Abstract
Mediterranean endorheic wetlands are strongly affected by local meteorological events, so they undergo frequent unpredictable disturbances, such as episodes of high salinity or desiccation. In this context, salinity and temperature may be crucial for determining the structure of zooplankton communities and regional biodiversity, since they may trigger the hatching of egg bank in different ways. The goal of this study is to assess the combined role of these two variables on the zooplankton assemblage emerging from the egg bank. We hypothesize that temperature and salinity affect the community structure in a non-linear way, that is, both factors interact and modify the magnitude of their effects. We performed a laboratory factorial design where the same sediment was incubated under different thermal and salinity conditions, reducing the potential effects of other possible confusion factors. Community structure was described by measuring cumulative abundances, species composition, richness, and diversity. Our results showed that the community structure was strongly determined by salinity at all experimental temperatures. In contrast, the magnitude of the temperature effect depended on salinity. The high variability among replicates when salinity and temperature increased suggests that climate change might lead to unpredictable patterns of the community emerging from the egg bank.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wetlands in drylands areas are strongly affected by local meteorological events, particularly precipitation and temperature patterns. Owing to this strong climatic influence, these ecosystems undergo frequent unpredictable disturbances, such as episodes of high salinity or desiccation. This changing environment translates into a marked year on year variability of both biotic and abiotic variables, which in turn results in zooplankton assemblages that change from a physically controlled pattern (saline stress) to a biological controlled pattern—dominance of grazing and competition—(Castro, 2004). Shortened hydroperiods may emphasize the issue of increased salinity, enhancing internal nutrient recycling and bringing about drastic changes to the composition of zooplankton communities and in the trophic status and food webs of these ecosystems (Beklioglu et al., 2007; Gilbert et al., 2017, 2021; Florencio et al., 2020).
The organisms living in these water bodies have developed strategies that allow them to survive and develop in very fluctuating and unpredictable conditions. One such strategy is the formation of resting structures that let the species escape in space (i.e., dispersal by currents, wind, waterfowl) and in time. Many organisms, from microbial eukaryotes (Esteban & Finlay, 2003; Galotti et al., 2014), to rotifers (Declerck & Papakostas, 2017) and to crustaceans (Lenormand et al., 2018), can withstand unfavorable environmental conditions in the form of inactive cysts, spores, diapausing eggs, or other dormant stages, until there are favorable conditions for growth. Entrance and exit in a dormancy stage can affect the ecosystem structure, both on a population and community level, influencing how these populations will respond to environmental changes. Egg banks extend the overlapping of generations, which can play an important role in maintaining diversity in a fluctuating environment when different types (species or genotypes) are favored at different times (Hairston & Kearns, 2002). Therefore, it is very important to know what environmental factors trigger each species to leave their dormant stages.
In literature, a great deal of research can be found on the role of temperature and photoperiod in the end of dormancy (see Gyllström & Hansson, 2004 and cites inside); somewhat less abundant are the works on the role of salinity (Newton & Mitchell, 1999; García-Roger et al., 2008; Waterkeyn et al., 2010, 2011; Branstrator et al., 2013; Mabidi et al., 2018). Data on the combined effect of temperature and salinity are even scarcer; they have been performed in other types of aquatic ecosystems or do not include the entire zooplankton community (Newton & Mitchell, 1999; Bailey et al., 2006; Conde-Porcuna et al., 2018).
In saline Mediterranean ponds, salinity is expected to be crucial in determining community structure as it fluctuates greatly, both seasonally and inter annually. Although high temperatures can increase salinity due to evaporation, the saline status depends mainly on the general precipitation regime and local hydrogeology. Thus, salinity can be as important as photoperiod and temperature in causing the hatching of resting eggs, as well as in determining the structure of the communities by encouraging one species to hatch instead of another (López-González et al., 1998). This might be particularly important in the current context of global change since an increase in both temperature and salinity is expected; consequently, the potential combined effects of both variables should be considered. On the other hand, a decrease in salinity of endorheic saline ponds is also possible due to the intensive irrigation of agricultural lands with water from other sub-basins.
Zooplankton is key to many ecological functions in wetlands; they are important in nutrient cycling (Merritt et al., 1984) and provide trophic support for many species. Knowing the mechanism that determines the behavior and dynamics of zooplankton egg banks could be crucial for preserving cryptic biodiversity and the resilience of wetlands, which play an important role in the maintenance of regional biodiversity (Gilbert et al., 2014, 2015) and are globally the most common and widespread inland bodies of water (Downing et al., 2006; Downing, 2010).
The main goal of our study was to analyze how the combined effect of temperature and salinity influence zooplankton assemblages emerging from egg banks in Mediterranean saline ponds. We hypothesized a reduction in zooplankton species abundance, richness, and diversity that emerge from the egg bank under stress conditions, with a heterogeneous and unpredictable emerging pattern. This hypothesis is based on previous results obtained by our research group in the same pond (López-González et al., 1998), in which environmental factors play a major role in shaping the plankton community. The changes in the community structure, which usually involve a simplification of the community during the drying period (with an increase of temperature and salinity), may be considered a consequence of stressing conditions that induces qualitative and quantitative changes in the community structure. Temporary saline ponds show more acute changes in environmental conditions (García & Niell, 1993), which can usually be assessed by analyzing diversity or some other population parameter (Hawkins et al., 1994). Moreover, López-González et al. (1998) also predicted the complex plankton dynamics and stochasticity inherent in hypersaline ponds that it is expected to manifest here, with a heterogeneous and unpredictable emerging pattern of the zooplankton assemblages. To test these, we performed an experimental design where the same sediment was incubated under different thermal and salinity conditions. We measured the abundances, richness, and diversity of the microcrustaceans and rotifers hatching in each treatment group. Unlike in field studies, this controlled scenario reduces the potential effects of possible confusion factors and enables us to gain a clearer insight into the role salinity and temperature play on the hatching pattern, as well as their potential interactive effect.
Methods
Study site and sampling
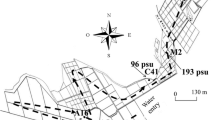
Sediment was collected from the Laguna Honda Natural Reserve (Alcaudete, Jaén: 37º 35′ 54″ N; 4º 8′ 34″ W), a non-coastal permanent athalasohaline shallow inland pond (460 m a.s.l.) located in an endorheic area in the Guadalquivir river watershed (Andalusia, southern Spain) and which has a basin surface of 9.94 ha (Castro et al., 2003). As naturally happens in most of the endorheic ponds of this region, there are no fishes in Laguna Honda. Its biological communities have adapted to an environment characterized by a marked year-on-year variability on both biotic and abiotic variables (Guerrero & Castro, 1997; López-González et al., 1998).
Laguna Honda is primarily mesosaline and is occasionally hypersaline or hyposaline. Its water is saline chloride, with a predominance of sodium chloride − Cl-Na-Mg-SO4 chemical subtype − (Castro, 2004). Maximum depth is 3.16 m, it is only reached in especially wet periods.
In extraordinary periods of drought, Laguna Honda can dry completely: the last times this happened was in the summer of 1995 and, more recently, in late 2021, that is, this pond has been permanent for more than two decades. Laguna Honda experiences large fluctuations in the water level (Fig. 1) and, therefore, in its salinity. The highest salinity recorded by our research team at Laguna Honda has been upper 300 g/L, in 1995 (Guerrero & Castro, 1997); in contrast, the lowest was 8.68 g/L, in July 2014. On the day the samples were taken, the salinity of the pond was 9.12 g/L.
Location of the five sampling stations. a Orthophotograph of Laguna Honda in a wet year (2013) and b the most current orthophotograph available (2019) (Sistema Cartográfico Nacional de España, 2013, 2019). c Bathymetric map; the interval between isolines is 0.5 m (modified from Castro et al., 2003)
As a consequence of the large fluctuations, dormant organisms with very different halotolerance ranges remain in the egg bank of Laguna Honda, waiting for years for favorable conditions that allow them to hatch. All these characteristics make Laguna Honda a perfect natural laboratory for studying the role of temperature and salinity in the structure of the community emerging from the egg bank.
In the first fortnight of August 2014, samples of sediment were collected with a KC Kajak Sediment Core sampler (5 cm internal diameter). To reduce the effect of spatial heterogeneity, 5 samples were randomly taken at a similar depth (2 to 2.5 m) and in patches without macrophytes (Fig. 1). The first 5 cm of each corer were collected. In order to have enough sediment, in each sampling station three pseudo-replicates were taken, which were mixed and homogenized manually in the storage bag. Sediment of each sampling station were separately stored. In this way, we have 5 replicates for the following experiments. Samples were transported under cold and dark conditions to the laboratory. Then in the lab, sediment was air-dried, separately, for at least 5 months to ensure that only resting structures remained before hatching experiments were set-up (Ning et al., 2008, 2010; Ning & Nielsen, 2011).
Experimental design
A fully crossed 2 × 3 factorial design was performed, with two levels for temperature factor (20ºC and 25ºC) and three levels for salinity factor: S1 (15 g/L; 14.4 mS/cm), S2 (26.7 g/L; 24.8 mS/cm), and S3 (45 g/L; 34.6 mS/cm). These ranges of salinity and temperature were chosen because they occur more frequently in Laguna Honda. Each combination (treatment) was replicated 5 times (each sampling station corresponds to one replicate). Water salinity was estimated by measuring water conductivity and looking for its equivalence in g/L in regression lines of conductivity versus salinity (Jiménez-Melero, 2007).
Water from Laguna Honda, previously filtered through a Whatman GFC filter, was taken to the experimental salinities by dilution-evaporation processes. When dilution was necessary, distilled water was added. For evaporation, water was gently warmed with a heater.
For each treatment, 10 g of dry sediment was placed in a one-liter glass beaker (10 cm Ø). Each beaker was filled with 500 mL of the previously prepared water at the desired salinity. Beakers were partially closed to prevent excessive evaporation and incubated in a cultivation chamber at the temperature of interest. Photoperiod was 12-h dark and 12-h light.
The water in the beakers was gently filtered (40-µm mesh size) and the captured individuals were examined under a binocular microscope (Leica MZ12.5) twice a week for two months. Water was oxygenated with air bubbles at every check and renewed fortnightly. In order to prevent reproduction, any newborns detected were immediately removed by means of a Pasteur pipette. They were individually placed on cell-tissue plates, incubated at the same experimental conditions that the origin beaker and fed on a mixed diet of Chlamydomonas reinhardtii and Tetraselmis suecica algae until they reached an age at which they could be identified taxonomically. Since in Iberian saline ponds there usually is only one species of ostracod, even though exceptionally a maximum of two coexist (Baltanás et al., 1990; Gilbert et al., 2023), to achieve a trade-off between estimates precision and time management, ostracods were not identified at the species level. We record the abundances of each species (or taxa) accumulated after 60 days of incubation.
Statistical analysis
A shade plot was used to visualize the abundance patterns of taxa on the 6 treatments after 60 days of incubation (Clarke & Gorley, 2015). A non-metric multi-dimensional scaling (nMDS) ordination plot based on the Bray–Curtis similarities among treatments was performed; the closer points are to each other the more similar is their community composition (Clarke & Gorley, 2015). Success of the ordination was measured by a stress coefficient (Clarke et al., 2014).
To detect differences in community composition between treatments after 60 days at different salinity and temperature conditions, a multivariate analysis of the community data was performed using a Permutational Analysis of Variance (PERMANOVA). This analysis was undertaken with 9999 permutations on Bray–Curtis similarity matrices. A significant result for a given factor from PERMANOVA signifies that the groups could differ in their location, in their dispersion or some combination of the two (Anderson et al., 2008). In order to measure and test homogeneity of multivariate dispersions among treatments, a test of homogeneity of dispersions (PERMDISP) was performed. In addition, the routine MVDISP provided by PRIMER v.7 software gave a description of relative multivariate variability within each of the groups in a single ordination. PERMDISP and MVDISP can help us to test our hypothesis that under stress conditions, such as increased salinity and temperature, the zooplankton assemblages (i.e., abundances and species composition) might become heterogeneous between replicates.
Finally, a Similarity Percentage Analyses (SIMPER) was performed to identify both the species characteristic of each treatment (i.e., the contribution that each species makes to the similarity within the group) and the discriminating species between treatments (i.e., which species contribute to the differences between zooplankton assemblages) (Clarke & Gorley, 2015).
PRIMER v7 software was used for performing all these analyses. Data were previously fourth root transformed in order to reduce the influence of more abundant species and increase the influence of rarer species in the analysis (Clarke et al., 2014).
Several two-way ANOVAs were performed to detect potential effects of salinity, temperature, and their interaction on the abundances of the characteristic species identified previously by SIMPER analysis. When necessary, data were transformed for fulfilling normality and homogeneity of variance assumptions. When no transformation satisfied these assumptions, a Generalized Linear Model (GLM), with Poisson distribution and log-link function, was performed (Quinn & Keouh, 2002).
Similarly, any possible effect of salinity, temperature, and their interaction on taxonomic richness and diversity (Shannon–Wiener’s diversity index) was separately tested with two-way ANOVA. Richness data were transformed to satisfy the normality assumption (Box-Cox: λ = 0.3775; Log likelihood = -22.595). STATISTICA 13.3 software was used for ANOVA and GLM analyses.
Results
In total 14 taxa were found (Fig. 2). Rotifers were the group with the highest taxonomic richness: Brachionus spp., Lecane luna (Müller, 1776), Lecane spp., Lepadella patella (Müller, 1773), Asplanchna spp., Ascomorpha spp., Hexarthra fennica (Levander, 1892), unidentified individuals of the superorder Gnesiotrocha, and other unidentified rotifers (from now on “taxa Rotifera”). Next in richness were branchiopods with three species: Pleuroxus letourneuxi (Richard, 1888), Daphnia mediterranea (Alonso, 1985), and Daphnia magna (Strauss, 1820). Just one copepod species was found, Arctodiaptomus salinus (Daday 1885). Before analyzing the effect of salinity and temperature on community structure, we checked that the sampling station was not a confounding factor; indeed, no differences were detected between samples (PERMANOVA: F4,25 = 0.6294; P = 0.896).
Role of temperature and salinity on zooplankton assemblage
Figure 2 shows the abundance of the 14 taxa in the different treatments. Note that data are fourth root transformed, this way 3 and 6 represent 81 and 1296 individuals, respectively. It should be stressed the singularity of the genus Daphnia in this research; only six D. mediterranea individuals were found, all of them at 20 °C. D. magna was even rarer and only three individuals appeared in one of the replicates at 25 °C and S1. In contrast, the other branchiopod, P. letourneuxi, was very abundant in some of the treatments. A. salinus, ostracods, and rotifers occurred in all the treatments.
The non-metric multi-dimensional scaling (nMDS) ordination plot based on the Bray–Curtis similarities (Fig. 3) shows a gradient in community structure from S3 to S1 (along MDS axis 1), from 20ºC to 25ºC (along MDS axis 2) and a potential interaction between salinity and temperature (MDS axis 3). The 2-d and 3-d stress level were low, 0.15, and 0.09, respectively. A greater dispersion is observed at S3, at both temperatures; while S1 and S2 at 20ºC have less dispersion. Indeed, a test of homogeneity of multivariate dispersions has shown that dispersion is not significant for salinity factor (PERMDISP: F2,27 = 3.3701; P = 0.0732) and neither temperature factor (PERMDISP: F1,28 = 0.9624; P = 0.3942). However, it is significant when both factors are combined (PERMDISP: F5,24 = 9.3558; P = 0.0002), owing to the replicates at S3 (Table 1). Specifically, the dispersion sequence—displayed by a multivariate dispersion analysis (MVDISP)—was 0.85, 0.898, and 1.252 for S2, S1, and S3, respectively, and 0.895 and 1.105 for 20ºC and 25ºC, respectively. Regrettably, this analysis does not allow working with two-way designs and, consequently, it is not possible to compare dispersion of cross-factors.
Zooplankton assemblages were affected by salinity (PERMANOVA: F2,24 = 8.6886; P = 0.0001) and temperature (F1,24 = 6.6257; P = 0.0001). The interaction was also significant (F2,24 = 2.0248; P = 0.0372). PERMANOVA pair-wise tests agree with the nMDS ordination: communities differ between temperatures at S1 (P = 0.0078) and S2 (P = 0.0073). In contrast, at S3 no differences between temperatures were detected (P = 0.2849). For a given temperature, either 20 or 25ºC, communities differ between salinities (P < 0.013).
An analysis of similarity percentages (SIMPER) shows both the typical taxa for each treatment (Table 2) as the discriminant taxa among groups (Table 3). SIMPER shows the overall average similarity for each treatment and defines the contribution that each species makes to the similarity within the group. For example, the average Bray–Curtis similarity between all pairs of replicates in treatment at S1-20ºC is 68.29, made up mainly of contributions from just two taxa: Ostracoda (36.93, i.e., 54.09% of total) and Pleuroxus letourneuxi (22.07, i.e., 32.32%), with a cumulative contribution of 86.41% of the total within-group similarity.
Average similarities agree with the nMDS plot and PERMDISP analysis: the groups with the highest similarity are S1-20ºC (i.e., 68.29) and S2-20ºC (i.e., 67.27), whereas the groups with the lowest ones are S3-20ºC (i.e., 45.64) and S3-25ºC (i.e., 27.03). Ostracoda is the main taxon in all treatments except for S3, where rotifers and A. salinus have the highest contribution (Table 2). P. letourneuxi is a characteristic species of S1, especially at 20ºC, while A. salinus is typical for S2 and S3.
Furthermore, SIMPER analysis allows defining the discriminatory species for each pair of groups, that is, it defines which species contribute to the differences between zooplankton assemblages (Table 3). For a better understanding of the results, the test was performed for the main factors rather than for each pair of treatments. That is, we looked for discriminatory taxa among salinities and between temperatures instead of among treatments. In this case, dissimilarity values was displayed instead of similarities. For a better interpretation of the Diss/SD ratio (column 6), abundances averages (fourth square transformed) of the compared pair are also provided (columns 3 and 4). When the aim is to identify species that contribute the most to the differentiation between groups, the focus should be in the Av. Diss. column; they will tend also to be the species with the larger abundances. But if we are looking for the best indicator of the differences between those groups the Diss/SD ratio should be also considered, since it can sometimes indicate species that are completely absent in one group and with very consistent presence in the other, but with low abundance (Clarke & Gorley, 2015). This is the case of P. letourneuxi, which is only present in S1 and in just one replicate of S2 (Fig. 2) and which become the most discriminant species between S1 and S2 and the second most discriminant between S1 and S3 (Table 3). Ostracoda is also a discriminant taxon since its abundances decrease when salinity increase (Table 3, Fig. 2 and 4). Regarding to temperature, most of the rotifers are discriminant species since they are more abundant at 25ºC than at 20ºC (Table 3, Fig. 2 and 4).
Ostracods abundance decreases with salinity and increases with temperature (Fig. 2 and Table 4.). Neither salinity nor temperature significantly affect the abundance of A. salinus, but it is higher at S3 than at S1 in the treatments at 20ºC (Tukey's pot-hoc test: P = 0.044; Fig. 4 and Table 4). Abundance of rotifers increases with temperature but it is not affected by salinity (Fig. 2 and Table 4). P. letourneuxi abundance increases with temperature and, in general, it is only present at the lowest salinity (Fig. 2, Fig. 4 and Table 4).
Effect of salinity and temperature on diversity
Table 5 and Fig. 5 show the results of several two-way ANOVAs testing the effect of salinity and temperature on richness and Shannon–Wiener’s diversity index. Richness was significantly affected by those variables, but in a non-linear way. Indeed, interaction was statistically significant: (i) richness reduced gradually at 25ºC when salinity increased, whereas at 20ºC salinity did not affect richness and (ii) richness was higher at 25ºC than at 20ºC, except at S3, in which not significant differences between temperatures were found. Consequently, temperature and salinity had a combined effect on taxonomic richness. In contrast, these variables had no observable effect on diversity. Nevertheless, even though there were not statistically significant differences among treatments, diversity displayed a similar trend to richness.
Interaction graphs comparing richness and diversity between treatments. Means predicted by two-way ANOVAs (Table 5). Asterisks in black indicate significant differences between main factors (salinity and temperature). Asterisks in gray indicate statistical significance between levels of a given factor (Bonferroni test). *P < 0.05; **P < 0.01. Bars show the standard error. Note that for a greater clarity the richness data shown in the figure are un-transformed, although in the statistical analysis they were previously transformed
Discussion
Mediterranean wetlands are a highly fluctuating environment both seasonally and year on year. Biota living in these water bodies have developed strategies to survive and develop in such variable and unpredictable conditions. In this context, we studied which zooplankton assemblages emerge from egg bank under different salinity and temperature scenarios. We analyzed the cumulative abundances and taxa composition after 60 days of incubation in the laboratory. The same sediment was incubated, which allowed us to focus on the effect of temperature, salinity, and its interaction, reducing the potential effects of other possible confusion factors, such as ionic composition, hydrological patterns, and geographical position.
Role of temperature and salinity on zooplankton assemblage
Temperature and salinity determined the species composition and abundances in a non-linear way. The composition of the community was strongly determined by salinity at both experimental temperatures. In contrast, the magnitude of the temperature effect depended on salinity; the communities at 20 and 25ºC differed between them at the two lowest salinities, whereas no clear effect of temperature was detected at the highest salinity. In general, salinity decreased total abundance but this decrease was especially marked at 25ºC.
The results obtained are schematized in Fig. 6. Overall, Ostracoda and P. letourneuxi dominated the community at the lowest salinity. At the opposite end A. salinus and rotifers became more important. At an intermediate salinity, Ostracoda and A. salinus dominated as transition taxa. If we focus on temperature, then the results suggest that the species most tolerant to salinity at high temperatures are the copepod A. salinus and the rotifers H. fennica and Brachionus spp.
Consequently, the cues triggering the end of the dormancy state may be crucial for determining the zooplankton assemblages in aquatic ecosystems. Once the community has emerged from the egg bank buried in the sediment, the succession is determined not only by the physicochemical variables but also by biological factors, that is, who and how many are at, a given time, can determine this ecological succession. In this sense, Castro (2004) found that in a very extreme dry hydrological cycle, with salinity values higher than 200 g/L and temperatures are above 30ºC, the plankton community structure in Laguna Honda was physically controlled, whereas in a wet period, with lower salinity ranges, the community structure was controlled by biological factors (i.e., dominance of grazing and competition).
The interaction observed between temperature and salinity, both on community composition and taxonomic richness, might be an emergent property from the physiology at species-level since temperature is known to have a species-specific interactive effect on salinity tolerance through changes in their metabolic rate (Lee & Bell, 1999) and osmoregulatory ability (Aladin & Potts, 1995).
Salinity may reduce the rate of population growth of the keystone species Daphnia pulex (Bezirci et al., 2012). In this respect, in previous research, a shift was seen from the dominance of large cladoceran species at low salinity, with a broad-sized feeding spectrum, to the dominance of smaller and less efficient grazer species at higher salinities, that is, copepods (mainly calanoids), small cladoceran, and rotifers (Heerkloss et al., 1991; Jeppesen et al., 1994, 2007). According to the findings in these studies, the calanoid A. salinus and the small branchiopod P. letourneuxi were, along with the Ostracoda and rotifers, the most abundant organisms to emerge from the sediment of this saline pond, whereas the presence of larger cladocerans (D. magna and D. mediterranea) is anecdotal. This result is corroborated by the data obtained by Castro (2004) in the same ecosystem; in which daphnids species appeared in Laguna Honda at low density with low salinity levels. A shift to the extensive dominance of rotifers when salinity increased was also observed in other enclosure experiments (Jeppesen et al., 2007).
Some authors suggest that climate change could modify the size structure of zooplankton to favor small-sized species (Molinero et al., 2006). In this sense, we have observed that rotifers are favored at 25ºC, although their abundance is drastically reduced to S3. Once again, the results agree with the observations of Castro (2004), who found a high abundance of rotifers in Laguna Honda in the summer season.
Not all the zooplankton species previously detected in Laguna Honda hatched in our experiment. Namely, the Andalusian Government found the rotifers Keratella tropica, K. quadrata, and Brachionus plicatilis, the copepods Copidodiaptomus numidicus and Acanthocyclops kieferi and the cladoceran Bosmina longirostris and Daphnia galeata (Junta de Andalucía, 2005). Our research group has previously observed the occurrence of the copepods Metacyclops minutus and Cletocamptus retrogressus and the cladoceran Moina salina and Alona sp. (López-González et al., 1998; Castro, 2004; Gilbert et al., 2015). In contrast, species or taxa not detected before in Laguna Honda hatched in our study. Namely, the rotifers Lecane luna, Lepadella patella, Asplanchna sp., Ascomorpha sp., and the superorder Gnesiotrocha.
There may be several reasons why those species did not hatch from our sediment. Firstly, cues triggering the end of dormancy for these species − higher salinity, for example − could be different to those implemented in our experiments. However, this would not explain the absence of M. salina since resting eggs of this cladoceran have been hatched in previous studies under conditions of salinity and temperature similar to those of our experiment (Rokneddine & Chentoufi, 2004). Chance may be a determining factor when a given species hatches: very abundant species in the water column − such as A. salinus in the last decades (personal observations and Junta de Andalucía, 2005) − will produce more resting eggs than less numerous species and, consequently, the probability that their eggs hatch will be higher. This might also explain why so few Daphnia individuals were observed in our experiments. Finally, the last time our team detected M. salina, Alona sp., M. minutus, and C. retrogressus in Laguna Honda was in 1998. Since then the excessive loads of sediment from erosion from nearby olive groves might have buried the active egg banks, thereby reducing chances of hatching. In this respect, Gleason and co-workers (2003) implemented sediment-load experiments and found that the total emergence of aquatic invertebrates reduced a 99.7% at burial depths of 0.5 cm. (Gleason et al., 2003). These researchers noticed that sediment entering wetlands from agricultural erosion may also hamper successional changes throughout inter-annual climate cycles.
Role of temperature and salinity on diversity
As happened to species composition and abundances, salinity and temperature determined richness of zooplankton in a non-linear way. Richness increased with temperature, except at the highest salinity where similar richness was detected at both temperatures. Similar results were reported by Kaya and co-workers who in a field study found that temperature positively affected the richness of rotifers (Kaya et al., 2010); although this effect was clear in freshwater habitats, it was not observed at higher salinities. In the same way, Conde-Porcuna and colleagues (2018) found also that the effect of salinity on hatching success of resting eggs of rotifers depended on the temperature. At 15ºC, the hatching success of most rotifers species decreased with salinity, whereas at 25ºC the relationship was quadratic with a maximum in the middle salinity (Conde-Porcuna et al., 2018). This last finding agrees with the rotifers abundances we have observed at the same temperature, 25ºC. Under controlled laboratory conditions, Bailey and colleagues (2006) studied the effect temperature and salinity had on cumulative abundance and the species richness that emerged from zooplankton resting eggs from different sediments in ship ballast. In a similar way to our results, they found that the salinity effect was temperature dependent, although the direction of the effect was case specific; interaction between these variables was only significant for half of the different type of sediments, and even in two trials the combination of high salinity and high temperatures led to more eggs hatching than at the other exposures (Bailey et al., 2006). In contrast, recent findings by Mabidi and colleagues (2018), from a lab incubation sediment experiment, are overwhelming and conclusive: a rise in salinity drastically reduced both the abundance and taxa richness of crustaceans emerging from egg banks in the wetlands of a semi-arid region in South Africa (Mabidi et al., 2018).
The richness of branchiopods in our experiments was very low and in concordance with field studies in similar endorheic areas, as explained below. Boronat and colleagues (2001) studied cladoceran assemblages from 44 endorheic bodies of water in Central Spain displaying a wide salinity gradient. At the boundary between the mesosaline and hypersaline status, the cladoceran assemblage they found consisted of exactly the same species that hatched from our sediment, that is, D. magna, D. mediterranea, and P. letourneuxi. As these researchers indicated, D. mediterranea and P. letourneuxi are typical in Spanish endorheic lakes and are of biogeographic interest due to their restricted circum-Mediterranean distribution. These are usually accompanied by other more widespread species, mainly the eurioic D. magna (Boronat et al., 2001).
While in several field studies, the specific richness of zooplankton has been observed to decline as a result of salinity (Boronat et al., 2001; Schallenberg et al., 2003; Brucet et al., 2009; Alcorlo et al., 2014; Lin et al., 2017), the same could not be said for temperature, the influence of which was not so clear. Some authors did not find any significant relationship between this variable and zooplankton richness (Lin et al., 2017) or on the hatching of resting eggs (Burian et al., 2016). In other studies, an inverse relationship has even been observed between zooplankton richness and temperature (Pomati et al., 2012). Consequently, the response of zooplankton resting eggs to temperature is not clear since different research show different trends.
It should be noted that although we found temperature and salinity have a significant effect on species richness, the same was not observed for diversity. This observation was in keeping with the findings of Paturej and Gutkowska (2015) who saw salinity had a significant negative effect on the richness and abundance of zooplankton, but they did not observe any relationship between salinity and diversity (measured with Shannon's index) (Paturej & Gutkowska, 2015).
Ecological implications
The richness of Laguna Honda, or similar saline ecosystems, might seem low if we only take a snapshot in time. However, its richness is very high in genetical terms, thanks to the egg bank. Different species coexist in the same habitat because of the dynamics of physical changes – i.e., “paradox of the plankton” (Hutchinson, 1961) – and the spatial heterogeneity – e.g., “hypothesis of the contemporaneous disequilibrium” (Richerson et al., 1970).
Many factors determine zooplankton communities, ranging from the merely physical (e.g., temperature and salinity) or biological (e.g., predation or competition), to stochastic (e.g., perturbations, spatial heterogeneity, or phenotypic plasticity). In the case at hand, competition and predation have not yet been allowed to act, so that physical and chemical changes should be the most important factors affecting the abundance and distributions of the planktonic species, as proposed by Por (1980). Consequently, in a pioneer phase, the emergence from the egg bank is strongly determined by the initial conditions of temperature and salinity. But spatial heterogeneity might be also important; it might explain part of the intra-treatment variability that we have observed.
In addition to physical factors, the pioneer community might be determined by the biological record or “history” of the pond, that is, by the communities that were present in the past and left resting structures in the sediment. As mentioned in previous paragraphs, abundant species in the water column, such as A. salinus, will produce more resting eggs than less numerous species and, consequently, the probability that their eggs hatch will be higher. In this way, according with the lottery hypothesis, species with similar patterns of resource use can coexist through chance recolonization of vacant space, provided there are temporal or spatial fluctuations in the relative abundance of recruits available to occupy vacant habitats (Chesson & Warner, 1981; Munday, 2004). Chance of occupying these vacant spaces can decrease due to anthropogenic perturbations, such as sedimentation because of soil loss, which may impair the species emergence from the egg bank.
Besides perturbations, temporal fluctuations, and spatial heterogeneity, phenotypic plasticity also introduces a stochastic component to the community structure. That is, although salinity causes certain crustaceans not to be suitable for hatching and development (Andreev et al., 1992; Hammer, 1993; Nielsen, 2003), some individuals of a population might hatch and survive even at the extremes of their tolerance ranges. All these stochastic components might explain why some species previously observed in the pond have not hatched in our experiment, even when the physical conditions are optimal for their hatching and development.
Conclusion
Our findings confirm the hypothesis that the coexistence of different species living together in a fluctuating saline pond was strongly determined by temperature and salinity, which have a combined effect. Salinity was more determining on community structure than temperature, such as López-González et al. (1998) observed in column water from the same ecosystem. Although the community was always dominated by smaller and less efficient grazer species, the zooplankton assemblage changes with salinity and temperature. Thus, Ostracoda and P. letourneuxi would dominate the community at the lowest salinity. At the opposite of the gradient A. salinus and rotifers would become more important. At an intermediate salinity, Ostracoda and A. salinus dominate as transition taxa. At high temperatures, the species most tolerant to salinity was the copepod A. salinus and the rotifers H. fennica and Brachionus spp. According to our field observations, A. salinus is a highly eurythermal and euryhaline species, allowing it to dominate the community during all seasons (Jiménez-Melero, 2007). Regarding richness, it was generally affected by salinity (decreasing) and temperature (increasing). In contrast, diversity was not affected.
The enormous heterogeneity we have observed at S3, for both within and inter-treatments, suggests that at a high salinity the structure of the emergent community is highly unpredictable. In this respect, Winder and Schindler (2004) warned that climate change affects biological processes in different ways, and it is therefore difficult to forecast how ecosystems will respond to this phenomenon (Winder & Schindler, 2004). The indirect effects of global warming, such as changes in salinity, will have a larger influence on brackish lagoon ecosystems than the increase in temperature per se (Brucet et al., 2009; Akbulut & Tavşanoğlu, 2018). In terms of population level, the phenotypic plasticity or individual variability of some life traits increases under conditions of stress (Carlotti & Nival, 1991; Jiménez-Melero et al., 2005, 2007, 2012). This phenomenon might be translated as an emergent property at community level, which gives rise to different taxonomic compositions under identical environmental conditions, as suggested by the high variability between replicates at the S3-25ºC incubations. Our results suggest that, under the current global warming scenario, increased temperature and salinity might lead to unpredictable changes in species composition and to a decrease in species richness of saline endorheic wetlands.
Data availability
Data are available from the lead author upon reasonable request: rmelero@ujaen.es.
References
Akbulut, N. E. & Ü. N. Tavşanoğlu, 2018. Impacts of environmental factors on zooplankton taxonomic diversity in coastal lagoons in Turkey. Turkish Journal of Zoology 42: 68–78.
Aladin, N. V. & W. T. W. Potts, 1995. Osmoregulatory capacity of the Cladocera. Journal of Comparative Physiology B 164: 671–683.
Alcorlo, P., S. Jiménez, Á. Baltanás & E. Rico, 2014. Assessing the patterns of the invertebrate community in the marshes of Doñana National Park (SW Spain) in relation to environmental factors. Limnetica 33: 189–204.
Anderson, M. J., R. N. Gorley & K. R. Clarke, 2008. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods. PRIMER-E, Plymouth, UK.
Andreev, N. I., I. S. Plotnikov & N. V. Aladin, 1992. The fauna of the Aral Sea in 1989. 2. The zooplankton. International Journal of Salt Lake Research 1: 111–116.
Bailey, S. A., K. Nandakumar & H. J. MacIsaac, 2006. Does saltwater flushing reduce viability of diapausing eggs in ship ballast sediment? Diversity and Distributions 12: 328–335.
Baltanás, A., C. Montes & P. Martino, 1990. Distribution patterns of ostracods in iberian saline lakes. Influence of Ecological Factors. Hydrobiologia 197: 207–220.
Beklioglu, M., S. Romo, I. Kagalou, X. Quintana & E. Bécares, 2007. State of the art in the functioning of shallow Mediterranean lakes: workshop conclusions. Hydrobiologia 584: 317–326.
Bezirci, G., S. B. Akkas, K. Rinke, F. Yildirim, Z. Kalaylioglu, F. Severcan & M. Beklioglu, 2012. Impacts of salinity and fish-exuded kairomone on the survival and macromolecular profile of Daphnia pulex. Ecotoxicology 21: 601–614.
Boronat, L., M. R. Miracle & X. Armengol, 2001. Cladoceran assemblages in a mineralization gradient. Hydrobiologia 442: 75–88.
Branstrator, D. K., L. J. Shannon, M. E. Brown & M. T. Kitson, 2013. Effects of chemical and physical conditions on hatching success of Bythotrephes longimanus resting eggs. Limnology and Oceanography 58: 2171–2184.
Brucet, S., D. Boix, S. Gascón, J. Sala, X. D. Quintana, A. Badosa, M. Søndergaard, T. L. Lauridsen & E. Jeppesen, 2009. Species richness of crustacean zooplankton and trophic structure of brackish lagoons in contrasting climate zones: north temperate Denmark and Mediterranean Catalonia (Spain). Ecography 32: 692–702.
Burian, A., M. Schagerl, A. Yasindi, G. Singer, M. N. Kaggwa & M. Winder, 2016. Benthic-pelagic coupling drives non-seasonal zooplankton blooms and restructures energy flows in shallow tropical lakes. Limnology and Oceanography 61: 795–805.
Carlotti, F. & S. Nival, 1991. Individual variability of development in laboratory-reared Temora stylifera copepodites: consequences for the population dynamics and interpretation in the scope of growth and development rules. Journal of Plankton Research 13: 801–813.
Castro, M. C., 2004. Caracterización limnológica y variabilidad temporal de la comunidad planctónica en Laguna Honda (Jaén). PhD Dissertation, Universidad de Jaén.
Castro, M. C., M. Rivera, M. Crespo, J. M. Martín-García & F. Guerrero, 2003. Morphological and sedimentological characterization of Honda temporary lake (southern Spain). Limnetica 22: 147–154.
Chesson, P. L. & R. R. Warner, 1981. Environmental variability promotes coexistence in lottery competitive systems. American Naturalist 117: 923–943.
Clarke, K. R., & R. N. Gorley, 2015. PRIMER v7: User Manual/Tutorial. PRIMER-E: Plymouth.
Clarke, K. R., R. N. Gorley, P. J. Somerfield, & R. M. Warwick, 2014. Change in marine communities: an approach to statistical analysis and interpretation. PRIMER-E Ltd, Plymouth.
Conde-Porcuna, J. M., C. Pérez-Martínez & E. Moreno, 2018. Variations in the hatching response of rotifers to salinity and waterbird ingestion. Journal of Plankton Research 40: 326–341.
Declerck, S. A. J. & S. Papakostas, 2017. Monogonont rotifers as model systems for the study of micro-evolutionary adaptation and its eco-evolutionary implications. Hydrobiologia 796: 131–144.
Downing, J. A., 2010. Emerging global role of small lakes and pond: little things mean a lot. Limnetica 29: 9–24.
Downing, J. A., Y. T. Prairie, J. J. Cole, C. M. Duarte, L. J. Tranvik, R. G. Striegl, W. H. McDowell, P. Kortelainen, N. F. Caraco, J. M. Melack & J. J. Middelburg, 2006. The global abundance and size distribution of lakes, ponds, and impoundments. Limnology and Oceanography 51: 2388–2397.
Esteban, G. F. & B. J. Finlay, 2003. Cryptic freshwater ciliates in a hypersaline lagoon. Protist 154: 411–418.
Florencio, M., R. Fernández-Zamudio, M. Lozano & C. Díaz-Paniagua, 2020. Interannual variation in filling season affects zooplankton diversity in Mediterranean temporary ponds. Hydrobiologia 847: 1195–1205.
Galotti, A., B. J. Finlay, F. Jiménez-Gómez, F. Guerrero & G. F. Esteban, 2014. Most ciliated protozoa in extreme environments are cryptic in the seed bank. Aquatic Microbial Ecology 72: 187–193.
García, C. M. & F. X. Niell, 1993. Seasonal change in a saline temporary lake (Fuente de Piedra, southern Spain). Hydrobiologia 267: 211–223.
García-Roger, E. M., X. Armengol-Díaz, M. J. Carmona & M. Serra, 2008. Assessing rotifer diapausing egg bank diversity and abundance in brackish temporary environments: an ex situ sediment incubation approach. Fundamental and Applied Limnology/Archiv für Hydrobiologie 173: 79–88.
Gilbert, J. D., I. de Vicente, R. Jiménez-Melero, G. Parra & F. Guerrero, 2014. Selecting priority conservation areas based on zooplankton diversity: the case of Mediterranean wetlands. Marine and Freshwater Research 65: 857–871.
Gilbert, J. D., I. de Vicente, F. Ortega, R. Jiménez-Melero, G. Parra & F. Guerrero, 2015. A comprehensive evaluation of the crustacean assemblages in southern Iberian Mediterranean wetlands. Journal of Limnology 74: 169–181.
Gilbert, J. D., I. de Vicente, R. Jiménez-Melero & F. Guerrero, 2017. Zooplankton body size versus taxonomy in Mediterranean wetlands: implications for aquatic ecosystem evaluation. Freshwater Science 36: 774–783.
Gilbert, J. D., I. de Vicente, F. Ortega & F. Guererro, 2021. Zooplankton community dynamics in temporary Mediterranean wetlands: which drivers are controlling the seasonal species replacement? Water 2021(13): 1447.
Gilbert, J. D., F. Ortega, R. Jiménez-Melero, A. Baltanás & F. Guerrero, 2023. La comunidad de ostrácodos en humedales del sudeste de la Península Ibérica. Limnetica 42(2): 000–000.
Gleason, R. A., N. H. Euliss Jr., D. E. Hubbard & W. G. Duffy, 2003. Effects of sediment load on emergence of aquatic invertebrates and plants from wetland soil egg and seed banks. Wetlands 23: 26–34.
Guerrero, F. & M. C. Castro, 1997. Chlorophyll-a of size-fractionated phytoplankton at a temporary hypersaline lake. International Journal of Salt Lake Research 5: 253–260.
Gyllström, M. & L.-A. Hansson, 2004. Dormancy in freshwater zooplankton: Induction, termination and the importance of benthic pelagic coupling. Aquatic Sciences 66: 274–295.
Hairston, N. G. & C. M. Kearns, 2002. Temporal dispersal: ecological and evolutionary aspects of zooplankton egg banks and the role of sediment mixing. Integrative and Comparative Biology 42: 481–491.
Hammer, U. T., 1993. Zooplankton distribution and abundance in saline lakes of Alberta and Saskatchewan, Canada. International Journal of Salt Lake Research 2: 111–132.
Hawkins, S. J., S. V. Proud, S. K. Spence & A. J. Southward, 1994. From the individual to the community and beyond: Water quality, stress indicators and key species in coastal ecosystems. In Sutcliffe, D. W. (ed), Water Quality and Stress Indicators in Marine and Freshwater Systems: Linking Levels of Organisation Freshwater Biological Association Special Publications, U.K: 35–62.
Hutchinson, G. E., 1961. The paradox of the plankton. American Naturalist 95: 137–145.
Heerkloss, R., W. Schnese & B. Adamkiewicz-Chojnacka, 1991. Seasonal variation in the biomass of zooplankton in two shallow coastal water inlets differing in their stage of eutrophication. Internationale Revue der Gesamten Hydrobiologie und Hydrographie 76: 397–404.
Jeppesen, E., A. R. Pedersen, K. Jürgens, A. Strzelczak, T. L. Lauridsen & L. S. Johansson, 2007. Salinity Induced Regime Shift in Shallow Brackish Lagoons. Ecosystems 10: 48–58.
Jeppesen, E., M. Søndergaard, E. Kanstrup, B. Petersen, R. B. Eriksen, M. Hammershøj, E. Mortensen, J. P. Jensen & A. Have, 1994. Does the impact of nutrients on the biological structure and function of brackish and freshwater lakes differ? Hydrobiologia 275: 15–30.
Jiménez-Melero, R. (2007) Population dynamics, demography and production of Arctodiaptomus salinus (Copepoda: Calanoida) in a saline endorheic pond. PhD Dissertation, Universidad de Jaén.
Jiménez-Melero, R., G. Parra & F. Guerrero, 2012. Effect of temperature, food and individual variability on the embryonic development time and fecundity of Arctodiaptomus salinus (Copepoda: Calanoida) from a shallow saline pond. Hydrobiologia 686: 241–256.
Jiménez-Melero, R., G. Parra, S. Souissi & F. Guerrero, 2007. Post-embryonic developmental plasticity of Arctodiaptomus salinus (Copepoda: Calanoida) at different temperatures. Journal of Plankton Research 29: 553–567.
Jiménez-Melero, R., B. Santer & F. Guerrero, 2005. Embryonic and naupliar development of Eudiaptomus gracilis and Eudiaptomus graciloides at different temperatures: comments on individual variability. Journal of Plankton Research 27: 1175–1187.
Junta de Andalucía, 2005. Caracterización ambiental de humedales en Andalucía. Consejería de Medio Ambiente de la Junta de Andalucía.
Kaya, M., D. Fontaneto, H. Seger & A. Altinda, 2010. Temperature and salinity as interacting drivers of species richness of planktonic rotifers in Turkish continental waters. Journal of Limnology 69: 297–304.
Lee, C. E. & M. A. Bell, 1999. Causes and consequences of recent freshwater invasions by saltwater animals. Trends in Ecology & Evolution 14: 284–288.
Lenormand, T., O. Nougué, R. Jabbour-Zahab, F. Arnaud, L. Dezileau, L.M. Chevin & M. I. Sánchez, 2018. Resurrection ecology in Artemia. Evolutionary Applications 11: 76–87.
Lin, Q., L. Xu, J. Hou, Z. Liu, E. Jeppesen & B.-P. Han, 2017. Responses of trophic structure and zooplankton community to salinity and temperature in Tibetan lakes: Implication for the effect of climate warming. Water Research 124: 618–629.
López-González, P. J., F. Guerrero & M. C. Castro, 1998. Seasonal fluctuations in the plankton community in a hypersaline temporary lake (Honda, southern Spain). International Journal of Salt Lake Research 6: 353–371.
Mabidi, A., M. S. Bird & R. Perissinotto, 2018. Increasing salinity drastically reduces hatching success of crustaceans from depression wetlands of the semi-arid Eastern Cape Karoo region, South Africa. Scientific Reports 8: 1–9.
Merritt, R. W., K. W. Cummins, & T. M. Burton, 1984. The role of aquatic insects in the processing and cycling of nutrients. In Resh, V. H., & D. M. Rosenburg (eds), The Ecology of Aquatic Insects. Praeger, New York: 134–163.
Molinero, J. C., O. Anneville, S. Souissi, G. Balvay & D. Gerdeaux, 2006. Anthropogenic and climate forcing on the long-term changes of planktonic rotifers in Lake Geneva, Europe. Journal of Plankton Research 28: 287–296.
Munday, P. L., 2004. Competitive coexistence of coral-dwelling fishes: The lottery hypothesis revisited. Ecology 85: 623–628.
Newton, G. M. & B. D. Mitchell, 1999. Egg dormancy in the Australian estuarine-endemic copepods Gippslandia estuarina and Sulcanus conflictus, with reference to dormancy of other estuarine fauna. Marine and Freshwater Research 50: 441–449.
Nielsen, D. L., M. A. Brock, K. Crossle & K., M. Healeyand, & I. Jarosinski, 2003. The effects of salinity on aquatic plant germination and zooplankton hatching from two wetland sediments. Freshwater Biology 48: 2214–2223.
Ning, N. S. P. & D. L. Nielsen, 2011. Community structure and composition of microfaunal egg bank assemblages in riverine and floodplain sediments. Hydrobiologia 661: 211–221.
Ning, N. S. P., D. L. Nielsen, T. J. Hillman & P. J. Suter, 2008. Evaluation of a new technique for characterizing resting stage zooplankton assemblages in riverine slackwater habitats and floodplain wetlands. Journal of Plankton Research 30: 415–422.
Ning, N. S. P., D. L. Nielsen, T. J. Hillman & P. J. Suter, 2010. The influence of planktivorous fish on zooplankton resting-stage communities in riverine slackwater regions. Journal of Plankton Research 32: 411–421.
Paturej, E. & A. Gutkowska, 2015. The effect of salinity levels on the structure of zooplankton communities. Archives of Biological Sciences 67: 483–492.
Pomati, F., B. Matthews, J. Jokela, A. Schildknecht & B. W. Ibelings, 2012. Effects of re-oligotrophication and climate warming on plankton richness and community stability in a deep mesotrophic lake. Oikos 121: 1317–1327.
Por, F. D., 1980. A classification of hypersaline waters, based on trophic criteria. Marine Ecology 1: 121–131.
Quinn, G. P. & M. J. Keough, 2002. Experimental Design and Data Analysis for Biologists, Cambridge University Press:
Richerson, P., R. Armstrong & C. R. Goldnman, 1970. Contemporaneous Disequilibrium, a New Hypothesis to Explain the “Paradox of the Plankton.” Proceedings of the National Academy of Sciences 67: 1710–1714.
Rokneddine, A. & M. Chentoufi, 2004. Study of salinity and temperature tolerance limits regarding four crustacean species in a temporary salt water swamp (Lake Zima, Morocco). Animal Biology Brill 54: 237–253.
Schallenberg, M., C. J. Hall & C. W. Burns, 2003. Consequences of climate-induced salinity increases on zooplankton abundance and diversity in coastal lakes. Marine Ecology Progress Series 251: 181–189.
Sistema Cartográfico Nacional de España, 2013. Ortofotos históricas del Plan Nacional de Ortofotografía Aérea: PNOA-ANUAL-2013-OF-ETRS89-HU30-H50–0968.ECW. Gobierno de España, Junta de Andalucía, URL: https://centrodedescargas.cnig.es/CentroDescargas/index.jsp#.
Sistema Cartográfico Nacional de España, 2019. Ortofotos históricas del Plan Nacional de Ortofotografía Aérea. PNOA-ANUAL-2019-OF-ETRS89-HU30-H50–0968.ECW. Gobierno de España, Junta de Andalucía, URL: https://centrodedescargas.cnig.es/CentroDescargas/index.jsp#.
Waterkeyn, A., B. Vanschoenwinkel, P. Grillas & L. Brendoncka, 2010. Effect of salinity on seasonal community patterns of Mediterranean temporary wetland crustaceans: A mesocosm study. Limnology and Oceanography 55: 1712–1722.
Waterkeyn, A., B. Vanschoenwinkel, H. Vercampt, P. Grillas & L. Brendonck, 2011. Long-term effects of salinity and disturbance regime on active and dormant crustacean communities. Limnology and Oceanography 56: 1008–1022.
Winder, M. & D. E. Schindler, 2004. Climatic effects on the phenology of lake processes. Global Change Biology 10: 1844–1856.
Acknowledgements
This study was supported by the University of Jaén (Plan de Apoyo a la I+D+I de la Universidad de Jaén 2012-2013; UJA2013/08/25) and co-founded by the Fundación Caja Rural de Jaén. D. Jarma was supported by a scholarship from Asociación Iberoamericana de Postgrado (AUIP). Our thanks go to the Consejería de Medio Ambiente (Junta de Andalucía) for granting us permission to undertake these field surveys.
Funding
Funding for open access publishing: Universidad de Jaén/CBUA. This study was supported by the University of Jaén (Plan de Apoyo a la I + D + I de la Universidad de Jaén 2012–2013; UJA2013/08/25) and co-founded by the Fundación Caja Rural de Jaén. D. Jarma was supported by a scholarship from Asociación Iberoamericana de Postgrado (AUIP).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. RJ and FG sought financial support. RJ, JMR, and FG designed the study. RJ and JDG did fieldwork. RJ, DJ, JDG, and JMR performed lab work. RJ and DJ did the statistical analyses. RJ and JMR prepared the figures. JDG and DJ realized the taxonomical identification of the organisms. RJ, DJ, and FG wrote the initial draft of the manuscript. All other authors critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Handling editor: Juan Carlos Molinero
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiménez-Melero, R., Jarma, D., Gilbert, J.D. et al. Cryptic diversity in a saline Mediterranean pond: the role of salinity and temperature in the emergence of zooplankton egg banks. Hydrobiologia 850, 3013–3029 (2023). https://doi.org/10.1007/s10750-023-05225-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05225-3