Abstract
Climate change is affecting the global hydrological cycle, causing drastic changes in precipitation patterns. Extreme climatic events are becoming more frequent and intense than in the past, leading to water-level fluctuations and affecting aquatic ecosystems. Semiarid regions are very susceptible to changing climate. We analyzed a 10 years dataset from a tropical semiarid reservoir during extreme hydrological events (heavy rains and prolonged drought), and evaluated phytoplankton functional responses to environmental conditions. We found, as hypothesized, that phytoplankton functional structure change in a temporal scale due to water–volume fluctuation induced by the rainfall pattern. Depth and inorganic material acted as environmental filters selecting phytoplankton groups. High water level seems to improve water quality and low water level worsen it. Colonial and filamentous cyanobacteria dominate the wet period; however, it may have a critical threshold during severe periods of drought, which will lead to dominance of groups well adapted to low light conditions and with mixotrophic metabolism. Phytoplankton functional approaches can simplify phytoplankton identification and reflect better the environmental conditions than the taxonomic approach. Therefore, these approaches can help to understand the shifts in aquatic ecosystems under extreme hydrological events and predict functional response of phytoplankton being an important tool to water management and conservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eutrophication is considered worldwide as a threat to aquatic biodiversity and water quality (Paerl & Huisman, 2009; Jeppesen et al., 2010). It can be characterized as an enrichment of aquatic ecosystems by increasing nutrients concentrations, mainly phosphorous and nitrogen, resulting in an exaggerated growth of phytoplankton and significant changes in ecosystems functioning (Dodds et al., 2009). The potential effects of eutrophication are the dominance of phytoplankton species, such as potentially toxic cyanobacteria, reduction of species diversity, increasing organic matter decomposition, and anoxia (Smith & Schindler, 2009). These lead to a degradation of water quality (Paerl & Otten, 2013). Phosphorous and nitrogen load is a topic that received special emphasis in publications related to eutrophication and phytoplankton ecology (Paerl, 2009; Moss et al., 2011; Chorus & Spijkerman, 2021; Vanderley et al., 2022). Moreover, an extended literature shows that phytoplankton resources (i.e., nutrients and light) and grazers’ pressure are important drivers of phytoplankton community (Soares et al., 2009; Gerea et al., 2018; Chorus & Spijkerman, 2021). However, several other factors can be linked to structuring phytoplankton, such as the physical environment and hydrological processes (Zohary et al., 2010; Naselli-Flores et al., 2021).
Phytoplankton is a diverse group of organisms with a wide variety of shapes and sizes, adapted to survive, reproduce, and grow under varying hydrodynamic conditions (Reynolds, 2006). The wide range of phytoplankton morphology is an adaptation to optimize their survival in highly heterogeneous environments and is influenced by intrinsic and extrinsic factors (Naselli-Flores et al., 2021). Phenotypic plasticity, the ability to produce alternative forms of morphology, physiological state, and/or behavior in response to environmental conditions, is a potent and flexible response to stressful environments (West-eberhard, 1989; Üveges et al., 2012; Boyd et al., 2016). Therefore, there is a selection pressure for important traits (morphological and physiological) that contribute to species' fitness and their responses to environmental changes. Physical disturbances are important in phytoplankton development and trait selection, and global environmental changes affect the physical environment that shapes phytoplankton assemblages (Zohary et al., 2010).
The most common climate-mediated stress factors that affect phytoplankton include increasing temperature, changes in the precipitation regime and intensification of extreme weather events, such as droughts, floods, strong winds, and heat waves (Kasprzak et al., 2017; Salmaso & Tolotti, 2021). The climate of the tropical pacific is influenced by El Niño–Southern Oscillation (ENSO) cycle, which is a climate phenomenon characterized by unusual precipitation fluctuations, leading to heavy rainy phase (El Niño) or extreme droughts phase (La Niña) and affecting patterns of weather variability worldwide (Gerten & Adrian, 2002; McPhaden et al., 2006). The precipitation pattern directly influences the water-level fluctuations, and indirectly the phytoplankton resources (Li et al., 2018). Water-level fluctuation can improve or worsen water quality and can be used as a tool for water management (Naselli-Flores & Barone, 2005; Yang et al., 2016). Increase or decrease water bodies’ volume can alter stratification patterns, nutrient concentrations, light availability along with other consequences such as changes in biological variables (Coops et al., 2003; Meerhoff et al., 2022).
The predicted scenarios of climate change for arid and semiarid climate foresee even an intensification of prolonged droughts and irregular rainfall cycles (Marengo et al., 2017; IPCC, 2023). As a consequence, lakes and reservoirs in these regions tend to have high water-level fluctuations and changes in the resources for phytoplankton, such as nutrients and light (Brasil et al., 2016; Costa et al., 2019; Amorim & Moura, 2022). Some studies already demonstrated that these predictions are, in fact, occurring in tropical environments (Devercelli, 2010; Costa et al., 2019; Cortez et al., 2022). Water-level fluctuation contributes widely to changes in stratification patterns, temperature, and temporal dynamics of nutrients (Zohary & Ostrovsky, 2011; Li et al., 2018) impacting directly and indirectly the phytoplankton communities (Devercelli, 2010; Kosten et al., 2012; Li et al., 2018). To understand how phytoplankton will respond to alteration on the rainfall patterns induced by climate changes is a key factor in aquatic ecology.
Phytoplankton functional- and trait-based approaches can capture the phytoplankton response to all species’ specific adaptation and environmental variability (Salmaso et al., 2015). Thereby, it increases the predictability of phytoplankton responses by aggregating information on many species without losing their key responses and impacts (Kruk et al., 2021). Different functional approaches are dealing with these adaptive strategies under distinct conditions by classifying taxa from different lineages into groups according to their similarities, providing good explanation and predictability (Reynolds et al., 2002; Salmaso & Padisák, 2007; Kruk et al., 2010; Salmaso et al., 2015). However, there are some misuses of ecological classification, and there is a need of strengthen functional knowledge to respond ecological questions (Padisák et al., 2009; Flynn et al., 2013; Salmaso et al., 2015). Phytoplankton classifications include information about species morphology, physiology, and ecological features such as phenology, habitat, and environmental conditions from molecular to ecosystem level (Salmaso et al., 2015). Evaluating phytoplankton functional response through time contributes to our knowledge about ecosystem dynamics (Reynolds, 2006).
This study is targeting to understand how the process of extreme hydrological events takes place in a semiarid tropical aquatic ecosystem and which environmental filters will select dominant species according to their functional roles and their morphological and physiological traits. We took advantage of a long temporal dataset (ten years) of phytoplankton and limnological variables of a reservoir in tropical semiarid region during extreme precipitation events (heavy rains and severe drought period with a complete water depletion). We aim to test the effect of hydrological events and physical variables on the selection of phytoplankton functional response according to their life history traits and adaptive advantages. We expect that distinct rainfall events will structure temporally the phytoplankton functional groups: (i) extreme rainy period will select traits related to high light requirement and (ii) extreme dry period will select traits related to light limitation. Moreover, we aim to evaluate how the different phytoplankton approaches reflect the most important changes that occur in the community in association with the hydrological variability.
Material and methods
Study area
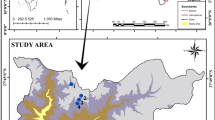
Gargalheiras Reservoir (06° 25′ 30′′ S/36° 36′ 09′′ W) was built in 1959 by the damming of the river Acauã, belonging to the watershed of the Piranhas-Açú River, located in the northeastern semiarid region of Brazil (Fig. 1). The reservoir has a maximum accumulation capacity of 44.4 million m3, a drainage area of 2120 km2 (ANA, 2020), and a maximum depth of 26.5 m (SEMARH, 2019). By Köppen's classification, the region's climate is tropical semiarid BS′h′—steppe type (Alvares et al., 2013). The rainy season runs from February to June; on average, the region receives 550 mm year−1 of rain (EMPARN, 2022). This region suffered a severe drought from 2012 to 2019, representing the worst and longest drought period in the last 60 years (Marengo et al., 2017; ANA, 2020). The reservoir, located in a rural area with little urbanization, is mostly used as water supply for humans (priority use) and animals, irrigation, fishing, and recreation. The diffuse pollution, resulting from agriculture and livestock seems to be the most relevant external source of plant nutrients. In addition, nutrient loading by intermittent rivers (i.e., flow only during the rainy season) is the source that maintains the high algal biomass during drought periods (Cavalcante et al., 2018).
Sampling and analysis
Water samples were collected quarterly from 2010 to 2021 at the deepest point of the reservoir near to the dam water intake (Fig. 1). In September 2018, the reservoir dried up completely, only returning to aquatic stage in the second half of 2019 (October). Due to the Covid-19 pandemic outbreak, samplings in 2020 were suspended in March according to biosecurity protocols by the State government and by the Federal University of Rio Grande do Norte, being continued only in February 2021.
Integrated water samples were taken from the epilimnion using a vertical Van Dorn bottle. Epilimnion was determined from vertical dissolved oxygen and temperature profiles. The profiles of temperature and dissolved oxygen (DO) were measured using a multiparametric probe (Hidrolab DS5) from 2010 to 2014, and with a portable oximeter (Instrutherm MO-900) from 2015 to 2021. The water transparency was measured by a Secchi disk. The bottles were previously washed with 10% hydrochloric acid (HCL) and deionized water, stored in thermoisolating boxes with ice during transport until carrying out the analyses. Phytoplankton samples were fixed with acidified Lugol’s solution for identification and counting.
In the laboratory, the turbidity (PoliControl AP2000), pH (BEL W3B pH meter) and the conductivity (Tec-4MP) were measured at 25 ± 1 °C. Total phosphorus (TP) was analyzed with the ascorbic acid method after oxidation with potassium persulfate (Valderrama, 1981). For inorganic nutrients (soluble reactive phosphorus (SRP) and nitrate), the water samples were filtered through fiberglass filters (Whatman GFC, ø = 47 mm; porosity of 1.2 μm). Subsequently, the analysis of SRP was carried out by using the ascorbic acid method (Murphy & Riley, 1962), as well as nitrate (NO3−), using sodium salicylate (Müller & Wiedemann 1955). Both analyses applied UV spectrophotometry. Filters were used for the analysis of suspended solids. Total and inorganic suspended solids (ISS) were determined by gravimetry after drying the filters overnight at 100 °C and the ignition of filters at 500 °C for 3 h (APHA, 2012). The organic suspended solids (OSS) were measured by the difference between total suspended solids and inorganic suspended solids (APHA, 2012).
Phytoplankton quantification according to Utermöhl (1958) was performed with inverted microscope Motic AE31 (× 400 magnification). Individuals (cells, colonies, filaments) were counted in random fields (Uehlinger, 1964), and at least 100 specimens of the most abundant species were counted (Lund et al., 1958). Biomass was estimated by biovolume calculation (mm3 l−1), using geometric approximations (Hillebrand et al., 1999), assuming the unit fresh weight, expressed as mass 1 mm3 l−1 = 1 mg l−1 (Wetzel & Likens, 2000), biovolume of the colonial taxa was estimated by their colony geometric forms approximations (Fonseca et al., 2014). Phytoplankton species were grouped into classic taxonomic approach (Adl et al., 2012; Komárek et al., 2014), morpho-functional groups (MFG) (Salmaso & Padisák, 2007), Reynolds functional groups (RFG) (Reynolds et al., 2002; Padisák et al., 2009; Kruk et al., 2017), and trait-based approaches (Litchman & Klausmeier, 2008) (Table 1). The traits selected in this study were based on phytoplankton size (i) microphytoplankton (> 20 μm) and (ii) nano-phytoplankton (2–20 μm), shape (i.e., filamentous, coloniality, round unicellular), movement (i.e., flagellates) and nutrition, i.e., if the species were capable of mixotrophy via engulfment of particles (phagotrophy) or not (autotrophy) (Table 1). Osmotrophy was neglected.
Data analysis
Data of rainfall, monthly, and historical data series were provided by the Agricultural Research Corporation of the state of Rio Grande do Norte (EMPARN, 2022). The water volumes stored in the reservoir throughout the study period were provided by the State Secretariat for the Environment and Water Resources of Rio Grande do Norte (SEMARH).
To understand the effect of temporal patterns on changes in the phytoplankton community, we used the k-means procedure to classify our data into distinct periods based on environmental variables. The time groups predicted by k-means were used to assess the period of change in the phytoplankton community and the phytoplankton classification method that best responds to environmental changes. Prior to all analyses, we tested for temporal autocorrelation using general least-squares regression, which assumes a first-order autocorrelation to account for time effects in the GLS residuals (Zuur et al., 2009). As we did not find temporal autocorrelation, we performed the procedures without an autocorrelation term. We also tested for collinearity using the variation inflation factor (assuming VIF < 10) (Kutner et al., 2004), and found collinearity only in the variables of maximum volume storage (%), Zmax, Secchi, Cond, pH, DO, TP, SRP, and ISS. All variables were standardized to unscale the measures' units.
K-means is a non-supervised analysis that searches for homogeneity and group samples using a similarity measure, such as Euclidian distance (Malik & Tuckfield, 2019). The k-means groups were used to compare the environmental variables and phytoplankton biomass changes among periods using the Wilcoxon-Mann–Whitney test. To access which environmental variables were responsible for the division of the groups and would affect the phytoplankton community, we used a logistic regression with the two groups proposed by k-means (0—wet, 1—dry) as our response variable and the environmental variables as predictors, transformed to z-score. We used the Model Averaging procedure to identify the variables that most effectively elucidate the temporal dynamics of phytoplankton groups. This method computes the mean slope values for each variable used in various model combinations, including maximum volume storage (%), Zmax, Secchi, Cond, pH, DO, TP, SRP, and ISS. However, we only considered variable combinations that produced models with delta AIC < 2 when calculating the mean. Models with delta AIC < 2 demonstrate strong evidence of fitting (Burnham & Anderson, 2002).
We also performed four canonical correspondence analyses (CCA) to examine the effect of environmental variables on the phytoplankton groups from each classification method (Tax, RFG, MFG, and Traits). We used environmental variables with VIF lower than 10, and all variables were transformed to z-score. The significance of the axes was tested using Monte Carlo simulation test (McCune & Mefford, 2011). The CCA was the best constrained ordination method according with the detrended correspondence analysis (DCA) (Ter Braak & Prentice, 1988). The scores of the first axes of the CCA of each CCA performed (Tax, RFG, MFG, and Traits) was used in a cluster analysis to determine which type of classification responds more similarly over time to environmental variations, thus, being an indication of similarity between classifications. To conduct this cluster analysis, we employed the Euclidean distance metric and the ward.D method for clustering. The Ward's method is a hierarchical clustering algorithm that groups similar observations into clusters using the minimum variance method. This robust method is often used in applications where the number of clusters is unknown in advance.
All analyses were performed in R software version 4.2.2 (R Development Core Team, 2022). We used the functions fviz_nbclust and kmeans from the factoextra and FactoMiner packages to perform the k-means procedure. The function glm for logistic regression, and wilcox.test for Wilcox-Mann–Whitney test. The function vif from car package, to verify collinearity, gls from nlme package. For CCA we used decorana, cca from vegan package, anova to verify the axis significance. For that we used the functions get_dist and hclust from factoextra and stats package, respectively.
Results
Environmental variables
The monthly and historical rainfall data and water volume of the reservoir recorded extreme hydrological events through the studied period (Fig. 2). In 2011, a heavy rainy season (above the historical average) filled the reservoir reaching 100% of maximum water volume capacity and the reservoir overflowed in May 2011 (Fig. 2). After 2012, a severe drought period was observed (rainfall below annual historical average) until 2019. This severe drought period decreased the water volume accumulation of the reservoir, resulting a complete emptying in 2018 (Fig. 2). The reservoir remained dry for up to 14 consecutive months. After that, with rainfall above historical average recorded (Fig. 2), the water volume of the reservoir increased in 2020/2021 (Fig. 2).
The k-means analysis identified three periods, (i) wet period (from June 2010 to September 2012), (ii) dry period (from March 2015 to February 2020) and (iii) transition periods (October 2013 to February 2015 and from November 2019 to January 2020; Fig. 3). Thus, the maximum water volume and maximum depth fluctuated throughout the study, following the identified periods, with an extreme decrease during the dry period (Fig. 3A, B).
Dynamics of environmental variables. Zmax maximum depth, DO dissolved oxygen, TP total phosphorous, SRP soluble reactive phosphorous, ISS inorganic suspended solids, OSS organic suspended solids. White areas represent the wet period, the transition period in light gray areas, and dry period in dark gray area
Water transparency decreased during dry period and increased in 2021 when water volume increased (Fig. 3C) and we observed an opposite pattern for the physical and chemical variables such as turbidity, conductivity, dissolved oxygen and pH (Fig. 3D–G). Nutrient concentrations (TP, SRP and nitrate) and inorganic and organic solids increased during dry period and decreased during wet periods (Fig. 3H–L).
Significant differences were detected in all environmental variables analyzed between dry and wet periods in the study (Fig. 4). Wet period showed higher values of water volume, maximum depth, water transparency, DO and pH; on the other hand, dry period showed higher values of conductivity, TP, SRP, organic and inorganic solids (OSS and ISS) (Fig. 4).
Box plots of some of the environmental variables - Water volume (%), Zmax = maximum depth, water transparency, conductivity, dissolved oxygen, pH, total phosphorous, soluble reactive phosphorous (SRP), inorganic (ISS) and organic solids (OSS) between dry (red) and wet (blue) periods. The box plot shows the median, minimum, maximum, first, and third quartiles. The p-value of the t-test are shown for each variable. Significant p-value < 0.005
Phytoplankton assemblages
A total of 65 taxa were identified in the reservoir through the studied period. However, only 22 species were considered as main descriptors of the phytoplankton community (i.e., representing at least five percent of each taxa in relation to total phytoplankton biomass). We identified six taxonomic groups, eleven RFGs, 9 MFGs and 8 traits (Fig. 5).
Phytoplankton taxonomic (A) and MFG (B) and RGF (C) functional groups, phytoplankton nutrition (D), shape (E) and size (F) dynamics. White areas represent the wet period, the transition period in light gray areas, and dry period in dark gray area. Those periods were identified by the k-means method of classification using the environmental variables dynamics
Phytoplankton biomass varied throughout the investigated period (Fig. 5). According to the taxonomic approach, Cyanobacteria dominated the wet period, Euglenophyta and Cryptophyta co-dominated during the dry period (Fig. 5A). The MFG approach (Fig. 5B) distinguished the cyanobacteria in the wet period with dominance of the groups of Nostocales (5e-Nostocales), represented mainly by Raphidiopsis raciborskii (Woloszynska) Aguilera et al. a high biomass of large vacuolated Chroococcales group (5b-LargeVacC), represented mainly by Microcystis panniformis (Komárek) Komárek, Komárková-Legnerová, Sant'Anna, M. T. P. Azevedo, & P. A. C. Senna and Microcystis protocystis Crow, before the reservoir reached its maximum capacity. When the reservoir was completely filled and overflowed, the thin filaments of Oscillatoriales (5a-FilaCyano) group, represented mainly by Planktolyngbya limnetica (Lemmermann) Komárková-Legnerová & Cronberg, reached a peak of biomass followed by 5e-Nostocales for the rest of wet and the transition period. However, the prolonged drought period was co-dominated by group of large euglenophytes (1c-LargeEugl), represented by species of Euglena spp., group of small euglenophytes (2c-SmallEugl), represented by Trachemolomas spp., and group of cryptophytes (2d-Crypto), mainly represented by Cryptomonas marssonii Skuja, C. brasiliensis A. Castro, C. E. M. Bicudo & D. Bicudo and C. ovata Ehrenberg.
The RFG (Fig. 5C) exhibited the higher number of functional groups. The cyanobacteria from the wet and transition period were distinguished in four groups, a dominance of the group SN (Raphidiopsis raciborskii) during the whole period, the group from colonial species M (Microcystis spp.) before the overflow, a co-dominance of the nitrogen fixing group H1, represented by Aphanizomenon gracile Lemmermann and Cuspidothrix sp., after the overflow and the thin filamentous groups S1, represented by Planktolyngbya limnetica and SN again, in the end of the studied period. With this approach it was possible to distinguish one more group (H1) reflecting an important functional role, which was not possible to reveal with MFG approach. During the dry period, RFG displayed a dominance of the W1 and W2, representing groups of high organic demand and bottom-dwelling groups, respectively. Meanwhile, in MFG these groups were separated morphologically by size (small and large euglenophytes). Moreover, during dry period RFG separated cryptophytes in Y and X2 groups, functionally representing phagotrophy characteristic for low light conditions and species from shallow mixed layers, respectively. In September 2017, both MFG and RFG traced a peak of unicellular cyanobacteria (4-UnicCyano) and L0 respectively, both represented by Synechocystis aquatilis Sauvageau. After the reflooding of the reservoir due to high accumulated rainfall in the middle of 2019, the mixotrophic and bottom-dwelling groups were replaced by the N-fixing H1 and the high light demanding M representatives of RFG, or the large vacuolated Microcystis (5b-LargeVacC) and the Nostocales (5e-Nostocales), representatives of MFG.
According to phytoplankton morphological and physiological traits we observed a change from colonial species to filamentous species during the wet period. Later, phago-mixotrophic and flagellated species dominated the dry period with a peak of spherical unicellular species, mainly represented by picocyanobacteria in September of 2017 (Fig. 5D). The second wet period was co-dominated by colonial and filamentous species (Fig. 5E). According to the size of the species, we observed a predominance of microphytoplankton in the wet period and nano-phytoplankton in the dry period, mainly represented by nanoflagellates (Fig. 5F).
The results of the ordination analysis (CCA) demonstrated temporal differences for all the four approaches, separating dry and wet periods (Fig. 6). The highest value of R-square was observed when we analyzed phytoplankton traits and environmental variables (R2 = 0.54); taxonomic, MFB and RFG showed similar R-square, 0.36, 0.37 and 0.35 respectively. In all CCA only the first axis was significant (p = 0.001). Maximum depth was the environmental variable that influenced most phytoplankton assemblages, followed by TP and ISS (Fig. 6). The results of the CCA for taxonomic groups showed that the wet period was related to Zmax, water transparency (Secchi), DO and pH and the dry period was related to conductivity, ISS TP, SRP and associated with euglenophytes and cryptophytes (Fig. 6A, suppl. material Table S1). For MFG, Zmax was also negatively related with the small euglenophytes (X2SmallEugle, r = − 0.55) and cryptophytes (X2dCrypto, r = − 0.58). We observed a similar pattern of taxonomic groups for environmental variables between wet and dry periods for MFG (Fig. 6B, suppl. material Table S2). According to the results of the CCA for RFG, we observe the groups W2, X2, Lo, W1 and Y associated to the environmental variables related to the dry period (i.e., TP, SRP, conductivity, and ISS), X2 and Y were negatively related to Zmax (Fig. 6C, suppl. material Table S3). The results of the CCA analyzing phytoplankton traits showed that Zmax was negatively related to nano-flagellates and mixo-phagotrophic species during the dry period. Microphytoplankton with filamentous and colonial shapes were associated to the wet period and positively correlated to Secchi, DO, pH and Zmax (Fig. 6D, suppl. material Table S4).
Models using cluster groups from k-means as response variable and environmental factors as predictor variable selected Zmax and ISS (AIC 19.57) as the best model to explain changes in phytoplankton through time (suppl. material Table S5) and the results of logistic regression using cluster groups as response variable and environmental factors as predictor variable from the main model selection according to AIC shows that Zmax was significant different for the best models (suppl. material Table S6). Significant differences of each phytoplankton functional group for each classification (taxonomic groups (suppl. material Fig. 1), MFG (suppl. material Fig. S2), RFG (suppl. material Fig. S3) and Traits (suppl. material Fig. S4) between the wet and dry periods are shown in the online resource (supplementary material).
The comparison of phytoplankton groups with each classification in relation to environmental variables demonstrated that taxonomic approach has marked dissimilarities with morphological and functional approaches (Fig. 7). The approaches MFG and RFG share more similarities and are more connected between themselves, and trait-based approach is closely related to morpho-functional groups (Fig. 7).
Discussion
Climatic fluctuation has effects on physical environment shaping phytoplankton community (Zohary et al., 2010). This study evaluated ten years of data of a reservoir in a tropical semiarid region during well-separated rainfall events. The region experienced rainfalls above the historical average in the rainy season and then a long period of severe drought reflecting in water volume and in the environmental conditions with consequences in biological communities. We took this opportunity to evaluate the phytoplankton ecological response related to extreme rainfall events, using different functional approaches. We found that, as hypothesized, phytoplankton community and functional structure change in a temporal-scale due to water volume fluctuation induced by the rainfall patterns. Understanding the phytoplankton functional response to changes in environmental variables due to extreme climate conditions will help make predictions about the future of aquatic ecosystems, mainly in semiarid regions, and improve the knowledge for water management (Salmaso et al., 2015).
Extreme hydrological conditions causing heavy rains and severe drought, such as Southern Oscillation (ENSO), i.e., El Niño and La Niña, were already recorded impacting phytoplankton dynamics. Studies reveled changes in phytoplankton structure, reduction of diversity, and biomass, mainly in El Niño events (i.e., in the heavy rainy period) (Devercelli, 2010; Sathicq et al., 2015; Tilahun & Kifle, 2019; de Oliveira et al., 2020). The hydrological pattern for the studied region was analyzed using the historical average (n = 60 years) and k-means analysis, which showed well-separated periods based on the accumulated rainfall per year. These periods had consequences for the reservoir's water volume and Zmax, which significantly altered the physical and chemical properties of the water. Short transition periods between the wet and dry seasons were also identified, during which the environmental conditions were changing. During the wet period, the high volume and maximum depth were recorded in the reservoir. This led to low values of turbidity, organic/inorganic substances, and consequently more light availability and nutrient concentration diluted in the water (Barbosa et al., 2012; Medeiros et al., 2015). On the other hand, with the reduction of accumulated rainfall in the subsequent years of the study, water volume and Zmax were gradually declining due to the prolonged drought. During dry periods, it intensified eutrophication symptoms: increased nutrient concentration, organic and inorganic turbidity, and conductivity and decreased water transparency and dissolved oxygen concentration (Costa et al., 2016; Vanderley et al., 2022). However, the drought in Brazilian semiarid region from 2012 to 2015 was considered unprecedented in the last six decades and affected water supply for the region (Marengo et al., 2017). The drought was so intense and severe that Gargalheiras’ reservoir, one of the largest reservoirs in the region, with the capacity to supply several cities in its surroundings completely dried out. In this way, we observe the reservoir going from its maximum volume capacity to complete water depletion within 5 years.
Water depletion frequently occurs during drought periods, and some studies show improvement of water quality after reflooding either by changes in the trophic structure (e.g., fish biomass reduction, vegetation cover; Teferi et al., 2014) or by dilutuion of chemical and biological variables of the water due to a bigger volume, thus, improving the trophic status (Leite & Becker, 2019). However, other studies have shown that reflooding may worsen eutrophication depending on the external nutrient load from the hydrographic basin into the reservoirs (Cortez et al., 2022). The rainfall recorded in 2019, recovered the reservoir volume, and improved the water quality status, despite the reservoir volume continued to be below 50% of its storage capacity, leading to changes in water properties and impacting biological communities, including phytoplankton which have a short generation time and respond quickly to environmental conditions (Reynolds, 1993).
Total phytoplankton biomass per each taxonomic and functional approaches followed the shifts in volume and depth and its consequences to water physical and chemical properties. Taxonomic identification of phytoplankton assemblages reflected well these changes, showing the Cyanobacteria dominance in wet period and the dry period co-dominated by euglenophytes and cryptophytes. Several studies have demonstrated that Cyanobacteria are often dominant under various environmental conditions (Kosten et al., 2012; Soares et al., 2013), likely due to their adaptive strategies, such as the presence of aerotopes (Reynolds et al., 1987), variability in size and shape (Naselli-Flores & Barone, 2011), potential cyanotoxin production (Princiotta et al., 2019), inedibility, poor nutritional quality, and P-storage capacity (Pettersson et al., 1993, Istvánovics et al., 2000; DeMott et al., 2001; Gliwicz & Lampert, 2017). However, taxonomic approach failed to characterize the chief environmental drivers in detail, potentially because taxa were grouped into phyla. This can be noticed observing phytoplankton taxonomic assemblages grouped in the center of the CCA ordination and the shape of the narrow ellipses representing the seasonality. On the other hand, functional approaches (i.e., MFG, RFG, and traits) responded similarly to shifts in environmental conditions and demonstrated a higher level of complexity reflecting their functional role in the aquatic ecosystems.
Morphological and physiological traits, such as size, shape, and nutritional strategy, were evaluated in the study. Cell size, shape, and coloniality are important to grazing susceptibility and resource acquisition (Naselli-Flores et al., 2007; Litchman & Klausmeier, 2008; Lürling, 2020). The wet period was characterized by high volume and stratified water column providing an ideal condition to the colonial and vacuolated species like Microcystis as these species are capable to maintain their populations in the upper epilimnion. The gas vesicles of Microcystis consist in an eco-physiological trait allowing them to regulate their buoyancy in stable water column (Walsby, 1991) and shading the water layers below, thus, gaining competitive advantage over other phytoplankton species (Reynolds & Walsby, 1975; Humphries & Lyne, 1988). However, with the continuous inflow of water from the rainfall, the reservoir completely filled up and overflowed in 2011 causing mixing and instability of the water column and flushing the vacuolated colonial species, Microcystis spp. as observed in other studies (Padisák et al., 1999). After the flushing, the mixed well-illuminated surface layers thrived the filamentous cyanobacteria, mainly represented by N-fixing cyanobacteria Aphanizomenon gracile and Raphidiopsis raciborskii. The physiological traits related to buoyancy regulation and light acquisition on mixed layers are well reported to favor these cyanobacteria dominance in tropical and subtropical lakes (Soares et al., 2013; Izaguirre et al., 2015, O’Farrell et al., 2019).
Regarding to cell size, we may infer that the microphytoplankton, for being bigger, leads them to have a competitive advantage to avoid predators (Ross & Munawar, 1981). On the other hand, the nano-phytoplankton, in general, are more easily predated (Ross & Munawar, 1981). This may explain the increased biomass of microphytoplankton during the study. However, several other environmental factors can explain which traits should be selected by environmental filters, such as the organisms’ nutritional strategy (Pomeroy, 2001; McKie-Krisberg et al., 2015).
Nutritional strategy is an important functional trait of phytoplankton and can be divided into autotrophy and mixotrophy. The mixotrophy is the capability of realizing autotrophy (i.e., via photosynthesis) and heterotrophy (via osmotrophy or phagotrophy) in the same organism (Sanders, 1991). Therefore, the mixotrophic organism can act both as producer and consumer, impacting biogeochemical cycles and ecosystem metabolism (Mitra et al., 2014). Because osmotrophy is widely distributed and common in almost all lineages of phytoplankton, ecologists are advising to refer to mixoplankton (i.e., mixotrophic organisms) as planktonic protists that have the potential to express phototrophy and phagotrophy (i.e., engulfment of particles) in the same organism (Flynn et al., 2019). The phagotrophic organisms, mainly represented by cryptophyte lineages appearing during the dry period, reflect the influence of the severe prolonged drought on water volume and available resources. Mixotrophy provides an adaptive advantage in environments with limiting conditions, mainly nutrient and light (Rothhaupt, 1996); in this case the low light availability due to critical depth, high inorganic material, and sediment resuspension, which provided an extreme condition even to cyanobacteria but conferred an ideal condition for mixotrophic organisms that in light-limiting condition changed their nutritional strategy to phagotrophy. Laboratory experiments with cryptophyte lineages, such as in our study, showed that mixotrophs rely on phagotrophy to supplement growth in low light conditions and high nutrient concentration associated with prey availability (Costa et al., 2022). Moreover, in situ studies in semiarid regions are showing an increase in the proportion of mixotrophic species in ecosystems with critical low depth and high inorganic material caused by the severe drought (Medeiros et al.; 2015; Costa et al., 2016, 2019). Therefore, the light limitation and the high nutrient concentration of the reservoir associated most probably with high bacterial density conferred the ideal environment for the mixotrophic groups.
Besides the phagotrophic behavior as an advantageous strategy to cope with low light conditions, cryptophytes are able for chromatic acclimatation, a phenotypic response trait to light absorption changes (Wehr & Sheath, 2003; Richardson, 2022). This could be one more factor allowing the cryptophytes to thrive during the dry season when turbidity was higher. The bottom-dwelling, flagellates, with osmotrophic nutritional strategy represented by Euglena spp., Trachelomonas spp., Phacus spp., accounted for a significant proportion in biomass due to their adaptive advantages under low light conditions and high dissolved organic material (Reynolds, 2006), especially in the dry period.
The comparison of the classification approaches allows us to identify common features of phytoplankton role in aquatic ecosystems (Salmaso et al., 2015). The use of classic taxonomic approach seems more distant from morphological and functional approaches. Thus, the MFG and RFG share more common similarities with each other and some similarities with trait-based approach. Moreover, using functional classifications can simplify the process of identification, however, in some approaches as RFG there is the need of familiarity with a high level of taxonomic knowledge (Kruk et al., 2021). In this case, using MFG or trait-based approaches proved to be appropriate with accurate information. To identify common similarities between phytoplankton taxa provide us knowledge to generalize and predict shifts in phytoplankton ecology and how this may affect ecosystems functioning (Salmaso et al., 2015), especially under a climate changing world. Usefulness of the functional approach for detecting environmental changes has been supported by emergence and application of functional group, guild or trait-based indices worldwide (Padisák et al., 2006; Borics et al., 2007; Stenger-Kovács et al., 2007; Wang et al., 2018; Soria et al., 2019).
In conclusion, hydrological variability such as extreme rainfall events can affect directly the water-level fluctuation of freshwater ecosystems altering physical and chemical properties of the water and indirectly the phytoplankton dynamics. High water level seems to improve water quality and low water level worsen it, based on phytoplankton biomass and species composition. There is a critical water-level threshold during severe drought periods which leads to dominance of groups well adapted to low light conditions and with mixotrophic metabolism. The environmental factors responsible for these scenarios are depth and inorganic turbidity, probably due to light depletion. Morphological and functional approaches can simplify phytoplankton identification and reflects the environmental conditions. Studies of phytoplankton ecology using functional approaches may help to understand the shifts in aquatic ecosystems under increasingly frequent extreme hydrological events and predict functional response of phytoplankton, being an important tool to water management and conservation.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adl, S. M., A. G. B. Simpson, C. E. Lane, J. Lukeš, D. Bass, S. S. Bowser, M. W. Brown, F. Burki, M. Dunthorn, V. Hampl, A. Heiss, M. Hoppenrath, E. Lara, L. Le Gall, D. H. Lynn, H. McManus, E. A. D. Mitchell, S. E. Mozley-Stanridge, L. W. Parfrey, J. Pawlowski, S. Rueckert, L. Shadwick, C. L. Schoch, A. Smirnov & F. W. Spiegel, 2012. The revised classification of eukaryotes. Journal of Eukaryotic Microbiology 59: 429–514. https://doi.org/10.1111/j.1550-7408.2012.00644.x.
Alvares, C. A., J. L. Stape, P. C. Sentelhas, J. L. M. Gonçalves & G. Sparovek, 2013. Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift 22: 711–721. https://doi.org/10.1127/0941-2948/2013/0507.
Amorim, C. A. & A. N. D. Moura, 2022. Habitat templates of phytoplankton functional groups in tropical reservoirs as a tool to understand environmental changes. Hydrobiologia 849: 1095–1113. https://doi.org/10.1007/s10750-021-04750-3.
ANA (Agência Nacional de Águas) 2020. Reservoir Monitoring System—SAR—Sistema de Acompanhamento de Reservatórios. https://www.ana.gov.br/sar/
APHA, 2012. Standard Methods for the Examination of Water and Waste Water, American Public Health Association:, 874.
Barbosa, J. E. D. L., E. S. F. Medeiros, J. Brasil, R. D. S. Cordeiro, M. C. B. Crispim & G. H. G. Da Silva, 2012. Aquatic systems in semi-arid Brazil: limnology and management. Acta Limnologica Brasiliensia 24: 103–118. https://doi.org/10.1590/S2179-975X2012005000030.
Borics, G., G. Várbíró, I. Grigorszky, E. Krasznai, S. Szabó & K. T. Kiss, 2007. A new evaluation technique of potamo-plankton for the assessment of the ecological status of rivers. Large Rivers 6: 466–486. https://doi.org/10.1127/lr/17/2007/466.
Boyd, P. W., C. E. Cornwall & A. Davison, 2016. Biological responses to environmental heterogeneity under future ocean conditions. Global Change Biology 22: 2633–2650. https://doi.org/10.1111/gcb.13287.
Brasil, J., J. L. Attayde, F. R. Vasconcelos, D. D. F. Dantas & V. L. M. Huszar, 2016. Drought-induced water-level reduction favors cyanobacteria blooms in tropical shallow lakes. Hydrobiologia 770: 145–164. https://doi.org/10.1007/s10750-015-2578-5.
Burnham, K. P. & D. R. Anderson, 2002. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed. Springer-Verlag, New York:
Cavalcante, H., F. Araújo & V. Becker, 2018. Phosphorus dynamics in the water of tropical semiarid reservoirs in a prolonged drought period. Acta Limnologica Brasiliensia 30: e105. https://doi.org/10.1590/S2179-975X1617.
Chorus, I. & E. Spijkerman, 2021. What Colin Reynolds could tell us about nutrient limitation, N:P ratios and eutrophication control. Hydrobiologia 848: 95–111. https://doi.org/10.1007/s10750-020-04377-w.
Coops, H., M. Beklioglu & T. L. Crisman, 2003. The role of water-level fluctuations in shallow lake ecosystems – workshop conclusions. Hydrobiologia 506–509: 23–27. https://doi.org/10.1023/B:HYDR.0000008595.14393.77.
Cortez, F., F. Monicelli, H. Cavalcante & V. Becker, 2022. Effects of prolonged drought on water quality after drying of a semiarid tropical reservoir Brazil. Limnologica 93: 125959. https://doi.org/10.1016/j.limno.2022.125959.
Costa, M. R. A., J. L. Attayde & V. Becker, 2016. Effects of water level reduction on the dynamics of phytoplankton functional groups in tropical semi-arid shallow lakes. Hydrobiologia 778: 75–89. https://doi.org/10.1007/s10750-015-2593-6.
Costa, M. R. A., R. F. Menezes, H. Sarmento, J. L. Attayde, L. S. L. Sternberg & V. Becker, 2019. Extreme drought favors potential mixotrophic organisms in tropical semi-arid reservoirs. Hydrobiologia 2: 43–54. https://doi.org/10.1007/s10750-018-3583-2.
Costa, M. R. A., H. Sarmento, V. Becker, I. Bagatini & F. Unrein, 2022. Phytoplankton phagotrophy across nutrients and light gradients using different measurement techniques. Journal of Plankton Research 44: 508–521. https://doi.org/10.1093/plankt/fbac035.
de Leite, J. N. C. & V. Becker, 2019. Impacts of drying and reflooding on water quality of a tropical semi-arid reservoir during an extended drought event. Acta Limnologica Brasiliensia 31: e15. https://doi.org/10.1590/S2179-975X6918.
de Oliveira, F. H., A. N. Moura & Ê. W. Dantas, 2020. Morphofunctional changes to the phytoplankton community in tropical ecosystems during the El Niño event of 2015–16. Marine and Freshwater Research 70: 1576–1584. https://doi.org/10.1071/MF19080.
DeMott, W. R., R. D. Gulati & E. Van Donk, 2001. Daphnia food limitation in three hypereutrophic Dutch lakes: Evidence for exclusion of large-bodied species by interfering filaments of cyanobacteria. Limnology and Oceanography 46: 2054–2060. https://doi.org/10.4319/lo.2001.46.8.2054.
Devercelli, M., 2010. Changes in phytoplankton morpho-functional groups induced by extreme hydroclimatic events in the Middle Paraná river (Argentina). Hydrobiologia 639: 5–19. https://doi.org/10.1007/s10750-009-0020-6.
Dodds, W. K., W. W. Bouska, J. L. Eitzmann, T. J. Pilger, K. L. Pitts, A. J. Riley, J. T. Schloesser & J. D. Thornbrugh, 2009. Eutrophication of U.S. freshwaters: analysis of potential economic damages. Environmental Science and Technology 4: 12–19. https://doi.org/10.1021/es801217q.
EMPARN - Empresa de Pesquisa Agropecuária do Rio Grande do Norte, 2022. https://meteorologia.emparn.rn.gov.br/
Flynn, K. J., D. K. Stoecker, A. Mitra, J. A. Raven, P. M. Glibert, P. J. Hansen, E. Granéli & J. M. Burkholder, 2013. Misuse of the phytoplankton-zooplankton dichotomy: The need to assign organisms as mixotrophs within plankton functional types. Journal of Plankton Research 35: 3–11. https://doi.org/10.1093/plankt/fbs062.
Flynn, K. J., A. Mitra, K. Anestis, A. A. Anschütz, A. Calbet, G. D. Ferreira, N. Gypens, P. J. Hansen, U. John, J. L. Martin, J. S. Mansour, M. Maselli, N. Medić, A. Norlin, F. Not, P. Pitta, F. Romano, E. Saiz, L. K. Schneider, W. Stolte & C. Traboni, 2019. Mixotrophic protists and a new paradigm for marine ecology: Where does plankton research go now? Journal of Plankton Research 41: 375–391. https://doi.org/10.1093/plankt/fbz026.
Fonseca, B. M., C. Ferragut, A. Tucci, L. O. Crossetti, F. Ferrari, D. D. Bicudo, C. L. Sant’Anna & C. E. Bicudo, 2014. Biovolume de cianobactérias e algas de reservatórios tropicais do Brasil com diferentes estados tróficos. Hoehnea 41: 9–30. https://doi.org/10.1590/S2236-89062014000100002.
Gerea, M., C. Queimalin & F. Unrein, 2018. Grazing impact and prey selectivity of picoplanktonic cells by mixotrophic flagellates in oligotrophic lakes. Hydrobiologia 831: 5–21. https://doi.org/10.1007/s10750-018-3610-3.
Gerten, D. & R. Adrian, 2002. Effects of climate warming, North Atlantic Oscillation, and El Niño-Southern Oscillation on thermal conditions and plankton dynamics in Northern Hemispheric lakes. The Scientific World Journal 2: 586–606. https://doi.org/10.1100/tsw.2002.141.
Gliwicz, Z. M. & W. Lampert, 2017. Food thresholds in Daphnia species in the absence and presence of blue-green filaments. Ecology 71: 691–702. https://doi.org/10.2307/1940323.
Hillebrand, H., C. D. Dürselen, D. Kirschtel, U. Pollingher & T. Zohary, 1999. Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology 35: 403–424. https://doi.org/10.1046/j.1529-8817.1999.3520403.x.
Humphries, S. E. & V. D. Lyne, 1988. Cyanophyte blooms: The role of cell buoyancy. Limnology and Oceanography 33: 79–91. https://doi.org/10.4319/lo.1988.33.1.0079.
IPCC, 2023. Climate Change 2023: Synthesis Report Summary Chapter for Policymakers. IPCC AR6 SYR. https://report.ipcc.ch/ar6syr/pdf/IPCC_AR6_SYR_SPM.pdf
Isvánovics, V., H. M. Shafik, M. Présing & S. Juhos, 2000. Growth and phosphate uptake kinetics of the cyanobacterium, Cylindrospermopsis raciborskii (Cyanophyceae) in throughflow cultures. Freshwater Biology 43: 257–275. https://doi.org/10.1046/j.1365-2427.2000.00549.x.
Izaguirre, I., M. L. Sánchez, M. R. Schiaffino, I. O’Farrell, M. P. Huber, N. Ferrer, J. Zunino, L. Lagomarsino & M. Mancini, 2015. Which environmental factors trigger the dominance of phytoplankton species across a moisture gradient of shallow lakes? Hydrobiologia 752: 47–64. https://doi.org/10.1007/s10750-014-2007-1.
Jeppesen, E., M. Meerhoff, K. Holmgren, I. González-Bergonzoni, F. Teixeira-de Mello, S. A. J. Declerck, L. De Meester, M. Søndergaard, T. L. Lauridsen, R. Bjerring, J. M. Conde-Porcuna, N. Mazzeo, C. Iglesias, M. Reizenstein, H. J. Malmquist, Z. Liu, D. Balayla & X. Lazzaro, 2010. Impacts of climate warming on lake fish community structure and potential effects on ecosystem function. Hydrobiologia 646: 73–90. https://doi.org/10.1007/s10750-010-0171-5.
Kasprzak, P., T. Shatwell, M. O. Gessner, T. Gonsiorczyk, G. Krillin, G. B. Selmeczy, J. Padisák & C. Engelhardt, 2017. Extreme weather event triggers cascade towards extreme turbidity in a clear-water lake. Ecosystems 20: 1407–1420. https://doi.org/10.1007/s10021-017-0121-4.
Komárek, J., J. Kaštovský, J. Mareš & J. R. Johansen, 2014. Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) 2014, using a polyphasic approach. Preslia 86: 295–335.
Kosten, S., V. L. M. Huszar, E. Bécares, L. S. Costa, E. van Donk, L. A. Hansson, E. Jeppesen, C. Kruk, G. Lacerot, N. Mazzeo, L. D. Meester, B. Moss, M. Lürling, T. Nõges, S. Romo & M. Scheffer, 2012. Warmer climates boost cyanobacterial dominance in shallow lakes. Global Change Biology 18: 118–126. https://doi.org/10.1111/j.1365-2486.2011.02488.x.
Kruk, C., V. L. M. Huszar, E. T. H. M. Peeters, S. Bonilla, L. Costa, M. Lürling, C. S. Reynolds & M. Scheffer, 2010. A morphological classification capturing functional variation in phytoplankton. Freshwater Biology 55: 614–627. https://doi.org/10.1111/j.1365-2427.2009.02298.x.
Kruk, C., M. Devercelli, V. L. M. Huszar, E. Hernández, G. Beamud, M. Diaz, L. H. S. Silva & A. M. Segura, 2017. Classification of Reynolds phytoplankton functional groups using individual traits and machine learning techniques. Freshwater Biology 62: 1681–1692. https://doi.org/10.1111/fwb.12968.
Kruk, C., M. Devercelli & V. L. Huszar, 2021. Reynolds functional groups: a trait-based pathway from patterns to predictions. Hydrobiologia 848: 113–129. https://doi.org/10.1007/s10750-020-04340-9.
Kutner, M. H., C. J. Nachtsheim & J. Neter, 2004. Applied Linear Regression Models, 4th ed. McGraw-Hill Irwin:
Li, Q., J. Xiao, T. Ou, M. Han, J. Wang, J. Chen, Y. Li & N. Salmaso, 2018. Impact of water level fluctuations on the development of phytoplankton in a large subtropical reservoir: implications for the management of cyanobacteria. Environmental Science and Pollution Research 25: 1306–1318. https://doi.org/10.1007/s11356-017-0502-4.
Litchman, E. & C. A. Klausmeier, 2008. Trait-based community ecology of phytoplankton. Annual Review of Ecology, Evolution, and Systematics 39: 615–639. https://doi.org/10.1146/annurev.ecolsys.39.110707.173549.
Lund, J. W. G., C. Kipling & E. D. Le Cren, 1958. The inverted microscope method of estimating algal num-ber and the statistical basis of estimating by counting. Hydrobiologia 11: 143–170. https://doi.org/10.1007/BF00007865.
Lürling, M., 2020. Grazing resistance of phytoplankton. Hydrobiologia 848: 237–249. https://doi.org/10.1007/s10750-020-04370-3.
Malik, A. & B. Tuckfield, 2019. Applied Unsupervised Learning with R: Uncover Hidden relationships and patterns with k-means clustering, hierarchical clustering, and PCA, Packt Publishing, Birmingham:
Marengo, J. A., R. R. Torres & L. M. Alves, 2017. Drought in Northeast Brazil—past, present, and future. Theoretical and Applied Climatology Theoretical and Applied Climatology 129: 1189–1200. https://doi.org/10.1007/s00704-016-1840-8.
Mc Cune, B. & M. J. Mefford, 2011. PC-ORD. Multivariate Analysis of Ecological Data. Version 6.0, MjM Software Design, Oregon:
McKie-Krisberg, Z. M., R. J. Gast & R. W. Sanders, 2015. Physiological responses of three species of Antarctic mixotrophic phytoflagellates to changes in light and dissolved nutrients. Microbial Ecology 70: 21–29. https://doi.org/10.1007/s00248-014-0543-x.
McPhaden, M. J., S. E. Zebiak & M. H. Glantz, 2006. ENSO as an integrating concept in earth science. Science 314: 1740–1745. https://doi.org/10.1126/science.1132588.
Medeiros, L., A. Mattos, M. Lürling & V. Becker, 2015. Is the future blue-green or brown? The effects of extreme events on phytoplankton dynamics in a semi-arid man-made lake. Aquatic Ecology 49: 293–307. https://doi.org/10.1007/s10452-015-9524-5.
Meerhoff, M., J. Audet, T. A. Davidson, L. De Meester, S. Hilt, S. Kosten, Z. Liu, N. Mazzeo, H. Paerl, M. Scheffer & E. Jeppesen, 2022. Feedback between climate change and eutrophication: revisiting the allied attack concept and how to strike back. Inland Waters 12: 187–204. https://doi.org/10.1080/20442041.2022.2029317.
Mitra, A., K. J. Flynn, J. M. Burkholder, T. Berge, A. Calbet, J. A. Raven, E. Granéli, P. M. Glibert, P. J. Hansen, D. K. Stoecker, F. Thingstad, U. Tillmann, S. Väge, S. Wilken & M. V. Zubkov, 2014. The role of mixotrophic protists in the biological carbon pump. Biogeosciences 11: 995–1005. https://doi.org/10.5194/bg-11-995-2014.
Moss, B., S. Kosten, M. Meerhoff, R. W. Battarbee, E. Jeppesen, N. Mazzeo, K. Havens, G. Lacerot, Z. Liu, L. De Meester, H. Paerl & M. Scheffer, 2011. Allied attack: climate change and eutrophication. Inland Waters 1: 101–105.
Müller, R. & O. Wiedemann, 1955. Die Bestimmung des Nitrat-Ions im Wasser. Vom Wasser 2: 247–271.
Murphy, J. & J. P. Riley, 1962. A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27: 31–36. https://doi.org/10.1016/S0003-2670(00)88444-5.
Naselli-Flores, L. & R. Barone, 2005. Water-level fluctuations in Mediterranean reservoirs: setting a dewatering threshold as a management tool to improve water quality. Hydrobiologia 548: 85–99. https://doi.org/10.1007/s10750-005-1149-6.
Naselli-Flores, L. & R. Barone, 2011. Fight on plankton! Or, phytoplankton shape and size as adaptive tools to get ahead in the struggle for life. Cryptogamie, Algologie 32: 157–204.
Naselli-Flores, L., J. Padisák & M. Albay, 2007. Shape and size in phytoplankton ecology: Do they matter? Hydrobiologia 578: 157–161. https://doi.org/10.1007/s10750-006-2815-z.
Naselli-Flores, L., T. Zohary & J. Padisák, 2021. Life in suspension and its impact on phytoplankton morphology: an homage to Colin S. Reynolds. Hydrobiologia 848: 7–30. https://doi.org/10.1007/s10750-020-04217-x.
O’Farrell, I., C. Motta, M. Forastier, W. Polla, S. Otaño, N. Meichtry, M. Devercelli & R. Lombardo, 2019. Ecological meta-analysis of bloom-forming planktonic Cyanobacteria in Argentina. Harmful Algae 83: 1–13. https://doi.org/10.1016/j.hal.2019.01.004.
Padisák, J., J. Köhler & S. Hoeg, 1999. The effect of changing flushing rates on development of late summer Aphanizomenon and Microcystis populations in a shallow lake, Müggelsee, Berlin, Germany. In Tundisi, J. G. & M. Straskraba (eds), Theoretical Reservoir Ecology. Backhuys Publishers, Leiden.
Padisák, J., G. Borics, I. Grigorszky & É. Soróczki-Pintér, 2006. Use of phytoplankton assemblages for monitoring ecological status of lakes within the water framework directive: the assemblage index. Hydrobiologia 553: 1–14. https://doi.org/10.1007/s10750-005-1393-9.
Padisák, J., L. O. Crossetti & L. Naselli-flores, 2009. Use and misuse in the application of the phytoplankton functional classification: a critical review with updates. Hydrobiologia 621: 1–19. https://doi.org/10.1007/s10750-008-9645-0.
Paerl, H. W., 2009. Controlling eutrophication along the freshwater-marine continuum: dual nutrient (N and P) reductions are essential. Estuaries and Coasts 32: 593–601. https://doi.org/10.1007/s12237-009-9158-8.
Paerl, H. W. & J. Huisman, 2009. Climate change: a catalyst for global expansion of harmful cyanobacterial blooms. Environmental Microbiology Reports 1: 27–37. https://doi.org/10.1111/j.1758-2229.2008.00004.x.
Paerl, H. W. & T. G. Otten, 2013. Harmful cyanobacterial blooms: causes, consequences, and controls. Microbial Ecology 65: 995–1010. https://doi.org/10.1007/s00248-012-0159-y.
Pettersson, K., E. Herlitz & V. Istvánovics, 1993. The role of Gloeotrichia echinulata in the transfer of phosphorus from sediments to water in Lake Erken. In Boers, P. C. M., T. E. Cappenberg & W. van Raaphorst (eds), Proceedings of the Third International Workshop on Phosphorus in Sediments, Vol. 84. Springer, Dordrecht.
Pomeroy, L. R., 2001. Caught in the food web: Complexity made simple? Scientia Marina 65: 31–40. https://doi.org/10.3989/scimar.2001.65s231.
Princiotta, S. D., S. P. Hendricks & D. S. White, 2019. Production of cyanotoxins by Microcystis aeruginosa mediates Interactions with the mixotrophic flagellate Cryptomonas. Toxins 11: 1–16. https://doi.org/10.3390/toxins11040223.
R Core Team, 2022. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna:
Reynolds, C. S., 1993. Scales of disturbance and their role in plankton ecology. Hydrobiologia 249: 157–171. https://doi.org/10.1007/978-94-017-1919-3_15.
Reynolds, C. S., 2006. The Ecology of Phytoplankton, Cambridge University Press:
Reynolds, C. S. & A. E. Walsby, 1975. Water blooms. Biological Reviews of the Cambridge Philosophical Society 50: 437–481.
Reynolds, C. S., R. L. Oliver & A. E. Walsby, 1987. Cyanobacterial dominance: the role of buoyancy regulation in dynamic lake environments. New Zealand Journal of Marine and Freshwater Research 21: 379–390. https://doi.org/10.1080/00288330.1987.9516234.
Reynolds, C. S., V. Huszar, C. Kruk, L. Naselli-Flores & S. Melo, 2002. Towards a functional classification of the freshwater phytoplankton. Journal of Plankton Research 24: 417–428. https://doi.org/10.1093/plankt/24.5.417.
Richardson, T. L., 2022. The colorful world of cryptophyte phycobiliproteins. Journal of Plankton Research 44: 806–818. https://doi.org/10.1093/plankt/fbac048.
Ross, P. E. & M. Munawar, 1981. Preference for nannoplankton size fractions in Lake Ontario zooplankton grazing. Journal of Great Lakes Research 7: 65–67. https://doi.org/10.1016/S0380-1330(81)72027-6.
Rothhaupt, K., 1996. Laboratorary experiments with a mixotrophic chrysophyte and obligately phagotrophic and photographic competitors. Ecology 77: 716–724. https://doi.org/10.2307/2265496.
Salmaso, N. & J. Padisák, 2007. Morpho-Functional Groups and phytoplankton development in two deep lakes (Lake Garda, Italy and Lake Stechlin, Germany). Hydrobiologia 578: 97–112. https://doi.org/10.1007/s10750-006-0437-0.
Salmaso, N. & M. Tolotti, 2021. Phytoplankton and anthropogenic changes in pelagic environments. Hydrobiologia 848: 251–284. https://doi.org/10.1007/s10750-020-04323-w.
Salmaso, N., L. Naselli-Flores & J. Padisák, 2015. Functional classifications and their application in phytoplankton ecology. Freshwater Biology 60: 603–619. https://doi.org/10.1111/fwb.12520.
Sanders, R., 1991. Mixotrophic protists in marine and freshwater ecosystems. Journal of Protozoology 38: 76–81. https://doi.org/10.1111/j.1550-7408.1991.tb04805.x.
Sathicq, M. B., D. E. Bauer & N. Gómez, 2015. Influence of El Niño Southern Oscillation phenomenon on coastal phytoplankton in a mixohaline ecosystem on the southeastern of South America: Río de la Plata estuary. Marine Pollution Bulletin 98: 26–33. https://doi.org/10.1016/j.marpolbul.2015.07.017.
SEMARH, 2019. Secretaria do Meio Ambiente e Recursos Hídricos do Estado do Rio Grande do Norte. https://www.semarh.rn.gov.br
Sieburth, J.M., V. Smetacek, & J. Lenz, J, 1978. Pelagic ecosystem structure: Heterotrophic compartments of the plankton and their relationship to plankton size fractions. Limnology and Oceanography 23: 1256-1263. https://doi.org/10.4319/lo.1978.23.6.1256
Smith, V. H. & D. W. Schindler, 2009. Eutrophication science: where do we go from here? Trends in Ecology and Evolution 24: 201–207. https://doi.org/10.1016/j.tree.2008.11.009.
Soares, M. C. S., M. I. De, M. M. Marinho, S. M. F. O. Azevedo, C. W. C. Branco & V. L. M. Huszar, 2009. Changes in species composition during annual cyanobacterial dominance in a tropical reservoir: physical factors, nutrients and grazing effects. Aquatic Microbial Ecology 57: 137–149. https://doi.org/10.3354/ame01336.
Soares, M. C. S., V. L. M. Huszar, M. N. Miranda, M. M. Mello, F. Roland & M. Lürling, 2013. Cyanobacterial dominance in Brazil: distribution and environmental preferences. Hydrobiologia 717: 1–12. https://doi.org/10.1007/s10750-013-1562-1.
Soria, J., D. Montagud, X. Soria, M. D. Sendra & E. Vicente, 2019. Phytoplankton reservoir trophic index (PRTI): new tool for ecological quality studies. Inland Waters 9: 301–308. https://doi.org/10.1080/20442041.2018.1494984.
Stenger-Kovács, K., É. H. Buczkó & J. Padisák, 2007. Epiphytic, littoral diatoms as bioindicators of shallow lake trophic status: Trophic Diatom Index for Lakes (TDIL) developed in Hungary. Hydrobiologia 589: 141–154. https://doi.org/10.1007/s10750-007-0729-z.
Teferi, M., S. A. J. Declerck, T. De Bie, P. Lemmens, A. Gebrekidan, T. Asmelash, T. Dejenie, K. Gebrehiwot, H. Bauer, J. A. Deckers, J. Snoeks & L. De Meester, 2014. Strong effects of occasional drying on subsequent water clarity and cyanobacterial blooms in cool tropical reservoirs. Freshwater Biology 59: 870–884. https://doi.org/10.1111/fwb.12312.
Ter Braak, C. J. F. & I. C. Prentice, 1988. A theory of gradient analysis. Advances in Ecological Research 18: 271–317.
Tilahun, S. & D. Kifle, 2019. The influence of El Niño-induced drought on cyanobacterial community structure in a shallow tropical reservoir (Koka Reservoir, Ethiopia). Aquatic Ecology 53: 61–77. https://doi.org/10.1007/s10452-019-09673-9.
Uehlinger, V., 1964. Étude statistique des méthodes de dénobrement planctonique. Archive Science 17: 121–123.
Utermöhl, H., 1958. Methods of collecting plankton for various purposes are discussed. SIL Communications 9: 1, 1–38. https://doi.org/10.1080/05384680.1958.11904091
Üveges, V., K. Tapolczai, L. Krienitz & J. Padisák, 2012. Photosynthetic characteristics and physiological plasticity of an Aphanizomenon flos-aquae (Cyanobacteria Nostocaceae) winter bloom in a deep oligo-mesotrophic lake (Lake Stechlin, Germany). Hydrobiologia 698: 263–272. https://doi.org/10.1007/s10750-012-1103-3.
Valderrama, J., 1981. The simultaneous analysis of total nitrogen and phosphorus in natural waters. Marine Chemistry 10: 109–122. https://doi.org/10.1016/0304-4203(81)90027-X.
Vanderley, R. F., V. Becker, R. Panosso, K. A. Ger & J. Padisák, 2022. The influence of trophic status and seasonal environmental variability on morpho-functional traits in tropical man-made shallow lakes. Environmental Monitoring and Assessment 7: 194–507. https://doi.org/10.1007/s10661-022-10091-y.
Walsby, A. E., 1991. The mechanical properties of the Microcystis gas vesicle. Microbiology 137: 2401–2408. https://doi.org/10.1099/00221287-137-10-2401.
Wang, C., V. Béres, C. Stenger-Kovács, X. Li & A. Abonyi, 2018. Enhanced ecological indication based on combined planktic and benthic functional approaches in large river phytoplankton ecology. Hydrobiologia 818: 163–175. https://doi.org/10.1007/s10750-018-3604-1.
Wehr, J. & R. G. Sheath, 2003. Freshwater Algae of North America: Ecology and Classification, Academic Press:, 917.
West-Eberhard, M. J., 1989. Phenotypic plasticity and the origins of diversity. Annual Review of Ecology and Systematics 20: 249–278.
Wetzel, R. & G. E. Likens, 2000. Limnological Analyses, Springer, New York:
Yang, J., H. Lv, J. Yang, L. Liu, X. Yu & H. Chen, 2016. Decline in water level boosts cyanobacteria dominance in subtropical reservoirs. Science of the Total Environment 557–558: 445–452. https://doi.org/10.1016/j.scitotenv.2016.03.094.
Zohary, T. & I. Ostrovsky, 2011. Ecological impacts of excessive water level fluctuations in stratified freshwater lakes. Inland Waters 1: 47–59. https://doi.org/10.5268/IW-1.1.406.
Zohary, T., J. Padisák & L. Naselli-Flores, 2010. Phytoplankton in the physical environment: Beyond nutrients, at the end, there is some light. Hydrobiologia 639: 261–269. https://doi.org/10.1007/s10750-009-0032-2.
Zuur, A., E. N. Ieno, N. Walker, A. A. Saveliev & G. M. Smith, 2009. Mixed effects models and extensions in ecology with R, Springer, New York:
Funding
This study was supported by grants provided Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES) – Finance Code 001. VB was supported by continuous funding through Research Productivity Grants provided by Brazilian National Council for Scientific and Technological Development (CNPq—www.cnpq.br), process 308652/2019-3. GBS and JP was supported by NKFIH KKP 144068 during manuscript writing. We are thankful to all staff members at the research group ELISA—Estudos Limnológicos do Semiárido for helping with fieldwork and laboratory analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work in this paper.
Additional information
Handling Editor: Sidinei M. Thomaz
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Viktória B-Béres, Luigi Naselli-Flores, Judit Padisák & Gábor Borics / Trait-Based Approaches in Micro-Algal Ecology
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Costa, M.R.A., Cardoso, M.M.L., Selmeczy, G.B. et al. Phytoplankton functional responses induced by extreme hydrological events in a tropical reservoir. Hydrobiologia 851, 849–867 (2024). https://doi.org/10.1007/s10750-023-05241-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05241-3