Abstract
Stream ecological theory predicts that the use of allochthonous resources declines with increasing channel width, while at the same time primary production and autochthonous carbon use by consumers increase. Although these expectations have found support in several studies, it is not well known how terrestrial runoff and/or inputs of primary production from lakes alter these longitudinal patterns. To investigate this, we analyzed the diet of filter-feeding black fly and caddisfly larvae from 23 boreal streams, encompassing gradients in drainage area, land cover and land use, and distance to nearest upstream lake outlet. In five of these streams, we also sampled repeatedly during autumn to test if allochthony of filter feeders increases over time as new litter inputs are processed. Across sites, filter-feeder autochthony was 21.1–75.1%, did not differ between black fly and caddisfly larvae, was not positively related to drainage area, and did not decrease with distance from lakes. Instead, lake and wetland cover promoted filter-feeder autochthony independently of stream size, whereas catchment-scale forest cover and forestry reduced autochthony. Further, we found no seasonal increase in allochthony, indicating low assimilation of particles derived from autumn litter fall. Hence, catchment properties, rather than local conditions, can influence levels of autochthony in boreal streams.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In aquatic systems, in situ (i.e., autochthonous) primary production provides basal resources that are high in essential nutrients, and thus, important for growth and physiology of consumers (Brett et al., 2009; Gladyshev et al., 2009). However, most aquatic systems are to some extent subsidized by terrestrially derived (i.e., allochthonous) organic matter. While this material is of poorer dietary quality than aquatic primary production, in some systems allochthonous resources constitute the majority of the resource base (Vannote et al., 1980; Collins et al., 2016). For example, in small streams, primary production is often limited by shading from the riparian canopy, which, at the same time, provides energy in the form of litter inputs (Hill et al., 1995; Wallace et al., 1997, 2015). Even in lakes and large rivers, which are often autochthonous based (Carpenter et al., 1985; Thorp et al., 1998), inputs of terrestrial dissolved organic matter (DOM) can serve as an important resource (Berggren et al., 2010; Fey et al., 2015). At the same time, ongoing browning of boreal and temperate waters linked to increased DOM in response to climate change may limit incident light and thereby primary production (Carpenter et al., 1998) and overall consumer growth (Karlsson et al., 2009). Further, because terrestrially derived resources are of relatively low quality to stream consumers, these resources differ in their ability to support production at higher trophic levels (Brett et al., 2017). It is, therefore, important to understand what drives the availability and use of these different types of resources.
In streams, food-web autochthony is predicted to be lowest in the headwaters and increase downstream (Vannote et al., 1980; Collins et al., 2016), and there should, therefore, be a simultaneous increase in secondary production downstream (Finlay, 2011; Kelly et al., 2014). However, in small, heterotrophic streams, a peak in secondary production—and therefore, consumer allochthony—can occur soon after the seasonal input of riparian plant litter, which therefore, often coincides with an increased abundance of macroinvertebrate detritivores (Richardson, 1991; Wallace et al., 1999). This response reflects a cascade of biological and ecological processes that ensue in response to pulses of litter. First, leaching of soluble compounds (i.e., DOM) takes place, and this DOM can represent approximately 30–42% of the total dissolved organic carbon pool during autumn (McDowell & Fisher, 1976; Meyer et al., 1988). This DOM tends to be of higher quality than that originating from terrestrial runoff making it more available to heterotrophic microbes (Dahm, 1981; Strauss & Lamberti, 2002), and thus more efficiently incorporated into stream food webs (e.g., Hall and Meyer, 1998). Second, coarse organic matter (i.e., leaf litter) itself constitutes a pulse of resources that can be rapidly consumed by macroinvertebrate detritivores, which in turn produce particles (i.e., frass and faeces) that may support multiple species of filter feeders (Short & Maslin, 1977; Wallace et al., 1977; Jonsson & Malmqvist, 2005). These consumers are, in turn, important prey for a variety of invertebrate and vertebrate predators (Wallace & Webster, 1996). Hence, there are good reasons to believe that the overall level of allochthony in stream communities should increase during autumn (Junker & Cross, 2014), even in systems that are already allochthonous based.
However, several studies have found surprisingly high levels of autochthony (i.e., aquatic diet) in small-stream consumers, despite low incident light and high allochthonous inputs (McCutchan & Lewis, 2002; Neres-Lima et al., 2016; Stenroth et al., 2015; Brett et al., 2017). This deviation from predictions occurs presumably because stream autotrophic production—even if occurring at low levels—is a much better resource than terrestrially derived organic matter, and would therefore, be favored by stream consumers (Brett et al., 2017). Unexpectedly high autochthony may also arise from terrestrial runoff of nutrients and subsequent stimulation of aquatic primary production, if nutrients rather than light are limiting (e.g., Seekell et al., 2015). In addition, lakes embedded within river networks can also supply streams, via their outlets, with high-quality organic resources derived from autochthonous production (Valett & Stanford, 1987). Indeed, lake outlets are known to be hotspots for stream macroinvertebrate production, particularly for filter-feeding taxa (Cushing, 1963; Armitage, 1976; Malmqvist & Eriksson, 1995). Thus, there are several attributes of catchments and embedded aquatic networks that may determine the availability and use of aquatic versus terrestrial resources in streams. How these attributes interact across catchments is not well known, but it is still assumed that downstream changes in stream width and canopy cover exert strong influences on patterns of basal resource use (Vannote et al., 1980; Collins et al., 2016).
To disentangle the many factors regulating the level of autochthony versus allochthony in stream consumers, we performed a study involving deuterium isotope (δD) analysis of filter-feeding black fly and caddisfly larvae collected in 23 streams representing a gradient in stream size (i.e., drainage area; Downing et al., 2012), land cover, land use, and distance to nearest upstream lake. At five of these sites, we also sampled once per month from September through December, to evaluate whether autumn litter inputs create short-term changes in the carbon sources used by filter feeders. Filter-feeding larvae were chosen because they are passive feeders (i.e., non-selective in their diet) and therefore, good indicators of the carbon sources available within the suspended particulate pool, which may in turn be shaped by a range of catchment-wide features and modifications (e.g., related to land use, lake cover, etc.). In particular, black fly larvae may be optimal for assessing the nature of dissolved particle loads from catchments, as they are able to feed on very small, suspended particles (Wotton, 1976), potentially even DOM (Hershey et al., 1996; Cibrowski et al., 1997). Moreover, filter-feeding larvae can be highly abundant and are, therefore, of ecological importance, both as larvae and as emerged adults (Wallace & Webster, 1996; Malmqvist et al., 2004; Jonsson et al., 2013). We hypothesized that stream size (i.e., drainage area) would be the primary determinant of autochthony versus allochthony, with a positive and negative correlation, respectively, and that the level of allochthony would increase throughout autumn, following the seasonal peak in riparian plant litter inputs.
Methods
Study area
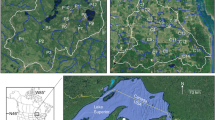
The study was performed in boreal northern Sweden (Fig. 1). This region is dominated by forest, of primarily coniferous tree species, and mires, lakes, and streams are common (Jonsson et al., 2017). In terms of land use in this region, large-scale forest management has been the dominant source of forest disturbance for over a century (Esseen et al., 1997; Laudon et al., 2011a, b), whereas agriculture is localized and rare.
In the study region, we selected sites in 23 different streams, ranging from 1st to 5th order, that each represented different catchments (Fig. 1). Stream width ranged from 1 to 10 m with bed slopes of ~ 0.1–2% exhibiting pool-riffle to plane bed morphologies; bed sediments were composed of sand, gravel, and cobbles. Thus, the sites spanned large gradients in drainage area, ranging from 50 to 40,030 ha, and in distance to nearest lake outlet, although three of the smallest catchments lacked lakes (Table 1). In terms of land cover and land use, coniferous forests dominated, wetlands were present in all catchments, and agriculture was rare, whereas forestry (i.e., logged forest) was much more common (Table 1). All sites were bordered by riparian vegetation dominated by coniferous forests (Norway spruce and/or Scots pine) but also including some deciduous species (e.g., birch and alder).
Land-use classification
To classify study sites based on land use and land cover, we used GIS to determine the percentage of different types of land use and land cover in the drainage area upstream of each sampling site. We delineated and calculated drainage areas using 50-m digital elevation models using the ArcHydro package in ArcMap 10. Using land-cover data obtained from the Swedish Land Survey that are based on CORINE Land Cover nomenclature, we analyzed a 25 × 25 m grid of 60 land-use and land-cover types, of which 39 were present in our study areas. Each land-cover type belonged to one of nine land-use or land-cover categories (Table 1), and the area representing each of these categories was recorded as a percentage of the total drainage area and was used in subsequent statistical analyses. There is a confirmed general positive relationship between drainage area and stream size (Downing et al., 2012). Hence, we used drainage area as proxy for stream size, as it is a more integrated measure of stream size than site-specific channel width. Distance from each site to nearest lake was measured on a map as the longitudinal (network) distance to the nearest upstream lake.
Sample collection
In October, we collected black fly (Diptera: Simuliidae) and/or caddisfly (Trichoptera: Hydropsyche spp.) larvae from cobbles at all 23 sites. October was chosen as the month for the main sampling, as the insect larvae were expected to be larger than earlier in the season. We recovered sufficient biomass to perform isotopic analyses from 21 sites for black fly larvae and 12 sites for caddisfly larvae. Ten sites had sufficient amounts of both black fly and caddisfly larvae.
In the study region, leaf senescence starts in early September (Lidman et al., 2017), and we, therefore, hypothesized that incorporation of terrestrially derived litter resources in filter-feeder biomass would increase throughout autumn. Hence, we selected five 2nd and 3rd order streams with similar local physical characteristics (i.e., width and channel slope), but of different types of catchment-scale land cover and land use, to sample filter-feeding black fly larvae once each month from September to December 2011. However, for one site (PEL) we could not find sufficient animal biomass in September, and for VEB we found a sufficient amount of larvae only in September and October.
We collected caddisfly and black fly larvae from cobbles manually, using forceps. Immediately after collection, all larvae (black flies and caddisflies, separately) were transferred to falcon tubes containing water from their home stream, and were left for 24 h to clear their guts, before being dried at 60°C for 48 h and weighed (350 μg) into silver capsules (3 individuals per taxon and stream).
In the five streams sampled repeatedly, we estimated black fly density (abundance and biomass), as a proxy for secondary production (Jenkins, 2015) from 10 randomly selected cobbles along a 50-m stretch in each site in September. The black fly larvae were preserved in 70% ethanol, and, together with the cobbles, brought back to the laboratory. Later, the black fly larvae were counted, dried at 60°C for 48 h, and weighed. To obtain area-based estimates of black fly larvae abundance and biomass, the upper surface of each cobble (i.e., the surface that was suitable habitat to black fly larvae) was covered with aluminum foil. By weighing the aluminum foil for each cobble from each stream to obtain a relationship between different aluminum foil areas and weights, we could convert obtained aluminum foil weights to cobble surface areas (Bergey & Getty, 2006). In September, we did not find any black fly larvae in one of the five sites (PEL), and therefore, could not calculate black fly larvae density there. We could not find caddisfly larvae in streams of similar size and physical characteristics, and therefore, did not estimate their density or potential temporal trends in their diet.
Stable isotope analyses
To obtain the isotopic signature of allochthonous food sources, stream-conditioned deciduous leaves were collected from 10 streams (ANG, BOS, DJA, FLA, GRA, KAL, OST, PEL, TAV, VEB) in mid-June, transported to the laboratory, and then frozen. Later, the leaves were thawed, washed, and dried in 60°C for 48 h, and leaf parts were weighed (350 μg) into silver capsules. Autochthonous food sources were represented by cobble biofilm that contains a mixture of algae and small particles (of autochthonous and allochthonous origin). To obtain biofilm, cobbles were collected from the stream bottom in seven streams (ANG, BOS, DJA, KAL, OST, PEL, TAV) in mid-June. In the laboratory, the biofilm was removed from the cobbles, using a metal brush and a small amount (a few ml) of water (Jonsson & Stenroth, 2016). The resulting biofilm mixture was dried at 60°C for 48 h and weighed (350 μg) into silver capsules.
A fraction of an organism’s nonexchangeable H comes from water (i.e., dietary water) rather than assimilated food. Hence, in October, stream water was collected from all study streams, and was filtered in the field with a 0.45-µm nylon membrane filter (Sarstedt, Nümbrecht, Germany). All water samples were stored in a cooling box with ice packs before being brought to the laboratory, where they were stored at 3°C in airtight glass vials without air bubbles until analysis. Hydrogen stable isotope samples of black fly and caddisfly larvae, and potential food sources, were analyzed at the Colorado Plateau Stable Isotope Laboratory, Northern Arizona University, following Wassenaar & Hobson (2003) and Doucett et al. (2007). The water samples were analyzed for δ2D at the Swedish University of Agricultural Sciences, Umeå, Sweden. The δ2D data are expressed in per mil (‰) notation relative to Vienna Standard Mean Ocean Water.
The relative contribution of allochthonous leaves and autochthonous (biofilm) algae to the diets of the black fly and the caddisfly larvae was estimated through a Bayesian mixing model using the SIAR package version 4.2 (Parnell et al., 2010) for R version 2.15.1 (R Core Team, 2016). SIAR incorporates several sources of variability within the model, such as variability of consumers, sources, and trophic fractionation factors and generates true probability distributions of potential food sources (Parnell et al., 2010). The dietary water was accounted for in the mixing models by correcting (δDcorr) all consumer δD values (δDcons) according to
where ω is the contribution of dietary water to consumer H (0.185), calculated as the mean of the estimates of ω published by Solomon et al. (2009) and Wang et al. (2009), and δDwater is the mean δD value for the water samples from the specific stream.
Statistical analyses
We used analysis of variance (ANOVA) with Tukey’s HSD test to compare means in δD values between black fly and caddisfly larvae, and means in black fly densities among the sites that were sampled repeatedly. To compare means in black fly δD values across time among repeatedly sampled sites, we used a hierarchical linear mixed effect model, with both month and site as fixed effects. Here, and in the first ANOVA, we used δD values rather than level of autochthony/allochthony, as results from the mixing models (based on δD values) only provided one value per site and could, therefore, not be used to calculate within-site variance. For the samples collected in October, we performed partial least squares (PLS) regression analyses on level of autochthony obtained from the mixing models, to explore how land use and land cover variables (Table 1) predicted the variation in dietary contribution of algae to black fly and caddisfly larvae among the sampled streams.
PLS regression relates two data matrices, predictor and dependent variables, to each other by a linear multivariate model and produces latent variables (PLS components) extracted from predictor variables that maximize the explained variance in the dependent variables. PLS regression is especially useful when predictor variables are correlated, and when there are more predictor variables than observations (Carrascal et al., 2009). Interpretations of the PLS regression models were based on the explanatory capacity of each component (R2), the weights of predictor variables, and the variable influence on projection (VIP). Weights describe the direction and relative strength in the relationship between predictor and dependent variables for each PLS component. VIP summarizes the importance of the predictor variables and we defined variables with VIP > 0.7 as significant for the model (Eriksson et al., 2006). All analyses were performed in R version 3.2.4 (R Core Team, 2016) using the standard package and the PLS package version 2.16-0 for the PLS models. Our variables were visually inspected for normality and were deemed fit for parametric testing (i.e., ANOVA). PLS regression analysis does not assume normally distributed data (Hulland et al., 2010).
Results
Allochthonous food sources estimated from leaf litter showed a δD value of − 153.58 ± 8.2‰ (n = 10), whereas the δD value of autochthonous food sources derived from scraping biofilms was − 212.86 ± 29.7‰ (n = 7) (mean ± 1 SD). Both estimates are within the ranges of δD values previously found for allochthonous and autochthonous resources, respectively (e.g., Doucett et al., 2007). For autochthony in the filter feeders, there was large variation among sites, ranging from 21.1 to 75.1% (Table 2), but there was no significant difference in overall mean autochthony between black fly larvae and caddisfly larvae (57.9 ± 13.7 and 59.4 ± 13.4%, respectively [mean ± 1 SD]; P > 0.05).
In contrast to our hypothesis, we found a significant (t = − 2.230, P = 0.026) negative trend in black fly larvae δD values (i.e., increasing autochthony) over time, but this was mainly driven by sites DJA and VEB (Fig. 2). There were strong differences in mean δD values among the repeatedly sampled sites (F4,5 = 42.869, P < 0.001), with significantly lower values (i.e., higher autochthony) in black fly larvae from sites with higher proportions of lakes in the catchments (DJA and OST; 13.5 and 8.8% lakes, respectively), compared to those with lower proportion of lakes (i.e., GRA, PEL, and VEB; 0.3, 0, and 1.1% lakes, respectively). Among the latter group, the site with 0% lakes in the catchment (PEL) had black fly larvae with significantly higher δD values (i.e., lower autochthony) than the other four sites (P < 0.05, in pairwise comparisons). Differences in proportion of lake in these catchments are, however, related to distance from nearest upstream lake, with the sites DJA and OST being considerably closer to a lake outlet than GRA and VEB, and PEL lacking lakes in its catchment (Table 1).
Both the abundance and biomass of black fly larvae differed among sites (F3,36 = 25.700, P < 0.001 and F3,36 = 12.920, P < 0.001, respectively), but the only difference was between site VEB and the other three sites where black flies were found (P < 0.05, in pairwise comparisons; Table 3). This difference was unrelated to among-site differences in mean δD in the black fly larvae, but the two sites (PEL and VEB) with black fly larvae showing the highest mean δD (i.e., the lowest autochthony) were the ones where we did not find black flies at all sampling events (Table 3).
Considering all the sites surveyed, the PLS regression model for black flies (two components, R2 = 0. 465) identified the proportions of wetlands and lakes in the catchment as the most important variables relating positively to autochthony. Conversely, low autochthony in black fly larvae was associated with a higher proportion of deciduous and mixed forest cover, and a high proportion of logged forests (Fig. 3). Distance to lake and proportional cover of coniferous forest showed only weak negative associations with black fly larvae autochthony, and drainage area and proportional cover of agricultural land failed to be included in the model.
The variable weights of the first component in the PLS models for percentage of autochthony in black fly and caddisfly larvae. Positive weights indicate a positive relationship between the predictor and response variables and vice versa. Variables with grey bars have a VIP > 0.7 and are, therefore, significant in the model, whereas white bars indicate a VIP < 0.7. The PLS model explained 46.5% (two components) of the variation in autochthony in the black fly larvae and 56.1% (two components) of the variation in autochthony in the caddisfly larvae
In the PLS regression model for caddisfly larvae (two components, R2 = 0.561), the proportion of lake and agricultural land use in catchments were ranked as the two most important variables for high autochthony, whereas drainage area, and proportional cover of coniferous, deciduous, and logged forests (i.e., forestry) were negatively associated with high autochthony (Fig. 3). Despite rather high loadings, distance from lake and proportional cover of wetland in catchments were only weakly, negatively related to autochthony in caddisfly larvae, and proportional cover of mixed forests in catchments was unrelated to difference in resource use.
Discussion
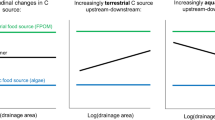
In contrast to our first hypothesis, i.e., that the level of autochthony would increase with increasing stream size (sensu Vannote et al., 1980), we found no systematic change in autochthony/allochthony with increasing drainage area. Instead, catchment-scale forest cover and forestry (i.e., logged forest) were the prime promoters of allochthony in stream filter feeders across sites, presumably via influences on the runoff of terrestrial organic matter. Moreover, consumer autochthony was promoted by a high proportion of lakes in the catchments, rather than by local canopy openness (i.e., stream size), and this was not only a local lake-outlet effect (sensu Malmqvist & Eriksson, 1995). Hence, for a better understanding of availability and use of autochthonous versus allochthonous resources in streams, it is necessary to also consider lateral (i.e., terrestrial runoff) and longitudinal (i.e., transport of lake production) resource transfers, and how they are influenced by catchment-scale land use and land cover.
Neither did we find support for our second hypothesis, i.e., that allochthony would increase as a consequence of seasonal riparian plant litter input, as allochthony in black fly larvae in fact decreased over time, despite substantial peaks in litter inputs in the study region during this time of the year (Lidman et al., 2017). Riparian plant litter resources may become available as food for black fly larvae via leaching of soluble compounds and/or after litter processing by microbes and detritivorous macroinvertebrates (Short & Maslin, 1977; Wallace et al., 1977; Jonsson & Malmqvist, 2005). Recent community-wide assessments have illustrated this shift toward allochthonous support in autumn and winter (e.g., Junker & Cross, 2014); however, the specific connection between plant-litter processing (i.e., particle production) and filter feeders has been questioned (Heard & Richardson, 1995), and neither do our results offer any support for such a link (cf. Carroll et al., 2016). One reason we failed to observe this link could be that dissolved and particulate OM originating from detrital inputs only make up a small fraction of the total organic-matter pool in boreal streams with naturally high concentrations of terrestrial DOM (Laudon et al., 2011a, b; Jonsson et al., 2017). Black fly larvae may use this DOM pool directly (Hershey et al., 1996; Cibrowski et al., 1997), making it a challenge to observe a change toward another terrestrial source (litter) throughout autumn. However, even the site that started the autumn with black fly larvae showing relatively high levels of autochthony showed no evidence of a shift toward more allochthonous support as litter processing ensued.
Autochthony in caddisfly larvae was positively associated with a high proportion of agriculture in catchments. This relationship is likely due to agricultural land use resulting in runoff of nutrients (nitrogen and phosphorus), which stimulate in-stream primary production and increases availability of autochthonous food sources to consumers (Stenroth et al., 2015; Jonsson & Stenroth, 2016). We found no similar association for autochthony in the black fly larvae, and this could possibly be explained by differences in diet between caddisfly and black fly larvae, with black fly larvae feeding on smaller sized particles (Wotton, 1976), potentially including DOM (Hershey et al., 1996; Cibrowski et al., 1997). By contrast, net-spinning caddisfly larvae are known to feed on fine particulate organic matter (i.e., phytoplankton and/or small prey items) (Merritt & Cummins, 1996). Nevertheless, forestry seemed to exert a stronger and more general influence on the resource base of filter feeders than did agriculture, which may be expected as forestry is much more widespread (i.e., represents a stronger gradient) than agriculture in the study region. Regardless, the associations between filter-feeder diets and both types of land use further emphasized that modifications in terrestrial environments can cause deviations from the expected relationship between stream size and autochthony versus allochthony in stream consumers (Finlay, 2011; Stenroth et al., 2015; Jonsson & Stenroth, 2016). In fact, when variability of a range of environmental factors was taken into account, stream size (assuming a positive relationship with drainage area) explained no variation in autochthony in the black fly larvae, and there was even a negative relationship between stream size and autochthony in the caddisfly larvae. This negative relationship could potentially reflect that these caddisflies (Hydropsyche spp.) specialize in feeding on lake primary production in small lake-outlet streams (Malmqvist & Eriksson, 1995). However, because distance to lake was not significant for autochthony in the caddisfly larvae, the support for this explanation is rather weak.
Besides proportional cover of agricultural land, lakes and wetlands also showed positive associations with filter-feeder autochthony. These results are intriguing as they suggest that, independent of a site’s position in the catchment, as defined by drainage area and distance from a lake outlet, lakes deliver sufficient amounts of autochthonous production to control the level of autochthony in filter-feeding organisms, at distances further away from lake outlets than previously thought (e.g., Malmqvist & Eriksson, 1995; Wotton et al., 1996). One alternative, or maybe complementary, explanation is that lakes (Jonsson & Jansson, 1997) and wetlands (Sponseller et al., 2017) deliver nutrients to streams, and that these nutrients in turn stimulate aquatic primary production (Jansson et al., 2001) that increases the autochthonous diet in downstream filter feeders. Lakes and wetlands may also modify flow and/or thermal regimes in ways that additionally promote primary production downstream (Dolph et al., 2017). Regardless of the mechanism, the catchment-scale importance of lakes for filter-feeder diet is further supported by the differences in autochthony in the black fly larvae from the streams that were repeatedly sampled; the highest black fly autochthony was found in streams with the highest occurrence of lakes in the catchment, whereas the stream without lakes showed the lowest black fly autochthony. Here, the level of autochthony in black fly larvae was also related to distance to nearest upstream lake, as proportion of lake in the catchment and distance to lake were related, but our analyses using all sites indicate that proportion of lake is more important than distance to nearest lake when both factors are taken into account. In this sense, the diet of filter feeders seems to better reflect landscape characteristics and processes occurring at the catchment scale than stream size and characteristics of the local riparian vegetation (sensu Vannote et al., 1980; Collins et al., 2016). Further, although we found no clear correspondence between standing stock of black fly larvae and their level of autochthony, failure to find black fly larvae at all sampling events in the two most allochthonous sites suggests, as one would expect, lowered total secondary production in these sites (Jenkins, 2015).
In conclusion, our results indicate that the autochthony versus allochthony in stream-living filter-feeding insect larvae in the boreal region is better explained by characteristics of the catchment rather than by local conditions, such as channel width and canopy openness. Whether or not similar patterns can also be seen in active feeders, such as shredders, collectors, and predators, is a matter for future studies. Irrespective, as filter feeders are widespread and often locally abundant, these results are generally relevant for in-stream secondary production (Brett et al., 2009, 2017), important ecosystem processes (Wallace & Webster, 1996; Malmqvist et al., 2004), and the quality of aquatic subsidies for riparian insectivores (Stenroth et al., 2015; Fritz et al., 2017). From an applied perspective, our results indicate that the diet of filter feeders can serve as a useful proxy for measuring broad-scale consequences of land use on downstream food webs, as it reflects upstream environmental change in a time-integrated fashion. In particular, the diet of caddisfly larvae seems suitable for measuring consequences of agricultural activity on the particulate-matter pool in catchments.
References
Armitage, R. D., 1976. A quantitative study of the invertebrate fauna of the River Tees below Cow Green Reservoir. Freshwater Biology 6: 229–240.
Bergey, E. & G. M. Getty, 2006. A review of methods for measuring the surface area of stream substrates. Hydrobiologia 556: 7–16.
Berggren, M., L. Ström, H. Laudon, J. Karlsson, A. Jonsson, R. Giesler, A.-K. Bergström & M. Jansson, 2010. Lake secondary production fueled by rapid transfer of low molecular weight organic carbon from terrestrial sources to aquatic consumers. Ecology Letters 13: 870–880.
Brett, M. T., M. J. Kainz, S. J. Taipale & H. Seshan, 2009. Phytoplankton, not allochthonous carbon, sustains herbivorous zooplankton production. Proceedings of the National Academy of Sciences USA 106: 21197–21201.
Brett, M. T., S. E. Bunn, S. Chandra, A. W. E. Galloway, F. Guo, M. J. Kainz, P. Kankaala, D. C. P. Lau, T. P. Moulton, M. E. Power, J. B. Rasmussen, S. J. Taipale, J. H. Thorp & J. D. Wehr, 2017. How important are terrestrial organic carbon inputs for secondary production in freshwater ecosystems? Freshwater Biology 62: 833–853.
Carpenter, S. R., J. F. Kitchell & J. R. Hodgson, 1985. Cascading trophic interactions and lake productivity. BioScience 35: 634–639.
Carpenter, S. R., J. J. Cole, J. F. Kitchell & M. L. Pace, 1998. Impact of dissolved organic carbon, phosphorus, and grazing on phytoplankton biomass and production in experimental lakes. Limnology and Oceanography 43: 73–80.
Carrascal, L. M., I. Galvan & O. Gordo, 2009. Partial least squares regression as an alternative to current regression methods used in ecology. Oikos 118: 681–690.
Carroll, T. M., J. H. Thorp & K. A. Roach, 2016. Autochthony in karst spring food webs. Hydrobiologia 776: 173–191.
Cibrowsk, J. J. H., D. A. Craig & K. M. Fry, 1997. Dissolved organic matter as food for blackfly larvae (Diptera: Simuliidae). Journal of the North American Benthological Society 16: 771–780.
Collins, S. M., T. J. Kohler, S. A. Thomas, W. W. Fetzer & A. S. Flecker, 2016. The importance of terrestrial subsidies in stream food webs varies along a stream size gradient. Oikos 125: 674–685.
Cushing, C. E., 1963. Filter-feeding insect distribution and plank- tonic food in the Montreal River. Transactions of the American Fisheries Society 92: 216–219.
Dahm, C. N., 1981. Pathways and mechanisms for removal of dissolved organic carbon from leaf leachate in streams. Canadian Journal of Fisheries and Aquatic Sciences 38: 68–76.
Dolph, C. L., A. T. Hansen & J. C. Finlay, 2017. Flow-related dynamics in suspended algal biomass and its contribution to suspended particulate matter in an agricultural river network of the Minnesota River Basin, USA. Hydrobiologia 785: 127–147.
Doucett, R. R., J. C. Marks, D. W. Blinn, M. Caron & B. A. Hungate, 2007. Measuring terrestrial subsidies to aquatic food webs using stable isotopes of hydrogen. Ecology 88: 1587–1592.
Downing, J. A., J. J. Cole, C. M. Duarte, J. J. Middelburg, J. M. Melack, Y. T. Prairie, P. Kortelainen, R. G. Striegl, W. H. McDowell & L. J. Tranvik, 2012. Global abundance and size distribution of streams and rivers. Inland Waters 2: 229–236.
Eriksson, L., E. Johansson, N. Kettaneh-Wold, J. Trygg, C. Wikström & S. Wold, 2006. Multi- and Megavariate Data Analysis Part I Basic Principles and Applications. Umetrics AB, Umeå.
Esseen, P. A., B. Ehnström, L. Ericson & K. Sjöberg, 1997. Boreal forests. Ecological Bulletins 46: 16–47.
Fey, S. B., A. N. Mertens & K. L. Cottingham, 2015. Autumn leaf subsidies influence spring dynamics of freshwater plankton communities. Oecologia 178: 875–885.
Finlay, J. C., 2011. Stream size and human influences on ecosystem production in river networks. Ecosphere 2: 1–21.
Fritz, K. A., L. J. Kirschman, S. D. McCay, J. T. Trushenski, R. W. Warne & M. R. Whiles, 2017. Subsidies of essential nutrients from aquatic environments correlate with immune function in terrestrial consumers. Freshwater Science 36: 893–900.
Gladyshev, M. I., M. T. Arts & N. N. Sushchik, 2009. Preliminary estimates of the export of Omega-3 highly unsaturated fatty acids (EPA + DHA) from aquatic to terrestrial systems. In Arts, M. T., M. T. Brett & M. Kainz (eds.), Lipids in Aquatic Ecosystems. Springer, New York: 179–209.
Hall, R. O. & J. L. Meyer, 1998. The trophic significance of bacterial in a detritus-based stream food web. Ecology 79: 1995–2012.
Heard, S. B. & J. S. Richardson, 1995. Shredder-collector facilitation in stream detrital food webs – is there enough evidence? Oikos 72: 359–366.
Hershey, A. E., R. W. Merritt, M. C. Miller & J. S. McCrea, 1996. Organic matter processing by larval black flies in a temperate woodland stream. Oikos 75: 524–532.
Hill, W. R., M. G. Ryon & E. M. Schilling, 1995. Light limitation in a stream ecosystem: responses by primary producers and consumers. Ecology 76: 1297–1309.
Hulland, J., M. J. Ryan & R. K. Rayner, 2010. Modeling customer satisfaction: a comparative performance evaluation of covariance structure analysis versus partial least squares. In V. Esposito Vinzi, W. W. Chin, J. Henseler & H. Wang (eds), Handbook of Partial Least Squares: Concepts, Methods and Applications. Springer, Berlin: 307–325.
Jansson, M., A. K. Bergström, S. Drakare & P. Blomqvist, 2001. Nutrient limitation of bacterioplankton and phytoplankton in humic lakes in northern Sweden. Freshwater Biology 46: 653–666.
Jenkins, D. G., 2015. Estimating ecological production from biomass. Ecosphere 6: 1–31.
Jonsson, A. & M. Jansson, 1997. Sediment and mineralisation of organic carbon, nitrogen and phosphorus in a large humic lake, northern Sweden. Archiv für Hydrobiologie 141: 45–65.
Jonsson, M. & B. Malmqvist, 2005. Species richness and composition effects in a detrital processing chain. Journal of the North American Benthological Society 24: 798–806.
Jonsson, M., P. Deleu & B. Malmqvist, 2013. Persisting effects of river regulation on emergent aquatic insects and terrestrial invertebrates in upland forests. River Research and Applications 29: 537–547.
Jonsson, M. & K. Stenroth, 2016. True autochthony and allochthony in aquatic-terrestrial resource fluxes along a land-use gradient. Freshwater Science 35: 882–894.
Jonsson, M., R. M. Burrows, J. Lidman, E. Fältström, H. Laudon & R. A. Sponseller, 2017. Land use influences macroinvertebrate community composition in boreal headwaters through altered stream conditions. Ambio 46: 311–323.
Junker, J. R. & W. F. Cross, 2014. Seasonality in the trophic basis of a temperate stream invertebrate assemblage: importance of temperature and food quality. Limnology and Oceanography 59: 507–518.
Karlsson, J., P. Byström, J. Ask, P. Ask, L. Persson & M. Jansson, 2009. Light limitation of nutrient-poor lake ecosystems. Nature 460: 506–509.
Kelly, P. T., C. T. Solomon, B. C. Weidel & S. E. Jones, 2014. Terrestrial carbon is a resource, but not a subsidy, for lake zooplankton. Ecology 95: 1236–1242.
Laudon, H., M. Berggren, A. Ågren, I. Buffam, K. Bishop, T. Grabs, M. Jansson & S. Köhler, 2011a. Patterns and dynamics of dissolved organic carbon (DOC) in boreal streams: the role of processes, connectivity, and scaling. Ecosystems 14: 880–893.
Laudon, H., R. A. Sponseller, R. W. Lucas, M. N. Futter, G. Egnell, K. Bishop, A. Ågren, E. Ring & P. Högberg, 2011b. Consequences of more intensive forestry for the sustainable management of forest soils and waters. Forests 2: 243–260.
Lidman, J., M. Jonsson, R. M. Burrows, M. Bundschuh & R. A. Sponseller, 2017. Composition of riparian litter input regulates organic matter decomposition: implications for headwater stream functioning in a managed forest landscape. Ecology and Evolution 7: 1068–1077.
Malmqvist, B. & Å. Eriksson, 1995. Benthic insects in Swedish lake-outlet streams: patterns in species richness and assemblage structure. Freshwater Biology 34: 285–296.
Malmqvist, B., P. H. Adler, K. Kuusela, R. W. Merrit & R. S. Wotton, 2004. Black flies in the boreal biome, key organisms in both terrestrial and aquatic environments: a review. Écoscience 11: 187–200.
McCutchan, J. H. & W. M. Lewis, 2002. Relative importance of carbon sources for macroinvertebrates in a Rocky Mountain stream. Limnology and Oceanography 47: 742–752.
McDowell, W. H. & S. G. Fisher, 1976. Autumnal processing of dissolved organic matter in a small woodland stream. Ecology 57: 561–569.
Merritt, R. W. & K. W. Cummins (eds.), 1996. An Introduction to the Aquatic Insects of North America, 3rd ed. Kendall/Hunt Publishing Company, Dubuque, IA.
Meyer, J. K., J. B. Wallace & S. L. Eggert, 1988. Leaf flitter as a source of dissolved organic carbon in streams. Ecosystems 1: 240–249.
Neres-Lima, V., E. F. Brito, F. A. M. Krsulović, A. M. Detweiler, A. E. Hershey & T. P. Moulton, 2016. High importance of autochthonous basal food sources for the food web of a Brazilian tropical stream regardless of shading. International Review of Hydrobiology 101: 132–142.
Parnell, A. C., R. Inger, S. Bearhop & A. L. Jackson, 2010. Source partitioning using stable isotopes: coping with too much variation. Plos ONE 5: e9672.
R Core Team, 2016. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
Richardson, J. S., 1991. Seasonal food limitation of detritivores in a montane stream: an experimental test. Ecology 72: 873–887.
Seekell, D. A., J.-F. Lapierre, J. Ask, A.-K. Bergström, A. Deininger, P. Rodríguez & J. Karlsson, 2015. The influence of dissolved organic carbon on primary production in northern lakes. Limnology and Oceanography 60: 1276–1285.
Short, R. A. & P. E. Maslin, 1977. Processing of leaf litter by a stream detritivore: effect on nutrient availability to collectors. Ecology 58: 935–938.
Solomon, C. T., J. J. Cole, R. R. Doucett, M. L. Pace, N. D. Preston, L. E. Smith & B. B. Weidel, 2009. The influence of environmental water on the hydrogen stable isotope ratio in aquatic consumers. Oecologia 161: 313–324.
Sponseller, R. A., M. Blackburn, M. B. Nilsson & H. Laudon, 2017. Headwater mires constitute a major source of nitrogen (N) to surface waters in the boreal landscape. Ecosystems 21: 31–44.
Stenroth, K., L. E. Polvi, E. Fältström & M. Jonsson, 2015. Land-use effects on terrestrial consumers through changed size structure of aquatic insects. Freshwater Biology 60: 136–149.
Strauss, E. A. & G. A. Lamberti, 2002. Effects of dissolved organic carbon quality on microbial decomposition and nitrification rates in stream sediments. Freshwater Biology 47: 65–74.
Thorp, J. H., M. D. Delong, K. S. Greenwood & A. F. Casper, 1998. Isotopic analysis of three food web theories in constricted and floodplain regions of a large river. Oecologia 117: 551–563.
Valett, H. M. & J. A. Stanford, 1987. Food quality and Hydropsychid caddisfly density in a lake outlet stream in Glacier National Park, Montana, USA. Canadian Journal of Fisheries and Aquatic Sciences 37: 77–82.
Vannote, R. L., G. W. Minshall, K. W. Cummins, J. R. Sedell & C. E. Cushing, 1980. The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 37: 130–137.
Wallace, J. B. & J. R. Webster, 1996. The role of macroinvertebrates in stream ecosystem function. Annual Review of Entomology 41: 115–1139.
Wallace, J. B., J. R. Webster & W. R. Woodall, 1977. The role of filter feeders in flowing waters. Archiv für Hydrobiologie 79: 506–532.
Wallace, J. B., S. L. Eggert, J. L. Meyer & J. R. Webster, 1997. Multiple trophic levels of a forest stream linked to terrestrial litter inputs. Science 277: 102–104.
Wallace, J. B., S. L. Eggert, J. L. Meyer & J. R. Webster, 1999. Effects of resource limitation on a detrital-based ecosystem. Ecological monographs 69: 409–422.
Wallace, J. B., S. L. Eggert, J. L. Meyer & J. R. Webster, 2015. Stream invertebrate productivity linked to forest subsidies: 37 stream-years of reference and experimental data. Ecology 96: 1213–1228.
Wang, Y. V., D. M. O’Brien, J. Jenson, D. Francis & M. J. Wooller, 2009. The influence of diet and water on the stable oxygen and hydrogen isotope composition of Chironomidae (Diptera) with paleoecological implications. Oecologia 160: 225–233.
Wassenaar, L. I. & K. A. Hobson, 2003. Comparative equilibration and online technique for determination of nonexchangeable hydrogen of keratins for use in animal migration studies. Isotopes in Environmental and Health Studies 39: 211–217.
Wotton, R. S., 1976. Evidence that blackfly larvae can feed on particles of colloidal size. Nature 261: 697.
Wotton, R. S., C. P. Joicey & B. Malmqvist, 1996. Spiralling of particles by suspension feeders in a small lake-outlet stream. Canadian Journal of Zoology 74: 758–761.
Acknowledgements
Funding was provided by the Swedish Research Councils Formas and Vetenskapsrådet to MJ and KS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Checo Colón-Gaud
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jonsson, M., Polvi, L.E., Sponseller, R.A. et al. Catchment properties predict autochthony in stream filter feeders. Hydrobiologia 815, 83–95 (2018). https://doi.org/10.1007/s10750-018-3553-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-018-3553-8