Abstract
The nitrogen availability, that affects the greenhouse gas emission and the trophic level of lakes, is controlled mainly by microbial processes. We measured in a boreal nitrate and iron rich lake how the rates of potential denitrification and dissimilatory nitrate reduction to ammonia (DNRA) are affected by degradability of organic matter and availability of aqueous ferrous iron. We also investigated the microbial community by using 16S rRNA gene and shotgun metagenomic sequencing approach, which allows taxonomic analyses and detection of metagenome-assembled genomes (MAGs) containing genes for both nitrate reduction and iron oxidation. The results show that truncated denitrification, leading to release of nitrous oxide, is favored over dinitrogen production in conditions where the degradability of the organic matter is low. DNRA rates were always minor compared to denitrification and appeared to be independent of the degradability of organic carbon. Reduced iron stimulated nitrate reducing processes, although consistently only DNRA. However, the proportion of MAGs containing DNRA genes was low suggesting chemistry driven stimulation by reduced iron. Nevertheless, the metagenomic analyses revealed unique taxa genetically capable of oxidizing iron and reducing nitrate simultaneously. Overall, the results highlight the spatial variability in microbial community and nitrous oxide emissions in boreal lake sediments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitrogen (N) loading to freshwater ecosystems continues to be a major driver of eutrophication globally, despite the efforts toward load reduction in the past decades (Galloway & Cowling, 2021). The negative effects of excess N loading can be partially mitigated by denitrification, where microbes reduce nitrate (NO3−) to nitrous oxide (N2O) and dinitrogen (N2) and anammox, where microbes oxidize ammonia (NH4+) with nitrite (NO2−) to N2, both processes eventually removing N from aquatic ecosystems. Boreal ecosystems are vulnerable to climate change and therefore it is important to understand their biogeochemical functioning in the context of the twin pressures of eutrophication and global warming. In boreal lake ecosystems, anammox is considered a minor process in the N cycle, as most of the nitrate is reduced into N2 through denitrification (Rissanen et al., 2011, 2013). The emissions of N2O, a potent greenhouse gas, are typically low from boreal lake ecosystems (Huttunen et al., 2003; Saarenheimo et al., 2015; Lauerwald et al., 2019); however, most of these measurements have been made during ice-free season when the N2O emissions from boreal lakes are the lowest (Kortelainen et al., 2020). Processes in the N cycle are also influenced by the quality of organic matter (e.g., Aalto et al., 2021). In many lakes, the quality of organic matter varies seasonally, but also with water depth, as the organic matter tends to be more bioavailable in the shallow areas (Den Heyer & Kalff, 1998). This means that the balance between nitrate removing and nitrate recycling processes varies between seasons and different lake areas. Denitrification competes for NO3− with dissimilatory nitrate reduction to ammonium (DNRA), in which microbes reduce NO3− via NO2− to NH4+ instead of the gaseous N species (Tiedje et al., 1984; Tiedje, 1988; Giblin et al., 2013). Hence, instead of NO3− being converted to non-reactive form, N remains bioavailable in DNRA and thus can accelerate eutrophication. DNRA does not leak intermediates, although there is evidence that some bacteria may carry both nrfA (marker gene for DNRA (Einsle et al., 1999) and nirK genes (nitrite reductase gene) on the same genome, suggesting that DNRA and canonical denitrification may be compatible (Sanford et al., 2012).
The NO3− reducing processes are controlled not only by NO3− availability but also by the availability of electron donors. Both denitrification and DNRA can be heterotrophic or chemolithotrophic processes. Studies from natural systems and chemostats have shown that denitrification tends to dominate NO3− reduction when the NO3− concentration is high and the availability of organic carbon low. Vice versa, DNRA dominates NO3− reduction when the NO3− concentration is low and the availability of organic carbon is high (van den Berg et al., 2015; Yoon et al., 2015; Aalto et al., 2021). Also, the nature of the organic electron donor influences the outcome of competition between DNRA and denitrification because DNRA can be driven both by direct oxidation of organic matter and by fermentation (Van den Berg et al., 2017). DNRA has been also shown to be stimulated by ferrous iron (Fe2+) in non-sulfidic sediments that are typical for freshwaters (Roberts et al., 2014; Robertson & Thamdrup, 2017; Kessler et al., 2019) although it is not entirely clear whether the stimulation is caused by Fe2+ acting as an electron donor in microbial NO3− reduction or a chemical reaction between Fe2+ and N compounds (Picardal, 2012; Klueglein & Kappler, 2013). Since the Fe and dissolved organic carbon (DOC) concentrations in boreal lakes have been increasing due to changing climate (Weyhenmeyer et al., 2014), the lacustrine N cycling is also likely be affected.
The aims of the study were to (i) determine the NO3− reduction rates in boreal lake sediments under naturally variable availability of organic compounds and elevated Fe2+ concentrations, and (ii) study the variability of microbial community and the relative abundance of potential denitrifying and DNRA microbes to see how the genetic potential for the NOx− reducing processes varies along a water depth gradient. Our hypothesis was that when the degradability and amount of organic matter decreases, the microbial community changes, DNRA becomes relatively more important, and that the microbes are more likely to use Fe2+ as an electron donor.
Materials and methods
Sampling site
Lake Pääjärvi is a deep oligo-mesotrophic lake located in the boreal region in southern Finland (61°04′N, 25°08′E) with an area of 13.4 km2 and maximal depth of 87 m. The lake has an ice cover for approximately four months during winter and the water column circulates twice per year and is always well oxygenated (Salonen 1981). Approximately 15% of Lake Pääjärvi’s large catchment area (244 km2) consists of agricultural land which causes the relatively high NO3− concentration in the lake. Despite the relatively high NO3− concentration, the denitrification rates (N2 production) are similar compared to other lakes in the area (Rissanen et al., 2013). Pääjärvi is characterized as a mesohumic lake with a DOC concentration of 10 mg C l−1 (Tulonen, 2004). In Finland, over half of lakes have a DOC concentration of similar or higher than that (Kortelainen, 1993). As typical for lakes in the boreal region, the DOC pool in Pääjärvi mainly consists of allochthonous C that is refractory to immediate bacterial utilization (Tulonen, 2004).
Porewater and sediment sampling
Vertical profiles of porewater and sediment samples were collected from three stations in Lake Pääjärvi on August 2017 (Table 1) using a hand-held HTH/Kajak corer with plexiglass tubes. The quality and quantity of organic matter in lake sediments varies with depth and distance from the shoreline creating a natural gradient in variability of organic matter. To investigate the effect of organic matter on NO3− reducing process and microbial communities, we collected samples from three stations that followed a water depth gradient, Station 1 being the shallowest and Station 3 the deepest. Core tubes were pre-drilled with vertical series of 4 mm holes (each at 2 cm resolution), then taped, in preparation for porewater sampling with Rhizons™. Rhizon sampling automatically filters the porewaters at 0.15 µm into attached plastic 10 ml syringes under vacuum. The vertical series of samples was taken for analysis of NO3−, Fe, lactate, and acetate. NO3−, lactate, and acetate subsamples were stored frozen at − 20°C until analyses. Fe subsamples were acidified to 1 M HNO3 and stored at room temperature. After porewater sampling, sediment cores were sliced into plastic bags at a resolution of 1 cm (0‒10 cm) or 2 cm (> 10 cm). Subsamples of 400 ‒ 500 µl wet sediment were collected from the 0‒10 cm interval of each site and stored frozen at − 20°C for DNA-based molecular microbiological analyses. The remaining wet sediment samples were stored frozen at − 20°C under N2 until further processing.
According to our standard protocols, all Rhizons were sequentially washed with 0.01 M HNO3 and MilliQ before field sampling. Later, it was noticed that the washing procedure caused background contamination in the porewater NO3− measurements. The magnitude and variability of this contamination was tested by sampling a solution of MilliQ water through Rhizons into plastic syringes and determining the NO3− concentration of the extracted sample. The same procedure was repeated using both factory-clean (9 replicates) and 0.01 M HNO3-washed rhizons (10 replicates). The difference between the mean NO3− values thus determined from the factory-clean and washed Rhizon samples (30.0 µmol l−1) was subtracted from all porewater NO3− data. Porewater NO3− concentrations were determined according to Miranda et al. (2001), and the samples from the HNO3 contamination test were analyzed by QuikChem®8000 flow injection analyzer (Lachat Instruments, Hach Co., Loveland, CO, USA) according to Wood et al. (1967).
Porewater Fe concentrations were determined by ICP-OES (Thermo iCAP 6000, Thermo Fisher Scientific, Waltham, MA, USA). The values represent total porewater concentrations. In this system, Fe is expected to be dominated by Fe2+ although other forms are possible. Porewater acetate and lactate were analyzed, in duplicates, using high performance liquid chromatography (HPLC) equipped with Shodex SUGAR column (Phenomenex, 300 mm × 8 mm, Torrance, CA, USA), autosampler (SIL-20AC HT, Shimadzu, Kyoto, Japan), refractive index detector (RID-10A, Shimadzu, Kyoto, Japan), and 0.01 M H2SO4 as the mobile phase. The HPLC samples were prepared as described in Salmela et al. (2018). The identification and quantification of the liquid metabolites was based on using external standards.
Separate sediment samples were prepared for the loss-on-ignition (LOI%, 4 h, 450°C) analysis and for the dry combustion of samples that were first freeze-dried, ground in an agate mortar, and weighed into tin cups for the C and N content determinations. In accordance with extensive previous studies on Finnish lake sediments (Pajunen, 2000; Kortelainen et al., 2004), acidification was not applied prior to the determinations. High levels of organic acidity from Finnish river catchments (Kortelainen & Saukkonen, 1995) maintain low annual mean pH values in most lakes and therefore there is negligible occurrence of carbonates in lake sediments. Hence our total C data are considered equivalent to organic C (Corg). C and N contents were determined using elemental analyzer (LECO TruSpec Micro, LECO Corp., St. Joseph, MI, USA). A simple two-component mixing model was applied for a first-order quantification of the relative contributions of terrestrial plant-derived and autochthonous phytoplankton-derived organic matter to the total sedimentary organic matter (percent of total organic C). The calculation uses the molar N:C ratio of organic matter, and end-member values, N/CEM, based on the studies of Goñi et al. (2003) and Jilbert et al. (2018)
where N/CEM-terr = 0.04, and N/CEM-phyt = 0.13. The calculation assumes that terrestrial plant matter and phytoplankton are the only sources of organic material present in the samples, that their N/C values are spatially and temporally fixed at the end-member values, and that these values do not alter significantly during sedimentation and burial of organic matter. Mineralization, however, may alter the values and therefore this calculation was applied only to the surface sediment layer. The porewater and sediment characteristics at the slurry collection depth (see below for incubation experiments) were determined by calculating average of the values from sediment and porewater samples collected at those depth layers (n = 2 for St1, and n = 3 for St2 and St3).
Incubation experiments
The sediment for the incubations was collected from the depth interval corresponding to the zone of Fe oxide reduction which indicates the onset of anaerobic remineralization processes. Four days prior to sampling, the porewater HPO42− concentration profile was determined at each site. Due to the strong correlation expected between porewater HPO42− and Fe2+ in the upper sediment layers (e.g., Jilbert et al., 2020) the depth of the first major increase in HPO42− was considered to indicate the depth of the first major increase in Fe2+ and hence reduction of Fe oxides. Based on the measured profiles, a 3 cm thick sediment slice was cut in the following depths: − 1 cm (St1), − 3 cm (St2), and − 3 cm (St3). Later analysis of porewater Fe2+ confirmed the validity of the approach.
The sediment collected from each station was mixed with water collected 50 cm above the sediment in 1:1 ratio. The sediment slurry was prepared in 1 l bottle and purged with N2 for 10 min. Thereafter, the bottle was quickly capped with a septum and the headspace was purged for 5 min. To ensure that the sediment slurry will be anoxic when the experiments begin, the bottle was placed overnight on a shaker table (120 rpm) at 5°C. The following morning, 1 l of water collected from 50 cm above the sediment–water interface was transferred into a glove bag with N2 atmosphere, and purged with N2 for 15 min. Also, 1 M Fe(II)SO4 solution and 20 mM K15NO3 (99% 15N, Cambridge isotope laboratories, Cambridge UK) solution were purged with N2 for 5 min. Thereafter, 2 ml of the sediment slurry was transferred into 12 ml Exetainer® vials (Labco Ltd, Lampeter, United Kingdom), and the rest of the vial was filled with bottom water. Fe(II)SO4 solution was added into the vials in a final concentration of 0 µM, 100 µM, 500 µM, 1000 µM (n = 10 for the 0 µM Fe2+ addition and n = 6 for other concentrations). The addition of Fe(II)SO4 was checked to reduce the pH by less than 0.5 units. K15NO3 was added to a final concentration of 100 µM in all samples. After all sample vials were closed, they were removed from the glove bag and placed on a shaker (120 rpm) in 5°C in dark climate-controlled chamber. After approximately 24 h, a 4 ml He headspace was created and 100 µl of ZnCl was injected in five (0 µM Fe2+) or three (100–1000 µM Fe2+ additions) samples from each treatment. Then the remaining three samples were uncapped and filtered through 0.8/0.2 µm double filter syringe (Sarsted AG & Co. KG, Nümbrecht, Germany) and frozen for DIN (NO2−, NO3−, NH4+) and 15NH4+ concentration analysis. The NO2− and NO3− concentrations were analyzed according to Miranda et al. (2001) and the NH4+ concentration according Fawcett & Scott (1960). Both analyzes were optimized for 1 ml sample volumes. The 15N2, 15N2O, and 15NH4+ were analyzed as per Jäntti et al. (2021; described in detail in Supplement 1).
Analysis of microbial community, and genetic potential for reduction of nitrogen oxides and oxidation of iron
DNA extraction
DNA was extracted from the frozen sediment samples using DNeasy PowerSoil Kit (Qiagen, Hilden, Germany). DNA concentration was measured using a Qubit 2.0 Fluorometer and a dsDNA HS Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA).
16S rRNA gene amplicon sequencing
PCR and 16S rRNA gene amplicon sequencing was performed by The Foundation for the Promotion of Health and Biomedical Research of Valencia Region (FISABIO, Valencia, Spain). In the PCR reactions, the V4 region of the bacterial and archaeal 16S rRNA genes were simultaneously targeted using primer pair 515FB (5′-GTGYCAGCMGCCGCGGTAA-3′)/806FB (5′-GGACTACNVGGGTWTCTAAT-3′) (Apprill et al., 2015; Parada et al., 2016). PCR, library preparation and sequencing (Illumina MiSeq, Illumina, San Diego, CA, USA) was performed as previously described (Myllykangas et al., 2020), except that, in PCR reactions, approximately 15 ng of DNA were used.
Shotgun metagenomic sequencing
The shotgun metagenomic sequencing analysis linked to metagenomic binning (i.e., construction of metagenome-assembled genomes) enables detection of microbes potentially coupling iron oxidation with NO3− reduction (i.e., those that possess genes for both iron oxidation and NO3− reduction). The shotgun metagenomic sequencing was done for the depth layers representing the incubation layers by Novogene Co.,Ltd (Hong Kong). The libraries were prepared from ~ 100 ng of DNA per sample using the NEBNext® Ultra™ DNA Library Prep Kit for Illumina® (New England Biolabs, Ipswich, MA, USA) according to the manufacturer´s protocol. The sequencing was done on Illumina HiSeq X ten (Illumina, San Diego, CA, USA) with paired-end mode and 150 bp read length producing from ~ 9 to ~ 12 Gb of raw data per sample.
Bioinformatic analysis of the 16S rRNA gene data
The quality assessment of the raw sequence reads, merging of paired-end reads, alignment, chimera removal, preclustering, taxonomic classification (and removal of chloroplast, mitochondria, and eukaryote sequences), and division of 16S rRNA gene sequences into operational taxonomic units (OTUs) at 97% similarity level was conducted as previously described (Rissanen et al., 2020). Singleton OTUs (OTUs with only 1 sequence) were removed, and the data were then normalized by subsampling to the same size, 77,340 sequences. One sample, representing the layer 8–9 cm depth at Station 3, was discarded from the analyses since it had only ∼10 000 sequences.
Bioinformatic analysis of shotgun metagenomic data
The raw data were trimmed using Trimmomatic (version 0.39) (Bolger et al., 2014). The trimmed data were assembled using Megahit (version 1.2.8) (Li et al., 2015). The quality-controlled reads were mapped to the assembly using BBmap (version 38.73) (https://sourceforge.net/projects/bbmap/). The mapping results were used to bin the contigs using Metabat2 (Kang et al., 2015, 2019). Genes of the obtained metagenomic bins were predicted and annotated using Prokka (version 1.14.5) (Seemann, 2014). Genes coding for iron cycling/usage enzymes were specifically predicted and annotated using FeGenie (Garber et al., 2020). Bins were also taxonomically annotated by CAT/BAT—tool using NCBI´s nr—database and taxonomy (von Meijenfeldt et al., 2019). Prokaryotic completeness and redundancy as well as the relative abundance of the bins, i.e., percent of community (estimate the proportion of a bin relative to the number of reads mapped to assembled contigs and adjusted for the size of the bin), were computed using CheckM (version v1.1.2) (Parks et al., 2015). However, as this metric of the relative abundance of bins only considers the reads that were mapped to contigs, which was 33–45% of reads, the relative abundance of bins was also calculated as percent of all reads. All the medium (completeness > 50% and contamination < 10%) and high quality (completeness > 90% and contamination < 5%) metagenomic bins, i.e., metagenome-assembled genomes (MAGs), were chosen for further analyses. Prokka annotations were specifically screened for MAGs that had genes coding for DNRA, i.e., nitrite reduction to ammonium (nrfA gene) and denitrification, i.e., nitrate reduction (narG and napA), nitrite reduction (nirS, nirK and aniA), nitric oxide reduction (norB), and nitrous oxide reduction (nosZ). In addition, Prokka and FeGenie annotations were used in combination to search for MAGs having genetic potential for both iron oxidation (cyc2 and MtoA genes) and denitrification or DNRA. NosZ genes were also specifically classified into clade I and clade II using blastP and a custom database consisting of deduced amino acid sequences of nosZ genes from a previously published phylogenetic tree (Altschul et al., 1990, 1997; Calderoli et al., 2018).
Sequence database accession
The sequences of the 16S rRNA gene amplicon dataset is deposited in NCBI’s Sequence Read Archive (SRA) under the accession number PRJNA627335. The sequences of the shotgun metagenomic dataset are deposited in European Nucleotide Archive (ENA) under the accession number PRJEB29513.
Statistical analyses
The differences between the NO3− reducing rates were analyzed by using Kruskal–Wallis test and determined significant at P < 0.05. The stimulating effect of Fe2+ on the NO3− reducing rates were analyzed by using linear regression and determined significant at P < 0.05. The differences between slopes were analyzed by using ANOVA and the residuals were checked for variance heterogeneity.
The multivariate analyses of the microbial community data (i.e., 16S rRNA gene—OTU data) were based on Bray–Curtis dissimilarities calculated among samples from the normalized data (see above). The differences between stations and between depths in the microbial communities were analyzed using two-way analysis of similarities (2-way ANOSIM) without replication and visualized using non-metric multidimensional scaling (NMDS). Furthermore, similarity percentage (SIMPER) analysis was carried out to assess which OTUs were responsible for the observed differences between stations. The multivariate analyses were conducted in PAST (version 3) (Hammer et al., 2001).
Results
Hypolimnion characteristics
The temperature in the hypolimnion decreased from 9.8°C at St 1 to 5.4°C at St3 and the hypolimnion was well oxidized throughout the water column at all stations (Table 1). The NO3− concentrations in the hypolimnion were relatively high (45.5–62.0-µmol l−1) and the NH4+ and NO2− concentrations were close to or below detection limit (0.01 µmol l−1) at all stations (Table 1).
Sediment characteristics
LOI% varied between 13.4% and 14.6% at the sediment surface. Surface-sediment C:N ratio was 12.8 at St1 and increased with water depth to 13.4 at St2 and 14.7 at St3 (Table 2). However, the C:N ratio was equal (12.7) at St 1 and St2 at the depths where the slurry samples were collected (Table 3). The estimated percentage of phytoplankton C in total organic carbon (TOC), indicating recently deposited organic matter, followed the expected gradient at the sediment surface by having the higher values at shallowest station (30.1) and lowest values in the deepest station (20.2) (Table 2). Acetate concentrations were higher at St1 (15.6 µmol l−1 surface, 12.0 µmol l−1 slurry collection depth) and St3 (11.2 µmol l−1 surface and 10.3 µmol l−1 slurry collection depth) than at St 2 (below detection limit at surface, 4.0 µmol l−1 slurry collection depth) (Tables 2 and 3). Lactate was present at concentrations above detection limit only at St1 both at the sediment surface (5.3 µmol l−1) and slurry collection depth (7.2 µmol l−1) (Table 3).
Incubation results
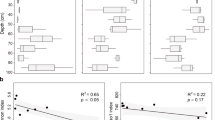
The total denitrification rate (N2 + N2O) was the highest at St 3 (1.038 µmol 15N l−1 day−1; SD 0.225) and the lowest at St2 (0.371 µmol 15N l−1 day−1; SD 0.052). Overall, N2O production dominated denitrification at all stations by constituting 48–95% of the total NO3− reduction rates and considerable N2 production was measured only at samples from St1 (0.253 µmol 15N l−1 day−1; SD 0.005) where it was approximately half of the total denitrification rate (0.570 µmol 15N l−1 day−1; SD 0.078). The N2O production at St3 (1.037 µmol 15N l−1 day−1; SD 0.225) was over 50% higher compared to St 1 and St2. DNRA rates were highest at St3 (0.019 µmol 15N l−1 day−1; SD 0.002) but overall minor compared to the other NO3− reducing processes. The highest relative DNRA rate was measured in samples from St2 (0.016 µmol 15N l−1 day−1; SD 0.003), where proportion of the DNRA from the total NO3− reduction rates was 4.1% (Fig. 1).
The Fe2+ additions increased linearly and significantly all NO3− reduction rates at St1 and the slope of 15N production per Fe2+ (15N h−1 (Fe2+)−1) was significantly higher (P < 0001) for 15N2O (1.8 × 10–4; SE 3.81 × 10–5) when compared to the slopes for 15N2 (1.58 × 10–6; SE 687 × 10–6) and 15NH4+ (7.23 × 10–6; SE 7.88 × 10–7). At St2, only 15NH4+ production (9.73 × 10–6; SE 1.59 × 10–6) was stimulated by Fe2+ additions. At St3 both 15N2 (6.36 × 10–6; SE 2.51 × 10–6) and 15NH4+ (2.19 × 10–5; SE 2.18 × 10–6) production rates were stimulated by Fe2+ and the slope of 15N production per µmol of Fe2+ for 15NH4+ was significantly higher (P < 0001) than the slope for 15N2. The only process that was consistently stimulated by Fe2+ additions at all stations was 15NH4+ production and the slope of the stimulation by the Fe2+ was significantly higher (P < 0001) at St3 than at St1 and St2. There was no significant difference (P > 0.05) in the slope of the stimulation by the Fe2+ in the 15N2 production between St1 and St3 (Fig. 2, Table 4).
Results of the 16S rRNA gene analysis
The microbial community changed with sediment depth (see NMDS figure in Supplement 2) (2-way ANOSIM; sediment depth R = 0.95, P < 0.001). The variation in the microbial communities between stations was lower but still significant and followed the water depth gradient from the shallow station 1 via station 2 to deep station 3 (NMDS figure in Supplement 2) (2-way ANOSIM; station R = 0.39, P < 0.05). Station 1 differed more from stations 2 and 3 than stations 2 and 3 differed from each other (SIMPER analysis: Overall average dissimilarity St 1 vs St 2 = 45.3%, St 1 vs St 3 = 46.9% and St 2 vs St 3 = 44.6%, Supplement 3). The contribution of single OTUs to the differences between stations remained low (< 1.7%). Especially OTUs that were affiliated with nitrifying archaea and bacteria (e.g., OTU 1: Nitrospira; OTU 2: Candidatus Nitrosoarchaeum, and OTU 5: Nitrosomonadaceae) had high relative abundance and contributed to the differences between the stations (Supplement 3).
Results of the shotgun metagenomic sequencing
Of the total of 337 metagenomic bins, 78 were of sufficient quality (i.e., completeness > 50% and contamination < 10%) and analyzed further for genes encoding NOx− reduction and Fe2+ oxidation. None of the MAGs that had genes coding for both NO3− reduction and Fe2+ oxidation possessed nrfA gene that encodes DNRA. MAGs containing genes coding for NO3− reduction and Fe2+ oxidation fell mainly to the bacterial FCB group and Proteobacteria. Particularly, NapA and nosZ together with cyc2 were common (Supplement 4). There were three MAGs belonging to unclassified bacterial groups of which two were in the candidate phylum Candidatus Eisenbacteria. There was also one MAG that was classified as Acidobacteria which had a gene encoding phototrophic Fe oxidation. MAGs which contained genes encoding NOx− reduction but not Fe2+ oxidation were also dominated by bacterial FCB group and Proteobacteria. Only two MAGs were detected that contained genes coding for Fe2+ oxidation but not for NOx− reduction.
The relative abundance of the MAGs was calculated in two ways: percent of community and percent of all reads (see “Materials and methods” section). The presence of MAGs containing the nrfA gene was low regardless of the station and calculation method and which matches the low measured 15NH4+ production rates at all stations (Fig. 3). There was no correlation between the relative abundance of the MAGs containing denitrification genes and the potential denitrification rates, or denitrification genes and N2O production rates (Fig. 3).
Average (+SD) proportion of MAGs containing the denoted genes, calculated relative to the number of reads mapped to assembled contigs and adjusted for the size of the bin (% of community) and relative to all shotgun metagenomic reads (% of all reads). Deni + Fe ox denotes for MAGs containing genes for both NOx− reduction and Fe2+ oxidation
Discussion
Quality of organic matter
Determining the quality and quantity of sediment organic matter is not standardized, and therefore often various parameters are used to characterize it. In this study, quality of organic matter is defined as an ease for microbes to degrade it. The LOI%, which is used as a robust indicator of the amount of organic matter in the sediment, had no clear differences between the sampling stations. The C:N ratio was increasing and the percent of C from phytoplankton was decreasing from St1 to St3, indicating that the degradability of organic matter was decreasing with water depth, as expected (Den Heyer & Kalff, 1998). The percent of C from phytoplankton indicates the biodegradability of the organic matter, thus the higher the percentage of C is from phytoplankton, the less it contains allochthonous C, that is recalcitrant for microbial mineralization. Organic acids (lactate + acetate) have been less frequently used as a quality parameter for organic matter. Organic acids are degradation products of organic substances but also act as a major electron donor for micro-organisms that use inorganic compounds as electron acceptors. Acetate acts as a major substrate for micro-organisms that use inorganic compounds as electron acceptors (Thamdrup, 2000). Thus, organic acids are intermediates in the carbon mineralization pathway and their concentration in the sediment reflects microbial activity and potential for microbial processes. The organic acid concentration was the highest at St1, indicating that both the carbon mineralization processes and the substrate availability for further processing was the highest.
The microbial communities in the sediment also responded to the quality of organic matter. The community composition differed between stations, although the sediment depth still caused the highest differences in the microbial community structure. At all stations, the dominant OTUs, that also contributed most to the differences between stations, contained several nitrifying taxa (Supplement 3), which reflects overall the low degradability of organic matter in this lake, thus creating favorable conditions for autotrophic microbes (Jäntti et al., 2011).
Denitrification and nitrous oxide emissions from boreal lakes
Generally, the highest denitrification rates are found in sediments rich in organic carbon and NO3− (Knowles, 1982) and therefore we expected that the highest denitrification rates would be found at St1, which had the lowest C:N ratio and highest amount of bioavailable C (highest percent of C from phytoplankton and organic acid concentrations). Indeed, the highest N2 production rates were measured from St1, but the highest total denitrification rates (N2 + N2O) were measured at St3, where the measured sediment characteristic (C:N ratio, percent of phytoplankton of C, organic acid concentrations) indicated a lower degradability of organic matter. However, denitrification at St3 was incomplete as over 90% of the end product was N2O. The lowest total denitrification rates were measured at St2, where the share of bioavailable C (i.e., the organic acid concentration) in the porewater was the lowest.
Overall, the N2O/N2 + N2O ratio in the slurry samples was high (48–95%), as typically N2O production is less than half of the N2 production (Seitzinger, 1988; Beaulieu et al., 2011; Myrstener et al., 2016). The dominance of N2O over N2 production at the sampled stations can be partially explained by the NO3− amendment to the slurries, because NO3− is preferred over N2O as an electron acceptor (Blackmer & Bremner, 1976; Richardson et al., 2009) and N2O/N2 + N2O ratio has been shown to increase in high NO3− concentrations (Oremland et al., 1984; Silvennoinen et al., 2008). Also, NO3− reduction to N2O proceeds faster than the N2O reduction to N2, especially when the quality of organic matter is low (Garcia-Ruiz et al., 1998a; b), suggesting that high N2O emissions are more likely to occur when the share of bioavailable carbon is low. Indeed, Aalto et al. (2018) observed previously that in boreal lake sediments in Central Finland, even poor-quality organic matter supported truncated denitrification and only the last step to N2 was inhibited.
The results of this experiment could explain the recently reported high N2O concentrations in boreal lakes during winter (Kortelainen et al., 2020). Since the N2O production does not respond to changes in temperature like N2 production (Saunders & Kalff, 2001; Silvennoinen et al., 2008) and even poor-quality organic matter can support truncated denitrification, the conditions for N2O production remain suitable even during winter months when availability of biodegradable carbon originating from phytoplankton is low. Also, it appears that profundal sediments could be important sites for N2O production unlike observed by Huttunen et al. (2003) who measured the highest N2O production rates in littoral sediments.
The 15NO3− reduction rates did not correlate with the relative abundance of organisms capable of denitrification, which is common (Dandie et al., 2008; Cuhel et al., 2010), because the presence of a denitrification genes does not always necessarily indicate NO3− respiration (Pandey et al., 2020). Nevertheless, according to the MAG data the microbes harboring the nosZ gene, of which low abundance has been linked to N2O emissions (Saarenheimo et al., 2015; Xu et al., 2020), consisted of the lowest percentage of the community at St3 where the N2O production was the highest. Interestingly, nosZ was dominated by ‘atypical’ clade II gene variants, which have been associated to organisms lacking enzymes for complete denitrification (Sanford et al., 2012). The high N2O emissions linked to dominance of nosZ clade II variant suggests that for the nosZ clade II variant to efficiently reduce the N2O to N2, easily degradable organic matter is required.
Dissimilatory nitrate reduction to ammonium (DNRA)
Overall DNRA rates are poorly quantified in boreal lake sediments. Of the few DNRA rates that are available, the results show that in general the DNRA rates are low compared to denitrification rates (Aalto et al., 2018). This is partially because the conditions for heterotrophic DNRA in typical boreal lakes are not optimal due to high NO3− to labile organic carbon ratio, which is known to favor denitrification over DNRA (Kelso et al., 1997; Kraft et al., 2014). Chemolithotrophic DNRA has also been thought to be negligible in freshwater ecosystem because of low availability of sulfides (Fenchel & Blackburn, 1979), which presence has been shown to favor DNRA over denitrification (Brunet & Garcia-Gil, 1996; Christensen et al., 2000). However, the recent discovery of Fe2+ driven DNRA in lake sediments (Robertson & Thamdrup, 2017; Cojean et al., 2020) suggests that DNRA could be an important process especially because transport of Fe to boreal lakes has been increasing during the past decades (Weyhenmeyer et al., 2014). Despite the increasing evidence of the linkage between N and Fe cycles, we found negligible DNRA rates compared to denitrification in this NO3− and Fe rich lake, even under elevated Fe2+ and NO3− availability in the sediment layers, where conditions should have been otherwise favorable for Fe2+ driven DNRA. Despite the overall low rates, the relative DNRA rates were the highest at St2, where the amount of bioavailable C was lowest, supporting to some degree our hypothesis that proportional DNRA rates increase, when the degradability of organic matter decreases. The low DNRA rates are further supported by the metagenomic results, showing that potential DNRA organisms were rare, as the MAGs of potential denitrifying NO2− reducers (i.e., having nirS, nirK, and aniA genes) significantly outnumbered them at all sites. Also, we could not detect any medium to good quality MAG that contained genetic potential for both DNRA (i.e., nrfA gene) and iron oxidation, which is a further indication of low potential for microbial Fe driven DNRA.
Effect of ferrous iron on nitrate reduction rates
Fe2+ can affect NO3− reduction rates through direct and indirect chemical reactions, microbially mediated processes, or by combination of all above. There are several thermodynamically favorable chemical reactions between N-species and Fe2+ (Picardal, 2012) of which one of the best documented is the reaction between Fe2+ and NO2−, resulting in N2O formation (Moraghan & Buresh, 1977). Nevertheless, the step from NO3 reduction to NO2− still must be mediated by microbes because the chemical reduction of NO3− by Fe2+ proceeds slowly without a suitable catalyst, such as Cu2+ (Buresh & Moraghan, 1976). Hence, the stimulation of 15N2O and 15N2 production rates by Fe2+ at St1 and 15N2 production at St3 are likely to be a result of combination of microbial and chemical reactions. Another, well documented chemical reaction between NO3− and Fe, is the formation NH4+ when green rusts act as a reactant (Hansen et al., 1994, 1996). The green rusts can be found in sediment, are highly reactive and consist of tri- octahedral Fe(II)–Fe(III)hydroxide layers separated by hydrated anionic interlayers. Although this can be competitive pathway for microbial DNRA (Picardal, 2012), green rusts were not visually detected in any of the slurry samples. Nevertheless, the additions of Fe2+ stimulated formation of 15NH4+ and the possibility of chemistry driven DNRA cannot be excluded, especially because of microbes that posses’ genes for both DNRA and Fe2+ oxidation were not detected.
Although, the microbial reactions between Fe2+ and NO3− have been known known to exist for more than two decades (Straub et al., 1996), the role of microbial Fe2+ oxidation with NO3− reduction in natural sediments has remained unclear. In an experiment with microbial mass from Lake Lugano sediments, N2 production was stimulated at low Fe2+ enrichment levels (Fe2+ ≤ 258 μM) but almost completely suppressed at higher levels (≥ 1300 μM) (Cojean et al., 2020). Because of the difference in experimental set ups, the results are not directly comparable, but stimulation of denitrification by Fe2+ at St1 and St3 appears to be in line with the results from Lake Lugano.
The role of organic carbon in Fe2+ driven NO3− reduction is not clear, because although purely chemolithoautotrophic, Fe2+ driven NO3− reduction has been shown to exist (Laufer et al., 2016), mixotrophic (i.e., organic carbon is required for growth of NO3− reducing Fe2+ oxidizing microbes) metabolism has been found to be common in freshwater sediments (Benz et al., 1998; Straub & Buchholz-Cleven, 1998). Based on the results from this experiment, we propose that Fe2+ oxidizing, NO3− reducing processes leading to denitrification are linked to the presence of fermentable carbon sources, such as lactate, because at St1, where lactate was present, all denitrifying processes were stimulated by Fe2+. DNRA in the other hand was stimulated by Fe2+ at all stations, regardless of the quality of organic matter, suggesting that Fe2+ driven DNRA is either mediated by chemolithotrophic microbes or is a purely chemical process.
Many Fe oxidizing, NO3− reducing bacteria have been intensively studied in cultures (i.e., Straub et al., 2004; Kumaraswamy et al., 2006; Weber et al., 2006) and it is known that the anaerobic NO3− reducing Fe oxidizers are not found exclusively in one class of the Proteobacteria, unlike most Fe oxidizing bacteria (Hedrich et al., 2011). However, their taxonomy in natural environments, especially in the boreal regions, is not yet well described. Our metagenomic analyses shed light to the taxonomy of Fe oxidizing, NO3− reducing microbes in boreal lake sediments by revealing several unidentified bacterial clades, for example in the FCB group and phylum Candidatus Eisenbacteria, which need further investigations on their ecological meaning. Candidatus Eisenbacteria was first detected in an aquifer adjacent to the Colorado River (USA) (Anantharaman et al., 2016) thus it is not unique to boreal region. It is noteworthy that nearly half of the MAGs which had genes for denitrification, also had genes for Fe oxidation, hence the genetic potential for Fe oxidation appears to be quite common among denitrifying bacteria. Genetic potential for Fe2+ driven DNRA, in the other hand, appears to be not common, mainly because of no microbes harboring genes for both DNRA and Fe2+ oxidation was detected. Also, the linear response of DNRA rates to Fe2+ additions regardless of the other environmental parameters is pointing toward chemistry driven Fe oxidation coupled to DNRA.
Conclusion
The relative proportion of N2O production compared to N2 production was one of the highest measured in lake sediments. The relative importance of N2 production decreased if easily degradable carbon sources were not present. These findings suggest that truncated denitrification might be an important sink for NO3− and lakes might be more important source of N2O than previously assumed, particularly during winter. The leakage of N2O appeared to be also linked to dominance of nosZ clade II variant, which is known to be harbored by non-traditional denitrifying microbes that do not perform the other steps of the denitrification pathway. Our results suggest that the denitrifying microbes utilizing Fe2+ as an electron donor are mixotrophic, whereas the Fe2+ driven DNRA is independent of the availability of organic substrates. This is either because the Fe2+ oxidizing, NO3− reducing DNRA microbes are chemolithotrophs, or because the reaction is purely driven by chemistry with no microbes involved. The taxonomic analyses revealed that the microbial communities vary along the depth gradient and quality of organic matter and that there are several previously unidentified bacterial groups capable of Fe oxidation and NO3− reduction.
Data availability
The sequences of the 16S rRNA gene amplicon dataset is deposited in NCBI’s Sequence Read Archive (SRA) under the accession number PRJNA627335. The sequences of the shotgun metagenomic dataset are deposited in European Nucleotide Archive (ENA) under the accession number PRJEB29513. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aalto, S. L., J. Saarenheimo, J. Ropponen, J. Juntunen, A. J. Rissanen & M. Tiirola, 2018. Sediment diffusion method improves wastewater nitrogen removal in the receiving lake sediments. Water Research 138: 312–322.
Aalto, S. L., E. Asmala, T. Jilbert & S. Hietanen, 2021. Autochthonous organic matter promotes DNRA and suppresses N2O production in sediments of the coastal Baltic Sea. Estuarine, Coastal and Shelf Science 255: 107369.
Altschul, S. F., W. Gish, W. Miller, E. W. Myers & D. J. Lipman, 1990. Basic local alignment search tool. Journal of Molecular Biology 215(3): 403–410.
Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller & D. J. Lipman, 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research 25(17): 3389–3402.
Anantharaman, K., C. T. Brown, L. A. Hug, I. Sharon, C. J. Castelle, A. J. Probst, et al., 2016. Thousands of microbial genomes shed light on interconnected biogeochemical processes in an aquifer system. Nature Communications 7(1): 1–11.
Apprill, A., S. McNally, R. Parsons & L. Weber, 2015. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquatic Microbial Ecology 75: 129–137.
Beaulieu, J. J., J. L. Tank, S. K. Hamilton, W. M. Wollheim, R. O. Hall, P. J. Mulholland, et al., 2011. Nitrous oxide emission from denitrification in stream and river networks. Proceedings of the National Academy of Sciences of the United States of America 108(1): 214–219.
Benz, M., A. Brune & B. Schink, 1998. Anaerobic and aerobic oxidation of ferrous iron at neutral pH by chemoheterotrophic nitrate-reducing bacteria. Archives of Microbiology 169(2): 159–165.
Blackmer, A. M. & J. M. Bremner, 1976. Potential of soil as a sink for atmospheric nitrous oxide. Geophysical Research Letters 3(12): 739–742.
Bolger, A. M., M. Lohse & B. Usadel, 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30(15): 2114–2120.
Brunet, R. C. & L. J. Garcia-Gil, 1996. Sulfide-induced dissimilatory nitrate reduction to ammonia in anaerobic freshwater sediments. FEMS Microbiology Ecology 21(2): 131–138.
Buresh, R. J. & J. T. Moraghan, 1976. Chemical Reduction of Nitrate by Ferrous Iron, Vol. 5, No. 3. American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America: 320–325.
Calderoli, P. A., F. J. Espínola, H. M. Dionisi, M. N. Gil, J. K. Jansson & M. Lozada, 2018. Predominance and high diversity of genes associated to denitrification in metagenomes of subantarctic coastal sediments exposed to urban pollution. PLoS ONE 13(11): e0207606.
Christensen, P. B., S. Rysgaard, N. P. Sloth, T. Dalsgaard & S. Schwærter, 2000. Sediment mineralization, nutrient fluxes, denitrification and dissimilatory nitrate reduction to ammonium in an estuarine fjord with sea cage trout farms. Aquatic Microbial Ecology 21(1): 73–84.
Cojean, A. N., M. F. Lehmann, E. K. Robertson, B. Thamdrup & J. Zopfi, 2020. Controls of H2S, Fe2+, and Mn2+ on Microbial NO3--reducing processes in sediments of an Eutrophic Lake. Frontiers in Microbiology 11: 1158.
Cuhel, J., M. Šimek, R. J. Laughlin, D. Bru, D. Chèneby, C. J. Watson & L. Philippot, 2010. Insights into the effect of soil pH on N2O and N2 emissions and denitrifier community size and activity. Applied and Environmental Microbiology 76(6): 1870–1878.
Dandie, C. E., D. L. Burton, B. J. Zebarth, S. L. Henderson, J. T. Trevors & C. Goyer, 2008. Changes in bacterial denitrifier community abundance over time in an agricultural field and their relationship with denitrification activity. Applied and Environmental Microbiology 74(19): 5997–6005.
den Heyer, C. & J. Kalff, 1998. Organic matter mineralization rates in sediments: a within- and among-lake study. Limnology and Oceanography 43(4): 695–705.
Einsle, O., A. Messerschmidt, P. Stach, G. P. Bourenkov, H. D. Bartunik, R. Huber & P. M. Kroneck, 1999. Structure of cytochrome c nitrite reductase. Nature 400(6743): 476–480.
Fawcett, J. K. & J. Scott, 1960. A rapid and precise method for the determination of urea. Journal of Clinical Pathology 13(2): 156–159.
Fenchel, T. & T. H. Blackburn, 1979. Bacteria and Mineral Cycling, Academic Press, London:
Galloway, J. N. & E. B. Cowling, 2021. Reflections on 200 years of Nitrogen, 20 years later. Ambio 50(4): 745–749.
Garber, A. I., K. H. Nealson, A. Okamoto, S. M. McAllister, C. S. Chan, R. A. Barco & N. Merino, 2020. FeGenie: a comprehensive tool for the identification of iron genes and iron gene neighborhoods in genome and metagenome assemblies. Frontiers in Microbiology 11: 37.
Garcia-Ruiz, R., S. N. Pattinson & B. A. Whitton, 1998a. Denitrification in river sediments: relationship between process rate and properties of water and sediment. Freshwater Biology 39(3): 467–476.
García-Ruiz, R., S. N. Pattinson & B. A. Whitton, 1998b. Kinetic parameters of denitrification in a river continuum. Applied and Environmental Microbiology 64(7): 2533–2538.
Giblin, A. E., C. R. Tobias, B. Song, N. Weston, G. T. Banta & V. H. Riviera-Monroy, 2013. The importance of dissimilatory nitrate reduction to ammonium (DNRA) in the nitrogen cycle of coastal ecosystems. Oceanography 26(3): 124–131.
Goñi, M. A., M. J. Teixeira & D. W. Perkey, 2003. Sources and distribution of organic matter in a river-dominated estuary (Winyah Bay, SC, USA). Estuarine, Coastal and Shelf Science 57(5–6): 1023–1048.
Hammer, O., D. A. Harper & P. Ryan, 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4: 1–9.
Hansen, H. C. B., O. K. Borggaard & J. Sørensen, 1994. Evaluation of the free energy of formation of Fe (II)-Fe (III) hydroxide-sulphate (green rust) and its reduction of nitrite. Geochimica Et Cosmochimica Acta 58(12): 2599–2608.
Hansen, H. C. B., C. B. Koch, H. Nancke-Krogh, O. K. Borggaard & J. Sørensen, 1996. Abiotic nitrate reduction to ammonium: key role of green rust. Environmental Science & Technology 30(6): 2053–2056.
Hedrich, S., M. Schlömann & D. B. Johnson, 2011. The iron-oxidizing proteobacteria. Microbiology 157(6): 1551–1564.
Huttunen, J. T., S. Juutinen, J. Alm, T. Larmola, T. Hammar, J. Silvola & P. J. Martikainen, 2003. Nitrous oxide flux to the atmosphere from the littoral zone of a boreal lake. Journal of Geophysical Research Atmospheres. https://doi.org/10.1029/2002JD002989.
Jäntti, H., F. Stange, E. Leskinen & S. Hietanen, 2011. Seasonal variation in nitrification and nitrate-reduction pathways in coastal sediments in the Gulf of Finland, Baltic Sea. Aquatic Microbial Ecology 63(2): 171–181.
Jäntti, H., S. L. Aalto & H. W. Paerl, 2021. Effects of ferrous iron and hydrogen sulfide on nitrate reduction in the sediments of an estuary experiencing hypoxia. Estuaries and Coasts 44(1): 1–12.
Jilbert, T., E. Asmala, C. Schröder, R. Tiihonen, J. P. Myllykangas, J. J. Virtasalo, et al., 2018. Impacts of flocculation on the distribution and diagenesis of iron in boreal estuarine sediments. Biogeosciences 15(4): 1243–1271.
Jilbert, T., S. Jokinen, T. Saarinen, U. Mattus-Kumpunen, A. Simojoki, S. Saarni, et al., 2020. Impacts of a deep reactive layer on sedimentary phosphorus dynamics in a boreal lake recovering from eutrophication. Hydrobiologia 847: 4401–4423.
Kang, D. D., J. Froula, R. Egan & Z. Wang, 2015. MetaBAT, an efficient tool for accurately reconstructing single genomes from complex microbial communities. PeerJ 3: e1165.
Kang, D. D., F. Li, E. Kirton, A. Thomas, R. Egan, H. An & Z. Wang, 2019. MetaBAT 2: an adaptive binning algorithm for robust and efficient genome reconstruction from metagenome assemblies. PeerJ 7: e7359.
Kessler, A. J., M. Wawryk, U. Marzocchi, K. L. Roberts, W. W. Wong, N. Risgaard-Petersen, et al., 2019. Cable bacteria promote DNRA through iron sulfide dissolution. Limnology and Oceanography 64(3): 1228–1238.
Kelso, B., R. V. Smith, R. J. Laughlin & S. D. Lennox, 1997. Dissimilatory nitrate reduction in anaerobic sediments leading to river nitrite accumulation. Applied and Environmental Microbiology 63(12): 4679–4685.
Klueglein, N. & A. Kappler, 2013. Abiotic oxidation of Fe (II) by reactive nitrogen species in cultures of the nitrate‐reducing Fe (II) oxidizer Acidovorax sp. BoFeN1-questioning the existence of enzymatic Fe (II) oxidation. Geobiology 11(2): 180–190.
Knowles, R., 1982. Denitrification. Microbiological Reviews 46(1): 43.
Kortelainen, P., 1993. Content of organic carbon in Finnish lakes and its relationship to catchment characteristics. Canadian Journal of Fisheries and Aquatic Sciences 50: 1477–1483.
Kortelainen, P. & S. Saukkonen, 1995. Organic vs. minerogenic acidity in headwater streams in Finland. Water, Air, & Soil Pollution 85: 559–564.
Kortelainen, P., H. Pajunen, M. Rantakari & M. Saarnisto, 2004. A large carbon pool and small sink in boreal Holocene lake sediments. Global Change Biology 10: 1648–1653.
Kortelainen, P., T. Larmola, M. Rantakari, S. Juutinen, J. Alm & P. J. Martikainen, 2020. Lakes as nitrous oxide sources in the boreal landscape. Global Change Biology 26(3): 1432–1445.
Kraft, B., H. E. Tegetmeyer, R. Sharma, M. G. Klotz, T. G. Ferdelman, R. L. Hettich, et al., 2014. The environmental controls that govern the end product of bacterial nitrate respiration. Science 345(6197): 676–679.
Kumaraswamy, R., K. Sjollema, G. Kuenen, M. van Loosdrecht & G. Muyzer, 2006. Nitrate-dependent [Fe(II)EDTA]2− oxidation by Paracoccus ferrooxidans sp. nov., isolated from a denitrifying bioreactor. Systematic and Applied Microbiology 29: 276–286.
Lauerwald, R., P. Regnier, V. Figueiredo, A. Enrich-Prast, D. Bastviken, B. Lehner, et al., 2019. Natural lakes are a minor global source of N2O to the atmosphere. Global Biogeochemical Cycles 33(12): 1564–1581.
Laufer, K., H. Røy, B. B. Jørgensen & A. Kappler, 2016. Evidence for the existence of autotrophic nitrate-reducing Fe (II)-oxidizing bacteria in marine coastal sediment. Applied and Environmental Microbiology 82(20): 6120–6131.
Li, D., C. M. Liu, R. Luo, K. Sadakane & T. W. Lam, 2015. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31(10): 1674–1676.
Miranda, K. M., M. G. Espey & D. A. Wink, 2001. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5(1): 62–71.
Moraghan, J. T. & R. J. Buresh, 1977. Chemical reduction of nitrite and nitrous oxide by ferrous iron. Soil Science Society of America Journal 41(1): 47–50.
Myllykangas, J.-P., A. J. Rissanen, S. Hietanen & T. Jilbert, 2020. Influence of electron acceptor availability and microbial community structure on sedimentary methane oxidation in a boreal estuary. Biogeochemistry 148: 291–309.
Myrstener, M., A. Jonsson & A. K. Bergström, 2016. The effects of temperature and resource availability on denitrification and relative N2O production in boreal lake sediments. Journal of Environmental Sciences 47: 82–90.
Oremland, R. S., C. Umberger, C. W. Culbertson & R. L. Smith, 1984. Denitrification in San Francisco Bay intertidal sediments. Applied and Environmental Microbiology 47(5): 1106–1112.
Pajunen, H., 2000. Carbon in Finnish Lake Sediments, Geological Survey of Finland, Espoo
Pandey, C. B., U. Kumar, M. Kaviraj, K. J. Minick, A. K. Mishra & J. S. Singh, 2020. DNRA: A short-circuit in biological N-cycling to conserve nitrogen in terrestrial ecosystems. Science of the Total Environment 738: 139710.
Parada, A. E., D. M. Needham & J. A. Fuhrman, 2016. Every base matters: assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environmental Microbiology 18: 1403–1414.
Parks, D. H., M. Imelfort, C. T. Skennerton, P. Hugenholtz & G. W. Tyson, 2015. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Research 25(7): 1043–1055.
Picardal, F., 2012. Abiotic and microbial interactions during anaerobic transformations of Fe (II) and NOx. Frontiers in Microbiology 3: 112.
Richardson, D., H. Felgate, N. Watmough, A. Thomson & E. Baggs, 2009. Mitigating release of the potent greenhouse gas N2O from the nitrogen cycle—could enzymic regulation hold the key? Trends in Biotechnology 27: 388–397.
Rissanen, A. J., M. Tiirola & A. Ojala, 2011. Spatial and temporal variation in denitrification and in the denitrifier community in a boreal lake. Aquatic Microbial Ecology 64(1): 27–40.
Rissanen, A. J., M. Tiirola, S. Hietanen & A. Ojala, 2013. Interlake variation and environmental controls of denitrification across different geographical scales. Aquatic Microbial Ecology 69(1): 1–16.
Rissanen, A. J., T. Saarela, H. Jäntti, M. Buck, S. Peura, S. L. Aalto, et al., 2020. Vertical stratification patterns of methanotrophs and their genetic controllers in water columns of oxygen-stratified boreal lakes. FEMS Microbiology Ecology 97(2): fiaa252.
Roberts, K. L., A. J. Kessler, M. R. Grace & P. L. Cook, 2014. Increased rates of dissimilatory nitrate reduction to ammonium (DNRA) under oxic conditions in a periodically hypoxic estuary. Geochimica Et Cosmochimica Acta 133: 313–324.
Robertson, E. K. & B. Thamdrup, 2017. The fate of nitrogen is linked to iron (II) availability in a freshwater lake sediment. Geochimica Et Cosmochimica Acta 205: 84–99.
Saarenheimo, J., A. J. Rissanen, L. Arvola, H. Nykänen, M. F. Lehmann & M. Tiirola, 2015. Genetic and environmental controls on nitrous oxide accumulation in lakes. PLoS ONE 10(3): e0121201.
Salmela, M., T. Lehtinen, E. Efimova, S. Santala & R. Mangayil, 2018. Metabolic pairing of aerobic and anaerobic production in a one-pot batch cultivation. Biotechnology for Biofuels 11(1): 1–13.
Salonen, K., 1981. The ecosystem of the oligotrophic Lake Pääjärvi 2. Bacterioplankton: With 3 figures in the text. Internationale Vereinigung Für Theoretische Und Angewandte Limnologie: Verhandlungen 21(1): 448–453.
Sanford, R. A., D. D. Wagner, Q. Wu, J. C. Chee-Sanford, S. H. Thomas, C. Cruz-García, et al., 2012. Unexpected nondenitrifier nitrous oxide reductase gene diversity and abundance in soils. Proceedings of the National Academy of Sciences of United States of America 109(48): 19709–19714.
Saunders, D. L. & J. Kalff, 2001. Nitrogen retention in wetlands, lakes and rivers. Hydrobiologia 443(1–3): 205–212.
Seemann, T., 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30(14): 2068–2069.
Seitzinger, S. P., 1988. Denitrification in freshwater and coastal marine ecosystems: ecological and geochemical significance. Limnology and Oceanography 33(4 Part 2): 702–724.
Silvennoinen, H., A. Liikanen, J. Torssonen, C. F. Stange & P. J. Martikainen, 2008. Denitrification and nitrous oxide effluxes in boreal, eutrophic river sediments under increasing nitrate load: a laboratory microcosm study. Biogeochemistry 91(2–3): 105–116.
Straub, K. L., M. Benz, B. Schink & F. Widdel, 1996. Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Applied and Environmental Microbiology 62(4): 1458–1460.
Straub, K. L., W. A. Schönhuber, B. E. Buchholz-Cleven & B. Schink, 2004. Diversity of ferrous iron-oxidizing, nitrate-reducing bacteria and their involvement in oxygen-independent iron cycling. Geomicrobiology Journal 21(6): 371–378.
Straub, K. L. & B. E. Buchholz-Cleven, 1998. Enumeration and detection of anaerobic ferrous iron-oxidizing, nitrate-reducing bacteria from diverse European sediments. Applied and Environmental Microbiology 64(12): 4846–4856.
Thamdrup, B., 2000. Bacterial manganese and iron reduction in aquatic sediments. In Advances in Microbial Ecology. Springer, Boston: pp. 41–84.
Tiedje, J. M., 1988. Ecology of denitrification and dissimilatory nitrate reduction to ammonium. Biology of Anaerobic Microorganisms 717: 179–244.
Tiedje, J. M., A. J. Sexstone, T. B. Parkin & N. P. Revsbech, 1984. Anaerobic processes in soil. Plant and Soil 76(1–3): 197–212.
Tulonen, T., 2004. Role of allochthonous and autochthonous dissolved organic matter (DOM) as a carbon source for bacterioplankton in boreal humic lakes. PhD Dissertation, University of Helsinki, Helsinki.
van den Berg, E. M., U. Van Dongen, B. Abbas & M. C. Van Loosdrecht, 2015. Enrichment of DNRA bacteria in a continuous culture. The ISME Journal 9(10): 2153–2161.
van den Berg, E. M., M. P. Elisário, J. G. Kuenen, R. Kleerebezem & M. van Loosdrecht, 2017. Fermentative bacteria influence the competition between denitrifiers and DNRA bacteria. Frontiers in Microbiology 8: 1684.
von Meijenfeldt, F. B., K. Arkhipova, D. D. Cambuy, F. H. Coutinho & B. E. Dutilh, 2019. Robust taxonomic classification of uncharted microbial sequences and bins with CAT and BAT. Genome Biology 20(1): 1–14.
Weber, K. A., J. Pollock, K. A. Cole, S. M. O’Connor, L. A. Achenbach & J. D. Coates, 2006. Anaerobic nitrate-dependent iron(II) bio-oxidation by a novel lithoautotrophic betaproteobacterium, strain 2002. Applied and Environmental Microbiology 72: 686–694.
Weyhenmeyer, G. A., Y. T. Prairie & L. J. Tranvik, 2014. Browning of boreal freshwaters coupled to carbon-iron interactions along the aquatic continuum. PLoS ONE 9(2): e88104.
Wood, E. D., F. A. J. Armstrong & F. A. Richards, 1967. Determination of nitrate in sea water by cadmium-copper reduction to nitrite. Journal of the Marine Biological Association of the United Kingdom 47(1): 23–31.
Xu, X., Y. Liu, B. P. Singh, Q. Yang, Q. Zhang, H. Wang, et al., 2020. NosZ clade II rather than clade I determine in situ N2O emissions with different fertilizer types under simulated climate change and its legacy. Soil Biology and Biochemistry 150: 107974.
Yoon, S., C. Cruz-Garcia, R. Sanford, K. M. Ritalahti & F. E. Loffler, 2015. Denitrification versus respiratory ammonification: environmental controls of two competing dissimilatory NO3-/NO2-reduction pathways in Shewanella loihica strain PV-4. ISME J. 9: 1093–1104.
Acknowledgements
We thank staff at the Lammi Biological Station for their support in field and laboratory work. This study was supported by Academy of Finland (Grant No. 286642 for AJR, 323214 for RM, 307331 for HJ, and 310302 for SLA), UEF Water joint funding by Olvi Foundation, Jenny and Antti Wihuri Foundation, and Saastamoinen Foundation (for HJ) and Kone Foundation (Grant No. 201803224 for AJR), and a Tenure Track starting package from University of Helsinki (TJ).
Funding
Open access funding provided by University of Eastern Finland (UEF) including Kuopio University Hospital.
Author information
Authors and Affiliations
Contributions
All authors declare participating for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Stefano Amalfitano
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jäntti, H., Jilbert, T., Aalto, S.L. et al. The role of organic matter and microbial community controlling nitrate reduction under elevated ferrous iron concentrations in boreal lake sediments. Hydrobiologia 849, 2145–2160 (2022). https://doi.org/10.1007/s10750-022-04858-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-04858-0