Abstract
Colin Reynolds exquisitely consolidated our understanding of driving forces shaping phytoplankton communities and those setting the upper limit to biomass yield, with limitation typically shifting from light in winter to phosphorus in spring. Nonetheless, co-limitation is frequently postulated from enhanced growth responses to enrichments with both N and P or from N:P ranging around the Redfield ratio, concluding a need to reduce both N and P in order to mitigate eutrophication. Here, we review the current understanding of limitation through N and P and of co-limitation. We conclude that Reynolds is still correct: (i) Liebig’s law of the minimum holds and reducing P is sufficient, provided concentrations achieved are low enough; (ii) analyses of nutrient limitation need to exclude evidently non-limiting situations, i.e. where soluble P exceeds 3–10 µg/l, dissolved N exceeds 100–130 µg/l and total P and N support high biomass levels with self-shading causing light limitation; (iii) additionally decreasing N to limiting concentrations may be useful in specific situations (e.g. shallow waterbodies with high internal P and pronounced denitrification); (iv) management decisions require local, situation-specific assessments. The value of research on stoichiometry and co-limitation lies in promoting our understanding of phytoplankton ecophysiology and community ecology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Now almost 30 years ago, in his classical paper on “What Vollenweider couldn’t tell us”, Colin Reynolds pointed out the importance of distinguishing between limitation of nutrient uptake rates, growth rates and the capacity for biomass formation. In order to effectively control eutrophication, he emphasised the practical “need for a consensus view of aquatic ecosystem functioning that is implicitly correct”, which at the time he assessed as “far from being the case” (Reynolds, 1992, p. 5). Some of the issues he listed as controversial may in part be resolved or better understood by now, i.e. the relative relevance of bottom-up versus top-down control, internal versus external P loads and internal nutrient recycling. However, there is a revival of the debate about whether or not reducing phosphorus loads is sufficiently effective for eutrophication control, or whether this, and in particular controlling cyanobacterial blooms, also requires reducing nitrogen concentrations. Some of this debate is general (see, e.g. Conley et al., 2009, the mini-review by Paerl & Otten, 2016; Paerl et al., 2019; Scott et al., 2019; Andersen et al., 2020) and some specific to the waterbody studied (e.g. Müller & Mitrovic, 2015). Parts of this debate appear to question the concept that carrying capacity is typically limited by one resource at a time, a concept clearly conveyed by Colin Reynolds in his books (Reynolds, 1984, 1999, 2006) and publications (e.g. 1992, 1998). The fascinating and growing research field of ecological stoichiometry (Sterner & Elser, 2002) is addressing nutrient limitation from a different angle (e.g. North et al., 2007), with numerous nutrient enrichment studies published (about 500–600; Elser et al., 2007) that quite frequently show more pronounced increases of biomass when both nutrients are added to natural populations as compared to adding only N or only P (see review in Elser et al., 2007). It is increasingly recognised that in many waterbodies summer phytoplankton is co-limited by P and N (e.g. Elser et al., 2007; Kolzau et al., 2014; Shatwell & Köhler, 2019; Andersen et al., 2020; Maberly et al., 2020), and N:P ratios are also quoted to argue for the need to reduce N loads.
Although numerous success stories of recovery from eutrophication and cyanobacterial blooms by reducing P loads and thus concentrations in the waterbody are now evident (discussed below), the debate at meetings and in publications about the need for a dual strategy, addressing both P and N, has been intensive and is ongoing (e.g. Schindler, 2012; Schindler et al., 2016; Paerl et al., 2019). For management it is important to understand whether it will be more effective to focus all efforts only on P, or in some cases perhaps on N, or to reduce both nutrients: only some of the available measures curb both nutrients (e.g. changing land use and agricultural practices); others, particularly in sewage treatment, require additional investment because removal techniques differ for N and P. However, in contrast to the meanwhile numerous cases of successful lake restoration by reducing phosphorus loads, to date there are only few examples of successful mitigation of eutrophication primarily achieved through reduced nitrogen loads, and these pertain to shallow lakes such as Müggelsee in Berlin, Germany (Shatwell & Köhler, 2019); Jeppesen et al. (2005) give further examples.
Some of the current debate about the role of N:P ratios and whether or not we need to reduce both nutrients seems surprising in face of the understanding of phytoplankton ecology that Colin Reynolds consolidated already decades ago. While the concept of functional groups that he introduced is widely used, it seems to be time to remind ourselves of the deep understanding of resource acquisition and resulting competitive advantages that he gave us—and to ensure that we incorporate this into our teaching of phytoplankton ecology. Colin Reynolds not only published clever analyses of the interplay of conditions that determine the growth of phytoplankton organisms and the outcomes of their competition. He was also gifted with empathy and imagination for what life is like for a phytoplankton cell entrained in the turbulence of a mixed epilimnion: in his lectures he could make us feel like a cell so small that the viscosity of water makes it feel like syrup, a cell facing the challenge of capturing photons for energy and nutrient molecules for division as we get moved around the mixed water layer. Also, he was fascinated by the biochemical mechanisms determining the rates of uptake of nutrients or capturing of photons in relation to the speed with which these resources can be processed inside the cell, and he endeavoured to explain the phenomena of resource limitation observed in the field or in continuous cultures in terms of the mechanisms understood at the time. It seems safe to say that the recent research on stoichiometry and co-limitation would have fascinated Colin Reynolds.
Can we reconcile outcomes of research on stoichiometry and co-limitation with the concepts he developed and taught? Or apply these concepts to the interpretation of the results of, e.g. nutrient enrichment experiments and N:P ratios? Is there a unifying view combining his approach with these to guide management in choosing the most effective approach to abating eutrophication?
In the following we first summarise the understanding Colin Reynolds gave us and then analyse how we can apply it to resolve the debate about the role of N (co-) limitation, N:P ratios (stoichiometry) and a need for N-load reduction. In line with the focus of his work, here we discuss the biological aspects of how nutrient limitation affects phytoplankton. The prerequisite for achieving nutrient concentrations that limit biomass is, of course, the appropriate management of external loads and, where concentrations in the waterbody do not respond to their reduction, an understanding of legacy loads from the sediment. This aspect requires a different discussion with a hydrological, physical and chemical focus, an aspect we can only touch upon here.
Colin’s key messages for understanding how nutrient limitation works
On the basis of his understanding of phytoplankton communities, Reynolds very elegantly reviewed, summarised, and explained nutrient limitation. He emphasised the need to be clear about “what is limited and when” (1992, p. 9), and for this purpose he differentiated three nutrient fractions that may exert limitation:
-
1.
Concentrations of dissolved inorganic nutrients limit uptake rates: From the body of experimental data available at the time for growth rates relative to nutrient concentrations, Reynolds derived residual concentrations (R* sensu Tilman et al., 1982) above which “it is most improbable that algal growth is limited” (1992, p. 12). Residual nutrient concentrations will allow nutrient uptake by phytoplankton and thus cell division, i.e. population growth. Following resource-based Monod curves for uptake rates relative to nutrient concentrations, Reynolds (1998, 1999) proposed P- or N-limitation to be unlikely above 3 µg/l soluble reactive phosphorus (SRP) and 100 µg/l dissolved inorganic nitrogen (DIN). These concentrations are widely quoted, often adjusted up to 10 µg/l SRP and 130 µg/l DIN (Dolman et al., 2012; Kolzau et al., 2014) to accommodate liberation of nutrient, e.g. by excretion. 3–10 µg/l SRP and 100–130 µg/l DIN thus serve as threshold concentrations above which limitation by the respective nutrient can be generally excluded (exceptions include waterbodies with high iron concentrations that form Fe–P complexes unavailable for phytoplankton; Spijkerman et al., 2018).
-
2.
The cellular nutrient content, i.e. cell quota sensu Droop (1973), limits cell division rates. Species differ in the minimum cell quotas (Q0) required for maintenance metabolism and in their maximum cell quotas for maximum growth rates (Finkel et al., 2010; Quigg et al., 2011), depending in particular on cell size. This trait is highly relevant in shaping phytoplankton communities (see, e.g. Klausmeier et al., 2007; Edwards et al., 2011, 2012). Thus, the N:P ratio within cells would best reflect which nutrient is limiting at a certain point in time, and for assessing competition in field communities it would be valuable if this could be differentiated by taxa. While cell quota can readily be determined in laboratory cultures (Quigg et al., 2011), preferably as ratio to carbon, in field samples they can at best be roughly estimated for the entire community using ratios of a measure of biovolume to TP and TN (or particulate P and N) as proxy. The concentration of chlorophyll-a (Chl.-a) is frequently used as a measure of biomass (although cellular contents of Chl.-a too can vary depending on growth conditions, particularly light availability). For example, Maberly et al. (2020) use thresholds of >0.3 µg Chl.-a per µg TP to indicate P-limitation and >0.042 µg Chl.-a per µg of DIN to indicate N-limitation. Unfortunately, other organic particles (difficult to separate from phytoplankton in a field sample) cloud the ratio more or less strongly, and moreover, trophic state affects the phytoplankton’s fraction of total seston (with that of phytoplankton being lower in eutrophic waterbodies; Gaedke et al., 2002).
-
3.
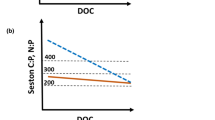
Concentrations of total P and total N limit the amount of phytoplankton biomass that can build up in a waterbody, i.e. the yield. Reynolds’ publications explain the concept of carrying capacity very clearly: the maximum possible plankton biomass that a given waterbody can attain depends on the total concentration of the limiting nutrient and the minimum intracellular amount with which a cell can function, i.e. the cell quota. Using Chl.-a as a measure of biomass, he shows conceptually and based on his own lake data that on a weight to weight basis, phytoplankton cells contain about as much Chl.-a as P, i.e. a ratio of 1:1 (this ratio may increase to 3:1 at low TP concentrations or under light limitation). Reynolds emphasises that in P-limited waterbodies, TP concentrations “set an upper limit” on carrying capacity for phytoplankton biomass, even if biomass often does not reach this maximum because of other constraints (e.g. light limitation or losses through grazing and sedimentation). In temperate climates the resource limiting carrying capacity typically shifts from light in winter to TP in spring and often throughout summer, potentially interrupted by phases of N-limitation during summer (Fig. 1; see also Lampert & Sommer, 2007).
Fig. 1 Reynolds’ concept of the carrying capacity for phytoplankton biomass: The dashed/dotted (blue) line shows the limit set by TP; the solid (grey) line that set by light, and the dotted (red) line the limit set by TN. The hatched grey area shows the potential carrying capacity at a given time of the year, i.e. which amount of chlorophyll-a would be possible in face of the limit set by these 3 resources at any given point in time. The solid green silhouette shows the actual concentration of Chl.-a in a theoretical lake (adapted from Reynolds, 1992)
Reynolds reminds us, quoting Schindler (1977) that “as no other proximal source of phosphorus is available to any phytoplankton, except the total phosphorus pool in the water, it follows that the ultimate chemical capacity is, indeed, of the size of that pool” (1992, p. 9). This is important, because rapid recycling of P released by decomposing biomass is sometimes misunderstood as source for further biomass; however, while it can indeed be a further source for phytoplankton growth, this is only possible if phytoplankton has not yet attained the maximum biomass, defined by the carrying capacity of the limiting resource.
All three nutrient fractions are relevant for understanding nutrient limitation of phytoplankton biomass. Understanding the concept of cell quota determining growth rates (discussed in more detail in “Stoichiometry of phytoplankton and implications of N:P ratios for nutrient limitation” section) is fundamental for studying species composition. Reynolds (1998) pointed out that the nutrient uptake capacities of cells far exceed their needs for growth. Concentrations of dissolved nutrients give information about the current limitation of uptake rates, provided they are in the range below 3–10 µg/l SRP or 100–130 µg/l DIN. However, even in this low range they do not necessarily prevent further cell division because cell quotas may (yet) be sufficiently high and be replenished from P or N liberated by excretion and biodegradation. Importantly, however, the inverse does apply: excess dissolved N and P above these thresholds indicates that the respective nutrient is not limiting at that point in time (light would be the next candidate limiting resource, particularly in a turbid mixed layer, or carbon if pH is extremely high). Therefore, non-limiting situations need to be excluded when drawing conclusions for nutrient limitation from N:P ratios or from growth responses in nutrient enrichment experiments (as done, e.g. by Maberly et al., 2020).
To visualise this, Reynolds once again takes the viewpoint of a phytoplankton cell striving to multiply, and he elegantly argues that the only relevant question for that cell is “whether there is enough of each of its various nutrient requirements available to sustain the next cell division”, emphasising that it does not matter whether the N:P ratio is 50:1 or 3:1 “if both nitrogen and phosphorus supersaturate the growth requirements for the current generation” (1992, p. 14). Of course if one nutrient is depleted, i.e. is fully incorporated in plankton biomass, more of the other will be left over; however, the mechanism setting a limit to further cell multiplication it is the depletion itself and not the ratio: “When both (nutrients) are ‘not limiting’, then neither is the growth of the cell ‘limited’ by these elements. … It is the resources themselves, each and individually, which are potentially crucial” (1999, p. 32).
The total fractions, i.e. TP and TN, are highly relevant for waterbody management because they set the theoretical upper limit for plankton biomass, i.e. they define carrying capacity. As reflected by the Vollenweider OECD annual means (Fig. 2), in practice, regressions derived from data for annual or seasonal means of Chl.-a (as measure of phytoplankton biomass) relative to TP give Chl.-a concentrations below the amount relative to TP that phytoplankton could attain if all of the TP were used to build phytoplankton cells. However, Vollenweider & Kerekes (1980) also show a regression for the maximum Chl.-a concentration relative to TP: with a slope of 0.64 this is steeper than that for the annual mean (slope of 0.28), thus coming closer to a 1:1 relationship of Chl.-a to TP, i.e. closer to full utilisation of the carrying capacity for biomass formation. The Vollenweider regressions and further similar ones (e.g. Dillon & Rigler, 1974; Smith, 2003; Jeppesen et al., 2005; Phillips, 2008) bridge the theoretical concept of biomass being limited by the carrying capacity with waterbody data that reflect the further constraints acting on phytoplankton development. They have served as basis for planning numerous successful cases of restoration of eutrophic waterbodies which demonstrate proof of the concept that managing the concentration of a limiting nutrient (so far usually P) can effectively control maximum phytoplankton biomass (discussed in further detail in part 5 below).
Recovery of 10 waterbodies from eutrophication in response to the reduction of their P load. Means for each waterbody are connected chronologically; white dotted lines show the Vollenweider regressions for the maximum (upper line) and annual mean (lower line) Chl.-a concentration. Revised from Fastner et al. (2016) and supplemented with data from Chorus et al. (2011)
Stoichiometry of phytoplankton and implications of N:P ratios for nutrient limitation
Redfield (1934) first published the ratio of N:P for the content of these two elements in marine phytoplankton of 16:1 as molar ratio (equivalent to 7:1 as mass ratio). Since then, rather than this being a fixed feature, further research has shown a wider range for the ratio in phytoplankton cells, in part due to variable pools of stored nutrients (Sterner & Elser, 2002). Also, differences between taxa and clones (Geider & La Roche, 2002) are pronounced and now recognised as specific traits developed during the species’ evolution (Quigg et al., 2011), characterising their performance in competition. Klausmeier et al. (2004) base their model of phytoplankton stoichiometry on the consideration that a cell’s ‘functional machinery’ has quite constant, species-specific nutrient requirements, high in N for building the proteins needed for nutrient uptake and for chloroplasts, but high in P for building ribosomes needed for cell division. In this model, competition for limiting nutrients selects for effective nutrient uptake (requiring high N:P ratios) while exponential growth requires more P and thus, depending on the limiting resource, the model of these authors predicts N:P ratios that range from 8.2 (by mass) at saturating resource levels up to 45 under P limitation. From empirical studies, others conclude a range of 16–30 (by mass) outside of which N or P limitation are likely (see discussion in Dolman & Wiedner, 2015) or a molar ratio of < 10 for N-limitation being likely and > 20 for a probable P limitation (Maberly et al., 2020).
Therefore within this range there is no ‘universally optimal value’; rather, the N:P ratio of a phytoplankton community at a given point in time is an average, resulting from the composition of species with ‘different structural N:P ratios’ (Klausmeier et al., 2004, p. 173). Geider & La Roche (2002) emphasise that the critical ratio of N:P within the cell at which growth switches from being limited by one nutrient to being limited by the other can be determined only from cell quotas. These authors show that for a given species, co-limitation by both nutrients only occurs at this critical ratio, and only at this ratio do the cellular contents of both nutrients need to increase in order to enable further growth. Furthermore, Edwards et al. (2011) showed a trade-off in species’ ability to compete for N and for P; thus, some species have a competitive advantage at low concentrations of P and others at low concentrations of N. This can be explained by the different structural N:P ratios proposed by Klausmeier et al. (2004), and it has consequences for the structure of phytoplankton communities along gradients of TN:TP.
The stoichiometry of N and P in phytoplankton organisms also determines nutrient fluxes between trophic levels (reviewed in Hessen et al., 2013). It is therefore of substantial interest for ecological research on drivers of plankton community structure. For assessing and managing eutrophication, i.e. for assessing a need to control the concentration of N, however, the criterion of the N:P ratio being below or above the molar ratio of 16 (or the mass ratio of 7) is of limited use for several reasons: (i) As discussed above, N:P ratios can be quite variable, even after excluding data with dissolved fractions above the thresholds for limitation of uptake, and even if the supply of N and P is in close balance with the demand of phytoplankton communities. In practice, the molar range of 10–20 outside of which N, respectively, P to be likely to be limiting is frequently used (e.g. Maberly et al., 2020). (ii) A further complication is the above-mentioned problem of separating phytoplankton from other seston particles to analyse their constituents. Thus, for field data TN:TP need to serve as a proxy—introducing further uncertainty. (iii) As visualised by Davidson & Howarth (2007), if the supply of N and P is near the range of the phytoplankton’s demand, this means that neither nutrient is available in substantial excess so that a slight increase in biomass is likely to consume that excess, then shifting limitation to the other nutrient.
As quoted above, Reynolds emphasised the primary relevance of absolute concentrations and questions that insights are to be gained from ratios. He does, however, recognise a value of analysing N:P ratios at the onset of the growth season to estimate which nutrient may become limiting during phases of population increase later in the course of the year, provided the absolute concentrations of TP and TN are low enough to potentially limit biomass.
Co-limitation of phytoplankton and implications for nutrient limitation
As discussed above, while cell quotas would be the most appropriate approach to identifying the nutrient limiting population growth at a given point in time, they are difficult to determine in field samples with mixed species of phytoplankton and other seston. Nutrient enrichment experiments provide an alternative approach, usually addressing the phytoplankton community as a whole. They have been widely employed to measure the growth response when adding N, P or both nutrients to phytoplankton communities freshly sampled from waterbodies. Hundreds of enrichment experiments have been conducted at different scales, i.e. in the laboratory, in mesocosms, and in some cases also in entire lakes (e.g. the whole lake additions of Schindler, 2009, confirming P as limiting nutrient), with methodological pros and cons reviewed by Elser et al. (1990). Growth responses are typically measured as an increase in a parameter reflecting biomass, such as the concentration of Chl.-a. Müller & Mitrovic (2015) used biovolume and thus were able to differentiate the impact of nutrient limitation between species. Because growth is slow and zooplankton, not totally removed from the sample of the mixed community, may reduce net growth, an interesting alternative is to assess the rapid response to nutrient addition through measuring the in vivo fluorescence of Chl.-a in photosystem 2 (Lachmann et al., 2019; Holland et al., 2004). This method is based upon the ecophysiology of the nutrient limited algal cell that, upon a spike of that nutrient, directs all its metabolic ‘efforts’ towards the uptake of that nutrient; this leads to a drain of electrons from photosynthesis, thus decreasing the Chl.-a fluorescence (Shelly et al., 2010).
A typical response pattern in enrichment experiments is an increase in growth relative to the controls when adding either N or P alone; however, in many publications a large fraction of the samples showed a more pronounced increase of biomass when adding both N and P, often 2–3 times that of adding either N or P alone (Davies et al., 2004; Elser et al., 2007; North et al., 2007; Sterner, 2008; Harpole et al., 2011; Mischler et al., 2014; Bracken et al., 2015; Müller & Mitrovic, 2015; Katkov et al., 2020). The conclusion drawn is that the phytoplankton in these waterbodies is co-limited by both N and P. Lachmann et al. (2019) show that this response to co-limitation might well occur within a time frame of few minutes. Davidson & Howarth (2007) interpret such findings as a result of N:P ratios in most natural communities being in fairly close balance around the Redfield ratio. In consequence, adding both nutrients merely increases the carrying capacity for biomass until it hits limitation by some other resource, which in eutrophic waterbodies typically is underwater light availability.
Factoring light availability (photon flux density) into an experimental design and extrapolating the experimental results to the situation in the waterbody is challenging and rarely done. Kolzau et al. (2014) give an example, and Maberly et al. (2020) use the approach suggested by Reynolds (1992) to calculate light limitation in lake by stating that at in situ mean epilimnetic light intensities above 20 μmol m−2 s−1 photosynthesis is likely to be light-saturated. To assess whether the growth responses in the culture vessels are relevant to the field situation, both research groups also use information about the concentrations of dissolved P (SRP) and N (DIN), as well as Chl.-a concentrations in relation to TP and TN in the waterbody.
In publications that explain basic principles and have reached almost textbook status Arrigo (2005) and Saito et al. (2008) define different types of co-limitation and explain how they work. Arrigo (2005) begins with describing situations in which it takes two nutrients to trigger growth because the levels of both are too low for further cellular uptake. This may be the case in very oligotrophic environments or if, for example, if in an already N-limited situation N-fixing cyanobacteria deplete the concentrations of P. However, Arrigo discounts this as co-limitation in the stricter sense because, although both nutrients are limiting, they are acting independently of each other. Along similar lines Saito et al. (2008) describe sequential limitation (termed ‘serial limitation‘ by Harpole et al., 2011), i.e. where alleviation of limitation by one resource, e.g. P, leads to a biomass increase up to the level limited by another, e.g. N or light. This is in line with the concept of oscillation between N- and P limitation proposed by Davidson & Howarth (2007; see above) and also with Liebig’s (1840) law of the limiting nutrient. Awareness of both of these mechanisms for pseudo-simultaneous limitation by two resources is important because they explain part of the frequently observed significantly higher growth response to adding both nutrients as compared to adding each one separately (see above).
On the cellular level, already in the 1970s, continuous cultures of algae have shown differences in the nutrient concentrations saturating their growth rates. This mechanism was a central focus of Colin Reynolds’ understanding of the differences between taxa in nutrient uptake kinetics and cell quotas. The resource limitation publications of Tilman and co-workers showed that these differences between species allow their coexistence when competing for resources because of differences in their uptake rates and cell quota. Community co-limitation thus is well in agreement with the theory of nutrient limitation described by Reynolds (e.g. 1992), a conceptual difference being that a few decades ago this was shown with algal culture strains in continuous culture competition experiments and now is addressed in the context of nutrient enrichment experiments analysing the growth responses of communities in field samples.
Also, more recent research is addressing different mechanisms of co-limitation within an algal cell: Saito et al. (2008) differentiates biochemical mechanisms for co-limitation: one is ‘biochemical substitution co-limitation’, i.e. two elements can serve the same function within the same enzyme or in different enzymes with the same function (usually metals in catalytic enzymes, but not N and P, which serve different functions). The other is ‘biochemically dependent limitation’, i.e. limitation by one element reduces the acquisition rate of another—e.g. if N-limitation reduces the cell’s machinery for P- uptake. Harpole et al. (2011) further discusses that different metabolic pathways in a cell may be limited by different resources, with independent or interactive mechanisms. Few experimental studies of different combinations of macro- and micronutrients have been published (Buitenhuis et al., 2003; Glass et al., 2010; Schrader et al., 2011; Spijkerman et al., 2011), and while these show intriguing interdependencies, the quantitative relevance of such mechanisms of biochemical co-limitation for phytoplankton communities in waterbodies remains to be further explored. While Colin Reynolds was fascinated by explaining phytoplankton growth patterns in the field with mechanisms on the cellular level, he could scarcely include these aspects of biochemically dependent co-limitation in his considerations of growth responses to resource availability.
The question raised by several workers, emphatically by Schindler (2012) and Schindler et al. (2016), is whether the small-scale short-term nutrient addition assays typically used in co-limitation research can provide relevant results for assessing long-term nutrient limitation in a waterbody. The aspect of scale is especially relevant in face of waterbodies not being homogeneous environments; rather they make nutrients available in patchy and pulsed patterns. Size and frequency of nutrient pulses determine the competitive advantages of different species (e.g. Spijkerman & Coesel, 1997; Moon & Carrick, 2007; Maberly et al., 2020). The study of Maberly et al. (2020) shows that co-limitation is more often detected in summer and autumn, which can be a result of chlorophytes and cyanobacteria competing for N and P (Brauer et al., 2012), i.e. community co-limitation (sensu Arrigo, 2005). Davidson & Howarth (2007) point to the relevance of scale for interpreting short-term nutrient enrichment experiments that show the response of the current community but do not reflect the adaptation of species composition in the somewhat longer term.
Co-limitation research, particularly that addressing biochemical mechanisms of co-limitation, is promoting our understanding of the drivers of phytoplankton community composition and thus of diversity. Whether N or P are limiting or how limitation oscillates between both nutrients affects diversity as well as the elemental composition of cells. The effect of P-limitation on phytoplankton species composition and food quality for higher trophic levels has been well studied and described (Lukas et al., 2011a, b; Sommer et al., 2012). Bergström et al. (2020) analysed N- and P-limitation in two Swedish mountain regions in the context of declining loads of macro-nutrients and found that a proportionally stronger decrease of N (leading to more phases of N-limitation) has a pronounced impact on phytoplankton quality (including as nutrition for higher trophic levels). Elser et al. (2009) pointed out that relatively few phytoplankton species have an enhanced ability to capture P, and where excess N shifts waterbodies to P-limitation, these will be favoured, outcompeting others. Thus, in P-limited systems excess N can have an additional impact on species composition. Following the predictions of a model by Brauer et al. (2012) this would be positive for water quality as it would disfavour cyanobacteria, many of which are superior competitors for N and light. Brauer et al. (2012) provide a unifying concept which contributes to resolving the controversy about the relevance of N:P ratios in shaping phytoplankton communities by including light as third major resource for which phytoplankton populations compete: these authors show that co-limitation of communities by two nutrients and light is most likely to occur in mesotrophic waterbodies, providing an opportunity for stable coexistence of species and thus the highest diversity. However, these authors emphasise that, depending upon the traits of the competing species, such situations may also result in alternative stable states.
Species composition is ecologically relevant, and a target for waterbody management can be a close-to-natural species composition and diversity (as, e.g. defined in the European Union’s Water Framework Directive’s criteria of a good ecological status). Functional traits of phytoplankton species, i.e. their differences in nutrient uptake affinities and minimum cell quota, allow community co-limitation, as elegantly shown by Edwards et al. (2011). Thus, a practical application of co-limitation research results is to understand at which concentrations of both nutrients, N and P,—or (at concentrations in the mesotrophic range) at which oscillations around a ratio of both—co-limiting conditions may favour diversity within phytoplankton communities.
Reducing P concentrations to reduce phytoplankton biomass
The relationship of phytoplankton biomass (measured in terms of the concentrations of Chl.-a) to concentrations of total phosphorus (TP) was first described by Vollenweider (1968) and later confirmed by numerous other studies with large data sets of further, in part very different waterbodies (Reynolds, 1992 and literature therein; Jones & Lee, 1986, Willén, 2001; Jeppesen et al., 2005; Phillips et al., 2005; Schindler, 2012; Fastner et al., 2016). For six deep stratified lakes Søndergaard et al. (2017) showed that during summer epilimnion concentrations of DIN typically exceeded limitation while those of SRP declined and ratios of TN:TP indicated P limitation.
Interestingly, regressions gleaned from annual means for a large number of lakes also hold for individual lakes as their trophic state develops across a range of TP concentrations (Fig. 2): Lake-specific plots of Chl.-a relative to TP show that in some lakes (e.g. Lake Washington, Arancia Reservoir, Wahnbach Reservoir, Mondsee), the decline of phytoplankton biomass (in terms of Chl.-a) followed that of TP quite closely. At the upper end of the TP-scale shallow Veluwemeer and subtropical Arancia Reservoir produced higher yields of phytoplankton than the large Lake Constance and the smaller, but also stratified Lakes Tegel and Schlachtensee. The data of the latter show thresholds below which TP needed to decline before phytoplankton biomass could respond.
At high TP concentrations, curves of Chl.-a to TP may level off because typically, “other limitations on capacity are imposed at some intermediate level of attainable yield” (Reynolds, 1992, p. 9). Reynolds illustrates this with the conceptual diagram of the OECD regression of mean Chl.-a against mean TP and two horizontal cut-off lines showing a TP-range above which light and one above which N determine carrying capacity. In other words, phytoplankton biomass limited by P can increase as TP increases until it hits a ceiling where another resource becomes limiting. Reynolds points out that this resource very often is the underwater light availability, particularly in deep mixed water layers in which “a substantial lower portion is dark or only dimly insolated…” (1992, p. 9) or in shallow waterbodies with turbidity through, e.g. mineral particles. Accordingly, in many years the phytoplankton in shallow but very turbid Neusiedler See did not attain the biomass that TP would have supported, and the examples of thermally stratified Schlachtensee and Lake Tegel in Fig. 2 show Chl.-a to be independent of TP at concentrations above the TP threshold range of 40–55 µg/l. Such threshold concentrations may depend somewhat on the depth of a shallow waterbody or mixing depth of the epilimnion in a stratified one (see Fastner et al., 2016 for further discussion). While Schauser & Chorus (2007) show that in the case of these two lakes the resource limiting biomass above the TP threshold was light, for a few of the OECD lakes evaluated by Vollenweider & Kerekes (1980) these authors propose N-limitation. However, in such cases also, if management measures reduce TP sufficiently, i.e. to where the carrying capacity supporting biomass is lower for P than for N, biomass declines through reduction of the P concentrations, and the seasonal or annual mean concentration of Chl.-a then relates to that of TP.
An argument brought forth against the predictive value of regressions of phytoplankton biomass against TP is their broad scatter—e.g. for the Vollenweider regression by about a factor of 10 within the 95% confidence its limits. However, as discussed by Jones & Lee (1986 and literature therein), if the TP concentration threshold is identified below which TP is likely to actually limit phytoplankton biomass for an individual waterbody, i.e. its “load/response coupling can be identified”, TP concentrations can predict those of Chl.-a within a factor of 1.5 to 2. Also, while regressions based on meta-analyses of a range of different waterbodies are bound to scatter considerably (see Vollenweider, 1968; Scott et al., 2019), Reynolds (1992) pointed out that scatter for the response of a single lake moving along the trajectories of Chl.-a and TP as it recovers from eutrophication may be much lower. Regressions calculated with the data for Lake Tegel demonstrate this: if we include only the years with summer mean epilimnion TP concentrations below 45–55 µg/l, TP explains 58–70% of the variation of the summer means of Chl.-a (i.e. R2 = 0.58–0.70, depending on whether we include data below 55 µg/l or only those below 45 µg/l). Above this threshold, Chl.-a is independent of TP (with light likely to have been the limiting resource). Data for Chl.-a relative to TP for a single lake may scatter less than those for a joint evaluation of several lakes because the other conditions affecting phytoplankton biomass may be less variable within one lake than between several lakes. However, for the two very shallow waterbodies in Fig. 2, wind-exposed Balaton and Neusiedler See, the data do show considerable scatter. This is likely due to their susceptibility to the stochastic nature of weather with events of wind-induced mixing causing a variable light climate due to resuspended sediment, thus rendering the mean seasonal yield of phytoplankton more variable. Furthermore, biological interactions, such as alternating stable states (Scheffer et al., 1993) or invasive species (such as the Dreissenid quagga mussel; Conroy et al., 2005) may superimpose their impact on phytoplankton biomass, further increasing the scatter of Chl.-a/TP relationships in lakes thus affected.
In summary, there is ample evidence of phosphorus either already being the nutrient limiting phytoplankton biomass in many waterbodies, or of it being successfully made to be the limiting nutrient through management measures that sustainably reduce external loads (Jeppesen et al., 2005; Schindler, 2012; Moss et al., 2013). Depending on water retention times and remaining (internal and external) P loads, it may take years or even decades for load reduction to lead to a concentration within the waterbody that is sufficiently low to control phytoplankton biomass (see Fastner et al., 2016 for further discussion).
A need for also reducing N loads to reduce phytoplankton biomass?
A key aspect in the debate about controlling N or P appears to be widespread confusion between acknowledging that N-limitation is currently relevant in many eutrophic waterbodies, as discussed above, particularly in shallow ones during summer (Kolzau et al., 2014; Maberly et al., 2020), and drawing the conclusion that the load of N needs to be reduced—in addition to or instead of that of P—in order to mitigate eutrophication or cyanobacterial blooms. One aspect is that, quite obviously, it is worthwhile to prevent the loss of a functioning mechanism: where N is currently limiting, any increase of N loads would likely increase phytoplankton biomass and therefore should be avoided. However, following Liebig’s law of the limiting nutrient, regardless of which nutrient is currently limiting, strongly reducing the concentration of one nutrient is likely to render that one to be limiting in the future. Numerous reports of successful trophic recovery through reduced P loads confirm this (see “Reducing P concentrations to reduce phytoplankton biomass” section), even in shallow lakes that are likely to have been N-limited during summer.
While this large body of experience with lake restoration is based on reducing the load and thus the concentration of TP, the basic principles also hold for limitation of carrying capacity by reducing TN, and focusing measures on reducing N can be equally successful. There are key differences between N and P for controlling phytoplankton biomass through nutrient limitation that need to be taken into account when making such a decision:
-
1.
Most of the technologies for nutrient removal in wastewater are specific either to N or to P, thus requiring different investments which would largely be additional if both nutrients are to be addressed;
-
2.
pathways from agricultural land to waterbodies differ, with N chiefly dissolved in water and thus leaching to the waterbody via drainage and inflow whereas P is mostly adsorbed to particles, reaching the waterbody via erosion (Carpenter, 2008);
-
3.
N-limitation can—in part—be compensated by N fixation, although this is less likely to be substantial than originally assumed (reviewed in Moss et al., 2013);
-
4.
both N and P can be lost from a waterbody by dilution (provided inflow concentrations are lower than those in the waterbody). However, the internal cycles of N and P differ: legacy N from previously high loads can be lost from the waterbody and its catchment through denitrification. While P can be lost from productive water layers through retention in the sediments, from these it may be remobilised, depending on its binding forms, redox conditions and sediment resuspension through turbulence as well as through bioturbation. Because denitrification can effectively remove N from the system while P may be cycled, responses to load reduction can be more immediate for N as compared to substantial delays (ranging from years to decades) of response to reduced P loads if legacy P from the sediments is remobilised (Shatwell & Köhler, 2019).
Points 1 and 2 suggest that focusing measures on reducing the load of only one nutrient (typically P) might be more effective. This typically applies if urban sewage is the main nutrient source: in that case, additional investment in treatment for more effective P stripping may be the measure of choice to achieve concentrations of TP that stringently limit biomass in the receiving waterbody, for example by adding a filtration step to remove the fine flocks that do not readily separate by sedimentation (Heinzmann & Chorus, 1994). However, such decisions require a thorough assessment of the overall situation: if, for example, a further target to protect groundwater where this is affected by infiltration from the waterbody may call for adding a denitrification step and for accepting such increased costs for sewage treatment. Likewise, for nutrient loads from agriculture, some measures, such as methods of tillage or use of tile drainage, target P or N more specifically while others—such as reducing stock density—can target both. Thus, it is important not to base decisions on a general paradigm of focusing load reduction on one of the two nutrients or on both, but rather on a comprehensive overall situation assessment and definition of targets.
For point 3, evidence is increasing that N fixation is of quite limited relevance in most waterbodies. Although N fixation may transiently have a role in importing N for phytoplankton growth and promoting the dominance of N-fixing cyanobacteria (Schindler et al., 2016), it requires much energy and thus fairly clear water—a condition scarcely given in eutrophic systems that are turbid from high phytoplankton density. Moreover, on an annual basis, denitrification often appears to outweigh N fixation (see discussions in Moss et al., 2013 and in Paerl et al., 2019). Scott and McCarthy (2010) estimate N fixation to sustain less than 50% of primary production, even at excess P supply. Scott et al. (2019) analysed 1964 USA lakes and show that in more than 80% of them, denitrification outweighs accumulation of N from N fixation, particularly for the eutrophic and hypertrophic ones. Shatwell & Köhler (2019) show that in shallow Müggelsee (Germany) the biomass of N-fixing cyanobacteria decreased to less than half in previous years. Even in recent years where nitrogen was clearly the limiting nutrient during summer, overall N fixation contributed far less N than the lake lost through denitrification although the fraction of heterocytes in cyanobacterial biovolume increased significantly. Dolman et al. (2012) even found N-fixing cyanobacteria to be more abundant in waterbodies with high N:P ratios.
For eutrophic waterbodies with an anoxic sediment and a fair amount of organic carbon “food” for the denitrifying bacteria, denitrification rates tend to be high during summer, particularly if waterbodies are shallow and temperatures above the sediment are high. In consequence, N-limitation may be achievable even if N-fixing cyanobacteria dominate, as in such situations while N input through N fixation is a possibility, more recent data evaluation indicates that this is not generally to be expected when reducing N loads (Scott & McCarthy, 2010).
Point 4, i.e. rapid loss of N from the waterbody through denitrification, is an enticing argument for controlling N loads in waterbodies in which internal nutrient cycling delays the recovery of concentrations after load reduction. There are, however, few examples of improved trophic state attributed only to reduced N concentrations. One is the shallow Müggelsee, mentioned above, where transient short phases of summer stratification quickly render the sediment surface anoxic, releasing P and thus maintaining high concentrations of TP and SRP: in Müggelsee N is now limiting for up to 100 days during summer, and the biomass of total phytoplankton has declined to less than ¼ of its previous levels; thus this lake would not have recovered from eutrophication in the near future had the N loads not strongly declined (Shatwell & Köhler, 2019). This example highlights an opportunity for controlling carrying capacity for summer phytoplankton biomass via N rather than P. The lesson is that for shallow lakes it may be easier and faster to get rid of legacy N as compared to legacy P.
There are, however, examples showing the opposite, i.e. that nitrate concentrations above 0.5 mg/l at the sediment-water interface can counteract P release from the sediment by maintaining a sufficiently high redox tension to prevent sulphate reduction; nitrate loads from treated sewage may also serve this purpose (discussed in Schauser et al., 2006). Nitrate products are sold on the market for this purpose and have been successful for controlling eutrophication (see Dokulil et al., 2000, Wauer et al., 2005 and Schauser et al., 2006 for examples). Regardless of an overall ecological assessment of this approach, such results highlight the need for careful site-specific evaluation of the sediment chemistry and the likely magnitude of P release when planning measures to reduce nitrate loads.
Excess N has been proposed to be the key cause of the deterioration of macrophytes (Moss et al., 2013). These are important in shallow lakes or in those with a large littoral area as they support keeping the water clear, provide habitats and enrich biodiversity. However, Søndergard et al. (2015) showed that elevated concentrations of nitrate are detrimental to macrophytes only in cases where TP concentrations exceed 100 µg/l. In their study of over 800 Danish lakes, these authors reported a loss of macrophytes at concentrations in the range of > 0.5 to 2 mg N/l if TP concentrations were above 100–200 µg/l. As macrophytes can improve the trophic state of shallow lakes, N concentrations below 0.5 mg/l may support achieving a macrophyte-dominated stable state. Søndergard et al. (2015) conclude that in general, reducing P has led to a more pronounced return of macrophytes than reducing only N.
Furthermore, the concentration of microcystins (cyanobacterial peptide toxins) in cyanobacterial cells has been proposed to relate to excess availability of nitrogen (e.g. Gobler et al., 2016; Wagner et al., 2019; Brandenburg et al., 2020), and this intuitively seems to be expected on the basis of the high N content of these molecules. However, the opposite has also been observed: experiments addressing the impact of N-limitation (and other growth conditions) on culture strains of various cyanobacteria have shown contradictory results, some of which may have methodological causes. A further aspect is that cyanobacteria contain numerous further oligopeptides other than microcystins, and their function for the cells is not yet understood. Therefore, meaningful results from experiments addressing the impact of N-limitation on the production of microcystins will need to include the production of the totality of oligopeptides. More importantly, the overall outcome of such experiments is that the impact of growth conditions on the microcystin production of a strain is in the range of not more than a factor of 2–4, while the key driver of microcystin concentrations in field populations is the biomass of producing species and clones (for overviews see Fastner & Humpage, in press, and the meta-analysis by Brandenburg et al., 2020). It follows that conditions favouring cyanobacterial dominance and blooms are the more relevant aspect to address. With Microcystis being an exception, many Cyanobacteria are recognised to be strong competitors for N (Tilman et al., 1982; Smith, 1990; Brauer et al., 2012), giving them a competitive advantage at low availability of N. Thus, reducing only the concentration of N in a waterbody would require reaching a sufficiently low level to ensure that the concentration of biomass supported by N is low.
Research on eutrophication and experience with reversing it (“re-oligotrophication”) has strongly focused on P limitation in temperate lakes, as demonstrated by the meta-analyses discussed above. Meanwhile, there is ample evidence from field data that N-limitation is frequently relevant during summer, particularly in shallow lakes (e.g. Jeppesen, 2007; Kolzau et al., 2014; Xu et al., 2010; Maberly et al., 2020). An important analysis of a large data set in this context is that of Søndergaard et al. (2017) using data for >3000 lake-years from >800 Danish lakes (most of which are quite eutrophic and 86% of which are shallow, i.e. with a mean depth < 3 m). These authors show that the carrying capacity for phytoplankton biomass (as Chl.-a) was limited by P if TN concentrations exceed 500 µg/l; vice versa it was limited by N if TP concentrations exceed 50–100 µg/l.
Conclusions and consequences from Colin’s concepts
Colin Reynolds has consolidated our understanding of phytoplankton nutrient uptake, cell division rates and how at any given point of time in the season one resource sets the upper limit to carrying capacity for biomass. He frequently pointed to the falacity of interpreting low N:P ratios as indication of N-limitation (Reynolds, 1998, 1999). In part based on this understanding, a large body of research on functional traits in relation to cell size and morphology has emerged, addressing how these features affect competition for nutrients and light. Numerous enrichment experiments have addressed nutrient limitation, often together with field data on N:P ratios, and their results have been interpreted to show that co-limitation between N and P occurs more frequently than expected from the well-studied relationship of phytoplankton biomass to TP. However, very few of these experiments included light limitation. This limits the applicability of their results to the field situation from which the experimental populations were sampled. The decisive role of light limitation in eutrophic and hypertrophic waterbodies is evident from the theoretical considerations by, e.g. Reynolds (1992). It is also seen in the trajectories of those lakes who showed no reduction of phytoplankton biomass (measured in terms of Chl.-a) along the course of their trophic recovery until the limiting nutrient (so far almost always TP) undercut a threshold below which limitation switched from light to TP (Lakes Constance, Tegel and Schlachtensee in Fig. 2).
One—trivial—conclusion from this state of knowledge is that the relevance of co-limitation is limited to situations in which not light is limiting, but both P and N are in a concentration range low enough to potentially be limiting. For this, two criteria need to be met, i.e.
-
1.
dissolved nutrient concentrations low enough to limit uptake rates, i.e. SRP < 3–10 µg/l and DIN <100–130 µg/l, and
-
2.
total nutrient concentrations low enough to limit the carrying capacity for biomass, with thresholds for this depending somewhat on the mixing depths of the epilimnion or waterbody and other (mineral) water constituent causing turbidity. For TP thresholds for limitation are above 50 µg/l only in shallow waterbodies or shallow epilimnia; for TN they will be about 7-fold higher.
Research about nutrient limitation—be it through enrichment experiments or evaluation of N:P ratios—needs to take this into account when deriving conclusions for the phytoplankton field population: even if N:P ratios are in a range around the Redfield ratio or growth responses are substantially higher when adding both N and P, such results do not indicate co-limitation if the concentration of either nutrient—or that of both—is well above a level at which N or P limitation is a realistic option. This is important for research on species composition and diversity, which tends to be highest at intermediate nutrient levels and intermediate N:P ratios; e.g. where community co-limitation supports coexistence (Brauer et al., 2012).
For eutrophication management recognising situations with pronounced N-limitation provides a basis for assessing whether this can be strengthened. However, while numerous authors (Elser et al., 1990; Moss et al., 2013; Kolzau et al., 2014; Søndergaard et al., 2017) emphasise the pronounced prevalence of extended phases of N-limitation in shallow lakes during summer (often due to denitrification on warm, organic-rich sediment surfaces), some of these authors explicitly point out that this does not argue against a focus on P-reduction which “will generally be a more reliable means to achieve lower standing stocks in the long term” (Elser et al., 1990, p. 1475; see also Moss et al., 2013). Even publications arguing a need to control N loads generally do not refute the need to control P, but rather call for a dual strategy of controlling both N and P. However, a key point to learn from Colin Reynolds is that from a current situation of N- or co-limitation it does not follow that we need to reduce both nutrients to mitigate eutrophication. Rather, reducing only one of the two will be effective when achieving sufficiently low N or P concentrations. Liebig’s law of the limiting nutrient continues to hold.
“Sufficient” is the magic word in this context. Achieving limiting P concentrations can take years or decades, even after drastic load reduction (e.g. Schauser & Chorus, 2007). Obstacles to achieving a pronounced P load reduction can be substantial, and a high internal load from the sediment coupled with a high retention time will extend the time it takes to export such legacy P. Reducing N loads in such situations may be an option supporting trophic recovery, and it can be particularly attractive if substantial denitrification supports loss of N from the system (Paerl et al., 2019). In specific situations a dual strategy might focus on P for longer-term control and combine this with targeted measures to reduce N loads during specific weeks or months of the year during which N-limitation can be effective (Kolzau et al., 2014). This requires a situation in the catchment in which, e.g. timing of the application of fertiliser or manure can be accordingly negotiated with the farmers. In practice, the local, site-specific feasibility of such approaches will be a most relevant criterion when planning measures to reduce nutrient loads.
“Site-specific assessments and decisions” is the other magic term in this context, quite in contrast to generalisations derived from the assessment of the current situation in a given waterbody or even from meta-analyses of many. Encouragingly, the need for management based on site-specific assessment is increasingly acknowledged in the development of regulations: e.g. the European Water Framework Directive requires basin-specific management plans. The World Health Organisation’s (WHO) concept of developing site-specific ‘Water Safety Plans’ specifies this approach, advising an assessment of the specific risks in a given water supply, from catchment to consumer, and the development of the locally most suitable measures to control them. This explicitly includes toxic cyanobacteria and eutrophication. Moreover, WHO advises to form a team for conducting the risk assessment and to develop the management approach which includes local stakeholders such as sewage treatment operators and farmers, but also those with the necessary scientific expertise, such as limnologists (Rickert et al., 2016). The concept of developing locally specific Water Safety Plans thus provides a framework for developing and applying specific measures to control N and P at times and at sites where their effect on phytoplankton control is most promising. This may facilitate the implementation of small-scale local measures, such as a local agreement with the farmers in the catchment area to reduce N loads during certain weeks in summer.
There is a wide range of reasons to reduce emissions of N to the environment, e.g. reducing greenhouse gases (including those from incomplete denitrification), preventing the acidification of soils and waterbodies (from ammonia precipitation), maintaining biodiversity and preventing the oxidation of toxic metals such as uranium by nitrate in the underground (which renders them soluble and can cause them to leach to drinking-water wells; van Berk & Fu, 2017). Per se, however, higher growth responses when enriching field phytoplankton communities with both N and P or N:P ratios around that of Redfield do not generally call for reducing the loads of N nor of both nutrients.
Research on nutrient stoichiometry, biochemical mechanisms of co-limitation and functional traits is promoting our understanding of ecological interactions within phytoplankton communities. The results so far are well aligned with the basic principles of how resource limitation shapes phytoplankton communities that Colin Reynolds so aptly consolidated—both in his theoretical considerations, his teaching as well as with data from the large number of phytoplankton community studies in which he was involved. His deep understanding of algal cells moved around in the mixed layer of a waterbody can still support research groups around the world for capturing ecological processes determining phytoplankton growth and yield.
References
Andersen, I. M., T. J. Williamson, M. J. González & M. J. Vanni, 2020. Nitrate, ammonium, and phosphorus drive seasonal nutrient limitation of chlorophytes, cyanobacteria, and diatoms in a hyper-eutrophic reservoir. Limnology and Oceanography 65: 962–978.
Arrigo, K. R., 2005. Marine microorganisms and global nutrient cycles. Nature 437: 349–355.
Bergström, A. K., A. Jonsson, P. D. Isles, I. F. Creed & D. C. Lau, 2020. Changes in nutritional quality and nutrient limitation regimes of phytoplankton in response to declining N deposition in mountain lakes. Aquatic Sciences 82: 1–16.
Bracken, M. E., H. Hillebrand, E. T. Borer, E. W. Seabloom, J. Cebrian, E. E. Cleland & J. E. Smith, 2015. Signatures of nutrient limitation and co-limitation: responses of autotroph internal nutrient concentrations to nitrogen and phosphorus additions. Oikos 124: 113–121.
Brandenburg, K., L. Siebers, J. Keuskamp, T. G. Jephcott & D. B. Van de Waal, 2020. Effects of nutrient limitation on the synthesis of N-rich phytoplankton toxins: a meta-analysis. Toxins 12: 221–235.
Brauer, V. S., M. Stomp & J. Huisman, 2012. The nutrient-load hypothesis: patterns of resource limitation and community structure driven by competition for nutrients and light. The American Naturalist 179: 721–740.
Buitenhuis, E. T., K. R. Timmermans & H. J. de Baar, 2003. Zinc-bicarbonate colimitation of Emiliania huxleyi. Limnology and Oceanography 48: 1575–1582.
Carpenter, S. R., 2008. Phosphorus control is critical to mitigating eutrophication. Proceedings of the National Academy of Sciences 105: 11039–11040.
Chorus, I., M. Dokulil, E. Lammens, M. Manca, L. Naselli-Flores, B. Nixdorf, G. Persson, D. Schindler, D. Straile, I. Tátrai & W. E. Tadonléké, 2011. Restoration responses of 19 lakes: are TP thresholds common? In Chorus, I. & I. Schauser (eds) Oligotrophication of Lake Tegel and Schlachtensee, Berlin – Analysis of System Components, Causalities and Response Thresholds Compared to Responses of Other Waterbodies. UBA Texte 45.
Conley, D. J., H. W. Paerl, R. W. Howarth, D. F. Boesch, S. P. Seitzinger, K. E. Havens, C. Lancelot & H. E. Likens, 2009. Controlling eutrophication: nitrogen and phosphorus. Science 323: 1014–1015.
Conroy, J. D., D. D. Kane, D. M. Dolan, W. J. Edwards, M. N. Charlton & D. A. Culver, 2005. Temporal trends in Lake Erie plankton biomass: roles of external phosphorus loading and dreissenid mussels. Journal of Great Lakes Research 31: 89–110.
Davidson, E. A. & R. W. Howarth, 2007. Nutrients in synergy. Nature 449: 1000–1001.
Davies, P. 2004. Nutrient processes and chlorophyll in the estuaries and plume of the Gulf of Papua. Continental Shelf Research 24: 317–2341.
Dillon, P. J. & F. H. Rigler, 1974. The phosphorus-chlorophyll relationship in lakes. Limnology and Oceanography 19: 767–773.
Dokulil, M. T., K. Teubner & K. Donabaum, 2000. Restoration of a shallow, groundwater fed urban lake using a combination of internal management strategies: a case study. Advances in Limnology 55: 271–282.
Dolman, A. M. & C. Wiedner, 2015. Predicting phytoplankton biomass and estimating critical N:P ratios with piecewise models that conform to Liebig’s law of the minimum. Freshwater Biology 60: 686–697.
Dolman, A. M., J. Rücker, F. R. Pick, J. Fastner, T. Rohrlack, U. Mischke & C. Wiedner, 2012. Cyanobacteria and cyanotoxins: the influence of nitrogen versus phosphorus. PLoS ONE 7: 38757.
Edwards, K. F., C. A. Klausmeier, & E. Litchman, 2011. Evidence for a three‐way trade‐off between nitrogen and phosphorus competitive abilities and cell size in phytoplankton. Ecology 92: 2085–2095.
Edwards, K. F., M. K. Thomas, C. A. Klausmeier & E. Litchman, 2012. Allometric scaling and taxonomic variation in nutrient utilization traits and maximum growth rate of phytoplankton. Limnology and Oceanography 57: 554–566.
Elser, J. J., E. R. Marnoif & C. R. Goldman, 1990. Phosphorus and nitrogen limitation of phytoplankton growth in the freshwaters of North America: a review and critique of experimental enrichments. Canadian Journal of Fisheries and Aquatic Science 47: 1468–1477.
Elser, J. J., M. E. Bracken, E. E. Cleland, D. S. Gruner, W. S. Harpole, H. Hillebrand & J. E. Smith, 2007. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecology Letters 10: 1135–1142.
Elser, J. J., T. Andersen, J. S. Baron, A. K. Bergstrom, M. Jansson, M. Kyle, K. R. Nydick, L. Steger & D. O. Hessen, 2009. Shifts in lake N:P stoichiometry and nutrient limitation driven by atmospheric nitrogen deposition. Science 326: 835–837.
Fastner, J. & A. R. Humpage, in press. Hepatotoxic cyclic peptides – microcystins and nodularins. In: Chorus, I. & M. Welker (eds), Toxic Cyanobacteria in Water, 2nd ed. World Health Organisation, Geneva.
Fastner, J., S. Abella, A. Litt, G. Morabito, L. Vörös, K. Pálffy & I. Chorus, 2016. Combating cyanobacterial proliferation by avoiding or treating inflows with high P load – experiences from eight case studies. Aquatic Ecology 50: 367–383.
Finkel, Z. V., J. Beardall, K. J. Flynn, A. Quigg, T. Rees & J. A. Raven, 2010. Phytoplankton in a changing world: cell size and elemental stoichiometry. Journal of Plankton Research 32: 119–137.
Gaedke, U., S. Hochstädter & D. Straile, 2002. Interplay between energy limitation and nutritional deficiency: empirical data and food web models. Ecological Monographs 72: 251–270.
Geider, R. & J. La Roche, 2002. Redfield revisited: variability of C:N:P in marine microalgae and its biochemical basis. European Journal of Phycology 37: 1–17.
Glass, J. B., F. Wolfe-Simon, J. J. Elser & A. D. Anbar, 2010. Molybdenum–nitrogen co-limitation in freshwater and coastal heterocystous cyanobacteria. Limnology and Oceanography 55: 667–676.
Gobler, C. J., J. M. Burkholder, T. W. Davis, M. J. Harke, T. Johengen, C. A. Stow & D. B. van de Waal, 2016. The dual role of nitrogen supply in controlling the growth and toxicity of cyanobacterial blooms. Harmful Algae 54: 87–97.
Harpole, W. S., J. T. Ngai, E. E. Cleland, E. W. Seabloom, E. T. Borer, M. Bracken, J. J. Elser, D. S. Gruner, H. Hillebrand, J. B. Shurin & J. E. Smith, 2011. Nutrient co-limitation of primary producer communities. Ecology Letters 14: 852–862.
Hessen, D. O., J. J. Elser, R. W. Sterner & J. Urabe, 2013. Ecological stoichiometry: an elementary approach using basic principles. Limnology and Oceanography 58: 2219–2236.
Heinzmann, B. & I. Chorus, 1994. Restoration concept for Lake Tegel, a major drinking and bathing water resource in a densely populated area. Environmental Science & Technology 28: 1410–1416.
Holland, D., S. Roberts, & J. Beardall, 2004. Assessment of the nutrient status of phytoplankton: a comparison between conventional bioassays and nutrient-induced fluorescence transients (NIFTs). Ecological Indicators 4: 149–159.
Jeppesen, E., M. Søndergaard, J. P. Jensen, K. E. Havens, O. Anneville, L. Carvalho, M. F. Coveney, R. Deneke, M. T. Dokulil, B. Foy, D. Gerdeaux, S. E. Hampton, S. L. Hilt, K. L. Kangur, J. Köhler, E. Lammens, T. L. Lauridsen, M. Manca, M. R. Miracle, B. Moss, P. Noges, G. Persson, G. L. Phillips, R. Portielje, S. Romo, C. L. Schelske, D. Straile, I. Tatrai, E. Willén & M. Winder, 2005. Lake responses to reduced nutrient loading – an analysis of contemporary long-term data from 35 case studies. Freshwater Biology 50: 1747–1771.
Jeppesen, E., M. Søndergaard, M. Meerhoff, T. L. Lauridsen, & J. P. Jensen, 2007. Shallow lake restoration by nutrient loading reduction — some recent findings and challenges ahead. In Shallow Lakes in a Changing World. Springer, Dordrecht: 239–252.
Jones, R. A. & G. F. Lee, 1986. Eutrophication modelling for water quality management: an update of the Vollenweider-OECD model. World Health Organization Water Quality Bulletin 11: 67–74.
Katkov, E., É. Low-Décarie & G. F. Fussmann, 2020. Intra-annual variation of phytoplankton community responses to factorial N, P, and CO2 enrichment in a temperate mesotrophic lake. Freshwater Biology 65: 960–970.
Klausmeier, C. A., E. Litchman, T. Daufresne & S. A. Levin, 2004. Optimal nitrogen-to-phosphorus stoichiometry of phytoplankton. Nature 429: 171–174.
Klausmeier, C. A., E. Litchman & S. A. Levin, 2007. A model of flexible uptake of two essential resources. Journal of Theoretical Biology 246: 278–289.
Kolzau, S., C. Wiedner, J. Rücker, J. Köhler, A. Köhler & A. M. Dolman, 2014. Seasonal patterns of nitrogen and phosphorus limitation in four German lakes and the predictability of limitation status from ambient nutrient concentrations. PLoS ONE 9: e96065.
Lachmann, S. C., T. Mettler-Altmann, A. Wacker & E. Spijkerman, 2019. Nitrate or ammonium: Influences of nitrogen source on the physiology of a green alga. Ecology and evolution 9: 1070–1082.
Lampert, W. & U. Sommer, 2007. Limnoecology: The Ecology of Lakes and Streams. Oxford University Press, Oxford.
Liebig, J., 1840. Die organische Chemie in ihrer Anwendung auf Agrikultur und Physiologie. Friedrich Vieweg Verlag, Braunschweig, Germany.
Lukas, M., E. Sperfeld & A. Wacker, 2011. Growth Rate Hypothesis does not apply across colimiting conditions: cholesterol limitation affects phosphorus homoeostasis of an aquatic herbivore. Functional Ecology 25: 1206–1214.
Maberly, S. C., J. A. Pitt, P. S. Davies & L. Carvalho, 2020. Nitrogen and phosphorus limitation and the management of small productive lakes. Inland Waters 100: 1–14.
Mischler, J. A., P. G. Taylor & A. R. Townsend, 2014. Nitrogen limitation of pond ecosystems on the plains of eastern Colorado. PLoS ONE 9: e95757.
Moon, J. B. & H. J. Carrick, 2007. Seasonal variation of phytoplankton nutrient limitation in Lake Erie. Aquatic Microbial Ecology 48: 61–71.
Moss, B., E. Jeppesen, M. Søndergaard, T. L. Lauridsen & Z. Liu, 2013. Nitrogen, macrophytes, shallow lakes and nutrient limitation: resolution of a current controversy? Hydrobiologia 710: 3–21.
Müller, S. & S. M. Mitrovic, 2015. Phytoplankton co-limitation by nitrogen and phosphorus in a shallow reservoir: progressing from the phosphorus limitation paradigm. Hydrobiologia 744: 255–269.
North, R. L., S. J. Guildford, R. E. H. Smith, S. M. Havens & M. R. Twiss, 2007. Evidence for phosphorus, nitrogen, and iron colimitation of phytoplankton communities in Lake Erie. Limnology and Oceanography 52: 315–328.
Paerl, H. W. & T. G. Otten, 2016. Duelling ‘CyanoHABs’: unravelling the environmental drivers controlling dominance and succession among diazotrophic and non-N2-fixing harmful cyanobacteria. Environmental Microbiology 18: 316–324.
Paerl, H. W., K. E. Havens, H. Xu, G. Zhu, M. J. McCarthy, S. E. Newell & B. Qin, 2019. Mitigating eutrophication and toxic cyanobacterial blooms in large lakes: the evolution of a dual nutrient (N and P) reduction paradigm. Hydrobiologia. https://doi.org/10.1007/s10750-019-04087-y.
Phillips, G., A. Kelly, J. A. Pitt, R. Sanderson & E. Taylor, 2005. The recovery of a very shallow eutrophic lake, 20 years after the control of effluent derived phosphorus. Freshwater Biology 50: 1628–1638.
Phillips, G, O. P. Pietiläinen, L. Carvalho, A. Solimini, A. Lyche Solheim & A. C. Cardoso, 2008. Chlorophyll-nutrient relationships of different lake types using a large European dataset. Aquatic Ecology 42: 213–226
Quigg, A., A. J. Irwin & Z. V. Finkel, 2011. Evolutionary inheritance of elemental stoichiometry in phytoplankton. Proceedings of the Royal Society 278: 526–534.
Redfield, A. C., 1934. On the proportions of organic derivatives in sea water and their relation to the composition of plankton. In: James Johnstone Memorial Voume. University of Liverpool, 176–192.
Reynolds, C. S., 1984. The Ecology of Freshwater Phytoplankton, 2nd ed. Cambridge University Press, Cambridge.
Reynolds, C. S., 1992. Eutrophication and the management of planktonic algae: what Vollenweider couldn’t tell us. In Sutcliffe, D. W. & J. G. Jones (eds), Eutrophication: Research and Application to Water Supply. The Freshwater Biological Association, Ambleside, Cumbria: 4–29.
Reynolds, C. S., 1998. What factors influence the species composition of phytoplankton in lakes of different trophic status? Hydrobiologia 369: 11–26.
Reynolds, C. S., 1999. Non-determinism to Probability, or N:P in the community ecology of phytoplankton. Archiv für Hydrobiologie 146: 23–35.
Rickert, B., I. Chorus & O. Schmoll, 2016. Protecting Surface Water for Health. Identifying Assessing and Managing Drinking-Water Quality Risks in Surface-Water Catchments. World Health Organization, Geneva.
Saito, M. A., T. J. Goepfert & J. T. Ritt, 2008. Some thoughts on the concept of colimitation: three definitions and the importance of bioavailability. Limnology and Oceanography 53: 276–290.
Schauser, I. & I. Chorus, 2007. Assessment of internal and external lake restoration measures for two Berlin lakes. Lake and Reservoir Management 23: 366–376.
Schauser, I., I. Chorus & J. Lewandowski, 2006. Effects of nitrate on phosphorus release: comparison of two Berlin lakes. Acta Hydrochimica et Hydrobiologica 34: 325–332.
Scheffer, M., S. H. Hosper, M.-L. Meijer, B. Moss & E. Jeppesen, 1993. Alternative equilibria in shallow lakes. Trends in Ecology and Evolution 8: 275–279.
Schindler, D. W. 1977. Evolution of phosphorus limitation in lakes. Science 195: 260–262.
Schindler, D. W., 2009. A personal history of the Experimental Lakes Project. Canadian Journal of Fisheries and Aquatic Sciences 66: 1837–1847.
Schindler, D. W., 2012. The dilemma of controlling cultural eutrophication of lakes. Proceedings of the Royal Society B: Biological Sciences 279: 4322–4333.
Schindler, D. W., S. R. Carpenter, S. C. Chapra, R. E. Hecky & D. M. Orihel, 2016. Reducing phosphorus to curb lake eutrophication is a success. Environmental Science & Technology 50: 8923–8929.
Schrader, P. S., A. J. Milligan & M. J. Behrenfeld, 2011. Surplus photosynthetic antennae complexes underlie diagnostics of iron limitation in a cyanobacterium. PLoS ONE 6: e18753.
Scott, J. T., M. J. McCarthy & H. W. Paerl, 2019. Nitrogen transformations differentially affect nutrient-limited primary production in lakes of varying trophic state. Limnology and Oceanography Letters 4: 96–104.
Scott, J. T. & M. J. McCarthy, 2010. Nitrogen fixation may not balance the nitrogen pool in lakes over timescales relevant to eutrophication management. Limnology & Oceanography 55: 1265–1270.
Shatwell, T. & J. Köhler, 2019. Decreased nitrogen loading controls summer cyanobacterial blooms without promoting nitrogen-fixing taxa: long-term response of a shallow lake. Limnology and Oceanography 64: 166–178.
Shelly, K., D. Holland & J. Beardall, 2010. Assessing nutrient status of microalgae using chlorophyll a fluorescence. In Suggett, D. J., M. A. Borowitzka & O. Prasil (eds), Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications. Springer, Dordrecht: 223–235.
Smith, V. H., 1990. Nitrogen, phosphorus, and nitrogen fixation in lacustrine and estuarine ecosystems. Limnology and Oceanography 35: 1852–1859.
Smith, V. H., 2003. Eutrophication of freshwater and coastal marine ecosystems a global problem. Environmental Science and Pollution Research 10: 126–139.
Sommer, U., R. Adrian, L. D. Domis, J. J. Elser, U. Gaedke, B. Ibelings, et al., 2012. Beyond the Plankton Ecology Group (PEG) model: mechanisms driving plankton succession. Annual Review of Ecology, Evolution and Systematics 43: 429–448.
Søndergaard, M., T. L. Lauridsen, L. S. Johansson & E. Jeppesen, 2017. Nitrogen or phosphorus limitation in lakes and its impact on phytoplankton biomass and submerged macrophyte cover. Hydrobiologia 795: 35–48.
Spijkerman, E. & P. F. M. Coesel, 1997. Growth kinetic parameters of two planktonic desmid species under fluctuating phosphorus conditions in continuous-flow culture. J. Plankton Res. 19(12): 1899–1912.
Spijkerman, E., F. De Castro & U. Gaedke, 2011. Independent colimitation for carbon dioxide and inorganic phosphorus. PLoS One 6: e28219.
Spijkerman, E., H. Behrend, B. Fach & U. Gaedke, 2018. Decreased phosphorus incorporation explains the negative effect of high iron concentrations in the green microalga Chlamydomonas acidophila. Science of The Total Environment 626: 1342–1349.
Sterner, R. W., 2008. On the phosphorus limitation paradigm for lakes. International Review of Hydrobiology 93: 433–445.
Sterner, R. W. & J. J. Elser, 2002. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. Princeton University Press, Princeton.
Tilman, D., S. S. Kilham & P. Kilham, 1982. Phytoplankton community ecology: the role of limiting nutrients. Annual Review of Ecology and Systematics 13: 349–372.
Wagner, N. D., F. S. Osburn, J. Wang, R. B. Taylor, A. R. Boedecker, C. K. Chambliss, B. W. Brooks & J. T. Scott, 2019. Biological stoichiometry regulates toxin production in Microcystis aeruginosa (UTEX 2385). Toxins 11: 601–618.
van Berk, W. & Y. Fu, 2017. Redox roll-front mobilization of geogenic uranium by nitrate input into aquifers: risks for groundwater resources. Environmental Science & Technology 51: 337–345.
Vollenweider, R. A., 1968. The Scientific Basis of Lake and Stream Eutrophication with Particular Reference to Phosphorus and Nitrogen as Factors in Eutrophication. Organization for Economic Cooperation and Development, Paris, France, DAS/CSI/67-27.
Vollenweider, R. A. & J. Kerekes, 1980. The loading concept as basis for controlling eutrophication: philosophy and preliminary results of the OECD programme on eutrophication. Progress in Water Technology 12: 5–38.
Wauer, G., T. Gonsiorczyk, K. Kretschmer, P. Casper & R. Koschel, 2005. Sediment treatment with a nitrate-storing compound to reduce phosphorus release. Water Research 39: 494–500.
Willén, E., 2001. Phytoplankton and water quality characterization: experiences from the Swedish large lakes Mälaren, Hjälmaren, Vättern and Vänern. A Journal of the Human Environment 30: 529–537.
Xu, H., H. W. Paerl, B. Qin, G. Zhu & G. Gao, 2010. Nitrogen and phosphorus inputs control phytoplankton growth in eutrophic Lake Taihu, China. Limnology and Oceanography 55: 420–432.
Acknowledgements
Open Access funding provided by Projekt DEAL. We thank two anonymous reviewers for very supportive and helpful comments and Uli Sommer for his review of the revised version.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Judit Padisák, J. Alex Elliott, Martin T. Dokulil & Luigi Naselli-Flores / New, old and evergreen frontiers in freshwater phytoplankton ecology: the legacy of Colin S. Reynolds
Ingrid Chorus, formerly Department of Drinking Water and Swimming Pool Hygiene, Federal Environment Agency, Berlin, Germany
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chorus, I., Spijkerman, E. What Colin Reynolds could tell us about nutrient limitation, N:P ratios and eutrophication control. Hydrobiologia 848, 95–111 (2021). https://doi.org/10.1007/s10750-020-04377-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-020-04377-w