Abstract
Ecosystems provide benefits to humans, including provisioning, regulating, and cultural services. However, invasive species can threaten ecosystem well-functioning and services provided. One invasive species with such potential is the New Zealand mud snail (NZMS) Potamopyrgus antipodarum. The aims of this study are focused on the quantitative review of (1) the NZMS impacts on ecosystem properties and their direct links with ecosystem services, and (2) the ecosystem services that can be affected by the NZMS. The high density reached by this species in most of the invaded ecosystems and its highly competitive ability affect ecosystem structure and functioning. However, some facilitation processes on native species may result in an improvement of some services. The NZMS tends to positively affect cultural services (88% positive cases) but negatively to provisioning services (77% of cases). Regarding, regulating and maintenance services, the proportions of positive and negative effects were similar (45% vs 36%, respectively). Therefore, the NZMS is a species with numerous negative impacts on ecosystem services. However, ecosystem services related to health (e.g., dilution effect against parasites) and research (e.g., biomonitoring) are cultural services that the NZMS can improve. No economic assessment of the impacts of the NZMS is available in the literature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ecosystem services are the benefits that ecosystems directly provide to human welfare through markets or other non-market values (Hooper et al., 2005; Millennium Ecosystem Assessment, 2005; Vilà & Hulme, 2017; Haines-Young & Potschin, 2018). These include provisioning services, i.e., goods obtained from ecosystems (e.g. food, water, fibers, or medicines), regulating services, i.e., the capacity of ecosystems to maintain their “homeostasis” and prevent catastrophic events (e.g. climate regulation, prevention of floods or pests, water purification), and cultural services, i.e., spiritual values that also contribute to human welfare (e.g. recreation, esthetic assets, and spiritual fulfillment) (Millennium Ecosystem Assessment, 2005; Haines-Young & Potschin, 2018). In recent decades, the growing demand of resources by humans is endangering ecosystem integrity, and thus, the capacity of ecosystems to provide services in a sustainable way (Millennium Ecosystem Assessment, 2005; Nishijima & Belgrano, 2020; O’Brien et al., 2021; Ashrafi et al., 2022). In fact, pollution, overexploitation of resources, and the introduction of exotic species are major threats to many ecosystem services (Millennium Ecosystem Assessment, 2005; Shin-ichiro et al., 2009; Lodge et al., 2012; Vilà & Hulme 2017; Cañedo-Argüelles, 2020).
Biological invasions represent one of the greatest threats to ecosystems and biodiversity (Gherardi, 2007; Shin-ichiro et al., 2009; Havel et al., 2015; Corrales et al., 2020). Specifically, aquatic ecosystems are exposed to a continuous increase in introductions of exotic species worldwide (Gherardi, 2007; Strayer, 2010; Lodge et al., 2012; Katsanevakis et al., 2014; Nunes et al., 2015; Corrales et al., 2020). Some introductions have been intentional, such as those fish, molluscs, and crayfish that have been intentionally introduced as food resources or to control disease vectors (Gofas & Zenetos, 2003; Gherardi, 2011; Deines et al., 2016; Heikal et al., 2018). Others have been accidental, such as those aquatic invertebrates introduced through ballast waters, attached to ships, or by inland canals (Gofas & Zenetos, 2003; Alonso & Castro-Díez, 2008; Nunes et al., 2015; Gilioli et al., 2017; Gallardo et al., 2019). In fact, invasive alien species are an important component of the global change that threatens the well-functioning of ecosystems and many of the services they provide (Gherardi, 2007; Strayer, 2010; Lodge et al., 2012; Katsanevakis et al., 2014; Corrales et al., 2020).
Aquatic ecosystems are particularly vulnerable to invasive species (Gherardi, 2007; Strayer, 2010; Nunes et al., 2015; Moorhouse & Macdonald, 2015; Corrales et al., 2020). That vulnerability is likely caused by (1) the higher dispersal ability of freshwater species as compared with terrestrial organisms, (2) the high rates of endemism (and often low biodiversity) of inland waters, and (3) the intense use of aquatic ecosystems by humans for recreational, commercial, and food resources (Gherardi, 2007). Invasive aquatic species may modify the structure and functioning of aquatic ecosystems (Shin-ichiro et al., 2009; Havel et al., 2015; Petsch, 2016; Corrales et al., 2020), which in turn may cause direct or indirect harmful impacts on human societies and economies (Shin-ichiro et al., 2009; Lodge et al., 2012; Laverty et al., 2015; Deines et al., 2016). However, biotic changes in aquatic ecosystem, including changes caused by exotic species, may increase some ecosystem services while simultaneously decreasing others (Hooper et al., 2005; Shin-ichiro et al., 2009; Limburg et al., 2010; Lodge et al., 2012; Katsanevakis et al., 2014; Laverty et al., 2015; Deines et al., 2016). For instance, some dreissenid mussels (Dreissena polymorpha (Pallas, 1771) and Dreissena burgensis (Andrusov, 1897)) can increase water clarity through filtration, but also increase nuisance algae (Limburg et al., 2010). Most introduced crayfish are a food supply for people, thus increasing a provisioning service; yet they are also important vectors of parasites and pathogens (Lodge et al., 2012). Thus, there is growing recognition that exotic species may either increase or decrease ecosystem services (Limburg et al., 2010; Lodge et al., 2012; Deines et al., 2016; Vilà & Hulme, 2017; Kourantidou et al., 2022). Many changes in ecosystem services are caused by the profound impacts of these species on the properties of aquatic ecosystems, mostly when they become invasive (Shin-ichiro et al., 2009; Limburg et al., 2010; Lodge et al., 2012; McLaughlan et al., 2014; Walsh et al., 2016). Therefore, the development of studies on the effects of exotic species on ecosystem services is important to aid and inform decision making by aquatic ecosystem managers and policymakers.
Ecosystem properties include both ecological compartments (e.g., pools of organic matter) and rates of processes (e.g., fluxes of energy among compartments), they are different across ecosystems, but not inherently “good” or “bad” (Hooper et al., 2005). By contrast, ecosystem services contribute to human welfare, so they are considered as “good” for human well-being (Hooper et al., 2005; Millennium Ecosystem Assessment, 2005). Some changes in ecosystem properties are directly related to certain ecosystem services. For example, the shells of dreissenid mussels on submerged surfaces can cause an increase in biofouling with a direct cost for the maintenance of infrastructure (Nakano & Strayer, 2014). However, other changes in ecosystem properties caused by invasive species are not so clearly related to ecosystem services, and their impact on services is difficult to discern (Charles & Dukes, 2007). Therefore, there is a need of knowledge on the links between changes of ecosystem properties by invasive species and their impact on ecosystem services (Walsh et al., 2016).
The impacts of various invasive alien species of crayfish (Procambarus clarkii (Girard, 1852), Pacifastacus leniusculus (Dana, 1852), Orconectes spp., Cherax spp.), bivalves (Dreissena polymorpha and D. burgensis), and gastropods (Pomacea spp.) on ecosystem services have been extensively studied (Limburg et al., 2010; Lodge et al., 2012; McLaughlan et al., 2014; Gilioli et al., 2017). However, alterations caused by other aquatic invertebrate species have been more studied from the perspective of their immediate ecological impacts than from the perspective of ecosystem services and “disservices.” This is the case of the New Zealand mudsnail (NZMS) Potamopyrgus antipodarum (Gray, 1853). The impacts of this successful invader on aquatic ecosystems, along with the causes of its ecological success, have been previously reviewed (Alonso & Castro-Díez, 2008, 2012) and recently updated (Geist et al., 2022). Even though this species is highly invasive, the benefits and harms of the NZMS on ecosystems services have received much less attention compared to other invasive invertebrates.
The NZMS is an aquatic gastropod of the family Tateidae; it is native to New Zealand but reported on most continents (except Antarctica) (Alonso & Castro-Díez, 2012; Taybi et al., 2021; Geist et al., 2022). This species presents a high reproductive rate and the ability to quickly monopolize invertebrate secondary production, helping to explain its high impact in most of the invaded ecosystems (Alonso & Castro-Díez, 2008). Previous studies showed that the NZMS highly affects both the structure and functioning of aquatic ecosystems (for more details, see reviews by Alonso & Castro-Díez, 2008, 2012; Geist et al., 2022). The secondary production reported for this species is one of the highest for a stream invertebrate (Hall et al., 2006). This finding is in accordance with the high densities found in some invaded ecosystems, reaching up to 800,000 individuals per square meter (Dorgelo, 1987). This species can consume up to 75% of the primary production of streams (Hall et al., 2003), successfully competing against native species while also benefiting a few other native fauna (Schreiber et al., 2002; Alonso & Castro-Díez, 2008; Riley et al., 2008; Rakauskas et al., 2016, 2018). Despite the severe impacts reported in the scientific literature for the NZMS, there is scarce information on how it increases or decreases specific ecosystem services.
The aim of this study is to review the scientific literature to identify both positive and negative effects of the NZMS on a wide range of ecosystem services throughout the introduced range. We also aim to identify how this species alters ecosystem properties and the direct effects of these changes on ecosystem services. This review provides (1) impacts of the NZMS on ecosystem properties and how these effects may be directly linked with improvements or impairments on the ecosystem services, and (2) information on the ecosystem services that can be potentially (directly or indirectly) affected by this invasive species.
Materials and methods
We focus on the effects of the NZMS on ecosystem properties and services in its non-native range. We used the term “effects” to document changes produced by the NZMS on aquatic ecosystem properties. Additionally, we attempt to estimate the type of the change caused by the NZMS on each ecosystem service (increase or decrease or non-change in the services).
Autoecology of the NZMS
Most of the non-native populations of the NZMS are composed of parthenogenetic females and no evidence of sexual reproduction has been reported outside of its native range (Alonso & Castro-Díez, 2008; Butkus et al., 2020). This species is ovoviviparous, and its sexual maturity is reached at shell length ranging from 2.0 to 3.5 mm (Lassen, 1979; Richards, 2002; Alonso & Castro-Díez, 2008; Gaino et al., 2008; McKenzie et al., 2013). In the brood pouch, which is formed by an elongated oviduct, adult females carry about 60 eggs and up to 147 embryos at different stages of development (Lassen, 1979; Gaino et al., 2008; Verhaegen et al., 2018, 2021). Up to six generations per year have been reported and the NZMS is a prolific reproducer that can produce a mean number of 230 offspring per adult female per year (Lassen, 1979; Richards, 2002). Field studies show the existence of drastic differences in space and time for fecundity of this species (Verhaegen et al., 2021). In any case, the NZMS is reported as a gastropod with a high reproductive effort, with a clear r-selected strategy (Lassen, 1979).
In its native New Zealand range, the NZMS not only is abundant in freshwater ecosystems (lakes, ponds, rivers, and streams) (Winterbourn, 1969, 1970; Alonso & Castro-Díez, 2008), but it also lives in water with up to 26‰ of salinity (Winterbourn, 1969, 1970; Alonso & Castro-Díez, 2008). The species can tolerate a wide range of environmental conditions in both native and invaded areas (Winterbourn, 1969; Alonso & Castro-Díez, 2008, 2012), but relative high temperature of water (> 30 °C) appears to restrict its distribution in New Zealand (Winterbourn, 1969). Clements et al. (2011) found populations of the NZMS in geothermal streams (Yellowstone National Park, USA) at water temperatures up to 35.3 °C. Low temperatures can also limit the species as Moffitt and James (2012) showed that the size of the NZMS populations is controlled by near-to-below freezing winter, with very low densities or lack of detection in field populations at freezing areas (Idaho, USA). Additionally, low water conductivity, low calcium ion concentration, and a high velocity of water have likewise been reported as limiting factors for the distribution of this snail (Vazquez et al., 2016, Verhaegen et al., 2019; Larson et al., 2020a). Acidic waters would also appear to limit species as low pH is a common limiting factor to molluscs (Okland, 1992; Levri et al., 2020).
Literature search
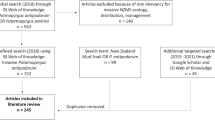
We performed a literature search of scientific publications on the NZMS using the Web of Science (https://www.webofscience.com/) in February 2022. The search covered the years from 1904 to 2022 and used different keywords and search strings (Table S1). With these combinations, scientific publications on ecosystem services and impacts on ecosystem properties were gathered. Additionally, technical reports and gray literature were also included. For this, Google Scholar search, and the Google search using the custom IGO (intergovernmental organizations), and NGO (non-governmental organizations) search tools were used in conjunction with expert knowledge by the authors regarding publications that cannot be found online.
Data compilation
The documents retrieved from the literature search were filtered according to the following procedure. (1) We checked whether the title was related with any target ecosystem properties/services (see below). (2) We read the abstract and, if the publication was clearly related with ecosystem properties and/or services, it was selected. (3) All selected publications were thoroughly checked to summarize all information on the potential effects of the NZMS on ecosystem properties and/or services in its non-native range. Ecosystem properties include both ecological compartments (e.g., pools of organic matter) and rates of processes (e.g., fluxes of energy among compartments) (Hooper et al., 2005). Changes on ecosystem properties were considered when authors of publications assessed any change caused by the NZMS in its non-native range (e.g., changes in secondary production). For each selected publication, the likely direct changes on ecosystem services (Provisioning, Regulation and Maintenance, and Cultural services) were also identified (Haines-Young & Potschin, 2018). The expert opinion of the authors was used to determine which ecosystem services would be directly affected by the changes in ecosystem properties. Since most of the available information does not allow a quantification of the ecosystem service, the collected data are largely qualitative.
For linking the variables reported by documents to ecosystem services, we used the Common International Classification of Ecosystem Services (CICES) (Haines-Young & Potschin, 2018). Three main groups of ecosystem services were analyzed: provisioning, regulation and maintenance, and cultural services. Each service is subsequently classified into several final sub-categories that are the ultimate benefits for people (e.g., food provisioning). Some of the outputs from ecosystems can be less tangible, such as the cultural services (e.g., recreational services or scientific value). In the compiled publications on ecosystem services, any aspect that can link with changes caused by the NZMS on ecosystem services, directly or indirectly, was selected. For that, the expert opinion of authors was followed (Drescher et al., 2013). The background ecological knowledge of the authors (e.g., parasitology, ecosystem function, autoecology, ecotoxicology, biomonitoring, behavior, community ecology, etc.) allowed an effective and rigorous classification of the possible impacts of the species on contrasting ecosystem services. Each effect on ecosystem services was classified in the most appropriate category (or categories) according to CICES (Haines-Young & Potschin, 2018). For each publication and service, the direction of the change was established (“+” the service increases, “−” the service decreases, and “0” the service is unchanged). The description of the change was established in a qualitative way.
Statistical analysis
The frequency of positive, neutral, and negative effects of the NZMS on the three main groups of ecosystem services (provisioning, regulation and maintenance, and cultural services) was compared by means of Fisher’s exact test (Field et al., 2012). When the frequency of positive, neutral, or negative cases is higher than expected by chance, the Fisher’s exact test reports a p value less than 0.05 (Field et al., 2012). Additionally, pairwise comparisons using Fisher's exact test for count data were used to compare the sign of cases among ecosystem services. Statistical analysis was conducted by means of the fisher.test and fisher.multcomp functions in R 4.0.5. Software (R Core Team, 2021).
Results
A total of 805 scientific publications were retrieved from the search in Web of Science. Among them, a total of 88 publications and documents, containing information of the effects of the NZMS on ecosystem properties and services, were finally selected. 37 out of them provided information on ecosystem properties (Supplementary S1) and 67 on ecosystem services (Supplementary S2). Table 1 summarizes the effects of the NZMS in its non-native area on ecosystem properties related to ecological compartments and rates of processes, and how these changes can cause direct effects on the main ecosystem services (provisioning, regulating and maintenance, and cultural services). In general, the NZMS affects the ecosystem properties in several ways, which can alter ecosystem services, mostly provisioning and cultural ones (Table 1). However, the revised literature shows that the three main ecosystem services could be directly affected by this species. Eight direct impacts of the NZMS on provisioning services, four in regulating and maintenance services, and eight in cultural services were identified (Table 1). Table 2 summarizes the effects of the NZMS for likely direct and indirect modifications on ecosystem services considering the CICES (Haines-Young & Potschin, 2018).
Across all publications and documents selected, a total of 90 case studies on the effects (positive, neutral, and negative) of the NZMS on the ecosystem services were collected (Fig. 1). For cultural services, most of the cases were positive (88%) (Fig. 1). On the contrary, for provisioning services, most of the cases were negative (77%). The regulating and maintenance services showed a relatively similar number of positive and negative effects (45% and 36%, respectively). Only provisioning, and regulating and maintenance services showed neutral cases, with 11 and 18% of cases, respectively (Fig. 1). The frequency of cases was different than expected by chance (Fisher’s exact test P < 0.001) (Fig. 1). The frequency of positive and negative cases was the main cause of the differences between services (P < 0.001; pairwise Fisher’s exact test).
The percentage of positive, neutral, and negative impacts of the NZMS on each ecosystem services (cultural, provisioning, and regulating and maintenance) are presented. The number of total cases and the percentage of cases for each service is showed in each column. Fisher’s exact test result is presented at the top of the graph, showing that the frequency of positive, neutral, or negative cases are higher than expected by chance. The frequencies of positive and negative cases were significantly difference of chance for each pair of ecosystem services (P < 0.001; pairwise Fisher’s exact test)
Discussion
Effects of the NZMS on ecosystem properties
Effects on native fauna
The NZMS reaches a high density in most of the invaded ecosystems with up to 800,000 individuals per square meter (Dorgelo, 1987; Alonso & Castro-Díez, 2012; Geist et al., 2022). These characteristics, along with its competitive ability, allow the NZMS to greatly affect both ecosystem structure and functioning (Dorgelo, 1987; Richards et al., 2001; Hall et al., 2003; Alonso & Castro-Díez, 2012; Geist et al., 2022). Both high reproductive potential and competitive superiority of the NZMS over native invertebrate species result in its dominance of invertebrate communities (Alonso & Castro-Díez, 2008, 2012; Geist et al., 2022 for reviews). The reduction in wild invertebrate populations of the native fauna supposes a direct deterioration in the provisioning services (e.g., reduction in invertebrate animals for food supply or/and as supporting vertebrate fauna), which can also cause a reduction in cultural services (i.e., reduction of bait for angling activities). However, the extreme density reached by the NZMS may have an indirect positive effect on regulating ecosystem services when the affected native species are vectors of diseases such as Bulinus truncatus (Audouin, 1827) and lymnaeids, gastropods which transmit liver flukes among them schistosomes (Jones et al., 2015; Mulero et al., 2021).
Several studies show that the NZMS may facilitate some native invertebrates (Schreiber et al., 2002; Brenneis et al., 2010). When these invertebrates have culinary interest or are used as bait for angling activities, the NZMS may be improving provisioning and cultural services. A field experiment conducted in an Australian stream shows that NZMS density correlates positively with some common aquatic invertebrates and with richness of native taxa (Schreiber et al., 2002; Rakauskas et al., 2018). In a North American estuarine system, no negative competitive impact of the NZMS on native benthic invertebrates is found, but a positive correlation is demonstrated between the NZMS and common native epibenthic invertebrates (Brenneis et al., 2010). Additionally, Brenneis et al. (2011) show, by means of an experimental approach that the native crayfish Pacifastacus leniusculus consumes and digests the NZMS, which could be an important food source for this species in areas invaded by the NZMS. Crayfish species are used as a source of food and disease control in many parts of the world (Gherardi, 2011; Nonaka, 2012; Heikal et al., 2018; Smietana et al., 2021). Thus, the NZMS may potentially increase the many ecosystem services reported for crayfish species (Gherardi, 2011), which can be important in aquatic ecosystems where the NZMS is an abundant prey. However, it is also possible that the benefit to crayfish may result in negative ecosystem effects as several species of crayfish are highly invasive (Gherardi et al. 2011), and NZMS facilitation of them may magnify their invasion capacity.
Even though the NZMS appears to be a good food source for crayfish, several studies demonstrate that the NZMS is likely to be a poor source of food for several species of fish. A low rate of assimilation and weight loss are associated with the consumption of the NZMS by fish (Cada, 2004; Vinson & Baker, 2008; Rakauskas et al., 2016). This may entail a direct reduction in provisioning services and in cultural services, since wild fish populations used for food provisioning or sport fishing could be affected (Vinson & Baker, 2008; Davis & Moeltner, 2010). The main reason for the low nutritional value of the NZMS for fish is the great resistance of the shell and the operculum that allows a large percentage of eaten individuals that to pass through the digestive tract of fish undigested, and even alive (Alonso & Castro-Díez, 2008; Vinson & Baker, 2008; Geist et al., 2022). However, there are also exceptions of fish species that can digest the NZMS (Hellmair et al., 2011; Rakauskas et al., 2016).
Effects on food webs, nutrient cycling, and water quality
The NZMS is well known for monopolizing the secondary production of the benthic macroinvertebrate community and for a high consumption of the primary production of the invaded ecosystems (Hall et al., 2003, 2006; Alonso & Castro-Díez, 2008, 2012; Riley et al., 2008; Geist et al., 2022). Consequently, the NZMS almost monopolizes the nitrogen cycle in some ecosystems since it excretes up to 65% of the ammonium demanded by primary producers and microbes (Hall et al., 2003). The NZMS consumes high amounts of green algae, which in turn may favor diatoms (Arango et al., 2009). As the latter can fix atmospheric nitrogen, the NZMS indirectly promotes this ecosystem function up to 50% in the periphyton of some streams (Arango et al., 2009). Additionally, the NZMS is reported to increase the rates of leaf litter decomposition (Geist et al., 2022). This can result in an increase of inorganic nutrient availability, especially if the organic matter comes from the terrestrial ecosystem. All this may cause various impacts on ecosystem services, related to possible effects of eutrophication and alteration of the physico-chemical properties of water, which can be detrimental to the use of water bodies for recreational uses (i.e., impact on cultural services) or for human consumption (i.e., impact on provisioning services). Previous studies highlight the impact that molluscs have on nutrient cycling, in many cases due to changes in the dominance of the different primary producers (McLaughlan et al., 2014; Gilioli et al., 2017). These changes caused by exotic species in the nutrient cycling can impact the freshwater quality by means of algal blooms (e.g., Dreissena polymorpha) or increase the phosphorous in the water column (e.g., Pomacea maculata (Perry, 1810)) (McLaughlan et al., 2014; Gilioli et al., 2017).
The high densities reached by the NZMS result in a considerable increase of shells on the substratum. As these shells accumulate calcium from the water in calcium carbonate (Medakovic et al., 2003; White et al., 2007), this may also result in a direct change in the chemical composition of water, which could be significant in extremely invaded ecosystems. However, this effect has not been well studied associated with mollusc invasions, so there is a high degree of uncertainty about its actual effects.
Effects of the NZMS on ecosystem services
Effects on provisioning services
In general, most of the revised studies show impacts of the NZMS on provisioning services. In fact, biomass, including that of primary producers, invertebrates, and fish is the division of provisioning services with the largest number of cases. The high rates of consumption of primary producers by the NZMS may lead to a drastic decrease in the biomass of some of those producers (Holomuzki et al., 2006; Krist & Charles, 2012; Laverty et al., 2015). Additionally, the NZMS provides a poor diet for many fish species, which can cause a decline in fish stocks (Cada, 2004; Vinson & Baker, 2008; Rakauskas et al., 2016). Moreover, the NZMS can host some parasites, posing another threat to the fish stock (Evans et al., 1981; Cichy et al., 2017; Gérard et al., 2017).
A “disservice” related to the high NZMS density is the high risk of biofouling for pipes and filters, causing a negative impact on provisioning services (Nakano & Strayer, 2014). This is a common effect caused by invasive species of molluscs, such as Dreissena spp., Corbicula spp., or Limnoperna fortunei (Dunker, 1857) (Nakano & Strayer, 2014). The damage to pipes and filters caused by the NZMS corresponds to an intermediate impact in comparison with other invasive invertebrates with less than ten cases reported in a literature review (Nakano & Strayer, 2014). Even so, the NZMS can cause an accumulation of living and non-living materials (e.g., shells) on and around water distribution pipes and filters, which may result in a relatively high economic cost (Nakano & Strayer, 2014). However, this issue needs further research for the NZMS, as most studies have focused on the biofouling effects of other species of molluscs.
Changes caused by other exotic molluscs on the trophic webs have been previously reported (Locke et al., 2014; Cattau et al., 2016; Zhang et al., 2019), including positive effects for terrestrial birds (Cattau et al., 2016). In fact, the facilitation of native species by trophic interactions with invasive species is not an unusual occurrence in the ecology of invasions (Rodriguez, 2006; Cattau et al., 2016). However, although the NZMS can be consumed by some species of fish and invertebrates, little is known of its nutritional value, so the effects on the improvement of the provisioning services of other components of the food webs (e.g., predators of fish or water birds) are largely unknown. However, the high density of the NZMS in some cases can produce a positive effect for several species of fish and crustaceans when successfully consumed (Brenneis et al., 2011; Hellmair et al., 2011).
Effects on regulation and maintenance services
The NZMS causes several changes in regulation and maintenance services related to water quality and pest/disease control. For instance, the NZMS may improve the water quality by reducing the presence of copper in water through bioaccumulation (Ramskov et al., 2015). As it can reach high densities, its bioaccumulation capacity may be a mechanism of metal removal from the water column and sediment. In any case, the NZMS shows bioaccumulation capacity of metals, as other invasive molluscs, which is useful in biomonitoring and water quality programs (Johns, 2012; Benito et al., 2017; Spyra et al., 2019). However, this direct improvement of water quality could become a problem as it may produce the biomobilization of metals through the food web, which could impact the provisioning services (Bray et al., 2015; Benito et al., 2017).
Pest and disease control can be improved by the NZMS by means of competition with native snail hosts of pathogens or as an unsuitable intermediate host for native pathogens (Jones et al., 2015; Marszewska et al., 2018; Mulero et al., 2021). The NZMS presents a potential dilution effect against native trematode parasites, consequently reducing infection level (both prevalence and abundance) of native intermediate and definitive host species, such as gastropods and vertebrates. Such a dilution effect has been demonstrated in Europe against the bird schistosome Trichobilharzia regenti (Horák, Kolářová & Dvořák, 1998) infecting lymnaeids and responsible for swimmer’s itch (Marszweska et al., 2018), but not against native trematodes of Physa spp., Galba spp., and Pyrgulopsis spp. in North America (Larson et al., 2020b). The dilution effect implies that native parasite species cannot develop in the NZMS, which, thus, constitutes a dead end with benefits for native host species. However, on evolutionary terms, the NZMS may become a new intermediate host allowing development of native parasite species, and thus, becoming a vector of disease (negative effect). Native parasite species are rarely recorded in the NZMS in its non-native areas [e.g., Fasciola hepatica (Linnaeus, 1758) infecting livestock (Jones et al., 2015), three echinostomes at metacercarial stage (Echinostoma revolutum (Fröhlich, 1802), Echinoparyphium aconiatum (Dietz, 1909), and Hypoderaeum conoideum (Block, 1872)) (Zbikowski and Zbikowska, 2009)], and up to now, it is unknown whether the NZMS is a dead end or a new intermediate host for these parasites (Marszewska et al., 2018; Larson et al., 2020b; Mulero et al., 2021). Therefore, the influence of the NZMS on ecosystem services associated with health presents a high degree of uncertainty. Even though they may currently be positive (e.g., dilution effect for native parasites), evolutionary processes may change towards disservices (e.g., transmission of native parasites). The risk for native species to become infected by exotic parasites introduced with the NZMS (i.e., new diseases) is limited, as the NZMS rarely harbors parasites native to New Zealand in its introduction areas. In fact, only two New Zealand parasite species have been reported so far, both in Europe: Notocotylus gippyensis (Beverley-Burton, 1958) infecting ducks as definitive hosts (Morley, 2008) and Aporocotylid I infecting fish as definitive hosts (Gérard et al., 2017). Only the Aporocotylid species has been proved to be persistent over time in Europe, but with very low prevalence (<< 1%) (Gérard et al., 2018).
Effects on cultural services
The cultural services provided by the NZMS are summarized in two groups. One “disservice” is related to the access restrictions that the authorities impose in NZMS-infested ecosystems (Proctor et al., 2007). Another disservice is the loss of recreational opportunities when stocking of fish from NZMS-positive facilities is limited to infested ecosystems (Hoyer & Myrick, 2012). Both facts prevent the recreational use of certain areas (bathing, fishing, boating, etc.), reducing the cultural services provided by aquatic ecosystems. Access restrictions to areas invaded by exotic species are proposed as a measure to avoid their dispersal to new ecosystems (Proctor et al., 2007; Pejchar & Mooney, 2009; Otero et al., 2013; USDI, 2016). However, natural vectors, such as water birds, terrestrial animals, fish, etc., can successfully contribute to the dispersion of the NZMS (Alonso & Castro-Díez, 2008; van Leeuwen & van der Velde, 2012). Therefore, access restriction measures may make sense at very early stages of invasion, but not when the species is widely distributed in the affected area.
Finally, a common cultural service provided by the NZMS is its use as a model organism for ecotoxicological and genetic studies, and as a biomonitoring species to assess the water quality of ecosystems. Several studies show the usefulness of the NZMS to assess the adverse effects of pollutants, including the development of a standardized OECD reproduction test (Geiss et al., 2017). Tests reveal that this species is a suitable organism for reproduction, growth, and behavioral bioassays in ecotoxicology (Pedersen et al., 2009; Geiss et al., 2017; Alonso & Valle-Torres, 2018). The maintenance of stable populations in laboratory is relatively feasible with several exotic species, which present a high reproductive capacity. Thanks to this, toxicological and genetic bioassays can be carried out under laboratory conditions (controlled temperature and water physico-chemical parameters, health conditions of the organisms, known age, etc.), which are not possible with wild populations (Orlova & Komendantov, 2013). Other invasive species of molluscs, such as Dreissena spp. or Corbicula fluminea (Müller, 1774), are amply used in ecotoxicology and biomonitoring studies (Binelli et al., 2008; Barenberg & Moffitt, 2018; Miserazzi et al., 2020). However, the NZMS is not always a sensitive species for all toxicants and environmental conditions (Jacobsen & Forbes, 1997; Alonso & Camargo, 2004), which must be considered when this species is used for biomonitoring.
Conclusions
Our review highlights that the NZMS, Potamopyrgus antipodarum, causes important impacts on several ecosystem properties. Most of them can be related to direct impacts on ecosystem services, mainly due to the high density and secondary production that this species can reach in invaded ecosystems. However, facilitation processes of the NZMS on native species may result in a direct improvement of some services. In general, there are few studies quantifying the relationships between the impacts of the NZMS on ecosystem properties and ecosystem services. Therefore, further studies linking ecosystem functions and services are necessary, in such a way that an impact assessment of exotic species on the ecosystem functioning is a good basis for the evaluation of the likely changes on ecosystem services. Most analyzed ecosystem services were negatively affected by the NZMS, which makes this invasive species a threat to the quality of ecosystem services provided by aquatic ecosystems. On the contrary, ecosystem services related to health (e.g., dilution effect) and research (e.g., biomonitoring, model species for ecotoxicological and genetic bioassays) are two groups of cultural services that the NZMS improves. However, health services present a high degree of uncertainty regarding their potential benefit. In general, the bibliography provides scarce quantification of the ecosystem services that the NZMS may provide/affect in the invaded ecosystems, with most studies showing qualitative results. Finally, we could not find economic assessment on the impact of the NZMS on ecosystem services (e.g., cost of biofouling), which would be highly relevant for managers and policymakers.
Data availability
Not applicable.
References
Alonso, A. & J. A. Camargo, 2004. Sub-lethal responses of the aquatic snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca) to unionized ammonia: a tolerant invading species. Fresenius Environmental Bulletin 13: 607–615.
Alonso, A. & P. Castro-Diez, 2008. What explains the invading success of the aquatic mud snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca)? Hydrobiologia 614: 107–116. https://doi.org/10.1007/s10750-008-9529-3.
Alonso, A. & P. Castro-Díez, 2012. The exotic aquatic mud snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca): state of the art of a worldwide invasion. Aquatic Sciences 74: 375–383. https://doi.org/10.1007/s00027-012-0254-7.
Alonso, A. & G. Valle-Torres, 2018. Feeding behavior of an aquatic snail as a simple endpoint to assess the exposure to cadmium. Bulletin of Environmental Contamination and Toxicology 100: 82–88. https://doi.org/10.1007/s00128-017-2230-3.
Arango, C. P., L. A. Riley, J. L. Tank & R. O. Hall, 2009. Herbivory by an invasive snail increases nitrogen fixation in a nitrogen-limited stream. Canadian Journal of Fisheries and Aquatic Sciences 66: 1309–1317. https://doi.org/10.1139/F09-079.
Ashrafi, S., R. Kerachian, P. Pourmoghim, M. Behboudian & K. Motlaghzadeh, 2022. Evaluating and improving the sustainability of ecosystem services in river basins under climate change. The Science of the Total Environment 806: 150702. https://doi.org/10.1016/j.scitotenv.2021.150702.
Barenberg, A. & C. M. Moffitt, 2018. Toxicity of aqueous alkaline solutions to New Zealand mudsnails, asian clams, and quagga mussels. Journal of Fish and Wildlife Management 9: 14–24. https://doi.org/10.3996/022017-JFWM-013.
Benito, M., R. Mosteo, E. Rubio, D. LaPlante, M. P. Ormad & P. Goni, 2017. Bioaccumulation of inorganic elements in Dreissena polymorpha from the Ebro river, Spain: could zebra mussels be used as a bioindicator of the impact of human activities? River Research and Applications 33: 718–728. https://doi.org/10.1002/rra.3126.
Binelli, A., L. Guzzella & C. Roscioli, 2008. Levels and congener profiles of polybrominated diphenyl ethers (PBDEs) in Zebra mussels (D. polymorpha) from Lake Maggiore (Italy). Environmental Pollution 153: 610–617. https://doi.org/10.1016/j.envpol.2007.09.007.
Bray, D. J., I. Green, D. Golicher & R. J. H. Herbert, 2015. Spatial variation of trace metals within intertidal beds of native mussels (Mytilus edulis) and non-native Pacific oysters (Crassostrea gigas): implications for the food web? Hydrobiologia 757: 235–249. https://doi.org/10.1007/s10750-015-2255-8.
Brenneis, V. E. F., A. Sih & C. E. De Rivera, 2010. Coexistence in the intertidal: interactions between the non-indigenous New Zealand mud snail Potamopyrgus antipodarum and the native estuarine isopod Gnorimosphaeroma insulare. Oikos 119: 1755–1764. https://doi.org/10.1111/j.1600-070.
Brenneis, V. E. F., A. Sih & C. E. de Rivera, 2011. Integration of an invasive consumer into an estuarine food web: direct and indirect effects of the New Zealand mud snail. Oecologia 167: 169–179. https://doi.org/10.1007/s00442-011-1962-8.6.2010.18471.x.
Butkus, R., L. Baltrūnaitė, K. Arbačiauskas & A. Audzijonytė, 2020. Two lineages of the invasive New Zealand mudsnail Potamopyrgus antipodarum spreading in the Baltic and Black sea basins: low genetic diversity and different salinity preferences. Biological Invasions 22: 3551–3559. https://doi.org/10.1007/s10530-020-02340-3.
Cada, C. A. 2004. Interactions Between the Invasive New Zealand Mud Snail, Potamopyrgus antipodarum, baetid Mayflies, and Fish Predators. Dissertation, Montana State.
Cañedo-Argüelles, M., 2020. A review of recent advances and future challenges in freshwater salinization. Limnetica 39: 185–211. https://doi.org/10.23818/limn.39.13.
Cattau, C. E., R. J. Fletcher Jr., B. E. Reichert & W. M. Kitchens, 2016. Counteracting effects of a non-native prey on the demography of a native predator culminate in positive population growth. Ecological Applications 26: 1952–1968. https://doi.org/10.1890/15-1020.1.
Charles, H. & J. Dukes, 2007. Impacts of invasive species on ecosystem services. In Nentwig, W. (ed), Biological Invasions, Ecological Studies, Vol. 193. Springer, Berlin: 217–237.
Cichy, A., A. Marszewska, J. Parzonko, J. Zbikowski & E. Zbikowska, 2017. Infection of Potamopyrgus antipodarum (Gray, 1843) (Gastropoda: Tateidae) by trematodes in Poland, including the first record of aspidogastrid acquisition. Journal of Invertebrate Pathology 150: 32–34. https://doi.org/10.1016/j.jip.2017.09.003.
Clements, W. H., J. L. Arnold, T. M. Koel, R. Daley & C. Jean, 2011. Responses of benthic macroinvertebrate communities to natural geothermal discharges in Yellowstone National Park, USA. Aquatic Ecology 45: 137–149. https://doi.org/10.1007/s10452-010-9342-8.
Corrales, X., S. Katsanevakis, M. Coll, J. J. Heymans, C. Piroddi, E. Ofir & G. Gal, 2020. Advances and challenges in modelling the impacts of invasive alien species on aquatic ecosystems. Biological Invasions 22: 907–934. https://doi.org/10.1007/s10530-019-02160-0.
Davis, A. & K. Moeltner, 2010. Valuing the prevention of an infestation: the threat of the New Zealand Mud Snail in Northern Nevada. Agricultural and Resource Economics Review 39: 56–74.
Deines, A. M., M. E. Wittmann, J. M. Deines & D. M. Lodge, 2016. Tradeoffs among ecosystem services associated with global Tilapia introductions. Reviews in Fisheries Science & Aquaculture 24: 178. https://doi.org/10.1080/23308249.2015.1115466.
Dorgelo, J., 1987. Density fluctuations in populations 1982–1986 and biological observations of Potamopyrgus-jenkinsi in two trophically differing lakes. Hydrobiological Bulletin 21: 95–110. https://doi.org/10.1007/BF02255459.
Drescher, M., A. H. Perera, C. J. Johnson, L. J. Buse, C. A. Drew & M. A. Burgman, 2013. Toward rigorous use of expert knowledge in ecological research. Ecosphere 83: 1–26. https://doi.org/10.1890/ES12-00415.1.
Evans, N. A., P. J. Whitfield & A. P. Dobson, 1981. Parasite utilization of a host community: the distribution and occurrence of metacercarial cysts of Echinoparyphium recurvatum (Digenea: Echinostomatidae) in seven species of mollusc at Harting Pond, Sussex. Parasitology 83: 1–12. https://doi.org/10.1017/S0031182000049982.
Field, A., J. Miles & Z. Field, 2012. Discovering Statistics Using R, Sage, Los Angeles:
Gaino, E., F. Scoccia, T. Lancioni & A. Ludovisi, 2008. The invader mudsnail Potamopyrgus antipodarum in the Tiber River basin (Central Italy). The Italian Journal of Zoology 75: 253–261. https://doi.org/10.1080/11250000701885513.
Gallardo, B., S. Bacher, B. Bradley, F. A. Comín, L. Gallien, J. M. Jeschke, C. J. B. Sorte & M. Vilà, 2019. InvasiBES: understanding and managing the impacts of invasive alien species on biodiversity and ecosystem services. NeoBiota 50: 109–122. https://doi.org/10.3897/neobiota.50.35466.
Geiss, C., K. Ruppert, C. Askem, C. Barroso, D. Faber, V. Ducrot, H. Holbech, T. H. Hutchinson, P. Kajankari, K. L. Kinnberg, L. Lagadic, P. Matthiessen, S. Morris, M. Neiman, O. Penttinen, P. Sanchez-Marin, M. Teigeler, L. Weltje & J. Oehlmann, 2017. Validation of the OECD reproduction test guideline with the New Zealand mudsnail Potamopyrgus antipodarum using trenbolone and prochloraz. Ecotoxicology 26: 370–382. https://doi.org/10.1007/s10646-017-1770-y.
Geist, J. A., J. L. Mancuso, M. M. Morgan, K. P. Bommarito, E. N. Bovee, D. Wendell, B. Burroughts, M. R. Luttenton, D. L. Strayer & S. D. Tiegs, 2022. The New Zealand mud snail (Potamopyrgus antipodarum): autecology and management of a global invader. Biological Invasions. https://doi.org/10.1007/s10530-021-02681-7.
Gérard, C., O. Miura, J. Lorda, T. H. Cribb, M. J. Nolan & R. F. Hechinger, 2017. A native-range source for a persistent trematode parasite of the exotic New Zealand mudsnail (Potamopyrgus antipodarum) in France. Hydrobiologia 785: 115–126. https://doi.org/10.1007/s10750-016-2910-8.
Gérard, C., M. Hervé & R. F. Hechinger, 2018. Long-term population fluctuations of the exotic New Zealand mudsnail Potamopyrgus antipodarum and its introduced aporocotylid trematode in northwestern France. Hydrobiologia 817: 253–266. https://doi.org/10.1007/s10750-017-3406-x.
Gherardi, F., 2007. Biological invasions in inland waters: an overview. In Gherardi, F. (ed), Biological Invaders in Inland Waters: Profiles, Distribution, and Threats Springer, Dordrecht: 3–25.
Gherardi, F., 2011. Towards a sustainable human use of freshwater crayfish (Crustacea, Decapoda, Astacidea). Knowledge and Management of Aquatic Ecosystems 401: 2–22. https://doi.org/10.1051/kmae/2011038.
Gherardi, F., L. Aquiloni, J. Dieguez-Uribeondo & E. Tricarico, 2011. Managing invasive crayfish: is there hope? Aquatic Sciences 73: 185–200. https://doi.org/10.1007/s00027-011-0181-z.
Gilioli, G., G. Schrader, N. Carlsson, E. van Donk, C. H. A. van Leeuwen, P. R. Martín, S. Pasquali, M. Vilà & S. Vos, 2017. Environmental risk assessment for invasive alien species: a case study of apple snails affecting ecosystem services in Europe. Environmental Impact Assessment Review 65: 1–11. https://doi.org/10.1016/j.eiar.2017.03.008.
Gofas, S. & A. Zenetos, 2003. Exotic molluscs in the Mediterranean basin: current status and perspectives. Oceanography and Marine Biology 41: 237–277.
Haines-Young, R. & M. B. Potschin, 2018. Common International Classification of Ecosystem Services (CICES) V5.1 and Guidance on the Application of the Revised Structure [available on www.cices.eu].
Hall, R. O., Jr., J. L. Tank & M. F. Dybdahl, 2003. Exotic snails dominate nitrogen and carbon cycling in a highly productive stream. Frontiers in Ecology and the Environment 1: 407–411. https://doi.org/10.1890/1540-9295(2003)001[0407:ESDNAC]2.0.CO;2.
Hall, R. O., M. F. Dybdahl & M. C. VanderLoop, 2006. Extremely high secondary production of introduced snails in rivers. Ecological Applications 16: 1121–1131.
Havel, J., K. Kovalenko, S. Thomaz, S. Amalfitano & L. Kats, 2015. Aquatic invasive species: challenges for the future. Hydrobiologia 750: 147–170. https://doi.org/10.1007/s10750-014-2166-0.
Heikal, M. N., M. Sanad, M. S. M. Shamseldean & S. A. Hamdi, 2018. The red swamp crayfish, Procambarus clarkii (Gerard, 1852) an invasive species in Egypt as a biocontrol agent of the mosquito, Culex quinquefasciatus Say, 1823. Bioscience Research 15: 839–849.
Hellmair, M., G. Goldsmith & A. P. Kinziger, 2011. Preying on invasives: the exotic New Zealand mudsnail in the diet of the endangered tidewater goby. Biological Invasions 13: 2197–2201. https://doi.org/10.1007/s10530-011-0054-3.
Holomuzki, J. R., R. L. Lowe & J. A. Ress, 2006. Comparing herbivory effects of stream macroinvertebrates on microalgal patch structure and recovery. New Zealand Journal of Marine and Freshwater Research 40: 357–367. https://doi.org/10.1080/00288330.2006.9517427.
Hooper, D. U., F. S. Chapin, J. J. Ewel, A. Hector, P. Inchausti, S. Lavorel, J. H. Lawton, D. M. Lodge, M. Loreau, S. Naeem, B. Schmid, H. Setälä, A. J. Symstad, J. Vandermeer & D. A. Wardle, 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecological Monographs 75: 3–35. https://doi.org/10.1890/04-0922.
Hoyer, S. A. & C. A. Myrick, 2012. Can copper-based substrates be used to protect hatcheries from invasion by the New Zealand mudsnail? North American Journal of Aquaculture 74: 575–583. https://doi.org/10.1080/15222055.2012.685213.
Jacobsen, R. & V. E. Forbes, 1997. Clonal variation in life-history traits and feeding rates in the gastropod, Potamopyrgus antipodarum: performance across a salinity gradient. Functional Ecology 11: 260–267. https://doi.org/10.1046/j.1365-2435.1997.00082.x.
Johns, C., 2012. Trends of total cadmium, copper, and zinc in the zebra mussel (Dreissena polymorpha) along the upper reach of the St. Lawrence River: 1994–2005. Environmental Monitoring and Assessment 184: 5371–5385. https://doi.org/10.1007/s10661-011-2346-6.
Jones, R. A., H. W. Williams, S. Dalesman & P. M. Brophy, 2015. Confirmation of Galba truncatula as an intermediate host snail for Calicophoron daubneyi in Great Britain, with evidence of alternative snail species hosting Fasciola hepatica. Parasites and Vectors 8:656. https://doi.org/10.1186/s13071-015-1271-x.
Katsanevakis, S., I. Wallentinus, A. Zenetos, E. Leppakoski, M. E. Cinar, B. Ozturk, M. Grabowski, D. Golani & A. C. Cardoso, 2014. Impacts of invasive alien marine species on ecosystem services and biodiversity: a pan-European review. Aquatic Invasions 9: 391–423. https://doi.org/10.3391/ai.2014.9.4.01.
Kourantidou, M., P. J. Haubrock, R. N. Cuthbert, T. W. Bodey, B. Lenzner, R. E. Gozlan, M. A. Nunez, J. M. Salles, C. Diagne & F. Courchamp, 2022. Invasive alien species as simultaneous benefits and burdens: trends, stakeholder perceptions and management. Biological Invasions. https://doi.org/10.1007/s10530-021-02727-w.
Krist, A. C. & C. C. Charles, 2012. The invasive New Zealand mudsnail, Potamopyrgus antipodarum, is an effective grazer of algae and altered the assemblage of diatoms more than native grazers. Hydrobiologia 694: 143–151. https://doi.org/10.1007/s10750-012-1138-5.
Larson, M. D., J. C. Dewey & A. C. Krist, 2020a. Invasive Potamopyrgus antipodarum (New Zealand mud snails) and native snails differ in sensitivity to specific electrical conductivity and cations. Aquatic Ecology 54: 103–117. https://doi.org/10.1007/s10452-019-09729-w.
Larson, M. D., E. P. Levri, S. V. Huzurbazar, D. J. Greenwood, K. L. Wise & A. C. Krist, 2020b. No evidence for a dilution effect of the non-native snail, Potamopyrgus antipodarum, on native snails. PLoS ONE 15: e0239762. https://doi.org/10.1371/journal.pone.0239762.
Lassen, H. H., 1979. Reproductive effort in Danish mudsnails (Hydrobiidae). Oecologia 40: 365–369. https://doi.org/10.1007/BF00345332.
Laverty, C., W. Nentwig, J. T. Dick & F. E. Lucy, 2015. Alien aquatics in Europe: assessing the relative environmental and socioeconomic impacts of invasive aquatic macroinvertebrates and other taxa. Aquatic Invasions 6: 341–350. https://doi.org/10.3391/mbi.2015.6.4.03.
Levri, E. P., N. Macelko, B. Brindle, J. E. Levri, T. J. Dolney & X. Li, 2020. The invasive New Zealand mud snail Potamopygus antipodarum (J.E. Gray, 1843) in central Pennsylvania. BioInvasions Records 9: 109–119. https://doi.org/10.3391/bir.2020.9.1.15.
Limburg, K. E., V. A. Luzadis, M. Ramsey, K. L. Schulz & C. M. Mayer, 2010. The good, the bad, and the algae: perceiving ecosystem services and disservices generated by Zebra and Quagga Mussels. Journal of Great Lakes Research 36: 86–92. https://doi.org/10.1016/j.jglr.2009.11.007.
Locke, S. A., G. Bulte, D. J. Marcogliese & M. R. Forbes, 2014. Altered trophic pathway and parasitism in a native predator (Lepomis gibbosus) feeding on introduced prey (Dreissena polymorpha). Oecologia 175: 315–324. https://doi.org/10.1007/s00442-014-2898-6.
Lodge, D. M., A. Deines, F. Gherardi, D. C. J. Yeo, T. Arcella, A. K. Baldridge, M. A. Barnes, W. L. Chadderton, J. L. Feder, C. A. Gantz, G. W. Howard, C. L. Jerde, B. W. Peters, J. A. Peeters, L. W. Sargent, C. R. Turner, M. E. Wittmann & Y. Zeng, 2012. Global introductions of crayfishes: evaluating the impact of species invasions on ecosystem services. Annual Review of Ecology, Evolution, and Systematics 43: 449–472. https://doi.org/10.1146/annurev-ecolsys-111511-103919.
Marszewska, A., A. Cichy, J. Bulantová, P. Horák & E. Zbikowska, 2018. Potamopyrgus antipodarum as a potential defender against swimmer’s itch in European recreational water bodies-experimental study. PeerJ 6: e5045. https://doi.org/10.7717/peerj.5045.
McKenzie, V. J., W. E. Hall & R. P. Guralnick, 2013. New Zealand mudsnails (Potamopyrgus antipodarum) in Boulder Creek, Colorado: environmental factors associated with fecundity of a parthenogenic invader. Canadian Journal of Zoology 91: 30–36. https://doi.org/10.1139/cjz-2012-0183.
McLaughlan, C., B. Gallardo & D. C. Aldridge, 2014. How complete is our knowledge of the ecosystem services impacts of Europe’s top 10 invasive species? Acta Oecologica 54: 119–130. https://doi.org/10.1016/j.actao.2013.03.005.
Medakovic, D., R. Slapnik, S. Popovic & B. Grzeta, 2003. Mineralogy of shells from two freshwater snails Belgrandiella fontinalis and B. kuesteri. Comparative Biochemistry and Physiology Part A Molecular & Integrative Physiology 134A: 121–127. https://doi.org/10.1016/S1095-6433(02)00218-0ER.
Millennium Ecosystem Assessment and Assessment ME, 2005. Ecosystems and Human Well-Being: Synthesis, Island Press, Washington, D.C.:
Miserazzi, A., M. Sow, C. Gelber, M. Charifi, P. Ciret, J. M. Dalens, C. Weber, S. Le Floch, C. Lacroix, P. Blanc & J. C. Massabuau, 2020. Asiatic clam Corbicula fluminea exhibits distinguishable behavioural responses to crude oil under semi-natural multiple stress conditions. Aquatic Toxicology 219: 105381. https://doi.org/10.1016/j.aquatox.2019.105381.
Moffitt, C. M. & C. A. James, 2012. Dynamics of Potamopyrgus antipodarum infestations and seasonal water temperatures in a heavily used recreational watershed in intermountain North America. Aquatic Invasions 7: 193–202. https://doi.org/10.3391/ai.2012.7.2.005.
Moorhouse, T. P. & D. W. Macdonald, 2015. Are invasives worse in freshwater than terrestrial ecosystems? Wiley Interdisciplinary Reviews-Water 2: 1–8. https://doi.org/10.1002/wat2.1059.
Morley, N. J., 2008. The role of the invasive snail Potamopyrgus antipodarum in the transmission of trematode parasites in Europe and its implications for ecotoxicological studies. Aquatic Sciences 70: 107–114. https://doi.org/10.1007/s00027-007-7052-7.
Mulero, S., E. Toulza, A. Loisier, M. Zimmerman, J. Allienne, J. Foata, Y. Quilichini, J.-P. Pointier, O. Rey & J. Boissier, 2021. Malacological survey in a bottle of water: a comparative study between manual sampling and environmental DNA metabarcoding approaches. Global Ecology and Conservation 25: e01428. https://doi.org/10.1016/j.gecco.2020.e01428.
Nakano, D. & D. L. Strayer, 2014. Biofouling animals in fresh water: biology, impacts, and ecosystem engineering. Frontiers in Ecology and the Environment 12: 167–175. https://doi.org/10.1890/130071.
Nishijima, S. & A. Belgrano, 2020. Towards ecological, socioeconomic and cultural sustainability of marine ecosystem services. Population Ecology 63: 14. https://doi.org/10.1002/1438-390x.12075.
Nonaka, T., 2012. Use of edible alien crayfish for human consumption. Crustacean Research Special 7: 115–124. https://doi.org/10.18353/crustacea.Special2012.7_115.
Nunes, A., E. Tricarico, V. Panov, A. Cardoso & S. Katsanevakis, 2015. Pathways and gateways of freshwater invasions in Europe. Aquatic Invasions 10: 359–370. https://doi.org/10.3391/ai.2015.10.4.01.
O’Brien, G., N. J. Smit & V. Wepener, 2021. Regional scale risk to the ecological sustainability and ecosystem services of an African floodplain system. Risk Analysis 41: 1925–1952. https://doi.org/10.1111/risa.13689.
Okland, J., 1992. Effects of acidic water on freshwater snails: results from a study of 1000 lakes throughout Norway. Environmental Pollution 78: 127–130. https://doi.org/10.1016/0269-7491(92)90020-B.
Orlova, M. I. & A. Y. Komendantov, 2013. Is the invader Potamopyrgus antipodarum (Gastropoda, Hydrobiidae) an object for maintenance in laboratory? Zoologichesky Zhurnal 92: 627–632. https://doi.org/10.7868/S004451341306010X.
Otero, M., E. Cebrian, P. Francour, B. Galil & D. Savini, 2013. Monitoring Marine Invasive Species in Mediterranean Marine Protected Areas (MPAs): A Strategy and Practical Guide for Managers, IUCN, Malaga:, 136.
Pedersen, S., H. Selck, D. Salvito & V. Forbes, 2009. Effects of the polycyclic musk HHCB on individual- and population-level endpoints in Potamopyrgus antipodarum. Ecotoxicology and Environmental Safety 72: 1190–1199. https://doi.org/10.1016/j.ecoenv.2008.10.012.
Pejchar, L. & H. A. Mooney, 2009. Invasive species, ecosystem services and human well-being. Trends in Ecology & Evolution 24: 497–504. https://doi.org/10.1016/j.tree.2009.03.016.
Petsch, D. K., 2016. Causes and consequences of biotic homogenization in freshwater ecosystems. International Review of Hydrobiology 101: 113–122. https://doi.org/10.1002/iroh.201601850.
Proctor, T., B. Kerans, P. Clancey, E. Ryce, M. Dybdahl, D. Gustafson, R. Hall, F. Pickett, D. Richards, R. D. Waldeck, J. Chapman, R. H. Wiltshire, D. Becker, M. Anderson, B. Pitman, D. Lassuy, P. Heimowitz, P. Dwyer & E. Levri, 2007. National Management and Control Plan for the New Zealand Mudsnail (Potamopyrgus antipodarum). Report Prepared by the New Zealand Mudsnail Management and Control Plan Working Group for the Aquatic Nuisance Species Task Force.
R Core Team, 2021. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria [available on https://www.R-project.org/].
Rakauskas, V., R. Butkus & E. Merkytė, 2016. Consumption of the invasive New Zealand mud snail (Potamopyrgus antipodarum) by benthivorous predators in temperate lakes: a case study from Lithuania. Hydrobiologia 775: 213–230. https://doi.org/10.1007/s10750-016-2733-7.
Rakauskas, V., E. Sidagyte, R. Butkus & A. Garbaras, 2018. Effect of the invasive New Zealand mud snail (Potamopyrgus antipodarum) on the littoral macroinvertebrate community in a temperate mesotrophic lake. Marine and Freshwater Research 69: 155–166. https://doi.org/10.1071/MF17059.
Ramskov, T., M. Croteau, V. E. Forbes & H. Selck, 2015. Biokinetics of different-shaped copper oxide nanoparticles in the freshwater gastropod, Potamopyrgus antipodarum. Aquatic Toxicology 163: 71–80. https://doi.org/10.1016/j.aquatox.2015.03.020.
Richards, D. C., 2002. The New Zealand mud snail invades the Western United States. Aquatic Nuisance Species 4: 42–44.
Richards, D. C., L. D. Cazier & G. T. Lester, 2001. Spatial distribution of three snail species, including the invader Potamopyrgus antipodarum, in a freshwater spring. Western North American Naturalist 61: 375–380.
Riley, L. A., M. F. Dybdahl & R. O. Hall Jr., 2008. Invasive species impact: asymmetric interactions between invasive and endemic freshwater snails. Journal of the North American Benthological Society 27: 509–520. https://doi.org/10.1899/07-119.1.
Rodriguez, L. F., 2006. Can invasive species facilitate native species? Evidence of how, when, and why these impacts occur. Biological Invasions 8: 927–939. https://doi.org/10.1007/s10530-005-5103-3.
Schreiber, E. S. G., P. S. Lake & G. P. Quinn, 2002. Facilitation of native stream fauna by an invading species? Experimental investigations of the interaction of the snail, Potamopyrgus antipodarum (Hydrobiidae) with native benthic fauna. Biological Invasions 4: 317–325.
Shin-ichiro, S. M., U. Nisikawa, T. Noriko & W. Izumi, 2009. Contrasting impacts of invasive engineers on freshwater ecosystems: an experiment and meta-analysis. Oecologia 158: 673–686. https://doi.org/10.1007/s00442-008-1180-1.
Smietana, N., R. Panicz, M. Sobczak, P. Smietana & A. Nedzarek, 2021. Spiny-Cheek crayfish, Faxonius limosus (Rafinesque, 1817), as an alternative food source. Animals 11: 59. https://doi.org/10.3390/ani11010059.
Spyra, A., A. Cieplok, M. Strzelec & A. Babczynska, 2019. Freshwater alien species Physella acuta (Draparnaud, 1805)—a possible model for bioaccumulation of heavy metals. Ecotoxicology and Environmental Safety 185: 109703. https://doi.org/10.1016/j.ecoenv.2019.109703.
Strayer, D. L., 2010. Alien species in fresh waters: ecological effects, interactions with other stressors, and prospects for the future. Freshwater Biology 55: 152–174. https://doi.org/10.1111/j.1365-2427.2009.02380.x.
Taybi, A. F., Y. Mabrouki & P. Glöer, 2021. First record of the New Zealand mudsnail Potamopyrgus antipodarum (J.E. Gray, 1843) (Tateidae, Mollusca) in Africa. Graellsia. https://doi.org/10.3989/graellsia.2021.v77.303.
USDI - The U.S. Department of the Interior, 2016. Safeguarding America’s Lands and Waters from Invasive Species: A National Framework for Early Detection and Rapid Response, USDI, Washington D.C.:, 55.
van Leeuwen, C. H. A. & G. van der Velde, 2012. Prerequisites for flying snails: external transport potential of aquatic snails by waterbirds. Freshwater Science 31: 963–972. https://doi.org/10.1899/12-023.1.
Vazquez, R., D. M. Ward & A. Sepulveda, 2016. Does water chemistry limit the distribution of New Zealand mud snails in Redwood National Park? Biological Invasions 18: 1523–1531. https://doi.org/10.1007/s10530-016-1098-1.
Verhaegen, G., M. Neiman & M. Haase, 2018. Ecomorphology of a generalist freshwater gastropod: complex relations of shell morphology, habitat, and fecundity. Organisms Diversity and Evolution 18: 425–441. https://doi.org/10.1007/s13127-018-0377-3.
Verhaegen, G., H. Herzog, K. Korsch, G. Kerth, M. Brede & M. Haase, 2019. Testing the adaptive value of gastropod shell morphology to flow: a multidisciplinary approach based on morphometrics, computational fluid dynamics and a flow tank experiment. Zoological Letters. https://doi.org/10.1186/s40851-018-0119-6.
Verhaegen, G., K. von Jungmeister & M. Haase, 2021. Life history variation in space and time: environmental and seasonal responses of a parthenogenetic invasive freshwater snail in northern Germany. Hydrobiologia 848: 2153–2168. https://doi.org/10.1007/s10750-020-04333-8.
Vilà, M. & P. E. Hulme (eds), 2017. Impact of Biological Invasions on Ecosystem Services. Vol. 12. Springer, Berlin.
Vinson, M. R. & M. A. Baker, 2008. Poor growth of rainbow trout fed New Zealand mud snails Potamopyrgus antipodarum. North American Journal of Fisheries Management 28: 701–709. https://doi.org/10.1577/M06-039.1.
Walsh, J. R., S. R. Carpenter & M. J. Vander Zanden, 2016. Invasive species triggers a massive loss of ecosystem services through a trophic cascade. Proceedings of the National Academy of Sciences 113: 4081–4085. https://doi.org/10.1073/pnas.1600366113.
White, M. M., M. Chejlava, B. Fried & J. Sherma, 2007. The concentration of calcium carbonate in shells of freshwater snails. American Malacological Bulletin 22: 139–142. https://doi.org/10.4003/0740-2783-22.1.139.
Winterbourn, M., 1969. Water temperature as a factor limiting the distribution of Potamopyrgus antipodarum (Gastropoda-Prosobranchia) in the New Zealand thermal region. New Zealand Journal of Marine and Freshwater Research 3: 453–458. https://doi.org/10.1080/00288330.1969.9515310.
Winterbourn, M., 1970. The New-Zealand species of Potamopyrgus gastropoda hydrobiidae. Malacologia 10: 283–321.
Zbikowski, J. & E. Zbikowska, 2009. Invaders of an invader—trematodes in Potamopyrgus antipodarum in Poland. Journal of Invertebrate Pathology 101: 67–70. https://doi.org/10.1016/j.jip.2009.02.005.
Zhang, H., E. S. Rutherford, D. M. Mason, M. E. Wittmann, D. M. Lodge, X. Zhu, T. B. Johnson & A. Tucker, 2019. Modelling potential impacts of three benthic invasive species on the Lake Erie food web. Biological Invasions 21: 1697–1719. https://doi.org/10.1007/s10530-019-01929-7.
Acknowledgements
This work was carried out within the sabbatical period of Álvaro Alonso as a professor in the University of Alcalá for the 2021–2022 academic year.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. Funds for this research came from the project EXARBIN RTI2018-093504-B-I00 (MCIU/AEI/FEDER, UE) of the Ministerio de Economía y Competitividad of Spain.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Ethical approval
Not applicable.
Consent for publication
Authors provide consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Manuel P. M. Lopes-Lima, Lyubov E. Burlakova, Ting Hui Ng, Alexandra Zieritz & Ronaldo G. Sousa / Biology and Impact of Invasive Freshwater Molluscs
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alonso, Á., Collado, G.A., Gérard, C. et al. Effects of the invasive aquatic snail Potamopyrgus antipodarum (Gray, 1853) on ecosystem properties and services. Hydrobiologia (2023). https://doi.org/10.1007/s10750-022-05116-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10750-022-05116-z