hofstede, 2006. Human impact on the hydrology of the Andean páramos. Earth-Science Reviews 79: 53–72. https://doi.org/10.1016/j.earscirev.2006.06.002.
Carayon, D., J. Tison-Rosebery & F. Delmas, 2019. Defining a new autoecological trait matrix for French stream benthic diatoms. Ecological Indicators 103: 650–658. https://doi.org/10.1016/j.ecolind.2019.03.055.
Carpenter, S. R., N. F. Caraco, D. L. Correll, R. W. Howarth, A. N. Sharpley & V. H. Smith, 1998. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecological Applications 8: 559–568.
Carter, J. L., V. H. Resh, D. M. Rosenberg, T. B. Reynoldson, G. Ziglio, M. Siligardi & G. Flaim, 2006. Biomonitoring in North American rivers: a comparison of methods used for benthic macroinvertebrates in Canada and the United States. Biological monitoring of rivers: applications and perspectives Wiley: Chichester, UK 203–228.
Castillejo, P., S. Chamorro, L. Paz, C. Heinrich, I. Carrillo, J. G. Salazar, J. C. Navarro & E. A. Lobo, 2018. Response of epilithic diatom communities to environmental gradients along an Ecuadorian Andean River. Comptes Rendus Biologies 341: 256–263.
Chang, F.-H., J. E. Lawrence, B. Rios-Touma & V. H. Resh, 2014. Tolerance values of benthic macroinvertebrates for stream biomonitoring: assessment of assumptions underlying scoring systems worldwide. Environmental Monitoring and Assessment 186(4): 2135–2149. https://doi.org/10.1007/s10661-013-3523-6. (Epub 2013 Nov 11).
Clarke, K.R. & R.N. Gorley. 2006. PRIMERv6: user Manual/Tutorial. PRIMER-E, Plymouth, 192 pp.
Clarke, K. R., P. J. Somerfield & R. N. Gorley, 2008. Testing of null hypotheses in exploratory community analyses: similarity profiles and biota-environment linkage. Journal of Experimental Marine Biology and Ecology 366: 56–69. https://doi.org/10.1016/j.jembe.2008.07.009.
De la Rey, P. A., J. C. Taylor, A. Laas, L. Van Rensburg & A. Vosloo, 2004. Determining the possible application value of diatoms as indicators of general water quality: a comparison with SASS 5. Water Sa Water Research Commission 30: 325–332. https://doi.org/10.4314/wsa.v30i3.5080.
Domínguez, E. & H. R. Fernández, 1998. Calidad de los ríos de la cuenca del Salí (Tucumán, Argentina) medida por un índice biótico. Serie Conservación De La Naturaleza Universidad Nacional De Tucumán Argentina 12: 1–40.
Dominguez, E. & H. Fernandez, 2009. Macroinvertebrados Bentónicos Sudamericanos. Sistemática y biología. Fundación Miguel Lillo, Tucumán.
Encalada, A. C., A. S. Flecker, N. L. Poff, E. Suárez, G. A. Herrera-R, B. Ríos-Touma, S. Jumani, E. I. Larson, & E. P. Anderson, 2019. A global perspective on tropical montane rivers. Science 365: 1124–1129. https://www.sciencemag.org/lookup/doi/10.1126/science.aax1682
European Commission, 2000. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. , 0001–0073, Retrieved from https://eur-lex.europa.eu/eli/dir/2000/60/oj.
Feio, M. J., S. F. P. Almeida & S. C. Craveiro, 2007. Diatoms and macroinvertebrates provide consistent and complementary information on environmental quality. Fundamental and Applied Limnology 169: 247–258. https://doi.org/10.1127/1863-9135/2007/0169-0247.
Feio, M. J., R. M. Hughes, M. Callisto, S. J. Nichols, O. N. Odume, B. R. Quintella, M. Kuemmerlen, F. C. Aguiar, S. F. P. Almeida, P. Alonso-EguíaLis, F. O. Arimoro, F. J. Dyer, J. S. Harding, S. Jang, P. R. Kaufmann, S. Lee, J. Li, D. R. Macedo, A. Mendes, N. Mercado-Silva, W. Monk, K. Nakamura, G. G. Ndiritu, R. Ogden, M. Peat, T. B. Reynoldson, B. Rios-Touma, P. Segurado & A. G. Yates, 2021. The Biological Assessment and Rehabilitation of the World’s Rivers: An Overview. Water 13: 371. https://doi.org/10.3390/w13030371.
Figueroa, R., C. Valdovinos, E. Araya & O. Parra, 2003. Macroinvertebrados bentónicos como indicadores de calidad de agua deríos del sur de Chile. Revista Chilena De Historia Natural 76: 275–285. https://doi.org/10.4067/S0716-078X2003000200012.
FONAG, 2012. Apoyo a la conservacion en la cuenca alta del río Pita. Retrieved from https://www.fonag.org.ec/doc_pdf/5.pdf
GAD Cantón Rumiñahui, 2015. Plan de Desarrollo y Ordenamiento Territorial Cantón Rumiñahui 2012–2022 Actualización 2015. Retrieved from http://www.ruminahui-aseo.gob.ec/wp-content/uploads/PDYOT-2020-2025.pdf
Gómez, N. & M. Licursi, 2001. The Pampean Diatom Index (IDP) for assessment of rivers and streams in Argentina. Aquatic Ecology 35: 173–181. https://doi.org/10.1023/A:1011415209445.
González-Paz, L., Delgado, C. & Pardo, I. 2020. Understanding divergences between ecological status classification systems based on diatoms. Science of the Total Environment, 734, 139418. https://doi.org/10.1016/j.scitotenv.2020.139418
Guerrero-latorre, L., B. Romero, E. Bonifaz, N. Timoneda, M. Rusiñol, R. Girones & B. Rios-Touma, 2018. Science of the Total Environment Quito‘s virome: Metagenomic analysis of viral diversity in urban streams of Ecuador ‘ s capital city. Science of the Total Environment 645: 1334–1343. https://doi.org/10.1016/j.scitotenv.2018.07.213.
Hamada, N. & Thorp. 2019. Thorp and Covich’s Freshwater Invertebrates Academic. In (Fourth E. Rogers (eds), Press: ii. https://doi.org/10.1016/C2010-0-65590-8
Hering, D., R. K. Johnson, S. Kramm & S. Schmutz, 2006. Assessment of European streams with diatoms, macrophytes, macroinvertebrates and fish: a comparative metric-based analysis of organism response to stress. Fresh Water Biology 51(9): 1757–1785. https://doi.org/10.1111/j.1365-2427.2006.01610.x.
Hodkinson, I. D. & J. K. Jackson, 2005. Terrestrial and aquatic invertebrates as bioindicators for environmental monitoring, with particular reference to mountain ecosystems. Environmental Management 35: 649–666. https://doi.org/10.1007/s00267-004-0211-x.
Hsieh, T. C., K. H. Ma & A. Chao, 2016. iNEXT: an R package for rarefaction and extrapolation of species diversity (H ill numbers). Methods in Ecology and Evolution 7: 1451–1456. https://doi.org/10.1111/2041-210X.12613.
Jacobsen, D., 1998. The effect of organic pollution on the macroinvertebrate fauna of Ecuadorian highland streams. Archiv Fur Hydrobiologie 143: 179–195. https://doi.org/10.1127/archiv-hydrobiol/143/1998/179.
Jamovi, 2021. The jamovi project. Retrieved from https://www.jamovi.org.
Josse, C., F. Cuesta, G. Navarro, E. Cabrera, E. Chacón Moreno, W. Ferreira, M. Peralvo, J. Saito & A. Tovar, 2009. Ecosistemas de los Andes del norte y centro. Bolivia, Colombia, Ecuador, Perú y Venezuela. Secretaría General de la Comunidad Andina, Programa Regional ECOBONA.
Kaesler, R. L., E. E. Herricks & J. S. Crossman, 1978. Use of Indices of Diversity and Hierarchical Diversity in Stream. Biological Data in Water Pollution Assessment: Quantitative and Statistical Analyses: a Symposium. American Society for Testing and Materials: 92–112. https://doi.org/10.1520/stp35659s
Karaouzas, I., C. Theodoropoulos, L. Vardakas, E. Kalogianni & NTh. Skoulikidis, 2018. A review of the effects of pollution and water scarcity on the stream biota of an intermittent Mediterranean basin. River Research and Applications 34: 291–299. https://doi.org/10.1002/rra.3254.
Kobayasi, H. & S. Mayama, 1982. Most pollution-tolerant diatoms of severely polluted rivers in the vicinity of Tokyo. Japanese Journal of Phycology 30: 188–196.
Lobo, E. & G. Leighton, 1986. Estructuras comunitarias de la fitocenosis planctónicas de los sistemas de desembocaduras de ríos y esteros de la Zona Central de Chile. Revista De Biología Marina 22(1): 1–29.
Lobo, A., S. Kitazawa & H. Konayasi, 1990. The use of scanning electron microscopy as a necesary complement of light microscopy diatom examination for ecological studies. Diatom 5: 33–43.
Lobo, E. A., C. E. Wetzel, M. Schuch & L. Ector, 2014. Diatomáceas epilíticas como indicadores da qualidade da água em sistemas lóticos subtropicais e temperados brasileiros, EDUNISC press, Santa Cruz do Sul, Brasil:
Lobo, E. A., C. G. Heinrich, M. Schuch, A. Dupont, A. B. Da Costa, C. E. Wetzel & L. Ector, 2015. Índice Trófico da Qualidade da Água Guia ilustrado para sistemas lóticos subtropicais e temperados Brasileiros.
Lobo, E. A., C. G. Heinrich, M. Schuch, C. E. Wetzel & L. Ector, 2016. Diatoms as bioindicators in rivers River algae. In Necchi JR, O. (ed) River Algae. Springer, Cham.
Luteyn, J. L., 1999. Paramos: a checklist of plant diversity, Geographic Distribution and Botanical Literature. Memoirs of the New York Botanical Garden 84: 1–278.
Madriñán, S., A. J. Cortés & J. E. Richardson, 2013. Páramo is the world’s fastest evolving and coolest biodiversity hotspot. Frontiers in Genetics Frontiers 4: 192. https://doi.org/10.3389/fgene.2013.00192.
MAE, 2012. Estrategia Nacional de Cambio Climatico en Ecuador (EMCC 2012–2025). Journal of Visual Languages & Computing 11: 55. Retrieved from https://www.ambiente.gob.ec/wp-content/uploads/downloads/2017/10/ESTRATEGIA-NACIONAL-DE-CAMBIO-CLIMATICO-DEL-ECUADOR.pdf
MAE, 2015. Registro Oficial Edición Especial 387. Retrieved from https://www.gob.ec/sites/default/files/regulations/2018-09/Documento_Registro-Oficial-No-387-04-noviembre-2015_0.pdf
Masabanda, M., B. Morales & A. Guzman, 2016. Hidrología y Sedimentología de la Cuenca Del Río Santa Clara. 1: 43–58. https://doi.org/10.24133/rcsd.v1n3.2016.06
Metzeltin, D. & F. García-Rodríguez, 2003. Las Diatomeas Uruguayas, DIRAC press, Montevideo, Facultad de Ciencias:
Metzeltin, D. & H. Lange-Bertalot, 1998. Tropical diatoms of South America I. In Lange-Bertalot, H. (ed.), Iconographia Diatomologica. Koeltz Scientific Books press, Königstein, Germany, 5:695 pp.
Metzeltin, D. & H. Lange-Bertalot, 2007. Tropical diatoms of South America II. In Lange-Bertalot, H. (ed.), Iconographia Diatomologica. Gantner Verlag press, Liechtenstein, 18:877 pp.
Metzeltin, D., H. Lange-Bertalot & F. García-Rodríguez, 2005. Diatoms of uruguay. In Lange-Bertalot, H. (ed.), Iconographia Diatomologica. Koeltz Scientific Books press, Königstein, Germany, 15: 736 pp.
Morin, S., N. Gómez, E. Tornés, M. Licursi & J. Rosebery, 2016. Benthic diatom monitoring and assessment of freshwater environments: standard methods and future challenges. In Romaní, A.M., H. Guasch & M.D. Balaguer (eds), Aquatic Biofilms: Ecology, Water Quality and Wastewater Treatment. Caister Academic Press: 111–124.
Pardo, I., M. Álvarez, J. Casas, J. L. Moreno, S. Vivas, N. Bonada, J. Alba-Tercedor, P. Jáimez-Cuéllar, G. Moyà, N. Prat, S. Robles, M. L. Suárez, M. Toro & M. del R. Vidal-Abarca, 2002. El hábitat de los ríos mediterráneos. Diseño de un índice de diversidad de hábitat. Limnetica 21: 115–133.
Ríos-Touma, B., R. Acosta & N. Prat, 2014. The Andean biotic index (ABI): revised tolerance to pollution values for macroinvertebrate families and index performance evaluation. Revista De Biologia Tropical 62: 249–273.
Ríos-Touma, B., C. Villamarín, G. Jijón, J. Checa, G. Granda-Albuja, E. Bonifaz & L. Guerrero-Latorre, 2022. Aquatic biodiversity loss in Andean urban streams. Urban Ecosystems 25: 1619–1629. https://doi.org/10.1007/s11252-022-01248-1.
Roldan, G., J. Builes, C. M. Trujillo & A. Suarez, 1973. Efectos de la contaminacion industrial y domestica sobre la fauna bentica del Rio Medellin. Actualidades Biologicas (medellin) 2: 54–64.
Rosero-López, D., J. Knighton, P. Lloret & A. C. Encalada, 2020. Invertebrate response to impacts of water diversion and flow regulation in high-altitude tropical streams. River Research and Applications 36: 223–233. https://doi.org/10.1002/rra.3578.
Rumrich, U., H. Lange-Bertalot & M. Rumrich, 2000. Diatoms of the Andes. From Venezuela to Patagonia/Tierra del Fuego and two additional contributions. In Lange-Bertalot, H. (ed.), Iconographia Diatomologica. Koeltz Scientific Books, Königstein, Germany, 9:673 pp.
Schneider, S. C., A. E. Lawniczak, J. Picińska-Faltynowicz & K. Szoszkiewicz, 2012. Do macrophytes, diatoms and non-diatom benthic algae give redundant information? Results from a case study in Poland. Limnologica Elsevier 42: 204–211. https://doi.org/10.1016/j.limno.2011.12.001.
Silva, D. R., A. T. Herlihy, R. M. Hughes & M. Callisto, 2017. An improved macroinvertebrate multimetric index for the assessment of wadeable streams in the neotropical savanna. Ecological Indicators 81: 514–525. https://doi.org/10.1016/j.ecolind.2017.06.017.
Smith, V. H., S. B. Joye & R. W. Howarth, 2006. Eutrophication of freshwater and marine ecosystems. Limnology and Oceanography 51: 351–355. https://doi.org/10.4319/lo.2006.51.1_part_2.0351.
Soininen, J. & K. Könönen, 2004. Comparative study of monitoring South-Finnish rivers and streams using macroinvertebrate and benthic diatom community structure. Aquatic Ecology 38: 63–75. https://doi.org/10.1023/B:AECO.0000021004.06965.bd.
Stone, W. W., R. J. Gilliom & K. R. Ryberg, 2014. Pesticides in US streams and rivers: occurrence and trends during 1992–2011. Environmental Science Technology 48(19): 11025–11030. https://doi.org/10.1021/es5025367.
Torrisi, M., S. Scuri, A. Dell’Uomo & M. Cocchioni, 2010. Comparative monitoring by means of diatoms, macroinvertebrates and chemical parameters of an Apennine watercourse of central Italy: The river Tenna. Ecological Indicators 10: 910–913. https://doi.org/10.1016/j.ecolind.2010.01.010.
Trimble, S. W., 1997. Contribution of stream channel erosion to sediment yield from an urbanizing watershed. Science American Association for the Advancement of Science 278: 1442–1444.
Vergara, W., 2007. The impacts of climate change in Latin America. Visualizing Future Climate in Latin America: Results from the application of the Earth Simulator, Latin America and Caribbean Region Sustainable Development. Working Paper 30: 1–18.
Villamarin, C., N. Prat, & M. Rieradevall, 2014. Caracterizacion fisica, quimica e hidromorfologica de los rios altoandinos tropicales de Ecuador y Peru. Latin American Journal of Aquatic Research 42: 1072–1086.
Vinueza, D., V. Ochoa-Herrera, L. Maurice, E. Tamayo, L. Mejía, E. Tejera & A. Machado, 2021. Determining the microbial and chemical contamination in Ecuador’s main rivers. Scientific Reports Nature Publishing Group 11: 1–14. https://doi.org/10.1038/s41598-021-96926-z.
Voss, K. A., Pohlman, A., Viswanathan, S., Gibson, D. & Purohit, J., 2012. A study of the effect of physical and chemical stressors on biological integrity within the San Diego hydrologic region. Environmental monitoring and assessment 184: 1603–1616. https://doi.org/10.1007/s10661-011-2064-0
Walteros, J. M. & A. pd. Ramírez, 2020. Urban streams in latin america: Current conditions and research needs. Revista de Biologia Tropical 68: S13–S28. https://doi.org/10.15517/rbt.v68is2.44330
Wang, L., D. M. Robertson, & P. J. Garrison, 2007. Linkages between nutrients and assemblages of macroinvertebrates and fish in wadeable streams: implication to nutrient criteria development. Environmental management Springer 39: 194–212.
Wetzel, C. E., E. A. Lobo, M. A. Oliveira, D. Bes, & G. Hermany, 2002. Diatomáceas epilíticas relacionadas a fatores ambientais em diferentes trechos dos rios Pardo e Pardinho, Bacia Hidrográfica do Rio Pardo, RS, Brasil: Resultados preliminares. Universidade de Santa Cruz do Sul.
Acknowledgements
We are grateful to Centro Jambatu for allowing us to sample in the lowest reach of the Pita River, as well as to Xavier Amigo and Nature Experience for their assistance in the field.
Funding
The authors have no relevant financial or non-financial interests to disclose. This study was performed under project numbers IBR.BIO.19.05 and AMB.BRT.22.01, financed by Universidad de Las Américas, Ecuador. The funders had no role in the present study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial or non-financial interest in the subject matter or materials discussed in this manuscript. The authors have no financial or proprietary interests in any material discussed in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Luiz Ubiratan Hepp, Frank Onderi Masese & Franco Teixeira de Mello / Stream Ecology and Environmental Gradients
Supplementary Information
Below is the link to the electronic supplementary material.
10750_2023_5276_MOESM3_ESM.xlsx
Appendix 3. Spearman’s Rank Correlation Matrix of Environmental Variables measured in all sites and both seasons. Supplementary file3 (XLSX 14 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Castillejo, P., Ortiz, S., Jijón, G. et al. Response of macroinvertebrate and epilithic diatom communities to pollution gradients in Ecuadorian Andean rivers. Hydrobiologia 851, 431–446 (2024). https://doi.org/10.1007/s10750-023-05276-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05276-6