Abstract
Chemical preservatives can alter stable isotope ratios in animal tissues. The effects of preservation on δ13C and δ15N values have been investigated in a variety of species, but not on δ2H values or on the freshwater pearl mussel (FPM, Margaritifera margaritifera) tissues. We evaluated the effect of ethanol preservation (unpreserved vs preserved tissues) over 6 months on the δ13C, δ15N and δ2H values of FPM foot and gonad tissues. Ethanol preservation significantly increased δ13C values (foot 0.4 ‰; gonad 0.3 ‰), whereas it did not significantly affect δ15N values (foot 0.2 ‰; gonad − 0.1 ‰). The positive effect of ethanol preservation on δ2H values (foot 7.1 ‰; gonad 14.5 ‰) and the negative effect on C:N ratios (foot − 0.1; gonad − 0.5) depended on the tissue type, with larger effects found on the lipid-rich gonad. Overall, ethanol preservation affected δ2H values more than the δ13C, δ15N or C:N ratios of FPM tissues. After 1 month of preservation, the isotope values remained rather stable, and significant changes were only observed in δ15N values. The results imply that ethanol-preserved FPM samples can be used if potential shifts in isotopic and elemental ratios are accounted for prior running mixing models for estimating dietary proportions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of stable isotope analysis (SIA), such as the ratios of naturally occurring stable isotopes of carbon (13C/12C; hereafter denoted as δ13C), nitrogen (15N/14N; δ15N) and hydrogen (2H/1H; δ2H), has improved our understanding of how aquatic ecosystems work (Fry, 2006). Stable isotope ratios are routinely used to study the structure and function of food webs and the energy sources of organisms within (Umbricht et al., 2018). Because primary producers vary in their isotopic composition, δ13C is generally used to determine the origins of carbon in food webs or to identify feeding areas (DeNiro & Epstein, 1978). As organisms are generally enriched in 15N relative to their diet, they show an increase in δ15N values with each trophic step in the food web. δ15N can also be used to assess nitrogen sources (Deniro & Epstein, 1981). δ2H values have been applied to differentiate terrestrial from aquatic inputs because δ2H varies between terrestrial and aquatic primary producers (Doucett et al., 2007) or to study dispersal of animals (Hobson & Wassenaar, 2008). C:N ratios in animals, including aquatic species, represent the overall macronutrient content in any given tissue and have been used to estimate lipid content of the tissue (Post et al., 2007).
Despite SIA being used successfully in ecological studies for over 3 decades, there are still many unknowns about the effects of sample treatment on isotope values (Lau et al., 2012). For SIA, fresh samples are preferred, but logistics often require the preservation of organisms for later analyses (Jesus et al., 2015). Preserved samples are also often found from museums or research institutes and SIA of such archived tissue samples may provide the only record about long-term ecosystem changes (Syväranta et al., 2008). Stable isotope values measured in animal tissues represent values generated by their diet during a given period (DeNiro & Epstein, 1978). However, many factors, including the preservation method and time, can affect the stable isotope ratios in animal tissues and therefore also the results of isotope mixing models used for estimation of consumer diets (Javornik et al., 2019). Since the isotope ratios are sensitive to even subtle variations, meticulous care must be taken to preserve samples (Yurkowski et al., 2017).
Numerous studies have examined preservation effects on stable isotope ratios of carbon and nitrogen, with inconsistent results across taxa, environments (e.g., marine vs freshwater), tissue types, preservation methods and duration of preservation (Vizza et al., 2013; Kiszka et al., 2014; Stallings et al., 2015; Hogsden & McHugh, 2017). Chemical preservatives can alter stable isotope ratios through a variety of mechani2004sms: they can either add carbon or nitrogen to tissues or they can leach macromolecules consisting of these elements (Sarakinos et al., 2002; Sweeting et al., ; Barrow et al., 2008; Ruiz‐Cooley et al., 2011). Ethanol is often used as a preservative to store animal tissues. Since ethanol does not contain nitrogen, it cannot add nitrogen to samples, but it may affect stable isotope ratios by breaking bonds with nitrogen atoms in tissues (Hetherington et al., 2019). Some studies reported that ethanol storage did not affect the δ15N values in sea stars, freshwater invertebrates and fish fin (Vizza et al., 2013; Hogsden & McHugh, 2017; Le Bourg et al., 2020). In contrast, other studies indicate that ethanol can increase δ15N values in bivalves and ray fishes (Syväranta et al., 2011; Burgess & Bennett, 2017; Umbricht et al., 2018) but decrease δ15N values in some other fishes (Olin et al., 2014). Carbon content of tissues and their δ13C values may also be affected by ethanol but the magnitude and direction of changes varies among studies. For instance, previous studies of marine and freshwater bivalves, invertebrates, fishes and mammals indicate that ethanol preservation can either have no effect (Lau et al., 2012; Kiszka et al., 2014; Burgess & Bennett, 2017) or lead to higher δ13C values of consumer tissues (Syväranta et al., 2011; Stallings et al., 2015; Hogsden & McHugh, 2017; Umbricht et al., 2018). Lipids are depleted in 13C and 2H as compared to proteins and carbohydrates (DeNiro & Epstein, 1978; Hobson et al., 1999). Thus, if ethanol extracts lipids from samples, a decrease in C:N ratio as well as an increase in the δ13C and δ2H values of preserved samples would be expected.
Freshwater bivalves are commonly used in biomonitoring (Farris & Van Hassel, 2007). They can be important components in freshwater food webs, provide valuable ecosystem services and have a large impact on ecosystem function, including nutrient cycling (Vaughn et al., 2008; Atkinson & Vaughn, 2014; Vaughn, 2018). Studies on the effect of ethanol preservation on stable isotope values of freshwater bivalves, Asiatic clams apart (Sarakinos et al., 2002; Syväranta et al., 2011), are practically non-existent. However, ethanol-preserved and archived samples of endangered freshwater mussels are indispensable sources of material for isotope analysis because they can be used to reconstruct historical energy and nutrient sources. Moreover, to the best of our knowledge, no studies have examined the effect of chemical preservation on δ2H values of animal tissues. Hydrogen stable isotopes are increasingly used to study aquatic food webs and animal migrations, reinforcing the need to understand how different storage and laboratory preparation methods affect δ2H values (Hobson & Wassenaar, 2008; Soto et al., 2013).
This study investigates the effect of ethanol preservation and its duration on δ13C, δ15N and δ2H values and C:N ratios of the endangered freshwater pearl mussel [FPM, Margaritifera margaritifera (Linnaeus, 1758)]. The obtained results will allow subsequent correction for potential preservation-induced changes in FPM isotope values prior to comparing or using data from preserved and non-preserved samples in isotope mixing models. We prepared FPM foot and gonad tissues for SIA to evaluate if the ethanol preservation effects varied among tissue types, with lipid-rich gonads expected to show larger changes. Specifically, we addressed the following questions: (1) How do isotope values and C:N ratios differ between unpreserved and ethanol-preserved tissues? (2) How does preservation time affect differences in isotope values and C:N ratios in FPM tissues? Based on previous studies, we hypothesized that ethanol preservation may increase δ13C and δ2H values, decrease C:N ratios of preserved samples and have no or minor effect on δ15N values. Further, we assumed that ethanol preservation would have a larger effect on the lipid-rich gonads (Jokela et al., 1993) than on the foot tissue of FPM. Moreover, we predicted that shifts in isotope values should increase with preservation time.
Materials and methods
Mussel collection and preparation
Twenty-four FPM individuals were collected from eight rivers, three individuals per river, in Northern Finland (64–68 °N, 27–29 °E) during August–September 2020. FPMs were collected under permission (KAIELY/296/2019 and 357/2019, LAPELY/1929/2019 and 2252/2019, POPELY/1276/2019 and 1490/2019) granted from the Centers for Economic Development, Transport and the Environment of Kainuu, Lapland and North Osthrobothnia, Finland, respectively. Upon collection, the mussels were packed in individual plastic bags, stored immediately in ice, transported to the laboratory and kept in a cool box under ice until dissection. Mussels were dissected and tissues (foot and gonad) were extracted. In previous studies (Syväranta et al., 2011; Xu et al., 2011; Liu et al., 2013), the ethanol preservation effects on δ13C and δ15N values have been shown to stabilize after 6 months preservation. Therefore, we decided to conduct this experiment for only 6 months. To examine the effects of ethanol preservation and preservation time (1, 2, 4, and 6 months) on δ13C, δ15N, δ2H values and C:N ratio, the foot and gonad samples dissected from each FPM individual were split in two parts; one stored at − 18 °C (‘unpreserved tissue’/time 0) overnight prior to freeze-drying, and another stored in 99.5% ethanol and kept at room temperature (~ 18 °C) (‘preserved’). The preserved samples of FPM foot and gonad tissues were removed from ethanol and dissected for subsamples after 1, 2, 4 and 6 months, rinsed with ultra-pure water and then freeze-dried. The unpreserved and preserved freeze-dried samples were ground to a fine powder with a mortar and pestle. Prior to final SIA, 0.500–0.700 mg of sample was weighed into a tin cup for δ13C and δ15N analyses and 0.350 mg of sample into a silver cup for δ2H analysis. Prior to folding, the silver cups were stored open in laboratory atmosphere with laboratory standards for at least five days to allow hydrogen exchange between the samples and laboratory air (Wassenaar & Hobson, 2003). To examine the effect of preservation time, the relative differences (Δ) in isotope values of preserved samples (time points 1–6 months) to the values of unpreserved samples (time 0) were calculated.
Stable isotope analysis
We measured δ13C, δ15N, δ2H values and C:N ratios at the Stable Isotope Laboratory at the University of Jyväskylä, Finland. We report stable isotope ratios in δ notation relative to Vienna Pee Dee belemnite (VPDB), atmospheric nitrogen (N2) and Vienna Standard Mean Ocean Water (VSMOW) for carbon, nitrogen, and hydrogen, respectively. δ13C and δ15N values and C:N ratios (by mass) were measured by continuous-flow stable isotope ratio mass spectrometer (CF-SIRMS) coupled with a FlashEA 1112 elemental analyzer (Thermo Electron Corporation, Waltham, MA, U.S.A.). δ2H values were measured using an Isoprime 100 CF-SIRMS (Isoprime Ltd, Stockport, U.K.) coupled with Elementar vario PYRO cube elemental analyzer (Elementar Analysensysteme GmbH, Hanau, Germany). In each run, multiple samples of two reference materials (caribou hoof [CBS], kudu horn [KHS]) were analyzed relative to VSMOW (Standard Mean Ocean Water) (Soto et al., 2017). Carbon, nitrogen and hydrogen stable isotope ratios are expressed as per mil (‰) and calculated following the equation:
where \({R}_{sample}\) and \({R}_{reference}\) are the 13C/12C, 15N/14N and 2H/1H ratios for the sample and international standards, respectively. \(X\) represents the heavy isotopes of 13C, 15N or 2H. Positive δ values indicate that the sample is isotopically enriched, meaning it contains a greater proportion of the heavy stable isotope (13C, 15N or 2H). The standard deviations of the delta-values of replicate reference materials in each run were always less than 0.14 ‰ for δ13C, 0.24 ‰ for δ15N and 2.41 ‰ for δ2H.
Statistical analyses
We used Generalized Linear Mixed Effect Models (GLMM) in the statistical software R (version 4.1.2; 2022-09-06; R Core Team, 2022) to test for the effects of ethanol preservation (unpreserved samples at time point 0 and preserved samples at time point 6; hereafter Preservation) and preservation time (preservation for 1, 2, 4, and 6 months; hereafter Time) on the δ13C, δ15N and δ2H values and C:N ratios of FPM foot and gonad tissues (hereafter Tissue). For the GLMMs, we used lme function with default settings in the nlme package (Pinheiro et al., 2021) and set the δ13C, δ15N and δ2H values and C:N ratios as the response variables and ethanol preservation (Preservation), preservation time (Time), tissue type (Tissue) and the Preservation x Tissue and Time x Tissue two-way interactions as the explanatory variables and FPM individual as the random factor (random = ~ 1|Individual). In the case of significant differences (P < 0.05), Tukey-tests were used for pairwise post hoc comparisons to compare the δ13C, δ15N and δ2H values and C:N ratios between different time points, using the emmeans package in R (Lenth et al., 2022).
Results
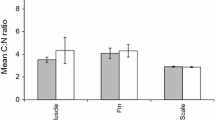
As indicated by the significant two-way interactions (Table 1), the positive effect of ethanol preservation on δ2H values and the negative effect on C:N ratios depended on the tissue type, with the effects being larger in the lipid-rich gonad than in the foot tissue (Fig. 1). However, no significant Preservation x Tissue interaction effects were detected for δ13C or δ15N, as gonads were consistently depleted in both 13C and 15N compared to foot tissue, and the significant positive main effect of Preservation on δ13C was comparable in both tissue types (i.e., 0.3–0.4 ‰; Tables 1, 2, Fig. 1).
Ethanol preservation increased δ13C values in both tissues but with a slightly higher magnitude in foot (0.4 ‰) than in gonad (0.3 ‰; Table 2). In contrast, preservation had minor and inconsistent effects on δ15N, with the shift in δ15N ranging from − 0.1 ‰ to 0.2 ‰ in gonad and foot, respectively (Table 2). Both tissues showed elevated δ2H values in preserved samples as compared to the unpreserved samples by a mean difference of 7.1 ‰ and 14.5 ‰ in foot and gonad (Table 2), respectively. C:N ratios were overall higher in gonad than foot and decreased 0.1 in foot and 0.5 in gonad after 6 months’ preservation.
The GLMM results indicated no significant Time x Tissue interaction effects for Δδ13C, Δδ15N, Δδ2H or ΔC:N ratios (Table 3). In contrast, the results show a significant main effect of Time on Δδ15N, but not for Δδ13C, Δδ2H and ΔC:N ratios (Table 3). However, as indicated by the pairwise comparisons, the effect of Time on Δδ15N was not significant over the preservation period and resulted from the lower Δδ15N values at months 2 (− 0.1 ‰) and 6 (− 0.2 ‰) (Fig. 2). Finally, gonads showed higher Δδ2H values (13.3 ‰) but lower ΔC:N ratios (0.5) than the FPM foot tissue at month 6 (Fig. 2, Table 3).
Boxplots showing the relative differences (Δ) in δ13C, δ15N, δ2H values and C:N ratio of preserved samples (time points 1–6 months) to the values of unpreserved samples (time 0) of freshwater pearl mussel. The central box spans the interquartile range with the middle line representing the median and whiskers defining minimum and maximum range
Discussion
Our results demonstrate that ethanol preservation can affect the δ13C and δ2H values and the C:N ratios of FPM, with the effects being particularly evident in the lipid-rich gonad tissues. The shifts in isotope values and C:N ratios were generally minor, with the largest shifts being observed in the δ2H values (ca. 7–14 ‰ increase).
The reasons for preservation-induced changes in isotope values are not fully understood, but they can be associated with lipid extraction (Syväranta et al., 2008), exchange of heavy and light isotopes between samples and preservatives (Hobson et al., 1997; Edwards et al., 2002), or protein hydrolysis during preservation (Arrington & Winemiller, 2002; Sarakinos et al., 2002; Lecea et al., 2011). Although this study was not designed to explain the causative mechanisms altering the stable isotope values in FPM tissues, most of the shifts in isotope values could be caused either by loss or uptake of materials during preservation. As compared to proteins and carbohydrates, lipids are depleted in 13C and 2H (Park & Epstein, 1961; DeNiro & Epstein, 1978; Hobson et al., 1999). Several studies have shown that the extraction of isotopically lighter lipids from the samples may increase the δ13C and δ2H values of an organism and decrease the C:N ratios (DeNiro & Epstein, 1978; McConnaughey & McRoy, 1979; Gloutney & Hobson, 1998; Kiljunen et al., 2006). The effect of ethanol preservation can arise through either hydrolysis of lipids (Hobson et al., 1997; Gloutney & Hobson, 1998; Bosley & Wainright, 1999) or uptake of ethanol into the tissues (Sarakinos et al., 2002). The increase in δ13C and δ2H values of preserved foot and gonad tissues may be explained by ethanol-induced lipid extraction as indicated by the decreased C:N ratios (Kaehler & Pakhomov, 2001; Arrington & Winemiller, 2002; Sweeting et al., 2004). C:N ratios in animals, including aquatic species, provide a strong predictor of lipid content within tissues. C:N ratios of > 4 typically indicate lipid-rich whereas C:N ratios of < 4 indicate lipid-poor tissues (Post et al., 2007). In our study, lipid-rich gonads showed lower δ13C values and higher C:N ratios in both unpreserved and preserved samples as compared to the foot. Surprisingly, δ2H values showed an opposite pattern with higher δ2H in lipid-rich gonads, but both tissues were clearly more enriched after 6 months’ preservation. 2H-enriched was larger in the gonads, suggesting that lipid-rich tissues are more likely to lose lipids which are depleted in 13C and 2H (Kaehler & Pakhomov, 2001; Sweeting et al., 2004; Carabel & Verísimo, 2009). Our results correspond with previous studies (Stallings et al., 2015; Hogsden & McHugh, 2017; Umbricht et al., 2018; Le Bourg et al., 2020), but contrast with the previously observed ethanol-induced decrease in δ13C values of a soft-shell clam species (Umbricht et al., 2018). While ethanol is known to alter isotopic values, recent reviews suggest that preservative effects on different tissues, species, and organisms can be variable and inconsistent across studies, taxa and environments (Hogsden & McHugh, 2017).
The differences in δ13C values between unpreserved and preserved FPM foot and gonad tissues were + 0.4 ‰ and + 0.3 ‰, respectively. This preservation effect was smaller in magnitude than previously reported for preserved fish muscle tissue (i.e., 1.3–1.4 ‰ increase; Vizza et al., 2013), probably due to differences in tissue composition. Similar enrichment was also found in an Asiatic clam [Corbicula fluminea (O. F. Müller, 1774)] (Sarakinos et al., 2002) with a higher magnitude of 2.2 ‰, while an increase of 0.8 ‰ was observed in dorsal muscle tissue of Arctic charr [Salvelinus alpinus (Linnaeus, 1758)] (Kelly et al., 2006). Thus, the degree of ethanol preservation effect on δ13C values varies among tissue types, species and preservation times. δ13C values in animal tissues usually provide information about the consumed food sources (McConnaughey & McRoy, 1979) and the trophic fractionation between the diet and consumer tissues is assumed to be ca. 0–1 ‰ per trophic level (Post, 2002). If preservation causes a shift that exceeds the trophic discrimination factor (TDF), this may cause mis-interpretations in food web studies (DeNiro & Epstein, 1978; Bosley & Wainright, 1999; Bugoni et al., 2008). Thus, the changes in δ13C observed here (i.e., 0.3–0.4 ‰ enrichment) are comparable to TDFs generally applied in ecological stable isotope studies (Post, 2002).
Ethanol preservation altered the δ15N values of FPM foot and gonad tissues in different directions. These shifts in δ15N values of foot (+ 0.2 ‰) and gonad (– 0.1 ‰) were relatively small when considering the analytical precision here (0.24 ‰ for δ15N) as well as the commonly used TDF values (i.e., 3–4 ‰ increase with each trophic level) (Deniro & Epstein, 1981; Minagawa & Wada, 1984). This suggests that the δ15N values of preserved FPM tissues can be used in food web studies if unpreserved samples are not available. Findings from our and previous studies (Hobson et al., 1997; Ponsard & Amlou, 1999; Sarakinos et al., 2002; Lau et al., 2012; Olin et al., 2014) demonstrate a minimal effect of ethanol on δ15N values. However, some other studies have found relatively high increase in δ15N values, such as 0.7 ‰ for squid muscle tissue (Ruiz‐Cooley et al., 2011) and 0.9–1.0 ‰ for Asiatic clams (Sarakinos et al., 2002; Syväranta et al., 2011).
The largest effects of ethanol preservation were observed in δ2H values, with a significantly higher 2H-enrichment in the lipid-rich gonad (+ 14.5 ‰) than in the foot tissue (+ 7.1 ‰). The significant increase of δ2H in both tissues is likely derived from the loss of 2H-depleted lipids (Hobson et al., 1999). It is possible that structural changes, such as shrinkage or hardening of the tissues, could have caused lipid-release and isotopic enrichment (Singhal et al., 2016; Panzacchi et al., 2019). The TDFs of δ2H are yet poorly studied, but there are substantial differences in δ2H values between terrestrial and aquatic primary producers, with an average difference of ~ 100 ‰ (Doucett et al., 2007). Although the ethanol-induced shifts in δ2H values of FPM foot and gonad tissues were relatively large as compared to those observed for δ13C and δ15N values, the marked differences between the terrestrial and aquatic isotopic endmembers may allow the use of δ2H values of ethanol-preserved FPM tissues in isotopic mixing models estimating the reliance of FPM on various food sources (Doucett et al., 2007; Brauns et al., 2021).
Some previous studies demonstrate that preservation time can significantly change stable isotope values (Sweeting et al., 2004; Ruiz‐Cooley et al., 2011; McConnaughey & McRoy, 1979), whereas other studies have found no significant effect on δ13C and δ15N values (Gloutney & Hobson, 1998; Barrow et al., 2008). Our results showed significant effects of preservation time only on Δδ15N. Our results indicated that the most preservation time-induced changes in Δδ13C happened after 1 month of storage for both tissues, although the effect on Δδ13C was not significant, as was the case also in some previous studies (Sweeting et al., 2004; Syväranta et al., 2008). Few studies have investigated temporal changes in δ13C and δ15N values of ethanol-preserved samples, but it appears that changes can occur almost instantly (e.g. an increase of δ13C within a week for freshwater clams; (Syväranta et al., 2011). The most striking and temporally stable shifts occurred in the Δδ2H values and ΔC:N ratios of the FPM gonad tissue after 6 months’ ethanol preservation. The Δδ13C and Δδ15N values showed inconsistent and unexplained fluctuations during the preservation experiment but the fluctuations were within the range of analytical error (i.e., 0.14 ‰ for δ13C and 0.24 ‰ for δ15N) and thus may simply represent instrument noise.
Our results indicate that ethanol preservation affects δ2H values more than δ13C and δ15N values of FPM foot and gonad tissues. Although significant and potentially ecologically relevant shifts were observed for all elements, the effects on δ15N seemed small enough to facilitate the use of ethanol-preserved FPM samples to infer e.g. the trophic position of consumers in food web studies. Considering our findings, we suggest that ethanol should be used with caution and potential isotopic shifts should be accounted for when using preserved bivalves as tracers of environmental changes (Glibert et al., 2018). While our findings are encouraging for samples preserved for short periods, more research is needed to determine whether archived samples of endangered and long-lived FPM can be used to evaluate long-term changes in freshwater ecosystems (Schöne, 2013; Fritts et al., 2017).
References
Arrington, D. A. & K. O. Winemiller, 2002. Preservation effects on stable isotope analysis of fish muscle. Transactions of the American Fisheries Society 131: 337–342.
Atkinson, C. & C. Vaughn, 2014. Biogeochemical hotspots: temporal and spatial scaling of freshwater mussels on ecosystem function. Freshwater Biology 60: 563–574.
Barrow, L. M., K. A. Bjorndal & K. J. Reich, 2008. Effects of preservation method on stable carbon and nitrogen isotope values. Physiological and Biochemical Zoology 81: 688–693.
Bosley, K. L. & S. C. Wainright, 1999. Effects of preservatives and acidification on the stable isotope ratios (15N:14N, 13C:12C) of two species of marine animals. Canadian Journal of Fisheries and Aquatic Sciences 56: 2181–2185.
Brauns, M., T. Berendonk, S. Berg, F. Grunicke, D. Kneis, S. Krenek, T. Schiller, J. Schneider, A. Wagner & M. Weitere, 2021. Stable isotopes reveal the importance of terrestrially derived resources for the diet of the freshwater pearl mussel (Margaritifera margaritifera). Aquatic Conservation: Marine and Freshwater Ecosystems 31: 2496–2505.
Bugoni, L., R. A. R. McGill & R. W. Furness, 2008. Effects of preservation methods on stable isotope signatures in bird tissues. Rapid Communications in Mass Spectrometry 22: 2457–2462.
Burgess, K. B. & M. B. Bennett, 2017. Effects of ethanol storage and lipid and urea extraction on δ15N and δ13C isotope ratios in a benthic elasmobranch, the bluespotted maskray Neotrygon kuhlii: treatment effects on n. kuhlii δ13C and δ15N. Journal of Fish Biology 90: 417–423.
Carabel, S. & P. Verísimo, 2009. Effects of preservatives on stable isotope analyses of four marine species. Estuarine Coastal and Shelf Science 82: 348–350.
DeNiro, M. J. & S. Epstein, 1978. Influence of diet on the distribution of carbon isotopes in animals. Geochimica Et Cosmochimica Acta 42: 495–506.
Deniro, M. J. & S. Epstein, 1981. Influence of diet on the distribution of nitrogen isotopes in animals. Geochimica Et Cosmochimica Acta 45: 341–351.
Doucett, R. R., J. C. Marks, D. W. Blinn, M. Caron & B. A. Hungate, 2007. Measuring terrestrial subsidies to aquatic food webs using stable isotopes of hydrogen. Ecology 88: 1587–1592.
Edwards, M. S., T. F. Turner & Z. D. Sharp, 2002. Short- and long-term effects of fixation and preservation on stable isotope values (δ13C, δ15N, δ34S) of fluid-preserved museum specimens. Copeia 4: 1106–1112.
Farris, J. L. & J. H. Van Hassel, 2007. Freshwater Bivalve Ecotoxicology. SETAC Books, CRC Press, Taylor & Francis, Boca Raton:
Fritts, A. K., M. W. Fritts, W. R. Haag, J. A. DeBoer & A. F. Casper, 2017. Freshwater mussel shells (Unionidae) chronicle changes in a North American river over the past 1000 years. Science of the Total Environment 575: 199–206.
Fry, B., 2006. Stable Isotope Ecology, Springer, New York, NY:
Glibert, P. M., J. J. Middelburg, J. W. McClelland & M. J. Vander Zanden, 2018. Stable isotope tracers: Enriching our perspectives and questions on sources, fates, rates, and pathways of major elements in aquatic systems. Limnology and Oceanography 64: 950–981.
Gloutney, M. L. & K. A. Hobson, 1998. Field preservation techniques for the analysis of stable-carbon and nitrogen isotope ratios in eggs. Journal of Field Ornithology 69: 223–227.
Hetherington, E. D., C. M. Kurle, M. D. Ohman & B. N. Popp, 2019. Effects of chemical preservation on bulk and amino acid isotope ratios of zooplankton, fish, and squid tissues. Rapid Communications in Mass Spectrometry 33: 935–945.
Hobson, K. A. & L. I. Wassenaar, 2008. Tracking Animal Migration with Stable Isotopes, Elsevier, Inc., San Diego, CA:
Hobson, K. A., M. L. Gloutney & H. L. Gibbs, 1997. Preservation of blood and tissue samples for stable-carbon and stable-nitrogen isotope analysis. Canadian Journal of Zoology 75: 1720–1723.
Hobson, K. A., L. Atwell & L. I. Wassenaar, 1999. Influence of drinking water and diet on the stable-hydrogen isotope ratios of animal tissues. Proceedings of the National Academy of Sciences 96: 8003–8006.
Hogsden, K. L. & P. A. McHugh, 2017. Preservatives and sample preparation in stable isotope analysis of New Zealand freshwater invertebrates. New Zealand Journal of Marine and Freshwater Research 51: 455–464.
Javornik, J., J. B. Hopkins III., S. Zavadlav, T. Levanič, S. Lojen, T. Polak & K. Jerina, 2019. Effects of ethanol storage and lipids on stable isotope values in a large mammalian omnivore. Journal of Mammalogy 100: 150–157.
Jesus, F. M., M. R. Pereira, C. S. Rosa, M. Z. Moreira & C. F. Sperber, 2015. Preservation methods alter carbon and nitrogen stable isotope values in crickets (Orthoptera: Grylloidea). PLOS ONE 10: e0137650.
Jokela, J., L. Uotila & J. Taskinen, 1993. Effect of the castrating trematode parasite Rhipidocotyle fennica on energy allocation of fresh-water clam Anodonta piscinalis. Functional Ecology 7: 332–338.
Kaehler, S. & E. Pakhomov, 2001. Effects of storage and preservation on the δ13C and δ15N signatures of selected marine organisms. Marine Ecology Progress Series 219: 299–304.
Kelly, B., J. B. Dempson & M. Power, 2006. The effects of preservation on fish tissue stable isotope signatures. Journal of Fish Biology 69: 1595–1611.
Kiljunen, M., J. Grey, T. Sinisalo, C. Harrod, H. Immonen & R. I. Jones, 2006. A revised model for lipid-normalizing δ13C values from aquatic organisms, with implications for isotope mixing models: revised lipid-normalization model for C isotope analysis. Journal of Applied Ecology 43: 1213–1222.
Kiszka, J., V. Lesage & V. Ridoux, 2014. Effect of ethanol preservation on stable carbon and nitrogen isotope values in cetacean epidermis: Implication for using archived biopsy samples. Marine Mammal Science 30: 788–795.
Lau, D. C. P., K. M. Y. Leung & D. Dudgeon, 2012. Preservation effects on C/N ratios and stable isotope signatures of freshwater fishes and benthic macroinvertebrates. Limnology and Oceanography: Methods 10: 75–89.
Le Bourg, B., G. Lepoint & L. N. Michel, 2020. Effects of preservation methodology on stable isotope compositions of sea stars. Rapid Communications in Mass Spectrometry 34: e8589.
Lecea, A. M., R. Cooper, A. Omarjee & A. J. Smit, 2011. The effects of preservation methods, dyes and acidification on the isotopic values (δ15N and δ13C) of two zooplankton species from the KwaZulu-Natal Bight. South Africa Rapid Communications in Mass Spectrometry 25: 1853–1861.
Lenth, R. V., P. Buerkner, M. Herve, J. Love, F. Miguez, H. Riebl, & H. Singmann, 2022. emmeans: Estimated marginal means, aka least-squares means. https://CRAN.R-project.org/package=emmeans.
Liu, B., Y. Liu, Y. Li, H. Wang & J. Xu, 2013. An assessment of sample preservation methods for the determination of stable carbon and nitrogen isotope ratios in mollusks. Analytical Letters Taylor & Francis 46: 2620–2634.
McConnaughey, T. & C. P. McRoy, 1979. Food-Web structure and the fractionation of carbon isotopes in the bering sea. Marine Biology 53: 257–262.
Minagawa, M. & E. Wada, 1984. Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochimica et Cosmochimica Acta 48: 1135–1140.
Olin, J. A., G. R. Poulakis, P. W. Stevens, J. A. DeAngelo & A. T. Fisk, 2014. Preservation effects on stable isotope values of archived elasmobranch fin tissue: comparisons between frozen and ethanol-stored samples. Transactions of the American Fisheries Society 143: 1569–1576.
Panzacchi, S., F. Gnudi, D. Mandrioli, R. Montella, V. Strollo, B. A. Merrick, F. Belpoggi & E. Tibaldi, 2019. Effects of short and long-term alcohol-based fixation on Sprague-Dawley rat tissue morphology, protein and nucleic acid preservation. Acta Histochemica 121: 750–760.
Park, R. & S. Epstein, 1961. Metabolic fractionation of C13 & C12 in Plants. Plant Physiology 36: 133–138.
Pinheiro, J., D. Bates, S. DebRoy, D.R Sarkar, & R Core Team 2021. nlme: linear and nonlinear mixed effects models. https://CRAN.Rproject.org/package=nlme
Ponsard, S. & M. Amlou, 1999. Effects of several preservation methods on the isotopic content of Drosophila samples. Comptes Rendus de L’academie des Sciences 322: 35–41.
Post, D., 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83: 703–718.
Post, D. M., C. A. Layman, D. A. Arrington, G. Takimoto, J. Quattrochi & C. G. Montaña, 2007. Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152: 179–189.
R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Ruiz-Cooley, R. I., K. Garcia & E. D. Hetherington, 2011. Effects of lipid removal and preservatives on carbon and nitrogen stable isotope ratios of squid tissues: Implications for ecological studies. Journal of Experimental Marine Biology and Ecology 407: 101–107.
Sarakinos, H. C., M. L. Johnson & M. J. V. Zanden, 2002. A synthesis of tissue-preservation effects on carbon and nitrogen stable isotope signatures. Canadian Journal of Zoology 80: 381–387.
Schöne, B. R., 2013. Arctica islandica (Bivalvia): A unique paleoenvironmental archive of the northern North Atlantic Ocean. Global and Planetary Change 111: 199–225.
Singhal, P., N. N. Singh, G. Sreedhar, S. Banerjee, M. Batra & A. Garg, 2016. Evaluation of histomorphometric changes in tissue architecture in relation to alteration in fixation protocol – an invitro study. Journal of Clinical and Diagnostic Research 10: ZC28–ZC32.
Soto, D. X., L. I. Wassenaar & K. A. Hobson, 2013. Stable hydrogen and oxygen isotopes in aquatic food webs are tracers of diet and provenance. Functional Ecology 27: 535–543.
Soto, D. X., G. Koehler, L. I. Wassenaar & K. A. Hobson, 2017. Re-evaluation of the hydrogen stable isotopic composition of keratin calibration standards for wildlife and forensic science applications. Rapid Communications in Mass Spectrometry 31: 1193–1203.
Stallings, C. D., J. A. Nelson, K. L. Rozar, C. S. Adams, K. R. Wall, T. S. Switzer, B. L. Winner & D. J. Hollander, 2015. Effects of preservation methods of muscle tissue from upper-trophic level reef fishes on stable isotope values (δ13C and δ15N). PeerJ 3: e874.
Sweeting, C. J., N. V. C. Polunin & S. Jennings, 2004. Tissue and fixative dependent shifts of δ13C and δ15N in preserved ecological material. Rapid Communications in Mass Spectrometry 18: 2587–2592.
Syväranta, J., S. Vesala, M. Rask, J. Ruuhijärvi & R. Jones, 2008. Evaluating the utility of stable isotope analyses of archived freshwater sample materials. Hydrobiologia 600: 121–130.
Syväranta, J., A. Martino, D. Kopp, R. Céréghino & F. Santoul, 2011. Freezing and chemical preservatives alter the stable isotope values of carbon and nitrogen of the Asiatic clam (Corbicula fluminea). Hydrobiologia 658: 383–388.
Umbricht, J., J. Dippner, B. Fry, I. Kröncke, I. Liskow, P. Nehmer, F. Thoms & M. Voss, 2018. Correction of the isotopic composition (δ13C and δ15N) of preserved Baltic and North Sea macrozoobenthos and their trophic interactions. Marine Ecology Progress Series 595: 1–13.
Vaughn, C. C., 2018. Ecosystem services provided by freshwater mussels. Hydrobiologia 810: 15–27.
Vaughn, C., S. Nichols & D. Spooner, 2008. Community and foodweb ecology of freshwater mussels. Journal of the North American Benthological Society 27: 409–423.
Vizza, C., B. L. Sanderson, D. G. Burrows & H. J. Coe, 2013. The effects of ethanol preservation on fish fin stable isotopes: does variation in C: N ratio and body size matter? Transactions of the American Fisheries Society 142: 1469–1476.
Wassenaar, L. I. & K. A. Hobson, 2003. Comparative equilibration and online technique for determination of non-exchangeable hydrogen of keratins for use in animal migration studies. Isotopes in Environmental and Health Studies 39: 211–217.
Xu, J., Q. Yang, M. Zhang, M. Zhang, P. Xie & L.-A. Hansson, 2011. Preservation effects on stable isotope ratios and consequences for the reconstruction of energetic pathways. Aquatic Ecology 45: 483–492.
Yurkowski, D. J., A. J. Hussey, N. E. Hussey & A. T. Fisk, 2017. Effects of decomposition on carbon and nitrogen stable isotope values of muscle tissue of varying lipid content from three aquatic vertebrate species. Rapid Communications in Mass Spectrometry 31: 389–395.
Acknowledgements
The authors would like to thank Nina Honkanen for her technical assistance in the laboratory. The Kolarctic Cross-Border Collaboration (CBC) Program (Project SALMUS/KO1017; JT), the Academy of Finland (COLDWEBS project/346293; APE), LIFE Revives (LIFE NAT/FI-000611; JT) and the Biological and Environmental Science Doctoral School of the JYU (MH) provided financial support for the study.
Funding
Open Access funding provided by University of Jyväskylä (JYU). The Kolarctic Cross-Border Collaboration (CBC) Program (Project SALMUS/KO1017; JT), the Academy of Finland (COLDWEBS project/346293; APE), LIFE Revives (LIFE NAT/FI-000611; JT) and the Biological and Environmental Science Doctoral School of the JYU (MH) provided financial support for the study.
Author information
Authors and Affiliations
Contributions
MH, JT, and MK all conceived of and designed the experiments, performed the experiments, and collected the data. MH, APE and MK analyzed the data. MH, JT, APE, and MK contributed to writing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Data availability
All the data and materials will be provided, if requested.
Code availability
Not applicable.
Ethical approval
Applicable.
Consent to participate
All authors have given their consent to participate.
Consent for publication
All authors have given their consent for publication.
Additional information
Handling editor: Manuel Lopes-Lima
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hajisafarali, M., Taskinen, J., Eloranta, A.P. et al. Ethanol preservation effects on stable carbon, nitrogen and hydrogen isotopes in the freshwater pearl mussel. Hydrobiologia 850, 1885–1895 (2023). https://doi.org/10.1007/s10750-023-05199-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05199-2