Abstract
Rodeo Lagoon, a low-salinity coastal lagoon in the Golden Gate National Recreation Area, California, United States, has been identified as an important ecosystem due to the presence of the endangered goby (Eucyclogobius newberri). Despite low anthropogenic impacts, the lagoon exhibits eutrophic conditions and supports annual episodes of very high phytoplankton biomass. Weekly assessments (February–December 2007) of phytoplankton indicated diatoms, Nodularia spumigena, Chaetoceros muelleri var. muelleri, flagellated protozoa, a mixed assemblage, and Microcystis aeruginosa dominated the algal community in successive waves. Phytoplankton succession was significantly correlated (r 2 = 0.37, p < 0.001) with averaged daily irradiance (max = 29.7 kW m−2 d−1), water column light attenuation (max = 14 m−1), and orthophosphate and dissolved inorganic carbon concentrations (max = 1.5 and 2920 μM, respectively). Negative effects of phytoplankton growth and decay included excessive ammonia concentrations (exceeded EPA guidelines on 77% of sampling days), hypoxia (<3 mg l−1 dissolved oxygen), and introduction of several microcystins, all in the latter half of the year. Our one-year study suggests that this coastal lagoon is a highly seasonal system with strong feedbacks between phytoplankton and geochemical processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Attempts have been made over the past several decades to predict the occurrence of phytoplankton blooms, especially harmful algal blooms (HABs), based on nutrient concentrations, particularly dissolved inorganic nitrogen (DIN) and dissolved inorganic phosphate (DIP) and irradiance in combination with temperature and salinity (Platt & Jasby, 1976; Cole & Cloern, 1987; Franks, 1997; Smayda & Reynolds, 2001). However, ecological processes such as competition and predation are also recognized as important determinants of algal succession (Hutchinson, 1961; Tilman, 1977; Lagus et al., 2004). Increasingly, site-specific models that take into account physical, chemical, and ecological influences are being developed to understand phytoplankton dynamics (Hartnett & Nash, 2004; Dube & Jayaraman, 2008; Sagert et al., 2008).
Several generalizations tend to be made about low-salinity coastal systems. First, it is often assumed that freshwater and low-salinity systems are phosphorus-limited, with this determination generally based on dissolved nutrient concentrations or short-term nutrient addition studies (Hecky & Kilham, 1988). However, longer-term studies are increasingly showing that this assumption is not always accurate. Second, pelagic primary production may be high in shallow systems due to coupling between the water column and sediments, or, conversely, may be limited by high light attenuation from riverine particulate inputs (Cole & Cloern, 1987; Paerl, 1988; Cloern, 1996). It appears that the direction of this assumption is highly site-specific. Third, nitrogen fixing phytoplankton prefer low DIN:DIP and stable water columns, but nuisance dinoflagellates do very well under high DIN:DIP conditions (Howarth et al., 1988; Paerl, 1988). When these organisms occur in the same system, competition pressures make predictions about HAB occurrences difficult.
Large brackish coastal systems such as the Baltic Sea in northern Europe, Peel-Harvey Estuary in Australia, and San Francisco Bay in the western United States have received much attention from phyco-ecologists (Cloern, 1996; Wasmund et al., 1998; Davis & Koop, 2006). These areas are important economic and recreational hubs that provide habitat for threatened and endangered aquatic organisms, which motivates studies on the effects of nutrient loading, whether natural or anthropogenic. Indeed, much of what is known about algal dynamics (primary productivity, nitrogen fixation, and toxin production) comes from studies of large estuaries.
Shallow coastal estuaries (SCEs), such as San Francisco Bay, share the unique distinction of being turbulence-driven systems with a strong coupling between pelagic and benthic processes (Cloern, 1996). Their shallow depth, rapid distribution of heat through the water column, and potential for high nutrient loading can lead to rapid proliferation of phytoplankton biomass. While some SCEs exhibit year-round linkage to the ocean, others may spend part of the year separated from direct ocean influences on temperature and salinity, water column mixing, and scouring of the bed (Roy et al., 2001). These tidally choked coastal lagoons can be considered to be relatively “closed systems” and may serve as excellent models, or “macrocosms,” for increased understanding of the environmental drivers of algal blooms in brackish systems (Smith et al., 1989).
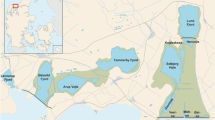
This study was conducted in Rodeo Lagoon, a brackish coastal lagoon located at the terminus of a small watershed in the Marin Headlands, part of the Golden Gate National Recreation Area (GGNRA) in northern California (Fig. 1). Detailed description of the lagoon is presented elsewhere (Cousins et al., 2010).
Potentially toxigenic cyanobacteria, such as Nodularia spumigena (Mertens) and Anabaena spp., have been noted in Rodeo Lagoon since 1993 (Podlech et al., 1993; Codemo et al., 1996). More recently, Microcystis aeruginosa (Kutzing), a potentially toxigenic colonial cyanobacterium, also has been observed. Fish die-offs in the lagoon have been reported since the 1980s (Fong, 1997; Rodriguez, 2004). These die-offs are especially notable because the endangered tidewater goby, Eucyclogobius newberryi (Girard), is found in Rodeo Lagoon; in fact, the lagoon is the last known location within the greater San Francisco Bay Area that retains the tidewater goby and is federally designated critical habitat (Verhey, 2008).
The aim of this study, as part of a larger examination of ecosystem drivers in this system, was to clarify the relations between temporal biogeochemical changes and phytoplankton succession in a small, relatively low-disturbance coastal lagoon. Although our study was motivated by the documented water column hypoxia and fish die-offs mentioned above, we focused on dynamics of phytoplankton as the source of conditions detrimental to higher trophic levels. The determination of bottom-up controls of phytoplankton succession in this unique system not only fills gaps in our knowledge of phytoplankton succession dynamics in small estuarine systems but also has conservation implications for local land managers.
Materials and methods
Field methods
Weekly surface water samples were collected from two stations near the main basin of the lagoon (Stations A and B; Fig. 1) from February to December 2007. Station A is located to the west of the main basin; station B is more central to the basin. Three depth samples were also collected each week from Station B. The maximum depth of the main basin is approximately 2 m. Fieldwork always took place between 09:00 and 11:30 A.M. In all, water samples from 44 consecutive weeks were analyzed. Depth samples at 50 and 1% surface photosynthetic photon flux density (PPFD, calculated from attenuation of surface irradiance values as determined by a digital scalar irradiance meter; Biospherical Instruments, Inc.) as well as bottom water samples were collected each week from Station B (41 weeks). Depth samples were collected using a 1-l Kemmerer bottle (LaMotte Water Sampler). Water samples were stored in polycarbonate bottles and carboys (Nalgene) and retained on ice during transport. Temperature, salinity, and dissolved oxygen of each sample were measured with YSI-30 and YSI-85 meter (YSI, Inc.). Density of surface and bottom water at Station B was calculated using temperature and salinity. The water column light attenuation coefficient, k d, was calculated from irradiance values determined by the digital scalar irradiance meter detailed above.
In addition to the coarse vertical information, fine-scale profiles were collected approximately monthly with a SEACAT 19+ conductivity/temperate/depth profiler (Seabird Electronics) equipped with an oxygen channel and a self-contained underwater fluorescence apparatus (SCUFA, Turner Designs). A slow profiling speed was used to obtain a resolution of 1 cm or less. Chlorophyll-a fluorescence data were calibrated to laboratory extractions of chlorophyll-a from surface water samples collected concurrently; no correction for turbidity was applied and no attempt was made to correct for photo-inhibition of fluorescence near the surface.
Laboratory methods
Both surface and bottom water samples (Stations A and B) were analyzed for dissolved inorganic nutrient concentration, phytoplankton biomass (as chlorophyll a), and phytoplankton species composition. Sample water was gently filtered (<100 mmHg) through a GF/F filter (Whatman, Inc.); filtrate was used for nutrient analysis and filters were retained for chlorophyll a analysis. Filtered water to be analyzed for ammonium, nitrate plus nitrite, orthophosphate, and silicic acid concentrations was stored in HDPE scintillation vials in a freezer (−20°C) for up to 1 year before analysis. Ammonium concentrations were measured fluorometrically (Turner Designs 10-AU) according to Holmes et al. (1999) and adjusted for matrix effects (Taylor et al., 2007). Nitrate plus nitrite (NO x ), orthophosphate, and silicic acid concentrations were measured colorimetrically on an AutoAnalyzer according to manufacturer methods (Bran-Lubbe, Buffalo Grove, IL). Detection limits for ammonium, NO x , orthophosphate, and silicic acid were 0.5, 0.1, 0.05, and 0.5 μM, respectively. Blanks were not included in these analyses. Filters retaining phytoplankton cells were stored frozen (−20°C) for less than 2 weeks. After extracting filters in 90% acetone for 24 h, chlorophyll a concentrations were determined fluorometrically (Turner Designs 10-AU) by bench top acidification methods of Holm-Hansen & Reimann (1978), and Arar & Collins (1997). Surface samples alone were used for nutrient analysis, while biomass and species composition were determined using surface and depth samples. Phytoplankton species composition was examined for whole-water surface samples preserved in acid Lugol’s solution. Units greater than 1 μm in diameter (as cells, filaments, or colonies depending on the species) were enumerated to genus in a nannoplankton counting chamber by light microscopy (Booth, 1993; Engstrom et al., 2002). Size classes of M. aeruginosa colonies were enumerated and converted to cells l−1 (Doers & Parker, 1988). In addition to species counts, both heterocysts and akinetes were enumerated for N. spumigena.
Water samples used in determination of primary production rates were not filtered for grazer removal because large aggregations of N. spumigena and M. aeruginosa cells may have been removed as well. Surface samples from Station B were analyzed each week for phytoplankton primary production rates by the 14C method (Joint Global Ocean Flux Study, 1996). Subsamples were inoculated with 50–125 μl of 40 μCu/ml 14C stock (PerkinElmer) and incubated in triplicate for 24 h at five light levels from 1 to 100% ambient PPFD plus a dark bottle in flow-through tables fed by San Francisco Bay water. Post incubation, samples were gently filtered onto GF/F filters and then acid-fumed. Radioassays of 14C-innoculated samples in OptiPhase Hi-Safe 3 scintillation cocktail (PerkinElmer) were performed on a Winspectral Guardian Liquid Scintillation Counter (PerkinElmer) with quench correction. Ambient dissolved inorganic carbon (DIC) of each water sample from Station B was taken into account when determining primary production rates; DIC was measured on a LI-COR 6252 Carbon Analyzer according to Freiderich et al. (2002). Heterotrophic production from dark bottles was accounted for in productivity calculations. Daily-integrated production rates, P ZT, were calculated by trapezoidal integration from particulate production rates. Water column production normalized to biomass, Λ, for each sample date was also calculated after integrating biomass with depth.
Unfiltered surface water samples from Stations A and B were analyzed for nitrogenase activity by the acetylene reduction assay (Capone, 1993). The headspace above subsamples contained in 15-ml glass bottles was at least 50% replaced with acetylene bubbled through deionized water to remove contaminants. Bottles were incubated at ambient surface PPFD or in darkness in small tanks manipulated to ambient lagoon temperature and repeatedly sampled for up to 4 h. Blanks were included. Ethylene concentrations produced during incubation were measured on a flame-ionization gas chromatograph (Shimadzu, Inc. Model GC-8A). The detection limit was 0.007 nmol ethylene. Gas solubility was accounted for when calculating ethylene production rates (Breitbarth et al., 2004). Acetylene reduction rates (i.e., ethylene production rates) were converted to N2-fixation rates by assuming a 4:1 acetylene:N2 molar ratio (Capone, 1993; Postgate, 1998).
Filtered and unfiltered samples collected at Station B between April 26 and December 11 were tested for the presence of 12 common cyanotoxins, including six microcystin congeners and nodularin, using an LC/MS/MS method with a detection limit of 0.01 ppb or μg l−1 (Loftin et al., 2008). Prior to analysis, samples were stored at −20°C.
Daily surface irradiance data, measured by a pyranometer (Li-Cor Biosciences) located at George Washington High school in San Francisco, CA, approximately 4 km southeast of Rodeo Lagoon, were obtained from the San Francisco Public Utilities Commission Solar Monitoring website (http://sfwater.org). These data were averaged for the week preceding each collection event.
Statistical analysis
Unless otherwise noted above, all parameters have been averaged between Stations A and B. Linear regressions and Student’s t-tests were performed in SPSS for Windows (SPSS, Inc.). Patterns in phytoplankton succession were examined in relation to abiotic variables using the BIOENV routine in Primer-E v6. (Plymouth Marine Laboratory, UK). In this program, the differences between biotic and abiotic matrices are compared and the best environmental parameters are chosen to explain biological variation (Clarke & Warwick, 2001). The seven biotic parameters encompassed the over-arching phytoplankton groups: N. spumigena, total centric diatoms, total pennate diatoms, M. aeruginosa, total dinoflagellates, total flagellated protozoa (excluding dinoflagellates), and chlorophytes. The thirteen abiotic parameters used were surface temperature, bottom temperature, surface salinity, bottom salinity, surface pH, k d, surface NO x , surface ammonium, surface orthophosphate, surface DIN:DIP, surface DIC, averaged daily irradiance, and density difference between surface and bottom. Thirty-one collection events (i.e., weeks) during 2007 had complete biotic and abiotic datasets for analysis of surface community succession. Additional analyses were performed on 8 weeks’ samples from May to December for bottom water succession; abiotic data described above were used with substitution of bottom water nutrient concentrations. Fourth-root transformations were applied to all biotic data, while abiotic parameters were converted to z-scores prior to analysis. Silicic acid concentration data were excluded from multivariate analyses for lack of a complete data set.
Results
The lagoon remained closed to exchange with the ocean from January to November 2007. Temperature, salinity, and volume of the lagoon, therefore, were not directly impacted by the nearby Pacific waters. The average surface water temperature of Rodeo Lagoon varied between 9.7°C (December 11) and 21°C (June 14) and was not significantly different between Stations A and B (Table 1). Surface salinities at A and B also were not significantly different from one another. Average surface salinity at A and B varied between 2.5 (March 14) and 6.5 (December 11, Table 1). Bottom water salinities at B show that the system was salt stratified in the early spring (until March 21), was well-mixed in terms of salinity through the summer and autumn, then became salt stratified again in the winter (after November 27, Table 1). Salinity gradually increased through the summer as a result of net evaporation.
Average daily irradiance was generally highest and most variable from week to week in summer months (Table 1). The average daily irradiance maximum was 30 kW m−2 and minimum was 7.3 kW m−2. Light attenuation in the water column was relatively high (Table 1). On only 59% of sampling dates did 1% PPFD penetrate 0.7–1.5 m to the bottom of the lagoon at station B. Greatest light attenuation, with a kd of 14.3 m−1, was observed on April 26. Lowest light attenuation values were seen in early spring and winter. As lagoon depth decreased by 47% through the late summer and fall, light continued to reach the bed on some dates despite high light attenuation.
Lagoon pH ranged from 7.5 to 10.3 (Table 1). Average pH was significantly higher before August and after September than during these months (p < 0.05). There was no significant difference in pH between Stations A and B.
Average surface ammonium concentrations ranged from below detection to 193 μM (Fig. 2A). From March through April, ammonium concentrations remained below 2 μM but exhibited a small peak in late May. A large peak in ammonium was observed from late August to October. Concentrations had not returned to their early spring values by December 18 when sampling was discontinued for the year. Average surface NOx followed a similar, less variable, and slightly time-delayed trend to ammonium (Fig. 2A). The spring NO x peak followed the ammonium peak by approximately 1 month; the autumn ammonium and NO x peaks were similarly spaced. NO x concentrations were generally much lower than ammonium concentrations, ranging from non-detectable to 40.3 μM. Like nitrogen, average surface orthophosphate concentrations were highly variable in 2007 (Fig. 2B). A decrease in surface orthophosphate was observed in April followed by a rebound in May. The peak in surface orthophosphate concentrations was forward-shifted relative to ammonium, with highest concentrations observed at 1.5 μM (August 9).
DIN:DIP suggests that nitrogen was more limiting than phosphorus until the final weeks of the N. spumigena proliferation (from Fig. 2A, B). Summer months showed variable DIN:DIP, averaging toward phosphorus-limitation based on Redfield ratios (Sterner & Elser, 2002). However, after early September, the lagoon was clearly a more phosphorus-limited system due to excessive ammonium concentrations.
Surface silicic acid concentrations averaged 158 μM with variations from 2.6 μM (June 21) to 426 μM (September 25, Fig. 2A). A distinct drawdown in silicic acid was evident during June and July.
DIC varied between 495 and 2920 μM (Fig. 2A), with higher concentrations in the second half of the year than in the first half. Linear regression of DIC with Λ was low but statistically significant (r = 0.49, p < 0.05), although regressions of DIC with P ZT and chlorophyll a concentration were not significant.
Although the standing stock of phytoplankton biomass in Rodeo Lagoon was relatively high throughout 2007 (lowest average biomass = 10.7 μg chlorophyll a l−1) there were six distinct peaks (Fig. 3A). Centric diatoms including Cyclotella spp. and Thalassiosira spp., and chlorophytes including Scenedesmus spp. dominated the first biomass peak in early March with abundances in excess of 4 × 106 cells l−1 (Fig. 4A). After its decline, the following peak in April and May was dominated by the cyanobacterium Nodularia spumigena, but Microcystis aeruginosa also proliferated during this time (Fig. 4A). Abundance of N. spumigena reached 1.5 × 106 filaments l−1 near the lagoon’s main basin. N. spumigena declined drastically in mid-May, during which time the observed biomass also decreased (from an average 1229 μg chlorophyll a l−1 on April 26 to 10.7 on μg chlorophyll a/l on May 24). An abrupt increase in biomass on May 31 coincided with a proliferation of centric diatoms, particularly the chain-forming Chaetoceros muelleri var. muelleri Lemmermann that reached 3.9 × 107 cells l−1 (Fig. 4A). The decline in centric diatoms at the beginning of July coincided with a biomass decrease. A fourth increase in biomass, centered on the middle of August (August 2–September 25), was dominated by flagellated protozoa including Cryptomonas spp., Colacium spp., and Euglena spp.; cell abundances exceeded 1.2 × 108 cells l−1 (Fig. 4A). M. aeruginosa, pennate diatoms, dinoflagellates, and chlorophytes also proliferated during this time (Fig. 4A). The presence of high concentrations of flagellated protozoa and high biomass persisted for over 2 months. Following the precipitous decline in protozoa and coincident decline in biomass, M. aeruginosa dominated the phytoplankton assemblage through October. After the decline in M. aeruginosa, biomass remained low for several weeks until resurgence of M. aeruginosa (3.0 × 1012 cells l−1) coincident with an increase in centric diatoms, again dominated by C. muelleri (2.3 × 106 cells l−1), and chlorophytes dominated by Scenedesmus spp. in late November and early December (Fig. 4A).
On most sampling days in 2007, biomass as represented by chlorophyll a at Station B did not show a significant variation between surface and 50 or 1% PPFD (p > 0.05, Fig 3). However, samples collected at the sediment–water interface were often enriched in biomass, especially in April through June, and occasionally displayed a markedly different species composition compared with the more uniform upper water column (Figs. 4B). In fact, September 2007 was the only month of observation where the total biomass at the surface and bottom closely tracked one another.
The increase in biomass at the bottom was not a gradual trend away from the surface, but rather often showed a distinctive vertical layering that reflected the density stratification in the lagoon. Detailed chlorophyll a profiles collected several times in 2007 show remarkable variation of biomass with depth on certain dates early and late in the season (Fig. 5A–C). These dates coincided with times of strong vertical density stratification (Table 1) as well as significant vertical variation in the ammonium and DO concentrations, where the vertical structure is known.
Specifically, profiles collected in winter frequently showed much higher concentrations of biomass within and immediately above the lower salty layer (Fig. 5A, C). In both cases, diatoms appear to be responsible for the elevated biomass. Pennate diatoms (Niztschia spp.) dominated the benthic algal community on February 16, while pennate and centric diatoms (not C. muelleri) dominated on December 11. On both February 16 and December 11, the bottom biomass peak was complemented by elevated ammonium in the bottom layer (179 μM vs. 2 μM at the surface on February 16; 80 μM vs. 28 μM at the surface on December 11). Similar trends were apparent in orthophosphate, probably reflecting contact with organic matter in the bed.
By contrast, conditions in the summer were more consistent with a well-mixed water column. In July, just a small, patchy remnant of the salty layer remained, and a sample from the bed showed elevated levels of N. spumigena akinetes and the chlorophyte Scenesdesmus spp. (Fig. 5B). From August to November, we did not observe vertical gradients in salinity, biomass, or nutrient concentration.
Depth-integrated phytoplankton primary production rates (P ZT) determined for Station B generally followed a similar trend to biomass through 2007 (Table 2). Highest rates coincided with C. muelleri in June (P ZT = 5000 mg C m−2 d−1) and the flagellated protozoa bloom in late August and early September (P ZT = 4800 mg C m−2 d−1). Lowest rates were observed in late April (447 mg C m−2 d−1) and September (303 mg C m−2 d−1). Average production from March to December was 2.0 g C m−2 d−1. Total primary production during the study was 550 g C m−2 (9-months)−1.
Water column primary production normalized to biomass, Λ, exhibited a very different trend than integrated production rates, P ZT (Table 2). Λ ranged from 2.7 mg C mg chlorophyll a −1 d−1 (April 26) to 103 mg C mg chlorophyll a −1 d−1 (October 2). Lowest Λ coincided with the peak in N. spumigena; highest Λ coincided with increases in M. aeruginosa and mixed assemblages of centric and pennate diatoms and chlorophytes (Fig. 4A).
Nitrogenase activity was only detected when N. spumigena filaments were observed in the lagoon (Figs. 4 and 6). Maximum rates of 78.1 nmol N2 l−1 h−1 were observed on April 12 for 100%-PPFD incubations. Heterocyst concentrations showed no relation with N2 fixation rates but were observed in greatest abundance when ammonium was below the detection limit; heterocyst abundance declined dramatically after ammonium concentration exceeded 5 μM. After May 24, when N. spumigena was no longer observed in the water column, nitrogenase activity was not detected for the remainder of 2007.
Dissolved oxygen (DO) in surface water, as measured in the late morning, was generally super-saturating (more than approximately 10 mg l−1) during times of high phytoplankton biomass and declined during large cell die-offs (Table 2). DO concentrations during presence of N. spumigena were near equilibrium with the air. In late summer, the high variability of DO is consistent with the lagoon’s hypereutrophic status (Barica & Mur, 1980), and is likely a reflection of strong diurnal variability and sample collection at slightly different times. Surface waters experienced hypoxia during the August–September flagellated protozoa bloom and decline, with a minimum concentration of 2.9 mg l−1 DO reached at the end of September. A minor fish kill was observed around August 6 when conditions were hypoxic but not at the seasonal minimum. DO concentrations rebounded in the autumn and into the winter when they were consistently saturating. In contrast to surface water, DO in the bottom water was generally hypoxic throughout the year (average 5.5 mg l−1).
Microcystin-RR and microcystin-YR were each detected on more than one sampling day, while microcystin-LR was detected in samples from 1 day only (Table 2). Positive samples ranged from 0.08 to 0.56 μg l−1 microcystin-RR in unfiltered water, and from 0.10 to 0.16 μg l−1 in filtered water. Despite the presence of N. spumigena, nodularin was not detected in filtered or unfiltered samples. Also, microcystin-LY, microcystin-LW, microcystin-LA, anatoxin-a, domoic acid, deoxycylindrospermopsin, cylindrospermopsin, and okadaic acid were not detected.
BIOENV analyses indicate that the variability in succession of the surface phytoplankton community exhibited a 37% correlation (p < 0.001) with surface irradiance, light attenuation, orthophosphate concentrations, and DIC concentrations for the 31 collection events with complete data sets (Fig. 7). Inclusion of other abiotic factors dramatically lowered biotic–abiotic correlations. Discriminant function (DF) 1 trended closest with DIC concentrations (r = 0.86, p < 0.001), and DF2 with orthophosphate concentrations (r = 0.93, p < 0.001).
Multi-Dimensional Scaling (MDS) plot of averaged daily irradiance, light attenuation, and orthophosphate and DIC concentrations for 31 collection events (weeks) with complete data sets. Symbols represent the most dominant phytoplankton group (by approximate biomass) during each event: (filled circle) chlorophyte and centric diatom mixed assemblage; (open circle) N. spumigena; (open triangle) C. muelleri var. muelleri; (filled triangle) flagellated protozoa; (cross) M. aeruginosa
Like surface succession, changes in the bottom water algal community from May to December were most closely linked by the BIOENV analyses to light attenuation and surface DIC; however, instead of orthophosphate, bottom water ammonium was the most closely linked nutrient. These three abiotic parameters showed a 43% correlation with bottom water succession (p < 0.001). Again, DF1 trended closest with surface DIC concentrations (r = 0.91, p < 0.001). DF2 regressed most closely with ammonium concentrations (r = 0.87, p < 0.001).
Discussion
Physical, chemical, and biological components of the system under study suggest a strong feedback arrangement in a highly seasonal system. Multivariate analyses indicate that light, phosphorus, and carbon were mostly closely associated with phytoplankton succession in 2007. In addition, phytoplankton activity strongly impacted the light, carbon, nitrogen, silicic acid, and oxygen processes in the system, as well as introducing cyanotoxins.
Most phytoplankton groups showed increased abundance with increasing irradiance. Notable exceptions were the initial proliferation of the C. muelleri-dominated centric diatoms in June and flagellated protozoa in July when dips in average daily irradiance were observed coincident with the beginning of each cell proliferation. This observed irradiance decrease occurred despite the longer summer days. In the case of the early summer centric diatom proliferation, made up mostly of C. muelleri, proliferation during a period of lower irradiance is interesting given the species’ decreased growth rate with decreased light (Leonardos & Geider, 2004). In contrast, the July–August bloom of flagellated protozoa likely represents a degree of uncoupling between a mixotrophic algal assemblage and irradiance. As the protozoa were likely less reliant on photosynthesis for energy, they may have been better nutrient competitors (Caron et al., 1990).
Coincident with decreased irradiance from the late summer through the end of the year, M. aeruginosa abundances were usually greatest at the surface of the lagoon. This species has light acquisition adaptations and can move relatively quickly through the water column in pursuit of light (Paerl, 1988). With wind generally coming from the west from August to November, it is not surprising that M. aeruginosa was often observed in thick “scums” at the east end of the lagoon during the late summer and fall (pers. obs.).
In addition to its effects on succession, light impacts the vertical structure of the phytoplankton assemblage though shading by surface cells (Shigesada & Okubo, 1981). For example, during late spring and summer, higher abundances of N. spumigena and centric diatoms were seen at 50% PPFD where they were shaded from photo-inhibition by cells at the surface. This suggests a degree of self-shading and a significant role for phytoplankton in distribution of light within the water column. In fact, a more extensive examination of chlorophyll a concentrations and light attenuation in Rodeo Lagoon from 2006 to 2008 indicated that chlorophyll a alone accounted for 73% of the observed variability in kd (Cousins et al., 2010). In 2007, a euphotic depth (the depth where PPFD is 1% of its surface value) as shallow as 0.5 m was frequently observed. As the water column was 1–1.5 m deep and well-mixed through most of the year, self-shading may have led to lowered phytoplankton productivity as the cells spent a significant amount of time in near-darkness.
Results of the nutrient analyses are consistent with several previous unpublished studies dating back to 1992 which show that the lagoon is enriched in DIN and DIP, and is particularly susceptible to events with high ammonia (Podlech et al., 1993; Silkie, 2008). For example, a 1992 study (BioSystems Analysis, Inc., 1993/unpublished) found approximately 6 μM orthophosphate and 60 μM ammonium but no nitrate or nitrite in the lagoon’s water column; the sediments were particularly rich in ammonium. Similarly, a 1-year sampling effort in 2006 by Silkie (2008) found a mean orthophosphate level of 2.4 μM and a mean ammonium level of 10 μM but did not detect NO x in the lagoon. High nutrient levels may reflect a large nutrient pool in the sediments that accumulate because density stratification limits scouring of the flocculent layer during winter rain events (Cousins et al., 2010).
Orthophosphate concentrations are of particular importance to the nitrogen-fixing toxigenic cyanobacterium, N. spumigena (Paerl, 1990). The significant decrease in orthophosphate concentrations during N. spumigena proliferation may have stressed the species. A negative relation is seen between orthophosphate concentration and akinete (i.e., resting cell) abundance near the main basin (r = −0.69, p < 0.05; Figs. 2 and 6), suggesting that as orthophosphate became limiting more resting cysts were produced. Phosphate concentrations below 0.6 μM have been shown to induce significantly lower growth rates in N. spumigena relative to higher phosphate concentrations (Lehtimaki et al., 1997).
Nitrogen fixation is energetically very expensive, with nitrogen fixers exhibiting reduced relative abundance following additions of ‘fixed’ inorganic nitrogen (Dixit et al., 1985; Postgate, 1998). In the system under study, ammonium concentrations exceeded 5 μM for the first time two weeks after the peak in N. spumigena abundance. After this point, both N. spumigena and heterocyst abundance declined rapidly, and centric diatom abundance increased, as ammonium concentrations remained above 1 μM and DIN:DIP increased above Redfield ratios. This suggests that increased DIN may have been a contributing factor to N. spumigena’s decline through its loss of competitive advantage.
Initial acetylene reduction rates of 367 nmol l−1 h−1 at Station B were on the order of those observed in the Peel-Harvey Estuary (initial rates of approximately 400 nmol l−1 h−1; Huber, 1986), with a steep decline after the first 3 weeks of observation of the species. Given the calculated N2 fixation rates observed in this study, and assuming constant rates with depth and light-only nitrogen fixation during a 12:12 light: dark cycle, the 9-week occurrence of N. spumigena cells potentially contributed 51 kg-N to the lagoon. This is equivalent to 0.26 g-N m−2 y−1, which is within the range previously estimated for estuaries (0.013–1.8 g-N m−2 y−1; Howarth et al., 1988).
By extrapolation, N. spumigena may have single-handedly raised the DIN concentration in the lagoon by 9.1 μM over the course of its presence in the phytoplankton community. An increase of a similar magnitude in ammonium was observed during this time (Fig. 2A). While on a short temporal scale this was important to the lagoon’s nutrient content, on a yearly scale this represented less than 1% percent of the DIN content of the lagoon. Thus, N. spumigena was a significant nitrogen contributor at the time of its proliferation but perhaps not significant in the overall yearly nutrient budget of the lagoon. The greater DIN source appears to be the sediments (Cousins et al., 2010), affecting algal succession in the bottom water.
Dominance by Uroglena sp. and other protozoa have been observed in assemblage studies under high DIN:DIP and elevated DIN concentration (Lagus et al., 2004). A dramatic increase in protozoa abundance in August was observed in Rodeo Lagoon following just such conditions. Significantly lower DO (p < 0.01, relative to before and after protozoa proliferation) during a time of high phytoplankton biomass, relatively low Λ, as well as proliferation of these cells coincident with a large dip in average daily irradiance suggests that these cells were mixotrophic (Caron et al., 1990). M. aeruginosa and pennate diatoms may have supported this mixotrophy at different points in the protozoa bloom.
With continuation of hypoxic conditions in the water column, the aerobic process of nitrification may have slowed or stopped in Rodeo Lagoon (Ward, 1986). During the period of nitrogen accumulation that spanned late August to mid-October, surface ammonium concentrations reached nearly 200 μM. There was an approximately 1-month delay between the peaks in ammonium and nitrate. Indeed, initial increases in nitrate following the flagellated protozoa bloom coincided with increased surface DO, suggesting that nitrification had resumed. Un-ionized ammonia concentrations, as calculated from ammonium concentrations, temperature, and pH drastically exceeded EPA standards for natural waters on 77% of sampling dates in 2007, and most of these dates occurred during and immediately following the flagellated protozoa bloom (Emmerson et al., 1975; U.S. EPA, 1999). However, a minor fish kill in early August preceded the increase in un-ionized ammonia and detection of microcystins and was probably linked to hypoxia or some other cause rather than ammonia toxicity. This is consistent with the results of a previous fish kill study in Rodeo Lagoon, which concluded that hypoxia was the most important factor in causing fish mortality (Martin et al., 2007).
The elevated orthophosphate concentrations in August were also likely linked to hypoxic water column conditions observed coincident with the flagellated protozoa bloom and crash. This observation is consistent with studies showing orthophosphate release under reducing conditions due to iron redox chemistry (Sǿndergaard et al., 2003), a problem exacerbated by the availability of sulfur in seawater (Rozan et al., 2002) like that found in the pore water of Rodeo Lagoon (Podlech et al., 1993).
Silicic acid concentrations were excluded from multivariate analyses because the data set was incomplete. However, this nutrient requires consideration because of the summer proliferation in centric diatoms, especially C. muellerii var. muellerii. Sustained high silicic acid concentrations were observed throughout the lagoon when diatom abundance was low but crashed during large diatom proliferations. Sediments in southern Marin County, CA and around the San Francisco Bay area contain large amounts of chert, basalt, and greywacke, all silica-rich rocks (Elder, 2001). Similar silicic acid concentrations have been found in San Francisco and San Pablo Bays, opposite the Marin Headlands from Rodeo Lagoon (Wilkerson et al., 2006). In addition, comparable concentrations have been observed previously in Rodeo Lagoon and the upstream wetland suggesting that concentrations observed during this study were not abnormal for this area (Podlech et al., 1993). Given the hypoxic conditions that immediately followed the summer diatom cell increase and crash, silicic acid concentrations should be considered an important parameter in this and similar systems.
Although DIC was not highly correlated with any primary productivity or biomass measures, it appears important to phytoplankton succession in the lagoon. Given the well-mixed status through much of the year, it is unlikely that the lagoon was inorganic carbon-limited (Schindler et al., 1972). Instead, DIN, DIP, or silicic acid limited phytoplankton growth at different times of year. However, the relatively high concentrations of these nutrients, sourced from the sediments or upstream and combined with the over-abundance of DIC allowed high rates of photosynthesis—greater than 500 mg C m−2 fixed per year—placing Rodeo Lagoon in the category of “hypereutrophic” (Pinckney et al., 2001). Rapid drawdown of the inorganic carbon pool in the lagoon was reflected in increased pH during phytoplankton biomass increases, with highest pH observed during when non-mixotrophic phytoplankton dominated. Like light, phytoplankton growth and DIC concentration are tightly coupled in this system.
The vertical phytoplankton biomass structure in the lagoon was well-mixed only in summer and early fall. By contrast, in February to April and December the bottom layer often had more biomass and displayed slightly different population dynamics than the upper water column. This vertical variability is surprising given that the lagoon depth is less than 2 m, and can be attributed to four causes, each of which occurred at different times in 2007: (a) sinking of dead/dying cells; (b) density stratification of the water column, which affects all aspects of the growth climate, especially nutrient concentrations and light; (c) nutrient supply from the organic-rich bottom sediments even when the lagoon is not density-stratified, encouraging growth at or near the bed; and (d) species-specific factors, such as the tendency to form surface scums in the case of M. aeruginosa or the presence of resting spores in the sediment in the case of N. spumigena.
Sustained presence of total microcystins and thick surface mats of M. aeruginosa, a known producer of microcystins, were observed from August through October, similar to patterns found in the San Francisco Estuary in recent years (Chorus & Bartram, 1999; Lehman et al., 2008). Microcystins-YR and -RR were detected in the lagoon from September 25 to December 12 with concentrations between 0.02 and 0.59 μg l−1. By comparison, the World Health Organization drinking water guideline for microcystin-LR is 1.0 μg l−1 (World Health Organization, 2008; no standard for microcystin-RR exists), recreational waters is 10 μg l−1 (Graham et al., 2009), and grazer toxicity is several orders of magnitude greater at 8000 μg l−1 (Blom et al., 2001). Interestingly, there was a significant lag between the peak M. aeruginosa colony count and microcystin concentration, perhaps indicating that toxin production was more closely associated with the decline of the species’ dominance than its peak. The presence of detectable microcystin-RR is also associated temporally with the increase in DIN in late October and decline in DIP from August onward. Previous studies have found that high nutrient levels favor the growth of toxic M. aeruginosa genotypes over non-toxic genotypes, though in the field this tends to occur at the beginning of the growth season rather than at the end (Kardinaal & Visser, 2005). Rodeo Lagoon may offer a counter-example in which high nutrient concentrations enhance microcystin production at the end of the growing season rather than the beginning. Introduction of microcystins to this system at a time when it already suffered from hypoxia and elevated ammonia levels could have detrimental effects on higher trophic levels, like the situation found in the upper San Francisco Estuary (Lehman et al. 2010). Continued study of this type of HAB in a macrocosm such as Rodeo Lagoon may aid in understanding its physical and nutritional drivers, with applications to larger systems such as San Francisco Bay and its delta waters.
Conclusions
In Rodeo Lagoon, physical and chemical factors have been shown to strongly influence phytoplankton seasonal succession. However, given the parameters measured in this study, the best correlation between abiotic data and surface phytoplankton succession was just 37%. The best fit of abiotic parameters was surface irradiance, orthophosphate and DIC concentrations, and light attenuation in the water column. Conversely, dissolved inorganic nutrients were strongly affected by phycological dynamics. This is especially true for DIN, which was heavily impacted by a spring N. spumigena proliferation and a summer flagellated protozoa bloom. In addition to temporal succession, a vertical structure to phytoplankton biomass was observed even when the water column was physically well-mixed.
In summary, sufficient irradiance—despite the San Francisco Bay area’s notorious summer fog—and plentiful nutrients allow individual phytoplankton groups to dominate Rodeo Lagoon’s algal assemblage and proliferate to the point where they overexploit nutritional conditions. Early in the year, low DIN:DIP supported dominance by N. spumigena until DIN exceeded 5 μM. Moderate DIN plus high silicic acid saw the proliferation of diatoms, particularly the chitonous C. muelleri var. muelleri, until nearly all silicic acid was locked in frustules. Flagellated protozoa took over during the higher irradiance portion of the year and likely exerted kleptoplasty on the abundant chlorophytes, pennate diatoms, and M. aeruginosa. M. aeruginosa then dominated during a period of very high DIN:DIP and introduced microcystins to the system. Finally, waning irradiance, falling DIN:DIP, and a resumption of high silicic acid saw the return of centric diatoms in general and the chitonous C. muelleri var. muelleri specifically.
References
Arar, E. J. & G. Collins, 1997. In vitro determination of chlorophyll and phaeophytin in marine and freshwater algae by fluorescence. U.S. Environmental Protection Agency, Cincinnati, OH.
Barica, J. & L. R. Mur, 1980. Hypertrophic ecosystems (Developments in Hydrology). Springer, The Netherlands.
Biosystems Analysis, Inc., 1993. Rodeo Lagoon nutrient analysis: sediment and water sampling November, 1992. Prepared for the National Park Service. Unpublished.
Blom, J. F., J. A. Robinson & F. Juttner, 2001. High grazer toxicity of [D-Asp3, (E)-Dhb7] microcystin-RR of Planktothrix rubescens as compared to different microcystins. Toxicon 39: 1923–1932.
Booth, B., 1993. Estimating cell concentration and biomass of autotrophic plankton using microscopy. In Kemp, P. F., E. B. Sherr & J. J. Cole (eds), Handbook of Methods in Aquatic Microbial Ecology. CRC Press, Boca Raton, FL: 199–204.
Breitbarth, E., M. Mills, G. Freidrichs & J. LaRoche, 2004. The Bunsen gas solubility coefficient of ethylene as a function of temperature and salinity and its importance for nitrogen fixation assays. Limnology and Oceanography: Methods 2: 282–288.
Capone, D., 1993. Determination of nitrogenase activity in aquatic samples using the acetylene reduction procedure. In Kemp, P. F., E. B. Sherr & J. J. Cole (eds), Handbook of Methods in Aquatic Microbial Ecology. CRC Press, Boca Raton, FL: 621–631.
Caron, D. A., K. G. Porter & R. W. Sanders, 1990. Carbon, nitrogen and phosphorus budgets for the mixotrophic phytoflagellate Poterioochromonas malhamensis (Chrysophyceae) during bacterial ingestion. Limnology and Oceanography 35: 433–442.
Chorus, I. & J. Bartram, 1999. Toxic cyanobacteria in water. Spon Press, UK.
Clarke, K. R. & R. M. Warwick, 2001. Change in marine communities: An approach to statistical analysis and interpretation, 2nd edn. PRIMER-E, Plymouth, U.K.
Cloern, J. E., 1996. Phytoplankton bloom dynamics in coastal ecosystems: a review with some general lessons from sustained investigation of San Francisco Bay, California. Reviews of Geophysics 34: 127–168.
Codemo, C.M., M. Podlech., R.J. Brown, & W.P. Jordan, 1996. Characterization of phytoplankton in Rodeo Lagoon and Rodeo Lake, GGNRA, during August 1996. University of San Francisco, San Francisco, CA.
Cole, B. E. & J. J. Cloern, 1987. An empirical model for estimating phytoplankton productivity in estuaries. Marine Ecology Progress Series 36: 299–305.
Cousins, M., M. Stacey & J. Drake, 2010. Effects of seasonal stratification on turbulent mixing in a hypereutrophic coastal lagoon. Limnology and Oceanography 55: 172–186.
Davis, J. R. & K. Koop, 2006. Eutrophication in Australian rivers, reservoirs and estuaries - a southern hemisphere perspective on the science and its implications. Hydrobiologia 559: 23–76.
Dixit, A., D. Amla & P. Saxena, 1985. On the structure cell differentiation and life cycle of blue-green alga Nodularia harveyana. Archiv fuer Hydrobiologie 104: 235–246.
Doers, M. P. & D. L. Parker, 1988. Properties of Microcystis aeruginosa and M. flos-aquae (Cyanophyta) in culture: taxonomic implications. Journal of Phycology 24: 502–508.
Dube, A. & G. Jayaraman, 2008. Mathematical modeling of the seasonal variability of & in a shallow lagoon. Non-linear Analysis: Theory, Methods, and Application 69: 850–865.
Elder, W. P., 2001. Geology of the Golden Gate Headlands. In Stoffer, P. W. & L. C. Gordon (eds), Geology and Natural History of the San Francisco Bay Area: A Field-trip Guidebook. U.S. Geological Survey Bulletin 2188.
Emmerson, K., R. Russo, R. E. Lund & R. V. Thurston, 1975. Aqueous ammonia equilibrium calculations: effect of pH and temperature. Journal of the Fisheries Research Board of Canada 32: 2379–2383.
Engstrom-Ost, J. K., K. Marja, M. Schmidt, S. H. Viitasalo, M. Jonasdottir, S. Kokkonen, K. Repka & Sivonen, 2002. Effects of toxic cyanobacteria on a plankton assemblage: community development during decay of Nodularia spumigena. Marine Ecology Progress Series 232: 1–14.
Fong, D., 1997. 1996 Fall Fish Kill Evaluation for Rodeo Lagoon, Golden Gate National Recreation Area, Marin Co. Sausalito, CA. National Park Golden Gate National Recreation Area Service Division of Resource Management and Planning.
Franks, P. J. S., 1997. Models of harmful algal blooms. Limnology and Oceanography 42: 1273–1282.
Freiderich, G. E., P. M. Walz, M. P. Burczynski & F. P. Chavez, 2002. Inorganic carbon in the central California upwelling system during the 1997–1999 El Nino-La Nina event. Progress in Oceanography 54: 185–203.
Graham, J. L., K. A. Loftin & N. Kamman, 2009. State monitoring programs for cyanobacterial toxins in recreational freshwaters of the United States. Lakeline 29: 18–24.
Hartnett, M. & S. Nash, 2004. Modeling nutrient and chlorophyll a dynamics in an Irish brackish waterbody. Environmental Modeling and Software 19: 47–56.
Hecky, R. E. & P. Kilham, 1988. Nutrient limitation of phytoplankton in freshwater and marine environments: a review of recent evidence on the effects of enrichment. Limnology and Oceanography 33: 796–822.
Holmes, R. M., A. Aminot, R. Keruoel, B. A. Hooker & B. J. Peterson, 1999. A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Canadian Journal of Fisheries and Aquatic Science 56: 1801–1808.
Holm-Hansen, O. & B. Reimann, 1978. Chlorophyll a determination: improvements in methodology. OIKOS 30: 438–447.
Howarth, R. W., R. Marino, J. Lane & J. J. Cole, 1988. Nitrogen fixation in freshwater, estuarine, and marine ecosystems. 1. Rates and importance. Limnology and Oceanography 33: 669–687.
Huber, A. L., 1986. Nitrogen fixation by Nodularia spumigena Mertens (Cyanobacteriaceae). 1: field studies and the contribution of blooms to the nitrogen budget of the Peel-Harvey Estuary, Western Australia. Hydrobiologia 131: 193–203.
Hutchinson, G. E., 1961. The paradox of the plankton. American Naturalist 95: 137–145.
Joint Global Ocean Flux Study, 1996. Chapter 19. Primary Production by 14C. Bergen, Norway, Scientific Committee on Oceanic Research, International Council of Scientific Unions: 123–134.
Kardinaal, W., & P.M. Visser, 2005. Dynamics of cyanobacterial toxins. Harmful Cyanobacteria. In Huisman, J., H. C. P. Matthijs, & P. M. Visser (eds), Springer, Norwell, MA.
Lagus, A., J. Suomela, G. Weithoff, K. Heikkila, H. Helminen & J. Sipura, 2004. Species specific differences in phytoplankton responses to N and P enrichments and the N:P ratio in the Archipelago Sea, northern Baltic Sea. Journal of Plankton Research 26: 779–798.
Lehman, P. W., G. Boyer, M. Satchwell & S. Waller, 2008. The influence of environmental conditions on the seasonal variation of Microcystis cell density and microcystins concentration in San Francisco Estuary. Hydrobiology 600: 197–204.
Lehman, P. W., S. J. Teh, G. L. Boyer, M. L. Nobriga, E. Bass & C. Hogle, 2010. Initial impacts of Microcystis aeruginosa blooms on the aquatic foodweb in the San Francisco Estuary. Hydrobiologia 637: 229–248.
Lehtimaki, J., P. Moisander, K. Sivonen & K. Kononen, 1997. Growth, nitrogen fixation, and nodularin production by two Baltic Sea cyanobacteria. Applied and Environmental Microbiology 63: 1647–1656.
Leonardos, N. & R. J. Geider, 2004. Effects of nitrate:phosphate supply ratio and irradiance on C:N:P stoichiometry of Chaetoceros muelleri. European Journal of Phycology 39: 173–180.
Loftin, K. A., M. T. Meyer, F. Rubio, L. Kamp, E. Humphries, & E. Whereat, 2008. Comparison of two cell lysis procedures for recovery of microcystins in water samples from Silver Lake in Dover, Delaware, with microcystin producing cyanobacterial accumulations. USGS Open-File Report 2008-1341.
Martin, B. A., M. K. Saiki & D. Fong, 2007. Relation between mortality of prickly sculpin and diurnal extremes in water quality in Rodeo Lagoon, Marin County, California. California Fish and Game 93: 214–223.
Paerl, H. W., 1988. Nuisance phytoplankton blooms in coastal, estuarine, and inland waters. Limnology and Oceanography 33: 823–847.
Paerl, H. W., 1990. Physiological ecology and regulation of N2 fixation in natural waters. Advances in Microbial Ecology 11: 305–344.
Pinckney, J., H. Paerl, P. Tester & T. Richardson, 2001. The role of nutrient loading and eutrophication in estuarine ecology. Environmental Health Perspectives 109: 699–706.
Platt, T. & A. D. Jasby, 1976. The relationship between photosynthesis and light for natural assemblages of coastal marine phytoplankton. Journal of Phycology 12: 421–430.
Podlech, M., C. Codemo, R. J. Brown & W. P. Jordan, 1993. Some physical, chemical, and biological characteristics of Rodeo Lagoon, Rodeo Lake, and Rodeo Creek: initial study. San Francisco, CA, Institute of Chemical Biology, University of San Francisco: 27.
Postgate, J., 1998. Nitrogen Fixation. Cambridge University Press, New York, NY.
Rodriguez, M. 2004. Marin County Watershed Management Plan Administrative Draft, April 2004. The Marin County Community Planning Agency, Planning Division, San Rafael, CA.
Roy, P. S., R. J. Williams, A. R. Jones, I. Yassini, P. J. Gibbs, B. Coates, R. J. West, P. R. Scanes, J. P. Hudson & S. Nichol, 2001. Structure and function of south-east Australian estuaries. Estuarine and Coastal Shelf Science 53: 251–384.
Rozan, T. F., M. Taillefert, R. E. Trouwborst, B. T. Glazer, S. Ma, J. Herszage, L. M. Valdes, K. S. Price & G. W. Luther III, 2002. Iron-sulfur-phosphorus cycling in the sediments of a shallow coastal bay: Implications for sediment nutrient release and benthic macroalgal blooms. Limnology and Oceanography 47: 1346–1354.
Sagert, S., T. Rieling, A. Eggert & H. Schubert, 2008. Development of a phytoplankton indicator system for ecological assessment of brackish coastal waters (German Baltic Sea coast). Hydrobiologia 611: 91–103.
San Francisco Public Utilities Commission Solar Monitoring Program. http://sfwater.org. Accessed 20 July 2009.
Schindler, D. W., G. J. Brunskill, S. Emerson, W. S. Broecker & T. H. Peng, 1972. Atmospheric carbon dioxide: its role in maintaining phytoplankton standing crops. Science 177: 1192–1194.
Shigesada, N. & A. Okubo, 1981. Analysis of the self-shading effect on algal vertical distribution in natural waters. Journal of Mathematical Biology 12: 311–326.
Silkie, S. S. 2008. Use of host-specific molecular markers in fecal source tracking. Ph.D. thesis. University of California, Berkeley.
Smayda, T. J. & C. Reynolds, 2001. Community assembly in marine phytoplankton: Application of recent models to harmful dinoflagellate blooms. Journal of Plankton Research 23: 447–461.
Smith, S. V., J. T. Hollibaugh, S. J. Dollar & S. M. Vink, 1989. Tomales Bay, California: a case for carbon-controlled nitrogen cycling. Limnology Oceanography 34: 37–52.
Sǿndergaard, M., J. P. Jensen & E. Jeppesen, 2003. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 506–509: 135–145.
Sterner, R. W. & J. J. Elser, 2002. Ecological Stoichiometry: the Biology of Elements from molecules to the biosphere. Princeton University Press, Princeton, NJ.
Taylor, B. W., C. F. Keep, R. O. Hall Jr, B. J. Koch, L. M. Tronstad, A. S. Flecker & A. J. Ulseth, 2007. Improving the fluorometric ammonium method: matrix effects, background fluorescence, and standard additions. Journal of the North American Benthological Society 26: 167–177.
Tilman, D., 1977. Resource competition between plankton algae: an experimental and theoretical approach. Ecology 58: 338–348.
United States Environmental Protection Agency, 1999. Update of Ambient Water Quality Criteria for Ammonia. Office of Research and Development, Duluth, MN: 150.
Verhey, D. M., 2008. Endangered and threatened wildlife and plants; revised designation of critical habitat for the tidewater goby (Eucyclogobius newberryi): final rule. 50 CFR Part 17. Federal Register 73: 5919–6005.
Ward, B.B. 1986. Nitrification in marine environments. In Prosser J. I. (ed.), Nitrification. IRL Press, Oxford, England.
Wasmund, N., G. Nausch & W. Matthaus, 1998. Phytoplankton spring blooms in the southern Baltic Sea – spatio-temporal development and long-term trends. Journal of Plankton Research 20: 1099–1117.
Wilkerson, F. P., R. Dugdale, V. E. Hogue & A. Marchi, 2006. Phytoplankton blooms and nitrogen productivity in San Francisco Bay. Estuaries and Coasts 29: 401–416.
World Health Organization, 2008. Guidelines for drinking-water quality, 3rd edn.
Acknowledgments
Field and laboratory support was provided by L. Drake, D. Fong, J. Herndon, C. Ikeda, A. Marchi, J. Murphy, M. Stacey, and J. Tirindelli. J. Johansen provided identification support. Financial support to J.L. Drake was provided by a student research award from the Department of Biology at San Francisco State University. Financial support to M. Cousins was provided by fellowships from the National Science Foundation Graduate Research Fellowship Program and the University of California Chancellor’s Opportunity Fellowship. The National Park Service, the University of California Toxic Substances Research and Teaching Program, and the U.S. Geological Survey Toxic Substances Hydrology Emerging Contaminant Program kindly provided additional funding for research. We also thank USGS and journal reviewers for their helpful comments. Use of trade names is for identification purposes and does not imply endorsement by the U.S. Government.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: David Philip Hamilton
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Drake, J.L., Carpenter, E.J., Cousins, M. et al. Effects of light and nutrients on seasonal phytoplankton succession in a temperate eutrophic coastal lagoon. Hydrobiologia 654, 177–192 (2010). https://doi.org/10.1007/s10750-010-0380-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-010-0380-y