Abstract
Flow-regulated discharges of water from control structures into estuaries result in hydrologic and water chemistry conditions that impact spatial and temporal variability in the structure and biomass of phytoplankton communities, including the potential for harmful algal blooms (HABs). The relationships between regulated Caloosahatchee River (i.e., C-43 Canal) discharges and phytoplankton communities in the Caloosahatchee Estuary and adjacent nearshore regions on the southwest coast of Florida were investigated during two study periods, 2009–2010 and 2018–2019. During periods of low to moderate discharge rates, when mesohaline conditions predominated in the estuary, and water residence times were comparatively long, major blooms of the HAB dinoflagellate species Akashiwo sanguinea were observed in the estuary. Periods of high discharge were characterized by comparatively low phytoplankton biomass in the estuary and greater influence of a wide range of freshwater taxa in the upper reaches. By contrast, intense blooms of the toxic dinoflagellate Karenia brevis in the nearshore region outside of the estuary were observed during high discharge periods in 2018–2019. The latter events were significantly associated with elevated levels of nitrogen in the estuary compared to lower average concentrations in the 2009–2010 study period. The relationships observed in this study provide insights into the importance of managing regulated discharge regimes to minimize adverse impacts of HABs on the health of the estuary and related coastal environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
River-dominated estuaries are characterized by highly variable salinity regimes, hydrologic conditions, and plankton communities (Jassby et al., 1997; Lucas et al., 1999a, b; Cloern, 2001; Bledsoe et al., 2004; Quinlan & Phlips, 2007; Cloern & Jassby, 2008; Sin & Jeong, 2015). The dynamics of river flow strongly affect the structure and function of biological communities in the estuary and nearshore environment (Bunn & Arthington, 2002; Poff & Zimmermann, 2010; Van Niekerk et al., 2019; Chilton et al., 2021), including phytoplankton populations (Snow et al., 2000; Dorado et al., 2015; Cloern et al., 2017; Phlips et al., 2020). These dynamic elements are strongly influenced by climatic conditions and the character of watershed inputs (Lancelot & Muylaert, 2011; Hallett et al., 2018; Chilton et al., 2021). In many estuaries around the world, high rainfall periods are associated with enhanced nutrient-enriched river discharges, which can influence phytoplankton composition and biomass in both the estuary and associated coastal regions (Eyre, 2000; Paerl et al., 2006; Quinlan & Phlips, 2007; Costa et al., 2009; Lancelot & Muylaert, 2011; Glibert et al., 2014; Winder et al., 2017; Bharathi et al., 2018). In hydrologically restricted estuaries, periods of nutrient-rich inflows can result in algal blooms due to extended water residence times (Knoppers et al., 1991; Monbet, 1992; Phlips et al., 2004, 2015, 2021; Robson & Hamilton, 2004; Ralston et al., 2015). By contrast, in well-flushed estuaries, high rates of river discharge can result in declines in water residence times and water clarity, reducing the buildup of phytoplankton biomass, despite elevated nutrient levels (Mallin et al., 1998; Phlips et al., 2004; Dix et al., 2013; Hart et al., 2015; Wang et al., 2016; Phlips et al., 2020). In some estuaries, river discharges can contain high freshwater phytoplankton biomass resulting in the introduction of freshwater harmful algal blooms (HABs) (Phlips et al., 2012; Rosen et al., 2018; Metcalf et al., 2021). The latter scenario is illustrated by the re-occurring toxic cyanobacteria blooms observed in the St. Lucie estuary on the east coast of Florida and Caloosahatchee Estuary on the west coast, both of which emanate from canals connected to Lake Okeechobee, a lake subject to frequent intense cyanobacteria blooms (Phlips et al., 2012, 2020; Rosen et al., 2018).
The already complex and dynamic nature of river-dominated estuaries is further complicated in ecosystems associated with rivers containing engineered water control structures designed to manage water levels, flows, and navigation (Doering & Chamberlain, 1999; Sin et al., 2013; Sin & Jeong, 2015; Jo et al., 2019; Kim & Kim, 2020). The Caloosahatchee River and estuary is one such system. In the nineteenth century, the Caloosahatchee River was channelized to create the C-43 Canal and the canal was extended to connect with Lake Okeechobee in order to manage water levels in the lake and provide a navigable passage from the east to the west coasts of Florida (SFWMD, 2014). Connection of the lake to the Caloosahatchee Estuary via the C-43 Canal has significantly altered salinity gradients in the estuary. Due to the eutrophic character of Lake Okeechobee and high nutrient levels in the watersheds that feed directly into the C-43 Canal, the connection has increased nutrient loads to the estuary and coastal environment, as well as increased inter- and intra-annual variability in salinity and water residence times in the estuary (Doering & Chamberlain 1999; SFWMD 2009; Wan et al. 2013; Mathews et al., 2015; Sun et al. 2022). For example, periods of high discharge from the C-43 Canal via the S-79 control structure can turn much of the upper estuary fresh or oligohaline and reduce water residence times (Mathews et al., 2015). The frequent occurrence of intense cyanobacteria blooms in Lake Okeechobee (Phlips et al., 1993; Havens et al., 2016; Rosen et al., 2018) elevates the potential for introduction of high levels of toxic cyanobacteria into the estuary via the canal (Phlips et al., 2012, 2020; Metcalf et al., 2021). Downstream of the Lake’s input, several tributaries from the watershed associated with the C-43 discharge water into the Canal, introducing additional nutrients to the canal (Doering & Chamberlain, 1999; Doering et al., 2006; SFWMD 2009). In addition, rapid human development in the watershed surrounding the Caloosahatchee Estuary proper has increased the area of impervious surface cover, adding to nutrient runoff into the estuary (Doering et al., 2006). Increases in nutrient loads to the estuary and nearshore regions of southwest Florida have raised concerns about the potential for intensification of harmful algal blooms, such as the toxic red tide events that frequent the eastern Gulf of Mexico (Heil et al., 2014b; Medina et al., 2021, 2022).
The objective of this study was to examine changes in the character of phytoplankton communities in the Caloosahatchee Estuary and adjacent nearshore regions within the context of variability in salinity and nutrient levels associated with different flow-regulated discharge levels from the C-43 Canal into the estuary. Salinity, nutrient levels, and water residence times all play major roles in defining spatial and temporal patterns in the composition and biomass of phytoplankton in the region, including harmful bloom-forming species, such as red tides dominated by the toxic dinoflagellate Karenia brevis (Steidinger, 2009; Heil et al., 2014). It was hypothesized that the range of residence times in the estuary, which are closely tied to discharge rates from control structures upstream of the estuary, would play a major role in defining phytoplankton biomass, composition, and the potential for blooms. By contrast, the volume and nutrient concentrations in water leaving the estuary would define the potential impact on major nearshore blooms, such as red tides that frequent the west coast of Florida. The relationships observed in this study provide insights into the importance of managing regulated discharge regimes to minimize the occurrence, and intensity of HABs, not just in the Caloosahatchee estuary, but in similar systems around the world. Other studies have highlighted the need to better understand the unique challenges posed by the presence of water control structures and their impact on phytoplankton dynamics in estuaries (Sin et al., 2013). Expected future changes in rainfall levels, storm frequencies, and sea level driven by climate change will exacerbate the need to better understand these relationships in order to inform future management efforts.
Methods
Sampling site description
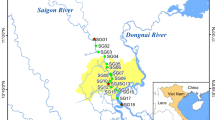
The Caloosahatchee Estuary is located on the southwest coast of Florida (Fig. 1) and has an area of 62 km2. The upper boundary of the estuary is defined by the Franklin lock and dam water control structure (S-79), which is the point of release of water from the C-43 Canal. The width of the estuary ranges from 160 m near S-79 to 2,500 m at the entrance to San Carlos Bay (Scarlatos, 1988). Upstream of the lock, the C-43 Canal extends up to Lake Okeechobee, where the S-77 water control structure regulates water releases from the lake into the canal. Between the S-77 and S-79, there is an additional control structure (S-78), and several tributaries from the C-43 basin watershed that discharge into the C-43 Canal. The mouth of the lower estuary is bordered by the San Carlos Bay, which is open to the Gulf of Mexico.
The average depths through the Caloosahatchee Estuary are near 2.0 m, and a narrow navigation channel of 3–4 m depth extends from the S-79 through the estuary (Scarlatos, 1988). The estuary experiences a combination of diurnal and mixed semi-diurnal tides with a mean tidal range of 0.3 m in the middle of the estuary near downtown Fort Myers (Scarlatos, 1988; NOAA, 2010).
Freshwater from the C-43 Canal is released into the estuary through S-79 in order to maintain prescribed water levels in Lake Okeechobee and control flooding in the watersheds (C-43 basin) between Lake Okeechobee and the Caloosahatchee Estuary (Fig. 1) (Flaig & Capece, 1998; Doering & Chamberlain, 1999). The C-43 basin watershed covers 3,625 km2 and is made up of agricultural areas in the east and urban areas surrounding the Caloosahatchee Estuary west of the S-79 (Knight & Steele, 2005). Additional sources of freshwater entering the estuary downstream of S-79 include surface water runoff and several regional tributaries, including Orange River, Popash Creek, and Whiskey Creek, which contribute to annual freshwater inputs to the estuary, although the inputs are relatively small compared to annual inputs from S-79 (Scarlatos, 1988; Flaig & Capece 1998; Knight & Steele, 2005; SFWMD, 2014).
Rainfall and S-79 discharge rates
Monthly rainfall levels at the Ft. Meyers Page Field meteorological station were obtained from the NOAA National Center for Environmental Data, Climatological Data—Florida (www.ncdc.noaa.gov). Daily rates of freshwater discharge from the S-79 Franklin Lock and Dam were obtained from DBHYDRO, a database maintained by the South Florida Water Management District (https://my.sfwmd.gov/dbhydroplsql/show_dbkey_info.main_menu). For statistical analyses of the relationships between discharge and phytoplankton biomass, mean discharge rates for the 14 days prior to water sampling dates were used.
Water sampling
Data used in this paper were based on two separate studies of the estuary and nearshore region, the first from February of 2009 to February of 2010 (funded by the South Florida Water Management District, W. Palm Beach, Florida), and the second from September of 2018 to November of 2019 (funded by the National Science Foundation, Washington DC).
The 2009–2010 study period included four sampling sites (1–4) (Fig. 1). Site 1 was located in the Upper Caloosahatchee Estuary just downstream of Beautiful Island. Site 2 was located in the Middle Estuary near downtown Fort Myers. Site 3 was located in the Lower Estuary. Site 4 was located in San Carlos Bay, just outside the estuary. Sampling was carried out just outside the main navigation channel. Depths at the sampling sites were between 2 and 3 m. The sites were sampled once a month from February 2009 to February 2010 (excluding March 2009).
The 2018–2019 study period included 13 sites sampling sites (Fig. 1). The locations of Sites 1–4 matched those used in the 2009/2010 study period. The depths of the sampling sites in San Carlos Bay (Sites 5–7) were between 2 and 3 m. Depths of Sites 8–13 ranged from 3 to 6 m. Because the sampling frequency was based on capturing responses to different flow regimes and sources, the samples were collected in Sept. 2018, Oct. 2018, May 2019, June 2019, August 2019, Sept. 2019, Oct. 2019, and Nov. 2019. The selection of the sampling dates was based on the priorities of the NSF-Rapid research grant that funded the 2018–2019 study period.
Water samples were collected with a vertically integrating pole sampler (Wetzel & Likens, 1991) that evenly collected water from the water column down to approximately 0.5 m from the bottom of sites up to 3.5 m in depth. For deeper sites (Sites 9–13), the top 3 m of the water column was sampled using the pole. A minimum of three pole draws of water were combined in a mixing vessel. From each collection, aliquots of sample water were bottled, stored, and preserved for chemical and phytoplankton analysis according to the protocols set forth in the Phlips Laboratory (University of Florida) National Environmental Laboratory Accreditation Program manual (NELAP Certification # E72883).
Field measurements
Water temperature and salinity were measured during each sampling event at the surface and near the bottom of the water column using a HACH (Loveland, Colorado) HjQ40D or YSI EXO2 sonde. Salinity was calibrated using a two-point calibration following the manufacturers' guidelines. Temperature and salinity data for the 2018–2019 study period included gaps in coverage from November 2018 to April 2019. The gaps were partially filled in with information from continuous water quality monitoring platforms equipped with YSI EXO2 sondes established by the Sanibel Captiva Conservation Commission RECON program. The platforms are located near Sites 1, 2, and 4.
Chemical analyses
Chemical analyses during the 2009–2010 study period were conducted at the Dr. Edward J. Phlips Laboratory at the University of Florida, using methods certified by the National Environmental Laboratory Accreditation Program (NELAP Certification # E72883). Aliquots of whole unfiltered sample water were transported on ice and stored frozen until chemical analysis, within NELAP-specified holding times. Samples for TN and TP analyses were digested using the persulfate oxidation method (Strickland & Parsons, 1972; APHA, 2005) and measured colorimetrically on a Bran-Luebbe (Norderstedt, Germany) AA3 auto-analyzer and a Hitachi (Tokyo, Japan) U2810 dual-beam spectrophotometer, respectively.
Chemical analyses during the 2018–2019 study period were conducted at Benchmark EA, using NELAP-certified methods (NELAP Certification #E85086). Aliquots of whole unfiltered water were fixed with sulfuric acid (pH < 2) and placed on ice (4 °C) for transportation to the laboratory. Samples were analyzed for TN and TP following EPA methods (http://www.benchmarkea.com). For TP, samples were analyzed following persulfate digestion (EPA 365.3), and for TN, a sulfuric acid digestion (EPA 351.2) followed by analysis on a Systea Easychem Plus (Systea, Italy).
Phytoplankton analyses
Integrated, whole water samples were preserved on-site with Lugol’s solution (APHA, 2005) and analyzed microscopically for phytoplankton abundance and species composition (S. Badylak & E. J. Phlips at the University of Florida). General phytoplankton abundance and composition was determined using the Utermöhl method (Utermöhl, 1958), as described in Badylak et al. (2014a). Samples preserved in Lugol's were settled in 19 mm diameter cylindrical chambers. Phytoplankton cells were identified and counted at 400 × and 100 × with a Leica phase contrast inverted microscope. At 400 × , a minimum of 100 cells of a single taxon and 30 grids were counted. If 100 cells were not counted by 30 grids, up to a maximum of 100 grids were counted until 100 cells of a single taxon were reached. At 100 × , a total bottom count was completed for taxa > 30 µm in size.
Picocyanobacteria abundances were determined using a Zeiss Axio compound microscope, using green and blue light excitation (Fahnenstiel & Carrick, 1992; Phlips et al., 1999). Samples were preserved with buffered glutaraldehyde. Subsamples of water were filtered onto 0.2 µm Nucleopore filters and mounted between a microscope slide and cover slip with immersion oil and picoplankton counted at × 1000 magnification.
Count data were converted to phytoplankton biovolume, using the closest geometric shape method (Smayda 1978; Sun & Liu 2003). Phytoplankton carbon values (as µg carbon l−1) were estimated by applying conversion factors for different taxonomic groups to biovolume estimates (expressed as 106 µm3 ml−1): i.e., 0.065 × biovolume of diatoms, 0.22 × biovolume for cyanobacteria, and 0.16 × biovolume for dinoflagellates or other taxa (Strathmann, 1967; Ahlgren, 1983; Sicko-Goad et al., 1984; Verity et al., 1992; Work et al., 2005).
Statistical methods
Non-parametric Spearman’s correlation analysis (P-value ≤ 0.10) was used to explore the relationships between the biomass of phytoplankton and the environmental factors for the estuary and nearshore regions.
Nonmetric multidimensional scaling (NMDS) was performed to investigate potential environmental predictors of phytoplankton assemblage patterns. NMDS is an indirect gradient analysis which creates an ordination based on a dissimilarity or distance matrix (Kruskal, 1964). NMDS is a non-parametric ordination technique based on the ranking of dissimilarity values rather than actual values of dissimilarity. NMDS is considered one of the most effective ordination methods for ecological data (McCune et al., 2002) and has been widely applied to phytoplankton composition analysis (Winter & Hunter, 2008; Rothenberger et al., 2009; Sun et al., 2011; Kim et al., 2020). Prior to the analysis, phytoplankton data were transformed by log (x + 1), and water temperature, salinity, TN, TP, TN:TP ratio, and mean discharge were included in the analysis. TN:TP ratios are expressed on a weight basis, and were used to examine deviations from the Redfield ratio (i.e., N:P, 7.2:1 by weight) (Reynolds, 2006), as an indicator of broad spatial and temporal shifts in relative nutrient levels. The NMDS ordinations were carried out using the statistical software R (R Core Team, 2022) with the “vegan” package (Oksanen et al., 2007). A Bray–Curtis distance matrix was used (Beals, 1984). NMDS was run for the samples collected in the estuary, and a separate NMDS was run for samples collected in the nearshore. NMDS arranged samples in ordination space according to their similarity or dissimilarity in species composition. Environmental variables or phytoplankton species were represented via vectors on the ordination maps to indicate the strength and direction of maximum increase (Rothenberger et al., 2009).
Results
Local rainfall and S-79 discharge
During the 2009–2010 study period, rainfall levels in the local region of the Caloosahatchee Estuary increased substantially from the beginning of the wet season in late May through September (Fig. 2). S-79 discharge rates from the S-79 control structure were below 2000 cfs from February through April 2009, then increased to between 2000 and 5000 cfs through September (Fig. 2). In October and November, discharges were negligible, then picked up to moderate levels (i.e., 500–2000 cfs) through February of 2010, in association with a moderate peak in rainfall. Discharge rates followed the same general pattern as local rainfall; however, during certain time periods, local rainfall levels were proportionally higher than discharge, such as October and December of 2009.
The 2018–2019 study period was primarily confined to the wet season in the region (May–October) (Fig. 2). The September/October 2018 sampling period was associated with high rainfall and the June–November 2019 sampling period was also characterized by high rainfall. S-79 discharge rates were high from July through September 2018, with most values between 2000 and 6000 cfs (Fig. 2). From October 2018 through May 2019, discharge rates were primarily below 2000 cfs, then increased in June through August to between 2000 to 6000 cfs. From September through November 2019, discharge rates were below 1000 cfs. However, during certain time periods, local rainfall levels were proportionally higher than discharge, such as May and October of 2019.
Temperature
The Caloosahatchee Estuary is located in the sub-tropical region of the eastern Gulf of Mexico along the south-western coast of Florida. As such, it is typically characterized by relatively modest seasonal range in water temperatures compared to estuaries in temperate latitudes. This is illustrated by water temperatures observed during the 2009–2010 and 2018–2019 study periods, which ranged from 15 °C in the winter of 2010 to near 30 °C during the summer months (Fig. 3). Temperatures did not drop below 20 °C except in mid-winter (Dec.–Feb.).
Surface water temperatures at the four sampling sites (Sites 1–4) during the 2009–2010 study period, and six sites (Sites 1–4, 7 and 12) during the 2018–2019 sampling period. Gaps in the time series at Sites 3, 7, and 12 in the 2018–2019 study period are because of the lack of in situ sampling events from November 2018 to April 2019. Temperatures for Sites 1, 2, and 4 during the latter time period were filled in with data from the RECON continuous water quality monitoring stations located near the sites
Salinity
During the 2009–2010 study period, salinities encountered at Sites 1–4 increased from the upper estuary to the nearshore reaches of the sampling region in San Carlos Bay (Site 4) (Fig. 4). All four sites exhibited similar temporal pattern of variability, with higher salinities during periods of low S-79 discharge and local rainfall levels, which were generally associated with the dry season in central Florida (November–April) (Fig. 2). The upper-most sites in the estuary (Sites 1) were the first to respond to changes in rainfall and S-79 discharge, reaching near freshwater surface water salinities (< 1 psu) from June through September of 2009. The largest range in salinity values was observed at Site 3 in the lower estuary, as illustrated by the decline in salinity from 28 psu in May to 3 psu in July. The smallest range in salinity was observed at Site 4 in San Carlos Bay near the mouth of the estuary, with a low of 25 psu in July and August, and a peak of 34 psu in May. Sites 1–3 exhibited periods of differences between surface and bottom water salinity during the wet season.
During the 2018–2019 study period, the salinities encountered at Sites 1–4 increased from the upper to the nearshore reaches of the sampling region (Fig. 5). All four sites exhibited similar temporal patterns of variability, with lower salinities during periods of high local rainfall and S-79 discharge (Fig. 2). All three sites in the estuary had near freshwater salinities (< 1 psu) in September 2018 and August 2019, reflecting the high S-79 discharge rates and local rainfall levels during the wet seasons of 2018 and 2019 (Fig. 2). The depressed salinity levels observed during these periods were even observed at the outer edge of San Carlos Bay (Site 7) (Fig. 5), demonstrating the extended influence of outflows from the Caloosahatchee Estuary on nearshore waters of the Gulf of Mexico. The nearshore sites outside of San Carlos Bay exhibited less salinity variation, as illustrated by Site 12 (Fig. 5).
Surface water salinities (psu) at six sampling sites during the 2018–2019 study period. Gaps in the time series at Sites 3, 7, and 12 are because of the lack of in situ sampling events during the dry season from November 2018 to April 2019. Salinities for Sites 1, 2, and 4 were filled in with data from the RECON continuous water quality monitoring stations located near the sites
Total nitrogen and total phosphorus
In the 2009–2010 study period, temporal trends in total nitrogen (TN) were similar at all four sites (Fig. 6). With the onset of the wet season in May, increases in local rainfall levels and discharge from the C-43 Canal coincided with increases in TN concentrations at all four sites until July–August, after which concentrations declined to near pre-wet season levels. Mean TN concentrations in the dry season ranged from 0.37 mg l−1 at Site 4 in San Carlos Bay to 0.90 mg l−1 at Site 1 in the upper Caloosahatchee Estuary near the S-79 structure (Table 1). In the wet season, mean TN concentrations were higher, ranging from 0.44 mg l−1 at Site 4 to 1.21 mg l−1 at Site 1.
Total phosphorus (TP) concentrations in the 2009–2010 study period exhibited the same general trend as TN (Fig. 6). TP concentrations increased at Sites 1 and 2 in the upper Caloosahatchee Estuary at the beginning of the wet season in May, followed by increases at Sites 3 and 4 in June. By June–July, TP concentrations increased by 2-fold-to-3-fold at all four sites, then declined to near pre-wet season levels by August–September. Mean TP during the dry season ranged from 0.04 mg l−1 at Site 4 in San Carlos Bay to 0.12 mg l−1 at Site 1 in the upper estuary (Table 1). Mean TP concentrations in the wet season were higher, ranging from 0.06 mg l−1 at Site 4 to 0.17 mg l−1 at Site 1.
The 2018–2019 study period was primarily confined to the wet periods of the 2 years. Temporal trends in TN concentrations in the estuary at Sites 1–3, and Site 4 in San Carlos Bay were similar to those observed in 2009–2010 (Fig. 7), with marked increases during the onset of the wet season in 2019. In the nearshore region outside of San Carlos Bay, TN concentrations were generally lower than in the Bay, as illustrated by Site 12 (Fig. 7). Mean TN concentrations for the wet seasons of 2018 and 2019 ranged from 0.88 mg l−1 at Site 7 at the southern edge of San Carlos Bay to 2.03 mg l−1 at Site 1 in the upper Caloosahatchee Estuary (Table 1). It is noteworthy that the mean wet season TN concentrations in the 2018–2019 period were nearly twice as high as the mean concentrations during the wet season of 2009–2010 at the shared sites (Table 1).
TP concentrations in the 2018–2019 study period exhibited the same general trends as TN (Fig. 7). Mean TP concentrations for the wet seasons of 2018/19 ranged from 0.04 mg l−1 at Site 7 to 0.14 mg l−1 at Site 1 (Table 1). Unlike mean TN concentrations, mean wet season TP concentrations for 2018–2019 were similar to those observed in 2009–2010 at all shared sites, suggesting that nitrogen loads to the estuary were higher in the former time period, but phosphorus loads remained similar (Table 1).
Mean TN/TP ratios in the 2009–2010 portion of the study period were similar at all four sites in both the dry and wet period, ranging from 6.5 to 8.9, near the Redfield ratio (i.e., 7.2 on a weight basis) (Table 1). In the 2018–2019 portion of the study period, TN/TP ratios for the wet period ranged from 14.5 to 24.2. Ratios at Sites 1–4 for the wet season were twice as high in 2018–2019 than in 2009–2010, largely due to higher TN values. The higher ratios in San Carlos Bay (i.e., Sites 4 and 7) than in the estuary reflect the incrementally larger decrease of TP than TN concentrations in the bay. The observed spatial and temporal changes in ratios provide broad insights into shifts in total nutrient loads to the ecosystem. However, it is premature to specifically define changes in nutrient limiting status for the phytoplankton community due to insufficient detailed information on the percent of each total that is in bioavailable forms.
Phytoplankton biomass and bloom composition
During the 2009–2010 study period, the range of phytoplankton biomass was highest at Sites 1 and 2 in the upper Caloosahatchee Estuary, with peak values of 7.2 and 4.4 mg carbon l−1, respectively (Fig. 8). Peak biomass at Site 3 in the lower estuary was 0.8 mg carbon l−1. The dominant species during the major bloom peaks at the three sites was the HAB dinoflagellate Akashiwo sanguinea (K. Hirasaka) Gert Hansen & Moestrup. Bloom levels of A. sanguinea were first observed at Site 1 in February 2009, then peaked in early May. Bloom levels of A. sanguinea were first observed at Site 2 in June, and subsequently in July at Site 3, suggesting movement of the bloom peaks downstream. Phytoplankton biomass levels at Site 4 in San Carlos Bay peaked in the mid-summer of 2009 at 0.25 mg carbon l−1 and were primarily dominated by diatoms (e.g., Skeletonema spp.) and picoplanktonic cyanobacteria (i.e., spherical picocyanobacteria and Synechococcus spp.). Diatoms and picoplanktonic cyanobacteria were also major contributors to total phytoplankton biomass at Sites 1–3 outside of periods of A. sanguinea blooms.
Biomass (mg carbon l−1) of phytoplankton by group in samples from the first collection time on the day of the monthly sampling in the Caloosahatchee Estuary (Sites 1–3) and San Carlos Bay (Site 4), i.e., pink—dinoflagellates (excluding K. brevis); red—Karenia brevis; orange—diatoms; light blue—picocyanobacteria; blue—other cyanobacteria; green—all other phytoplankton taxa. Letters designate dominant species in sample with major bloom, i.e., A—Akashiwo sanguinea; E—Euglena
During the 2018–2019 study period, the highest biomass levels in the Caloosahatchee Estuary were observed at Sites 2 and 3, with peak values of 7.2 and 4.0 mg carbon l−1, respectively (Fig. 8). The peaks were dominated by A. sanguinea and were only observed the last month of the sampling period in November 2019. In the other months of the sampling period, total phytoplankton biomass levels at Sites 1–3 were generally less than 0.5 mg carbon l−1 and dominated by cyanobacteria, nanoplanktonic eukaryotes, or diatoms. At Site 4 in San Carlos Bay, biomass levels were consistently below 0.25 mg carbon l−1 and most months were dominated by picoplanktonic cyanobacteria, nanoplanktonic eukaryotes, or diatoms. At the end of the sampling period, low concentrations of the toxic ‘red tide’ dinoflagellate Karenia brevis (C.C. Davis) Gert Hansen & Moestrup were observed.
At the nearshore sites outside of the boundary of the Caloosahatchee Estuary (7–13), a major red tide event dominated by the dinoflagellate K. brevis was observed in September of 2018 (as illustrated by representative sites, Fig. 9). Sites 7–13 were all involved in the event, with biomass levels up to 6.0 mg carbon l−1 (cell density of 5.6 × 106 cells l−1). Another K. brevis bloom event was observed in the same region in November 2019, with peak biomass of 5.1 mg carbon l−1 (Fig. 9). During the rest of the sampling dates, total phytoplankton biomass values were below 0.5 mg carbon l−1 and generally dominated by picoplanktonic cyanobacteria and/or diatoms.
Mean and median total phytoplankton biomass levels were higher during the wet than dry season in the 2009–2010 study period (Table 2). Mean and median biomass levels were higher in the upper (Sites 1 and 2) than the lower estuary, in part reflecting the disproportionate effect of large A. sanguinea blooms in the early months of the wet season on seasonal mean values. During the wet period of the 2018–2019 study period, mean biomass levels in the upper estuary were lower than in 2009–2010, while the reverse was true in the lower estuary, largely reflecting differences in the distribution of A. sanguinea between the two study periods. In San Carlos Bay, mean and median biomass levels were similar in the wet periods of both study periods. In the nearshore region outside of the Bay mean wet season, total biomass levels were higher than in either the estuary or Bay, reflecting the presence of an intense red tide dominated by K. brevis.
Dominant phytoplankton taxa in different salinity regimes
Oligohaline (≤ 5 psu) conditions in surface water were primarily limited to Sites 1 and 2 in the upper estuary during the wet season (May–October), with the exception of two months at Site 3 in the lower estuary, i.e., July and August of 2009 and 2019 (Figs. 4, 5). The Top-50 list was predominantly populated by freshwater taxa during oligohaline conditions, including cyanobacteria, dinoflagellates, euglenophytes, diatoms, and chlorophytes (Table 3). Also prominently represented on the list is the marine diatom Skeletonema costatum (Greville) Cleve, which is known for its tolerance to low salinities (Brand 1984). Several euryhaline dinoflagellates were also on the list, including A. sanguinea, Peridinium quinquecorne T. H. Abé, Karlodinium veneficum (D. Ballantine) J. Larsen, and Scrippsiella sp. In the cases involving the latter taxa, water below the pycnocline fell within the lower mesohaline range, suggesting that the prominent presence of these taxa may have in part been based on the presence of higher salinities below the pycnocline.
During mesohaline conditions (salinities > 5–18 psu), the Top-50 list was dominated by the HAB dinoflagellate A. sanguinea and the diatom S. costatum, both of which are euryhaline species (Table 3). Several other marine dinoflagellates were well represented, including the mixotrophic/heterotrophic species Polykrikos schwartzii Bütschli, Protoperidinium pp., and Scrippsiella trochoidea (F. Stein) A. R. Loeblich III. The diatom genus Chaetoceros was also present under mesohaline conditions.
During polyhaline conditions (salinities > 18–30 psu), the Top-50 list was dominated by the HAB dinoflagellates A. sanguinea and K. brevis, both of which were observed at major bloom levels of biomass (i.e. , > 4 mg carbon l−1) (Table 3). The list also contained several euryhaline diatoms, including S. costatum, Rhizosolenia setigera Brightwell and Coscinodiscus, as well as six mixotrophic/heterotrophic species of dinoflagellates; Gyrodinium spirale (Bergh) Kofoid & Swezy, P. quinquecorne, Gonyaulax, Gyrodinium cf. pingue (F. Schütt) Kofoid & Swezy, and the toxic HAB species K. veneficum.
Under euhaline conditions (salinities > 30 psu), the Top-50 list was dominated by the HAB dinoflagellate K. brevis, with numerous observations of major bloom biomass levels (Table 3). The other noteworthy taxa on the list were dinoflagellates, including P. quinquecorne, Protoperidinium, and HAB species A. sanguinea and Prorocentrum minimum (Pavillard) J. Schiller.
Picocyanobacteria were an important component of the phytoplankton communities of the Caloosahatchee Estuary, San Carlos Bay and the nearshore community, as highlighted by the large presence of the group in the Top-50 list of individual taxa biomass contributions across all salinity regimes from oligohaline (salinities ≤ 5 psu) to euhaline (salinities > 30 psu) (Table 3). While picocyanobacteria were not observed at major bloom biomass levels (i.e., > 2 mg carbon l−1), as some other species were, they were generally the most numerically abundant photoautotrophic taxa in all salinity regimes (Table 3) and sampling sites, reaching cell densities of up to 1.6 × 109 cells l−1.
Relationships between environmental factors and phytoplankton biomass
Overall, the two taxa that reached the highest biomass levels over the study period were A. sanguinea and K. brevis (Figs. 8, 9; Table 3). From a salinity perspective, they dominated two ends of the spectrum with some overlap, with major blooms of A. sanguinea primarily observed under mesohaline conditions in the Caloosahatchee Estuary, and K. brevis under euhaline conditions in nearshore regions outside of the estuary (Fig. 10). In terms of potential drivers of blooms of the two taxa, differences were also observed in the relationships with discharge rates from the S-79 into the estuary. The major blooms in the estuary (i.e., carbon biomass values > 2 mg l−1) were dominated by A. sanguinea and limited to periods of low to moderate S-79 discharge (i.e., < 2000 cfs) (Fig. 11).
The relationships between mean discharge rates prior to the sampling dates and biomass of Akashiwo sanguinea in the Caloosahatchee Estuary and Karenia brevis in the nearshore region. In the top panel (A. sanguinea), colored circles represent the Site 1 (red), Site 2 (blue), and Site 3 (green). In the bottom panel (K. brevis), colored circles represent 2018 blooms (red) and 2019 blooms (orange). Open circles represent dates in each region when neither species was observed
The negative relationship between S-79 discharge and A. sanguinea was further indicated by the results of Spearman’s correlation analysis, which revealed a negative correlation between mean discharge and dinoflagellate biomass for the estuarine region (Fig. 12a). For cyanobacteria, biomass was positively correlated with temperature, discharge, and TN concentrations in the estuary. In the case of the nearshore region, where blooms of K. brevis were the major feature, dinoflagellate biomass was positively correlated to TN concentrations (Fig. 12b). No significant correlations were observed for cyanobacteria or diatoms for the nearshore region, but “All Other” group biomass, which was dominated by nanoplanktonic eukaryotes (such as cryptophytes), was positively correlated to TN and TP concentrations (Fig. 12b).
Correlation matrix of Spearman’s correlation coefficients between phytoplankton biomass and environmental variables for the estuary (a) and nearshore (b) regions. Blue squares correspond to positive correlations and red squares to negative correlations. The color intensity is proportional to the correlation coefficient value. When a correlation coefficient was not significantly different from ‘0,’ the corresponding cell in the matrix was left blank
NMDS analysis was used to further examine potential environmental predictors of phytoplankton taxa/species biomass patterns in the two major regions of the study, i.e., Caloosahatchee Estuary and adjacent nearshore region (Fig. 13).
The ordination of samples by NMDS for the Caloosahatchee Estuary revealed that higher A. sanguinea, dinoflagellates, and cryptophytes biomass were associated with higher TP concentrations, but lower TN concentration, TN:TP ratio, mean discharge, and temperature (Fig. 13a, b). By contrast, higher picocyanobacteria and other cyanobacteria (predominantly filamentous forms) biomass were associated with higher TN:TP ratio and temperature.
The ordination of samples by NMDS for the nearshore region revealed that K. brevis and dinoflagellates biomass were higher for higher discharge, salinity, and TN:TP ratio (Fig. 13c, d). Picocyanobacteria and other cyanobacteria biomass were higher for higher temperature, while Rhizosolenia spp. and other diatoms (including Pseudo-Nitzschia spp. and Chaetoceros spp.) biomass were higher for higher TP. However, the length of other diatom vectors was less important than the vector for Rhizosolenia.
Discussion
The relationships between environmental variables and phytoplankton biomass and composition were different in the Caloosahatchee Estuary and the adjacent nearshore environment. In the Caloosahatchee Estuary, salinity regimes are affected by micro-tidal mixing with the Gulf of Mexico, and inflows from local watersheds, of which discharges from the S-79 control structure are major components. Low rainfall and low discharge levels generally coincide with the dry season, during which mesohaline (salinities 5–18 psu) or polyhaline (18–30 psu) conditions prevail through most of the estuary. From the perspective of the phytoplankton community, this is reflected in the major roles that euryhaline marine dinoflagellates, diatoms and picocyanobacteria play in phytoplankton community biomass under these salinity regimes. The highest phytoplankton biomass levels in the estuary were observed in the periods of transition from low to high discharge, suggesting a dual influence of inputs of nutrients in early stages of elevated rainfall, and sufficient water residence time to allow for biomass accumulation. In another study of an estuary associated with a water control structure in South Korea, peak phytoplankton biomass levels were also correlated to periods of low discharge (Sin et al. 2013).
By contrast, during periods of high S-79 discharge into the Caloosahatchee estuary oligohaline (≤ 5 psu) and lower mesohaline (> 5–10 psu), conditions are common in the estuary, as reflected by the frequent presence of freshwater taxa and some exceptionally low salinity tolerant marine taxa, such as Skeletonema costatum (Brand, 1984). The prominence of cyanobacteria during the summer season is also reflected in the positive correlation with temperature, which comports with the preference of many bloom-forming cyanobacteria for high temperatures (Paerl et al., 2016). While high discharge periods during this study were generally associated with relatively low phytoplankton biomass in the estuary, high biomass of cyanobacteria can be periodically introduced to the estuary via regulated discharges of water into the C-43 Canal from Lake Okeechobee, which is regularly subject to intense cyanobacteria blooms (Rosen et al., 2018; Phlips et al., 2020; Metcalf et al., 2021).
In the nearshore region outside of the Caloosahatchee Estuary, euhaline (> 30 psu) conditions predominate, although near the mouth of the estuary in San Carlos Bay, strong estuarine outflows can result in polyhaline conditions, particularly in the wet season. Euhaline conditions in the nearshore regions are favorable for the influx of red tides dominated by the toxic dinoflagellate Karenia brevis, which is not tolerant of low salinities (Heil et al., 2014a; Steidinger, 2009; Vargo, 2009). During this study period, there were noteworthy differences in the dominant species in phytoplankton blooms in the Caloosahatchee Estuary compared to nearshore regions, as described below.
Phytoplankton blooms in the estuary
During the 2009–2010 study period, major blooms of the dinoflagellate A. sanguinea were observed in the upper Caloosahatchee Estuary during the periods of low to moderate discharges in the Spring. The highest TP concentrations in the upper estuary also occurred in the late Spring, indicating that the initial flushes from the watershed during the beginning of the wet season yielded high phosphorus concentrations. The importance of the period of elevated phosphorus levels is further indicated by the close proximity of TP and A. sanguinea biomass vectors on the NMDS analysis axes for the Caloosahatchee Estuary (Fig. 13a, b). The NMDS relationship suggests that high phosphorus levels in Spring S-79 discharges may have contributed to the intensity of A. sanguinea blooms. By contrast, sustained high S-79 discharge rates in the Summer shorten water residence times (WRT), which limit the time needed for substantial biomass accumulation of marine taxa, thereby limiting the potential for bloom formation. The importance of WRT is supported by the negative correlation between mean discharge rate and dinoflagellate biomass (Fig. 12a), as well as the opposing directions of the A. sanguinea biomass and mean discharge vectors on the NMDS analysis axis for the estuary (Fig. 13a, b). The effect of S-79 discharge on WRT in the Caloosahatchee Estuary has been investigated by Wan et al. (2013). In the upper estuary at the City of Ft. Myers (i.e., Site 2), their study estimated that at high discharge rates (i.e., 2000 to 6000 cfs), WRT values range from < 1 to 10 days. Given that maximum growth rates of most phytoplankton in the natural environment largely range between 0.1 and1.0 doubling per day (Stolte & Garcés, 2006), the latter range of WRTs have the potential to limit the formation of major blooms. By contrast, periods of low discharge (i.e., 0 to 1,000 cfs) are associated with WRT from 30 to 80 days, a range suitable for potential bloom development, given sufficient nutrient levels and acceptable salinity levels. Similar observations of the importance WRT in defining phytoplankton biomass were found during chlorophyll a modeling efforts by Sun et al. (2022).
During the spring of 2009, A. sanguinea blooms first appeared in the upper-most region of the estuary in February, then peaked in early May before substantial increases in S-79 discharges late in May. After May, lower levels of A. sanguinea were found further downstream in the estuary until July. These observations support the hypotheses of an earlier study of the estuary, which increases in water temperature in the spring enhance algal growth rates, allowing for increases in phytoplankton biomass in the estuary (Buzzelli et al., 2014). As observed in our study, increases in S-79 discharge rates in the summer push chlorophyll maxima progressively further downstream in the estuary, while sustained increases in S-79 discharge rates in summer lower the potential for blooms in the estuary, as reflected by the comparatively low phytoplankton biomass observed in the summer of both study periods. The latter observations highlight the major role water residence time plays in defining the potential for HAB development of marine taxa in the estuary. The importance of water residence times and flushing rates in defining bloom potential has been documented for other Florida ecosystems (Murrell et al., 2007; Phlips et al., 2004, 2012, 2020, 2021; Dix et al., 2013; Hart et al., 2015), and estuaries around the world (Knoppers et al., 1991; Monbet, 1992; Eyre, 2000). In the Caloosahatchee Estuary, low discharge levels from the S-79 provide both the time and salinity levels needed for accumulation of marine phytoplankton biomass. Blooms of A. sanguinea, and other marine taxa, are supported by the nutrient levels sustained in the upper estuary, even under comparatively low rainfall and S-79 discharges, manifesting the nutrient-enriched character of watershed inputs to the Caloosahatchee Estuary (Doering & Chamberlain, 1999; Doering et al., 2006; Medina et al., 2021, 2022). A. sanguinea also benefits from its mixotrophic capabilities, which allows it to acquire organic carbon and nutrients through phagotrophy (i.e., consumption of particulates) (Burkholder et al., 2008).
The major role played by A. sanguinea in blooms observed in the Caloosahatchee Estuary during this study period is in part related to the euryhaline nature of the species (Matsubara et al., 2007; Badylak et al., 2014a), which is an advantage in the upper estuary where salinities generally vary within the mesohaline to lower polyhaline range (5–25 psu). Another key factor in the prominence of A. sanguinea is the apparent presence of a seed bank of resting cysts in the upper estuary, as evidenced by previous observations of all major developmental life stages of the species during the beginning of the 2009 bloom in the upper estuary (Badylak et al., 2017). A. sanguinea is a globally important HAB species (Hallegraeff et al., 2003; Lassus et al., 2016) and common to many Florida estuaries (Badylak et al., 2007, 2014b, 2017; Quinlan & Phlips, 2007; Phlips et al., 2010; Hart et al., 2015). While A. sanguinea is not known to be toxic, it has been associated with mass mortality events of marine animals (Harper & Guillen, 1989; Shumway 1990; Kahru et al., 2004), in part due to the potential for the development of hypoxic conditions during intense blooms (Hallegraeff et al., 2003). A. sanguinea is also known to produce large quantities extracellular carbohydrate polymer (Badylak et al., 2014b), as shown for other taxa linked to ecosystem disruption associated with polymer production (Phlips et al., 1989, 1999; Liu & Buskey, 2000; Botes et al., 2002; Smayda, 2002; Gobler & Sunda, 2012).
Nearshore blooms
In the nearshore environment outside the Caloosahatchee Estuary, major phytoplankton blooms observed in 2018–2019 involved the toxic red tide dinoflagellate Karenia brevis. K. brevis red tides occur on a regular basis in the Gulf of Mexico (Tester et al., 1991; Steidinger, 2009; Vargo, 2009; Brand et al., 2012; Heil et al. 2014a). The blooms have been the subject of extensive research, in part because of threats to human health and marine resources (Kirkpatrick et al., 2004; Fleming et al., 2005; Landsberg et al., 2009; Heil & Muni-Morgan, 2021). Unlike A. sanguinea blooms, K. brevis blooms generally start offshore on the continental shelf of the Gulf and then are distributed along the west coast of Florida by prevailing circulation patterns (Weisberg et al., 2019). The more stenohaline character of K. brevis than A. sanguinea is reflected in its preference for salinities above 30 psu (i.e., euhaline conditions), although historical observations of blooms frequently extend to salinities down to 20 psu (Steidinger, 2009). The latter trends are supported by the relationship between salinity and K. brevis biomass observed in this study.
The potential influence of outflows from the Caloosahatchee Estuary on the intensity of red tide events has been an important topic of concern for decades, particularly as it relates to effects of nutrient loads from the estuary (Steidinger, 2009; Vargo, 2009; Heil et al., 2014b; Medina et al., 2021, 2022). The Caloosahatchee Estuary is one of a number of major sources of nutrient inputs to the southwest coast of Florida (Heil et al., 2014b). However, quantifying the specific role of these inputs in red tide events has been challenging because of the multitude of potential sources of nutrients for red tides, such as deep-water upwelling from the edge of the shelf (Walsh et al., 2003), nutrient inputs from shelf sediments (Dixon et al., 2014b), nitrogen fixation by diazotrophic cyanobacteria (e.g., Trichodesmium) (Mulholland et al., 2014), and re-cycling of nutrients from decomposing biomass (Bronk et al., 2014), including the decomposition of biomass from animal mortality caused by the toxic red tides (Killberg-Thoreson et al., 2014b). Much of the focus has been on nitrogen because phytoplankton production in the coastal environment near the Caloosahatchee Estuary is most often nitrogen limited (Dixon et al 2014a; Heil et al. 2014b; Killberg-Thoreson et al., 2014a), as reported for several other coastal regions along the west and east coast of Florida (Bledsoe & Phlips, 2000; Phlips et al., 2002; Bledsoe et al., 2004). In terms of the general role of nitrogen availability for blooms, the positive correlation between TN concentration and dinoflagellate biomass observed in our study (Fig. 12b) further supports the importance of nitrogen supply. The potential importance of nitrogen from Caloosahatchee estuary discharges in terms of red tide intensity is also suggested by the close proximity of K. brevis and both mean discharge and TN:TP ratio vectors on the NMDS analysis axes for the Caloosahatchee Estuary (Fig. 13c, d).
Medina et al., (2021) used non-linear time series analyses to show a relationship between the seasonal timing of peaks of NOx (nitrate + nitrite) concentrations in water entering the Caloosahatchee Estuary from the S-79 control structure, and peak seasons of K. brevis blooms in the coastal region near the Caloosahatchee Estuary, between 26° and 27°N latitude, and up to 16 km from the shore. While this is a valuable observation about the seasonality of the two variables, many questions remain about the specific causal linkages. For example, how much is the temporal pattern of K. brevis biomass in the region related to autochthonous primary production versus allochthonous introductions of biomass from outside the region? In addition, what is the relative role of NOx in supporting K. brevis blooms, by comparison to other soluble and particulate, inorganic and organic source of nitrogen? The latter question is complicated by the mixotrophic character of the species, and by the potential contribution of other nitrogen forms, including dissolved organic nitrogen (DON) (Pisani et al., 2017), as observed in many other ecosystems (Glibert et al., 2006; Bronk et al., 2007; Ivey et al., 2020; Kang & Kang, 2022). It is noteworthy that the peak K. brevis biomass values observed in this study were 6 mg carbon l−1, which equates to roughly 1 mg nitrogen l−1, based on estimates using the Redfield ratio, or five times higher than peak NOx values reported by Medina et al., (2021), further suggesting that a range of different nitrogen types and sources help drive blooms.
During this study, major K. brevis blooms (i.e., > 106 cells l−1) were observed in the latter half of the 2018 wet season and the end of the 2019 wet season. The period was associated with mean wet season total nitrogen concentrations in the Caloosahatchee Estuary approximately twice as high as in the wet season of 2009–2010. The high TN:TP ratios observed during the wet seasons of 2018–2019 further suggest a role for nitrogen inputs from the estuary in the high intensity of red tides in 2018 and 2019, compared to 2009–2010. The Bloom Severity Index (BSI) recently developed by Stumpf et al. (2022) reported a moderate to high index value of 8 for 2018 (i.e., the highest value over the 1953–2019 recording period) and 2.2 for 2019. By comparison, the 2009 BSI was 0.4, and < 0.1 for 2010, both among the lowest values for the period of record. Differences in TN concentrations in the S-79 outflow may in part reflect temporal variability in the relative volumes of water from Lake Okeechobee versus other tributaries that introduce water to the C-43 Canal from watersheds downstream of Lake Okeechobee, as well as inputs from watersheds in the immediate vicinity of the estuary. It is known that specific sources of nutrient inputs differ in nutrient composition and concentrations spatially and temporally (Heil et al., 2014b; SFWMD, 2014; Medina et al., 2021, 2022). Similar observations have been made for discharges from Lake Okeechobee and regional watersheds associated with the St. Lucie estuary on the east coast of Florida (Moncada et al., 2021). The character and quantity of these external inputs to the Caloosahatchee and St. Lucie estuaries also vary in relation to the timing and levels of rainfall levels and changes in regulatory discharges from water control structures (e.g., S-77, S-79). The difference in TN concentrations in the Caloosahatchee Estuary between the 2009–2010 and 2018–2019 time periods could also be partially attributable to changes in watershed characteristics over the ten-year gap between the 2009–2010 and 2018–2019. The 10-year period was subject to substantial economic growth in the region after the depressing effects of the”Great Recession” of 2007–2009. World-wide development in coastal watersheds has accelerated cultural eutrophication and increased the potential for algal blooms (Glibert et al., 2005; Paerl et al., 2006, 2008; Heisler et al., 2008; Moore et al., 2020; Anderson et al., 2021).
Other important taxa
Outside of time periods dominated by A. sanguinea blooms in the Caloosahatchee Estuary and K. brevis blooms in the nearshore environment, one characteristic shared by all regions and salinity regimes observed in this study was the importance of picoplanktonic cyanobacteria. Although biomass levels for picocyanobacteria did not reach the peaks encountered for the former two taxa, they were generally the most numerically abundant phytoplankton group, reflecting their importance in microbial loop processes (Stockner, 1988; Pomeroy et al., 2007; Fenchel, 2008). For example, the mixotrophic red tide species K. brevis has been shown to graze on the picoplanktonic cyanobacteria (Glibert et al., 2009). Similarly, the strong presence of other mixotrophic and heterotrophic dinoflagellates on the Top-50 list of major biomass contributors to phytoplankton biomass in our study highlights the potential importance of the microbial loop in the dynamics of phytoplankton in the Caloosahatchee Estuary and nearshore environment. The opposing direction of vectors for picoplanktonic cyanobacteria and K. brevis/dinoflagellates biomass in the NMDS plots for the nearshore region (Fig. 13c, d) seems to support this observation.
Another noteworthy feature of the Top-50 list during oligohaline conditions in the Caloosahatchee Estuary is the prominence of a wide range of freshwater taxa that are introduced to the estuary from the C-43 Canal via the S-79. These include a number of cyanobacteria genera that contain potentially toxic species. Previous studies of HAB cyanobacteria in the canal systems emanating from Lake Okeechobee that flow into the Caloosahatchee and St. Lucie estuaries have observed a number of toxin-producing filamentous cyanobacteria, such as Raphidiopsis raciborskii (Woloszynska) Aguilera & al. and Dolichospermum circinale (Rabenhorst ex Bornet & Flahault) Wacklin, Hoffmann & Komárek (Rosen et al., 2018; Metcalf et al., 2021). These taxa were observed during our study, but at low levels of biomass. Another potentially toxic freshwater taxa observed the Top-50 list was the dinoflagellate genus Peridinium (Adachi, 1965; Rengefors & Legrand, 2001; Carty, 2014). Peridinium species have been linked to ichthyotoxic events (Hashimoto et al., 1968), and algicidal effects against some other algal taxa (Wu et al., 1998). There is a range of potential allochthonous threats to the estuary from introductions of freshwater HAB species from the C-43 Canal, particularly given the role of inputs from Lake Okeechobee.
One important HAB phenomena in the Caloosahatchee Estuary not encountered during our study period was the introduction of intense toxic cyanobacteria blooms from Lake Okeechobee into the estuary via the C-43 Canal. Discharges of water containing high concentrations of toxic cyanobacteria have been observed in both the Caloosahatchee Estuary via C-43 Canal inputs (Metcalf et al., 2021) and the St. Lucie estuary on the east coast of Florida via inputs from the St. Lucie Canal (Phlips et al., 2012). One of the most common toxic species involved in the HAB events in both estuaries has been Microcystis aeruginosa (Kützing) Kützing (Phlips et al., 2012; Rosen et al., 2018; Metcalf et al., 2021), a prolific producer of the hepatotoxin microcystin (Black et al., 2011). Large volume discharges of bloom-laden water into the estuaries from Lake Okeechobee not only represent a risk of exposure to cyanobacterial toxins, but, as in the case of K. brevis blooms, these events also represent a risk for hypoxia and disruption of food web structure and function (Phlips et al., 2012; Milbrandt et al., 2021). The introductions can result in widespread surface scums of M. aeruginosa in the estuaries, which can contain microcystin concentrations over 1000 µg l−1 (Phlips et al., 2012; Metcalf et al., 2021); very high considering WHO guideline for human recreational exposure is 24 µg l−1 (WHO, 2020).
Future directions
Local, state, and federal environmental organizations are actively pursuing research to find ways to control and/or mitigate exposure of the Caloosahatchee estuaries, and nearshore regions, to HAB events (Heil & Muni-Morgan, 2021; U.S.E.P.A., 2021). It is likely that future rates, duration, and timing of discharges from S-79 into the Caloosahatchee Estuary will play key roles in defining the character and impacts of freshwater and marine HABs on the estuary and associated nearshore environments. Predicting the future impacts of HABs on the Caloosahatchee Estuary (Buzzelli et al., 2014; Julien & Osborne, 2018), and other estuaries around the world, will be complicated by the effects of cultural eutrophication on nutrient loads and climate change on temperature, rainfall, and tropical storms (Howarth et al., 2000; Heisler et al., 2008; Glibert et al., 2014; Thompson et al., 2015; Paerl et al., 2016; Gobler, 2020; Phlips et al., 2020; Anderson et al., 2021). It has been predicted that there will be increased in all four factors in the near and long term, creating additional challenges for managing nutrient loads, hydrology, and biology of the estuary and nearshore regions. Efforts to mitigate the intensity and frequency of major HAB events in coastal ecosystems around the world have often focused on the control of nutrient availability (Nixon, 1995; Cloern, 2001; Heisler et al., 2008), which is certainly an important target for management. However, in many ecosystems like the Caloosahatchee Estuary, hydrologic considerations are also of central importance. The presence of water control structures in the Caloosahatchee ecosystem present challenges to satisfying multiple and sometimes conflicting objectives, including the control of Lake Okeechobee water levels to avoid catastrophic flooding, providing water for agriculture activities, maintaining navigational needs, and preserving the health of the lake, estuary, and adjacent coastal regions (SFWMD, 2014). At the same time, the presence of these structures provides opportunities to manage HABs through targeted regulation of discharge regimes, ideally with recognition of future changes in climatic conditions and cultural eutrophication. As additional temporally intensive data on the dynamics of phytoplankton communities and nutrient concentrations in the Caloosahatchee ecosystem become available, it should be possible to develop quantitative models that help to define optimal discharge regimes to minimize ecosystem disruptions from HABs.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adachi, R., 1965. Studies on a dinoflagellate Peridinium polonicum Woloszynska. 1. The structure of skeleton. Journal of the Faculty of the Fisheries Prefectural, University of Mie 6: 318–326.
Ahlgren, G., 1983. Comparison of methods for estimation of phytoplankton carbon. Archives Hydrobiologia 98: 489–508.
Anderson, D., E. Fensin, C. Gobler, A. Hoeglund, K. Hubbard, D. Kulis, J. Landsberg, K. Lefebvre, P. Provoost, M. Richlen, J. Smith, A. Solow & V. Trainer, 2021. Marine harmful algal blooms (HABs) in the United States: history, current status and future trends. Harmful Algae 102: 101975. https://doi.org/10.1016/j.hal.2021.101975.
APHA, 2005. Standard Methods for the Examination of Water and Wastewater, 21st ed. American Public Health Association, Washington, D.C:
Badylak, S., E. J. Phlips, P. Baker, J. Fajans & R. Boler, 2007. Distributions of phytoplankton in Tampa Bay, USA. Bulletin of Marine Science 80: 295–317.
Badylak, S., E. J. Phlips & A. L. Mathews, 2014a. Akashiwo sanguinea (Dinophyceae) blooms in a sub-tropical estuary: an alga for all seasons. Plankton and Benthos Research 9: 1–9.
Badylak, S., E. J. Phlips, A. L. Mathews & K. Kelley, 2014b. Observations of Akashiwo sanguinea (Dinophyceae) extruding mucous from pores on the cell surface. Algae 29: 1–5.
Badylak, S., E. J. Phlips, A. L. Mathews & K. Kelley, 2017. In situ observations of Akashiwo sanguinea (Dinophyceae) displaying life cycle stages during blooms in a subtropical estuary. Botanica Marina 60: 653–664.
Beals, E. W., 1984. Bray-Curtis ordination: an effective strategy for analysis of multivariate ecological data. Advances in Ecological Research 14: 1–55.
Bharathi, M. D., V. V. S. S. Sarma & K. Ramaneswari, 2018. Intra-annual variations in phytoplankton biomass and its composition in the tropical estuary: influence of river discharge. Marine Pollution Bulletin 129: 14–25.
Black, K., M. Yilmaz & E. J. Phlips, 2011. Growth and toxin production by Microcystis aeruginosa PCC 7806 (Kutzing) Lemmerman at elevated salt concentrations. Journal of Environmental Protection 2: 669–674.
Bledsoe, E. & E. J. Phlips, 2000. Nutrient versus light limitation of phytoplankton in the Suwannee River and Estuary. Estuaries 23: 458–473.
Bledsoe, E., E. J. Phlips, C. Jett & K. Donnelly, 2004. The relationships among phytoplankton biomass, nutrient loading and hydrodynamics in an inner-shelf estuary, the Suwannee River estuary, Florida, USA. Ophelia 58: 29–47.
Botes, L., A. J. Smit & P. A. Cook, 2002. The potential threat of algal blooms to the abalone (Haliotis midae) mariculture industry situated around the South African coast. Harmful Algae 2: 4247–4259.
Brand, L. E., 1984. The salinity tolerance of forty-six marine phytoplankton isolates. Estuarine, Coastal and Shelf Science 18: 543–556.
Brand, L. E., L. Campbell & E. Bresnan, 2012. Karenia: the biology and ecology of a toxic genus. Harmful Algae 14: 156–178.
Bronk, D. A., J. See, P. Bradley & L. Killberg, 2007. DON as a source of bioavailable nitrogen for phytoplankton. Biogeosciences 4: 283–296.
Bronk, D. A., L. Killberg-Thoreson, M. R. Mulholland, R. E. Sipler, Q. N. Roberts, P. W. Bernhardt, M. Garrett, J. M. O’Neil & C. A. Heil, 2014. Nitrogen uptake and regeneration (ammonium regeneration, nitrification and photoproduction) in waters of the west Florida shelf prone to blooms of Karenia spp. Harmful Algae 38: 50–62.
Bunn, S. E. & A. Arthington, 2002. Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environmental Management 30(4): 492–507.
Burkholder, J. M., P. Glibert & H. Skelton, 2008. Mixotrophy, a major mode of nutrition for harmful algal species in eutrophic waters. Harmful Algae 8: 77–93.
Buzzelli, C., B. Boutin, M. Ashton, B. Welch, P. Gorman, Y. Wan & P. Doering, 2014. Fine-scale detection of estuarine water quality with managed freshwater releases. Estuaries and Coasts 37: 1134–1144. https://doi.org/10.1007/s12237-013-9751-8.
Carty, S., 2014. Freshwater Dinoflagellates of North America, Cornell University Press, Ithaca:
Chilton, D., D. Hamilton, I. Nagelkerken, P. Cook, M. Hipsey, R. Reid, M. Sheaves, N. Waltham & J. Brookes, 2021. Environmental flow requirements of estuaries: providing resilience to current and future climate and direct anthropogenic changes. Frontiers of Environmental Science 9: 764218. https://doi.org/10.3389/fenvs.2021.764218.
Cloern, J. E., 2001. Our evolving conceptual model of the coastal eutrophication problem. Marine Ecology Progress Series 210: 223–253.
Cloern, J. E. & A. Jassby, 2008. Complex seasonal patterns of primary producers at the land-sea interface. Estuaries and Coasts 33: 230–241.
Cloern, J. E., A. Jassby, T. Schraga, E. Nejad & C. Martin, 2017. Ecosystem variability along the estuarine salinity gradient: examples from long-term study of San Francisco Bay. Limnology and Oceanography 62(S1): S272–S291.
Costa, L. S., L. M. Huszar & A. R. Ovalle, 2009. Phytoplankton functional groups in a tropical estuary: hydrological control and nutrient limitation. Estuaries Coasts 32: 508–521.
Dix, N., E. J. Phlips & P. Suscy, 2013. Factors controlling phytoplankton biomass in a subtropical coastal lagoon: relative scales of influence. Estuaries Coasts 36: 981–996.
Dixon, L. K., G. Kirkpatrick, E. Hall & A. Nissanka, 2014a. Nitrogen, phosphorus and silica on the West Florida Shelf: patterns and relationships with Karenia spp. occurrence. Harmful Algae 38: 8–19.
Dixon, L. K., P. Murphy & C. Charniga, 2014b. The potential of benthic nutrient flux in support of Karenia brevis blooms off of west central Florida USA. Harmful Algae 38: 30–39.
Doering, P. H. & R. H. Chamberlain, 1999. Water quality and the source of freshwater discharge to the Caloosahatchee Estuary, Florida. Journal of the American Water Resources Association 35: 793–806.
Doering, P. H., R. Chamberlain & K. Haunert, 2006. Chlorophyll a, and its use as an indicator of eutrophication in the Caloosahatchee Estuary, Florida. Florida Scientist 69: 51–72.
Dorado, S., T. Booe, J. Steichen, A. McInnes, R. Windham, A. Shepard, A. Lucchese, H. Preischel, J. Pinckney, S. Davis, D. Roelke & A. Quigg, 2015. Towards an understanding of the interactions between freshwater inflows and phytoplankton communities in a subtropical estuary in the Gulf of Mexico. PLoS ONE 10(7): e0130931. https://doi.org/10.1371/journal.pone.0130931.
Eyre, B. D., 2000. Regional evaluation of nutrient transformation and phytoplankton growth in nine river-dominated sub-tropical east Australian estuaries. Marine Ecology Progress Series 205: 61–83.
Fahnenstiel, G. L. & H. Carrick, 1992. Phototrophic picoplankton in Lakes Huron and Michigan: abundance, distribution, composition and contribution to biomass and production. Canadian Journal of Fisheries and Aquatic Sciences 49: 379–388.
Fenchel, T., 2008. The microbial loop - 25 years later. Journal of Experimental Marine Biology and Ecology. 366: 99–103.
Flaig, E. & J. Capece, 1998. Water use and runoff in the Caloosahatchee Watershed. In: Treat, S. F. (ed.), Proceedings of the Charlotte Harbor public conference and technical symposium, March 15–16, 1997, Punta Gorda, Florida. Charlotte Harbor National Estuary Program Technical Report No. 98-02, South Florida Water Management District, West Palm Beach, Florida, 73–80
Fleming, L. E., B. Kirkpatrick, L. Backer, J. Bean, A. Wanner, D. Dalpra, R. Tamer, J. Zaias, Y. Cheng, R. Pierce, J. Naar, W. Abraham, R. Clark, Y. Zhou, M. Henry, D. Johnson, G. Van De Bogart, G. Bossart, M. Harrington & D. Baden, 2005. Initial evaluation of the effects of aerosolized Florida red tide toxins (brevetoxins) in persons with asthma. Environmental Health Perspectives 113: 650–657.
Glibert, P. M., S. Seitzinger, C. Heil, J. Burkholder, M. Parrow, L. Codispoti & V. Kelly, 2005. The role of eutrophication in the global proliferation of harmful algal blooms, New perspectives and new approaches. Oceanography 18: 196–207.
Glibert, P. M., J. Harrison, C. Heil & S. Seitzinger, 2006. Escalating worldwide use of urea: a global change contributing to coastal eutrophication. Biogeochemistry 77: 441–463. https://doi.org/10.1007/s10533-005-3070-5.
Glibert, P. M., J. Burkholder, T. Kana, J. Alexander, H. Skelton & C. Shilling, 2009. Grazing by Karenia brevis on Synechococcus enhances its growth rate and may help to sustain blooms. Aquatic Microbial and Ecology 55: 17–30.
Glibert, P. M., J. Allen, Y. Artioli, A. Beusen, L. Bouwman, J. Harle, R. Holmes & J. Holt, 2014. Vulnerability of coastal ecosystems to changes in harmful algal bloom distribution in response to climate change: projections based on model analysis. Global Change Biology 20: 3845–3858. https://doi.org/10.1111/gcb.12662.
Gobler, C. J., 2020. Climate change and harmful algal blooms: insights and perspective. Harmful Algae 91: 101731. https://doi.org/10.1016/j.hal.2019.101731.
Gobler, C. J. & W. G. Sunda, 2012. Ecosystem disruptive algal blooms of the brown tide species, Aureococcus anophagefferens and Aureoumbra lagunensis. Harmful Algae 14: 36–45. https://doi.org/10.1016/j.hal.2011.10.013.
Hallegraeff, G. M., 2003. Harmful algal blooms: a global overview. In Hallegraeff, G. M., D. M. Anderson & A. D. Cembella (eds), Manual on Harmful Marine Microalgae UNESCO, Paris: 25–49.
Hallett, C. S., A. J. Hobday, J. R. Tweedley, P. A. Thompson & K. McMahon, 2018. Observed and predicted impacts of climate change on the estuaries of South-Western Australia, a Mediterranean climate region. Regional Environmental Change 18: 1357–1373. https://doi.org/10.1007/s10113-017-1264-8.
Harper, D. E. & G. Gullen, 1989. Occurrence of a dinoflagellate bloom associated with an influx of low salinity water at Galveston Texas, and coincident mortalities of demersal fish and benthic invertebrates. Contributions to Marine Science 31: 147–161.
Hart, J. A., E. J. Phlips, S. Badylak, N. Dix, K. Petrinec, A. Mathews, W. Green & A. Srifa, 2015. Phytoplankton biomass and composition in a well-flushed sub-tropical estuary: the contrasting effects of hydrology, nutrient loads and allochthonous influences. Marine Environmental Research 112: 9–20.
Hashimoto, Y., T. Okaichi, D. Dang & T. Noguchi, 1968. Glenodinine, an ichthyotoxic substance produced by a dinoflagellate, Peridinium polonicum. Bulletin of the Japanese Society of Scientific Fisheries 34: 528–533.
Havens, K., H. Paerl, E. J. Phlips, M. Zhu, J. Beaver & A. Srifa, 2016. Extreme weather events and climate variability provide a lens into how shallow lakes may respond to climate change. Water 8: 229. https://doi.org/10.3390/w8060229.
Heil, C. A. & A. Muni-Morgan, 2021. Florida’s harmful algal bloom (HAB) problem: escalating risks to human environment and economic health with climate change. Frontiers in Ecology and Evolution 9: 646080. https://doi.org/10.3389/fevo.2021.646080.
Heil, C. A., D. Bronk, L. Dixon, G. Hitchcock, G. Kirkpatrick, M. Mulholland, J. O’Neil, J. Walsh, R. Weisberg & M. Garrett, 2014a. The Gulf of Mexico ECOHAB: Karenia Program 2006–2012. Harmful Algae 38: 3–7.
Heil, C. A., L. Dixon, E. Hall, M. Garrett, J. Lenes, J. O’Neil, B. Walsh, D. Bronk, L. Killberg-Thoreson, G. Hitchcock, K. Meyer, M. Mulholland, L. Procise, G. Kirkpatrick, J. Walsh & R. Weisberg, 2014b. Blooms of Karenia brevis (Davis) G. Hansen & Ø. Moestrup on the West Florida Shelf: nutrient sources and potential management strategies based on a multi-year regional study. Harmful Algae 38: 127–140. https://doi.org/10.1016/j.hal.2014.07.016.
Heisler, J. P., P. Glibert, J. Burkholder, D. Anderson, W. Cochlan, W. Dennison, Q. Dortch, C. Gobler, C. Heil, E. Humphries, A. Lewitus, R. Magnien, H. Marshall, K. Sellner, D. Stockwell, D. Stoecker & M. Suddleson, 2008. Eutrophication and harmful algal blooms: a scientific consensus. Harmful Algae 8: 3–13.
Howarth, R. W., D. Swaney, T. Butler & R. Marino, 2000. Climatic control on eutrophication of the Hudson River estuary. Ecosystems 3: 210–215.
Ivey, J. E., J. Wolny, C. Heil, S. Murasko, J. Brame & A. Parks, 2020. Urea inputs drive picoplankton blooms in Sarasota Bay, Florida, USA. Water 12: 2755. https://doi.org/10.3390/w12102755.
Jassby, A. D., B. Cole & J. Cloern, 1997. The design of sampling transects for characterizing water quality in estuaries. Estuarine Coastal Shelf Science 45: 285–302.
Jo, H., E. Jeppesen, M. Ventura, T. Buchaca, J. Gim, J. Yoon, D. Kim & G. Joo. 2019. Responses of fish assemblage structure to large-scale weir construction in riverine ecosystems. Science of the Total Environment 67: 1334–1342.
Julien, P., II. & T. Osborne, 2018. From lake to estuary, the tale of two waters: a study of aquatic continuum biogeochemistry. Environmental Monitoring and Assessment 190: 96. https://doi.org/10.1007/s10661-017-6455-8.
Kahru, M., G. Mitchell, A. Diaz & M. Miura, 2004. MODIS detects a devastating algal bloom in Paracas Bay, Peru. Eastern Transactions, American Geophysical Union 85: 493–509.
Kang, Y. & C. Kang, 2022. Reduced forms of nitrogen control the spatial distribution of phytoplankton communities: the functional winner, dinoflagellates in an anthropogenically polluted estuary. Marine Pollution Bulletin 177: 113528. https://doi.org/10.1016/j.marpolbul.2022.113528.
Killberg-Thoreson, L., M. Mulholland, C. Heil, M. Sanderson, J. O’Neil & D. Bronk, 2014a. Nitrogen uptake kinetics in field populations and cultured strains of Karenia brevis. Harmful Algae 38: 73–85.
Killberg-Thoreson, L., R. Sipler, C. Heil, M. Garrett, Q. Roberts & D. Bronk, 2014b. Nutrients released from decaying fish support microbial growth in the eastern Gulf of Mexico. Harmful Algae 38: 40–49.
Kim, J. Y. & G. Kim, 2020. Effects of regulated dam discharge on plants and migratory waterfowl are mediated by salinity changes in estuaries. International Review of Hydrobiology 106: 58–63.
Kim, H. G., S. Hong, D. K. Kim & G. J. Joo, 2020. Drivers shaping episodic and gradual changes in phytoplankton community succession: taxonomic versus functional groups. Science of the Total Environment 734: 138940.
Kirkpatrick, B., L. Fleming, D. Squicciarini, L. C. Backer, R. Clark, W. Abraham, J. Benson, Y. Cheng, D. Johnson, R. Pierce, J. Zaias, G. Bossard & D. Baden, 2004. Literature review of Florida red tide: implications for human health effects. Harmful Algae 3: 99–115.
Knight, R. & J. Steele, 2005. Caloosahatchee River/Estuary Nutrient Issue White Paper, Wetland Solutions Inc, Gainesville:
Knoppers, B., B. Kjerfve & J. Carmouze, 1991. Trophic state and water turn-over time in six choked coastal lagoons in Brazil. Biogeochemistry 14: 147–166.
Kruskal, J. B., 1964. Nonmetric multidimensional scaling: a numerical method. Psychometrika 29: 115–129.
Lancelot, C. & K. Muylaert, 2011. Trends in estuarine phytoplankton ecology. In Wolanski, E. & D. McLusky (eds), Functioning of Ecosystems at the Land-Ocean Interface, Vol. 7. Elsevier Inc, Lueven: 5–15.
Landsberg, J. H., L. Flewelling & J. Naar, 2009. Karenia brevis red tides, brevetoxins in the food web, and impacts on natural resources: decadal advancements. Harmful Algae 8: 598–607.
Lassus, P., N. Chomérat, P. Hess & E. Nézan, 2016. Toxic and Harmful Microalgae of the World Ocean, IOC Manuals and Guides #68, Paris:
Liu, H. & E. Buskey, 2000. The exopolymer secretions (EPS) layer surrounding Aureoumbra lagunensis cells affects growth, grazing and behavior of protozoa. Limnology and Oceanography 45: 1187–1191.
Lucas, L. V., J. R. Koseff & J. E. Cloern, 1999a. Processes governing phytoplankton blooms in estuaries. I. The local production-loss balance. Marine Ecology Progress Series 187: 1–15.
Lucas, L. V., J. R. Koseff & J. E. Cloern, 1999b. Processes governing phytoplankton blooms in estuaries. II. The role of horizontal transport in global dynamics. Marine Ecology Progress Series 187: 17–30.
Lundholm, N., C. Churro, S. Fraga, M. Hoppenrath, M. Iwataki, L. Larsen, J. Mertens, K.;Moestrup, Ø. Zingone (eds), 2009 onwards. IOC-UNESCO Taxonomic Reference List of Harmful Micro Algae. [available on internet at https://www.marinespecies.org/hab].
Mallin, M. A., L. B. Cahoon, M. R. McIver, D. C. Parsons & G. C. Shank, 1998. Alteration of factors limiting phytoplankton production in the Cape Fear River estuary. Estuaries 22: 825–838.
Mathews, A. L., E. J. Phlips & S. Badylak, 2015. Modeling phytoplankton productivity in a shallow, microtidal, subtropical estuary. Marine Ecology Progress Series 531: 63–80.
Matsubara, T., S. Nagasoe, Y. Yamasaki, T. Shikata, Y. Shimasaki, Y. Oshima & T. Honjo, 2007. Effects of temperature, salinity, and irradiance on the growth of the dinoflagellate Akashiwo sanguinea. Journal of Experimental Marine Biology and Ecology 342: 226–230.
McCune, B., J. B. Grace & D. L. Urban, 2002. Analysis of Ecological Communities, Vol. 28. MjM software design, Gleneden Beach:
Medina, M., R. Huffaker, J. Jawitz & R. Muñoz-Carpena, 2021. Seasonal dynamics of terrestrially sourced nitrogen influenced Karenia brevis blooms off Florida’s southern Gulf Coast. Harmful Algae 98: 101900.
Medina, M., D. Kaplan, E. Mibrandt, D. Tomasko, R. Huffaker & C. Angelini, 2022. Nitrogen-enriched discharges from a highly managed watershed intensify red tide (Karenia brevis) blooms in southwest Florida. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2022.154149.
Metcalf, J., S. Banack, R. Wessel, M. Lester, J. Pim, J. Cassani & P. Cox, 2021. Toxin analysis of freshwater cyanobacteria and marine harmful algal blooms on the west coast of Florida of Florida and implications for estuarine environments. Neurotoxicity Research 39: 27–35.
Milbrandt, E. C., A. Martignette, M. Thompson, R. Bartleson, E. Phlips, S. Badylak & N. Nelson, 2021. Geospatial distribution of hypoxia associated with a Karenia brevis bloom. Estuarine, Coastal and Shelf Science 259: 107446. https://doi.org/10.1016/j.ecss.2021.107446.
Monbet, Y., 1992. Control of phytoplankton biomass in estuaries: a comparative analysis of microtidal and macrotidal estuaries. Estuaries 15: 563–571.
Moncada, A., A. Melesse, J. Vithanage & R. Price, 2021. Long-term assessment of surface water quality in a highly managed estuary basin. International Journal of Environmental Research and Public Health 18: 9417. https://doi.org/10.3390/ijerph18179417.
Moore, S., S. Dreyer, J. Ekstrom, K. Moore, K. Norman, T. Klinger, E. Allison & S. Jardine, 2020. Harmful algal blooms and coastal communities: Socioeconomic impacts and actions taken to cope with the 2015 U.S. west coast domoic acid event. Harmful Algae 96: 101799. https://doi.org/10.1016/j.hal.2020.101799.
Mulholland, M. R., P. W. Bernhardt, I. Ozmon, L. A. Procise, M. Garrett, J. O’Neil, C. Heil & D. Bronk, 2014. Contributions of N2 fixation to N inputs supporting Karenia brevis blooms in the Gulf of Mexico. Harmful Algae 38: 20–29.
Murrell, M., J. Hagey III., E. Lores & R. Greene, 2007. Phytoplankton production and nutrient distributions in a subtropical estuary: importance of freshwater flow. Estuaries and Coasts 30: 390–402.
Nixon, S. W., 1995. Coastal marine eutrophication. A definition, social consequences, and future concerns. Ophelia 41: 199–220.
NOAA, 2010. Tides and currents. National Oceanic Atmospheric Administration. Retrieved December 1, 2010. [available on internet at http://tidesandcurrents.noaa.gov].
Oksanen, J., R. Kindt, P. Legendre, B. O’Hara, M. Stevens, M. Oksanen & M. Suggests, 2007. The vegan package. Community Ecology Package 10(631–637): 719.
Paerl, H., L. Valdes, B. Peierls, J. Adolf & L. Harding, 2006. Anthropogenic and climatic influences on the eutrophication of large estuarine systems. Limnology and Oceanography 51: 448–462.
Paerl, H. W., J. Joyner, A. Joyner, K. Arthur, V. Paul, J. O’Neil & C. Heil, 2008. Co-occurrence of dinoflagellate and cyanobacterial harmful algal blooms in southwest Florida coastal waters: dual nutrient (N and P) input controls. Marine Ecology Progress Series 371: 143–153. https://doi.org/10.3354/meps07681.
Paerl, H. W., W. Gardner, K. Havens, A. Joyner, M. McCarthy, S. Newell, B. Qin & J. T. Scott, 2016. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 54: 213–222.
Phlips, E. J., C. Zeman & P. Hansen, 1989. Growth, photosynthesis, nitrogen fixation and carbohydrate production by a unicellular cyanobacterium, Synechococcus sp. (Cyanophyta). Journal of Applied Phycology 1: 137–145.
Phlips, E. J., F. Aldridge, P. Hansen, P. V. Zimba, J. Ihnat, M. Conroy & P. Ritter, 1993. Spatial and temporal variability of trophic state parameters in a shallow subtropical lake (Lake Okeechobee, Florida, USA). Archive Fur Hydrobiologia 128: 437–458.
Phlips, E. J., S. Badylak & T. Lynch, 1999. Blooms of the picoplanktonic cyanobacterium Synechococcus in Florida Bay. Limnology and Oceanography 44: 1166–1175.
Phlips, E. J., S. Badylak & T. Grosskopf, 2002. Factors affecting the abundance of phytoplankton in a restricted subtropical lagoon, the Indian River Lagoon, Florida, USA. Estuarine Coastal and Shelf Science 55: 385–402.
Phlips, E. J., N. Love, S. Badylak, P. Hansen, C. John & R. Gleeson, 2004. A comparison of water quality and hydrodynamic characteristics of the Guana Tolomato Matanzas National Estuarine Research Reserve and the Indian River Lagoon in Florida. Journal of Coastal Research, Special Issue 45: 93–109.
Phlips, E. J., S. Badylak, M. Christman & M. Lasi, 2010. Climatic trends and temporal patterns of phytoplankton composition, abundance and succession in the Indian River Lagoon, Florida, USA. Estuaries and Coasts 33: 498–513.
Phlips, E. J., S. Badylak, J. Hart, D. Haunert, J. Lockwood, H. Manley, K. O’Donnell, D. Sun, P. Viveros & M. Yilmaz, 2012. Climatic influences on autochthonous and allochthonous phytoplankton blooms in a subtropical estuary, St. Lucie Estuary, Florida, USA. Estuaries and Coasts 35: 335–352.
Phlips, E. J., S. Badylak, M. Lasi, R. Chamberlain, W. Green, L. Hall, J. Hart, J. Lockwood, J. Miller & J. Steward, 2015. From red tides to green and brown tides: bloom dynamics in a restricted subtropical lagoon under shifting climatic conditions. Estuaries and Coasts 38: 886–904. https://doi.org/10.1007/s12237-014-9874-6.
Phlips, E. J., S. Badylak, N. Nelson & K. Havens, 2020. Hurricanes, El Niño, and harmful algal blooms in two sub-tropical Florida estuaries: direct and indirect impacts. Scientific Reports 10: 1910. https://doi.org/10.1038/s41598-020-58771-4.
Phlips, E. J., S. Badylak, N. Nelson, L. Hall, C. Jacoby, M. Lasi, J. Lockwood & J. Miller, 2021. Cyclical patterns and a regime shift in the character of phytoplankton blooms in a restricted sub-tropical lagoon, Indian River Lagoon, Florida, USA. Frontiers of Marine Science 8: 730934. https://doi.org/10.3389/fmars.2021.730934.
Pisani, O., J. Boyer, D. Podgorski, C. Thomas, T. Coley & R. Jaffé, 2017. Molecular composition and bioavailability of dissolved organic nitrogen in a lake flow-influenced river in south Florida, USA. Aquatic Sciences 79: 891–908. https://doi.org/10.1007/s00027-017-0540-5.
Poff, N. L. & J. Zimmerman, 2010. Ecological responses to altered flow regimes: a literature review to inform the science and management of environmental flows. Freshwater Biology. 55: 194–205.
Pomeroy, L. R., P. J. Williams, F. Azam & J. E. Hobbie, 2007. The microbial loop. Oceanography 20: 28–33.
Quinlan, E. L. & E. J. Phlips, 2007. Phytoplankton assemblages across the marine to low-salinity zone in a blackwater dominated estuary. Journal of Plankton Research 29: 410–416.
R Core Team, 2022. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [available on internet at https://www.R-project.org/].
Ralston, D. K., M. Brosnahan, S. Fox, K. Lee & D. Anderson, 2015. Temperature and residence time controls on an estuarine harmful algal bloom: modeling hydrodynamics and Alexandrium fundyense in Nauset estuary. Estuaries and Coasts 38: 2240–2258.
Rengefors, K. & C. Legrand, 2001. Toxicity in Peridinium aciculiferum - an adaptive strategy to outcompete other winter phytoplankton. Limnology and Oceanography 46: 1990–1997.
Reynolds, C. S., 2006. Ecology of Phytoplankton, Cambridge University Press, Cambridge:
Robson, B. J. & D. P. Hamilton, 2004. Three-dimensional Modelling of a Microcystis bloom event in the Swan River Estuary, Western Australia. Ecological Modelling 174: 203–222. https://doi.org/10.1016/j.ecolmodel.2004.01.006.
Rosen, B. H., T. Davis, C. Gobler, B. Kramer & K. Loftin, 2018. Cyanobacteria of the 2016 Lake Okeechobee Waterway harmful algal bloom: U.S. Geological Survey Open-File Report 2017–1054, 34. https://doi.org/10.3133/ofr20171054.
Rothenberger, M. B., J. M. Burkholder & T. R. Wentworth, 2009. Use of long-term data and multivariate ordination techniques to identify environmental factors governing estuarine phytoplankton species dynamics. Limnology and Oceanography 54: 2107–2127.
Scarlatos, P. D., 1988. Caloosahatchee Estuary hydrodynamics. Technical Publication 88–7, South Florida Water Management District, West Palm Beach, Florida.
SFWMD, 2009. South Florida Water Management District. Caloosahatchee River Watershed protection plan. West Palm Beach, Florida.
SFWMD, 2014. Document to support a water reservation rule for the CERP Caloosahatchee River (C-43) West Basin Storage Reservoir Project. South Florida Water Management District, W. Palm Beach, Florida. [available on internet at www.evergladesplan.org/pm/projects/docs_04_c43_pir_final.aspx].
Shumway, S. E., 1990. A review of the effects of algal blooms on shellfish and Aquaculture. Journal of the World Aquaculture Society 21: 65–104.
Sicko-Goad, L. M., C. L. Schelske & E. F. Stoermer, 1984. Estimation of intracellular carbon and silica content of diatoms from natural assemblages using morphometric techniques. Limnology and Oceanography 29: 1170–1178.
Sin, Y., B. Hyun, B. Jeong & H. Y. Soh, 2013. Impacts of eutrophic freshwater inputs on water quality and phytoplankton size structure in a temperate estuary altered by a sea dike. Marine Environmental Research 85: 54–63.
Sin, Y. & B. Jeong, 2015. Short-term variations of phytoplankton communities in response to anthropogenic stressors in a highly altered temperate estuary. Estuarine, Coastal and Shelf Science 156: 83–91.
Smayda, T. J., 1978. From phytoplankters to biomass. In Sournia, A. (ed), Phytoplankton Manual UNESCO, Paris: 273–279.
Smayda, T. J., 2002. Turbulence, water mass stratification and harmful algal blooms: an alternative view and frontal zones as “pelagic seed banks.” Harmful Algae 1: 95–112.
Snow, G. C., J. Adams & G. Bate, 2000. Effect of river flow on estuarine microalgal biomass and distribution. Estuarine, Coastal and Shelf Science 51: 255–266.
Steidinger, K. A., 2009. Historical perspective on Karenia brevis red tide research in the Gulf of Mexico. Harmful Algae 8: 549–561.
Stockner, J. G., 1988. Phototrophic picoplankton: an overview from marine and freshwater ecosystems. Limnology Oceanography 33: 765–775.
Stolte, W. & E. Garcés, 2006. Ecological aspects of harmful algal in situ population growth rates. In Graneli, E. & J. T. Turner (eds), Ecological Studies 189: Ecology of Harmful Algae Springer, Berlin: 139–152.
Strathmann, R. R., 1967. Estimating the organic carbon content of phytoplankton from cell volume or plasma volume. Limnology and Oceanography 12: 411–418.
Strickland, J. & T. Parsons, 1972. A Practical Handbook of Seawater Analysis, 2nd ed. Fisheries Research Board of Canada, Ottawa: 9–14.
Stumpf, R. P., L. Yizhen, B. Kirkpatrick, R. Litaker, K. Hubbard, R. Currier, K. Harrison & M. Tomlinson, 2022. Quantifying Karenia brevis bloom severity and respiratory irritation impact along the shoreline of Southwest Florida. PLoS ONE 17(1): e0260755.
Sun, J. & D. Liu, 2003. Geometric models for calculating cell biovolume and surface area for phytoplankton. Journal of Plankton Research 25: 1331–1346.
Sun, C. C., Y. S. Wang, M. L. Wu, J. D. Dong, Y. T. Wang, F. L. Sun & Y. Y. Zhang, 2011. Seasonal variation of water quality and phytoplankton response patterns in Daya Bay, China. International Journal of Environmental Research and Public Health 8: 2951–2966.
Sun, D., M. Barton, M. Parker & Y. P. Sheng, 2022. Estuarine water quality: one-dimensional model theory and its application to a riverine estuary in Florida. Estuarine, Coastal and Shelf Sciences 277: 108058. https://doi.org/10.1016/j.ecss.2022.108058.
Tester, P. A., R. Stumpf, F. Vukovich, P. Fowler & J. Turner, 1991. An expatriate red tide bloom: transport, distribution, and persistence. Limnology and Oceanography 36: 1053–1061.
Thompson, P. A., T. O’Brien, H. Paerl, B. Peierls, P. Harrison & M. Robb, 2015. Precipitation as a driver of phytoplankton ecology in coastal waters: a climatic perspective. Estuarine and Coastal Shelf Science 162: 119–129.
U.S.E.P.A., 2021. EPA needs an agencywide strategic action plan to address harmful algal blooms. Office of the Inspector General, U.S. Environmental Protection Agency, Report No.21-E-0264.EPA, Washington, D.C. [internet on available at www.epa.gov/org].
Utermohl, H., 1958. Zur vervollkommnung der quantitative phytoplankton-methodik. Mitteilungen. Internationale Vereinigung Fur Theoretische Und Angewandte Limnologie 9: 1–38.
Van Niekerk, L., S. Taljaard, J. Adams, S. Lamberth, P. Huizinga, J. Turpie & T. Wooldridge, 2019. An environmental flow determination method for integrating multiple scale ecohydrological and complex ecosystem processes in estuaries. Science of the Total Environment 656: 482–494. https://doi.org/10.1016/j.scitotenv.2018.11.276.
Vargo, G. A., 2009. A brief summary of the physiology and ecology of Karenia brevis (Davis) Hansen and Moestrup red tides on the West Florida shelf and of hypotheses posed for their initiation, growth, maintenance, and termination. Harmful Algae 8: 573–584.
Verity, P. G., C. Robertson, C. Tronzo, M. Andrews, J. Nelson & M. Sieracki, 1992. Relationships between cell volume and the carbon and nitrogen content of marine photosynthetic nanoplankton. Limnology and Oceanography 37: 1434–1446.
Walsh, J. J., R. Weisberg, D. Dieterle, R. He, B. Darrow, J. Jolliff, K. Lester, G. Vargo, G. Kirkpatrick, K. Fanning, T. Sutton, A. Jochens, D. Biggs, B. Nababan, C. Hu & F. Muller-Karger, 2003. The phytoplankton response to intrusions of slope water on the West Florida shelf: models and observations. Journal of Geophysical Research 108: 21–31.
Wan, Y., C. Qiu, P. Doering, M. Ashton, D. Sun & T. Coley, 2013. Modeling residence time with a three-dimensional hydrodynamic model: linkage with chlorophyll a in a subtropical estuary. Ecological Modelling 268: 93–102.
Wang, C., Y. Yu, P. Wang. Q. Sun, J. Hou & J. Qian. 2016 Assessment of the ecological reservoir operation in the Yantze Estuary based on the salinity requirements of the indicator species. River Research and Applications 32: 946–957.
Weisberg, R. H., Y. Liu, C. Lembke, C. Hu, K. Hubbard & M. Garrett, 2019. The coastal ocean circulation influence on the 2018 West Florida Shelf K. brevis red tide bloom. Journal of Geophysical Research Oceans 124: 2501–2512. https://doi.org/10.1029/2018JC014887.
Wetzel, R. G. & G. E. Likens, 1991. Limnological Analyses, 2nd ed. Springer-Verlag, New York:
WHO, 2020. Cyanobacterial toxins: microcystins. Background document for development of WHO Guidelines for drinking-water quality and Guidelines for safe recreational water environments. Geneva: World Health Organization. (WHO/HEP/ECH/WSH/2020.6). License: CC BY-NC-SA 3.0 IGO.
Winder, M. & D. A. Hunter, 2008. Temporal organization of phytoplankton communities linked to physical forcing. Oecologia 156: 179–192.
Winder, M., J. Carstensen, A. Galloway, H. Jakobsen & J. Cloern, 2017. The land-sea interface: a source of high-quality phytoplankton to support secondary production. Limnology and Oceanography 62(S1): S258–S271. https://doi.org/10.1002/Ino.10650.
Work, K., K. Havens, B. Sharfstein & T. East, 2005. How important is carbon to planktonic grazers in a turbid, subtropical lake? Journal of Plankton Research 27: 357–372.
Wu, J., L. Kuo-Huang & J. Lee, 1998. Algicidal effect of Peridinium bipes on Microcystis aeruginosa. Current Microbiology 37: 257–261.
Acknowledgements
The research presented in this paper was in part funded by grants from the South Florida Water Management District (West Palm Beach, Florida), NSF CBET Award #1853041, and institutional support from the University of Florida. Natalie Nelson is supported by the USDA National Institute of Food and Agriculture, Hatch Project 1016068. Edward Phlips, Susan Badylak, and Ben Stelling are supported by the USDA National Institute of Food and Agriculture, Hatch Project 1017098.
Funding
The authors have no financial or proprietary interests in any material discussed in this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any potential conflict of interest.
Ethical approval
The research did not involve human participants and/or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Erik Jeppesen, Miguel Cañedo-Argüelles, Sally Entrekin, Judit Padisák & S.S.S. Sarma / Effects of induced changes in salinity on inland and coastal water ecosystems
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Phlips, E.J., Badylak, S., Mathews, A.L. et al. Algal blooms in a river-dominated estuary and nearshore region of Florida, USA: the influence of regulated discharges from water control structures on hydrologic and nutrient conditions. Hydrobiologia 850, 4385–4411 (2023). https://doi.org/10.1007/s10750-022-05135-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-05135-w