Abstract
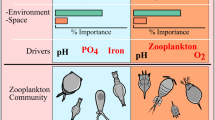
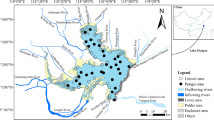
A bloom of the cyanobacteria Microcystis aeruginosa was sampled over the summer and fall in order to determine if the spatial and temporal patterns in cell density, chlorophyll a (chl a) concentration, total microcystins concentration, and percent microcystins composition varied with environmental conditions in San Francisco Estuary. It was hypothesized that the seasonal variation in Microcystis cell density and microcystin concentration was ecologically important because it could influence the transfer of toxic microcystins into the aquatic food web. Sampling for Microcystis cell density, chl a concentration, total microcystins concentration and a suite of environmental conditions was conducted biweekly at nine stations throughout the freshwater tidal and brackish water regions of the estuary between July and November 2004. Total microcystins in zooplankton and clam tissue was also sampled in August and October. Microcystis cell density, chl a concentration and total microcystins concentration varied by an order of magnitude and peaked during August and September when \( {\text{P}}^{{\text{B}}}_{{\text{m}}} \) and αB were high. Low streamflow and high water temperature were strongly correlated with the seasonal variation of Microcystis cell density, total microcystins concentration (cell)−1 and total microcystins concentration (chl a)−1 in canonical correlation analyses. Nutrient concentrations and ratios were of secondary importance in the analysis and may be of lesser importance to seasonal variation of the bloom in this nutrient rich estuary. The seasonal variation of Microcystis density and biomass was potentially important for the structure and function of the estuarine aquatic food web, because total microcystins concentration was high at the base of the food web in mesozooplankton, amphipod, clam, and worm tissue during the peak of the bloom.







Similar content being viewed by others
References
American Public Health Association, American Water Works Association, & Water Environment Association, 1998. Standard Methods for the Examination of Water and Wastewater. 20th edn. American Public Health Association, Washington, D.C., USA.
Carmichael, W. W., 1995. Toxic Microcystis in the environment. In Watanabe, M. F., K. Harada, W. W. Carmichael & H. Fujiki (eds), Toxic Microcystis. CRC Press: New York, 1–12.
Christian, R. R., W. L. Bryant Jr. & D. W. Stanley, 1986. The relationship between river flow and Microcystis aeruginosa blooms in the Neuse River, North Carolina. Water Resources Research Institute Report 223. North Carolina State University.
De Bernaradi, R. & G. Giussani, 1990. Are blue-green algae a suitable food for zooplankton? An overview. Hydrobiologia 200/201: 29–41.
DeMott, W. R. & F. Moxter, 1991. Foraging on cyanobacteria by copepods: responses to chemical defenses and resource abundance. Ecology 72: 1820–1834.
DeMott, W. R. & D. C. Muller-Navarra, 1997. The importance of highly unsaturated fatty acids in zooplankton nutrition: evidence from experiments with Daphnia, a cyanobacterium and lipid emulsions. Freshwater Biology 38: 649–664.
Donaghay, P. L. & T. R. Osborn, 1997. Toward a theory of biological-physical control of harmful algal bloom dynamics and impacts. Limnology and Oceanography 42: 1283–1296.
Fastner, J., U. Neumann, B. Wirsing, J. Weckesser, C. Wiedner, B. Nixdorf & I. Chorus, 1999. Microcystins (hepatotoxic heptapeptides) in german fresh water bodies. Environmental Toxicology 14: 13–22.
Federal Environmental Agency, 2005. Current approaches to cyanotoxin risk assessment, risk management, regulations in different countries. In Chorus, I. (ed.), Federal Environmental Agency, Berlin.
Fulton, R. S. III & H. W. Paerl, 1987. Effects of colonial morphology on zooplankton utilization of algal resources during blue-green algal (Microcystis aeruginosa) blooms. Limnology and Oceanography 32: 634–644.
Huisman, J., J. Sharples, J. M. Stroom, P. M. Visser, W. E. A. Kardinaal, J. M. H. Verspagen & B. Sommeijer, 2004. Changes in turbulent mixing shift competition for light between phytoplankton species. Ecology 85: 2960–2970.
Ibelings, B. W., K. Bruning, J. de Jonge, K. Wolfstein, L. M. Dionisio Pires, J. Postma & T. Burger, 2005. Distribution of microcystins in a lake foodweb: no evidence for biomagnification. Microbial Ecology 49: 487–500.
Jacoby, J. M., D. C. Collier, E. B. Welch, F. J. Hardy & M. Crayton, 2000. Environmental factors associated with a toxic bloom of Microcystis aeruginosa. Canadian Journal of Fisheries and Aquatic Science 57: 231–240.
Jassby, A. D., 2005. Phytoplankton regulation in a eutrophic tidal river (San Joaquin River, California). San Francisco Estuaries Watershed Science 3: 1–2.
Jassby, A. D., J. E. Cloern & B. E. Cole, 2002. Annual primary production: patterns and mechanisms of change in a nutrient-rich tidal ecosystem. Limnology and Oceanography 47: 698–712.
Kotak, B. G., R. W. Zurawell, E. E. Prepas & C. F. B. Holmes, 1996. Microcystin-LR concentration in aquatic food web compartments from lakes of varying trophic status. Canadian Journal of Fisheries and Aquatic Sciences 53: 1974–1985.
Latour, D., O. Sabido, M. Salencon & H. Giraudet, 2004. Dynamics and metabolic acitivity of the benthic cyanobacterium Microcystis aeruginosa in the Grangent reservoir (France). Journal of Plankton Research 26: 719–726.
Lehman, P. W., 2004. The influence of climate on mechanistic pathways that impact lower food web production in northern San Francisco Bay estuary. Estuaries 27: 311–324.
Lehman, P. W., G. Boyer, C. Hall, S. Waller & K. Gehrts, 2005. Distribution and toxicity of a new colonial Microcystis aeruginosa bloom in the San Francisco Bay Estuary, California. Hydrobiologia 541: 87–99.
Lehman, P. W., T. Sommer & L. Rivard, 2007. The influence of floodplain habitat on the quantity and quality of riverine phytoplankton carbon produced during the flood season in San Francisco Estuary. Aquatic Ecology. DOI 10.1007/s10452–007–9102–6.
Lyck, S., 2004. Simultaneous changes in cell quotas of microcystin, chlorophyll a, protein and carbohydrate during different growth phases of a batch culture experiment with Microcystis aeruginosa. Journal of Plankton Research 26: 727–736.
Malbrouck, C. & P. Kestemont, 2006. Effects of microcystins on fish. Environmental Toxicology and Chemistry 25: 72–86.
O’Brien, K. R., D. L. Meyer, A. M. Waite, G. N. Ivey & D. P. Hamilton, 2004. Disaggregation of Microcystis aeruginosa colonies under turbulent mixing:laboratory experiments in a grid-stirred tank. Hydrobiologia 519: 143–152.
Orr, P. T., G. J. Jones & G. B. Douglas, 2004. Response of cultured Microcystis aeruginosa from the Swan River, Australia, to elevated salt concentration and consequences for bloom and toxin management in estuaries. Marine and Freshwater Research 55: 277–283.
Ouellette, A. J., S. M. Handy & S. W. Wilhelm, 2006. Toxic Microcystis is widespread in Lake Erie: PCR detection of toxin genes and molecular characterization of associated cyanobacterial communities. Microbial Ecology 51: 154–165.
Paerl, H. W., 1988. Nuisance phytoplankton blooms in coastal, estuarine and inland waters. Limnology and Oceanography 33: 823–847.
Pearl, H. W., R. S. Fulton III, P. H. Moisander & J. Dyble, 2001. Harmful freshwater algal blooms, with an emphasis on cyanobacteria. The Scientific World 1: 76–113.
Pickney, J. L., D. F. Millie, B. T. Vinyard & H. W. Paerl, 1997. Environmental controls of phytoplankton bloom dynamics in the Neuse River Estuary, North Carolina, USA. Canadian Journal of Fisheries and Aquatic Science 54: 2491–2501.
Pietsch, C., C. Wiegand, M. V. Ame, A. Nicklisch, D. Winderlin & S. Pflugmacher, 2001. The effects of cyanobacterial crude extract on different aquatic organisms: evidence for cyanobacterial toxin modulating factors. Environmental Toxicology 16: 535–542.
Prepas, E. E., B. G. Kotak, L. M. Campbell, J. C. Evans, S. E. Hrudey & C. F. B. Holmes, 1997. Accumulation and elimination of cyanobacterial hepatotoxins by the freshwater clam Anodonta grandis simpsoniana. Canadian Journal of Fisheries and Aquatic Sciences 54: 41–46.
Regel, R. H., J. D. Brookes, G. G. Ganf & R. W. Griffiths, 2004. The influence of experimentally generated turbulence on the Mash01 unicellular Microcystis aeruginosa strain. Hydrobiologia 517: 107–120.
Reinikainen, M., J. Hietala & M. Walls, 1999. Reproductive allocation in Daphnia exposed to toxic cyanobacteria. Journal of Plankton Research 21: 1553–1564.
Reynolds, C. S., 1997. Vegetation processes in the pelagic: a model for ecosystem theory. In O. Kinne (ed.), Excellence in Ecology. Ecology Institute, Germany.
Robarts, R. D. & T. Zohary, 1987. Temperature effects on photosynthetic capacity, respiration, and growth rates of bloom-forming cyanobacteria. New Zealand Journal of Marine and Freshwater Research 21: 391–399.
Robson, B. J. & D. P. Hamilton, 2003. Summer flow event induces a cyanobacterial bloom in a seasonal Western Australia estuary. Marine and Freshwater Research 54: 139–151.
Rocha, C., H. Galvão & A. Barbosa, 2002. Role of transient silicon limitation in the development of cyanobacteria blooms in the Guadiana estuary, south-western Iberia. Marine Ecology Progress Series 228: 35–45.
Rohrlack, T., K. Christoffersen, E. Dittmann, I. Nogueira, V. Vasconcelos & T. Borner, 2005. Ingestion of microcystins by Daphnia: intestinal uptake and toxic effects. Limnology and Oceanography 50: 440–448.
SAS Institute, Inc., 2004. SAS/STAT User’s Guide, Version 8. SAS Institute Inc., SAS Campus Drive, Cary, North Carolina, USA.
Sedmak, B. & T. Elersek, 2005. Microcystins induce morphological and physiological changes in selected representative phytoplanktons. Microbial Ecology 51: 508–515.
Sellner, K. G., D. C. Brownleee, M. H. Bundy, S. G. Brownlee & K. R. Braun, 1993. Zooplankton grazing in a Potomac River cyanobacteria bloom. Estuaries 16: 859–872.
Sellner, K. G., R. V. Lacouture & K. G. Parlish, 1988. Effect of increasing salinity on a cyanobacteria bloom in the Potomac River Estuary. Journal of Plankton Research 10: 49–61.
Shapiro, J., 1990. Current beliefs regarding dominance of blue-greens: the case for the importance of CO2 and pH. Verhandlungen der Internationalen Vereinigung für Theoretische und Angewandte Limnologie 24: 38–54.
Smith, A. D. & J. J. Gilbert, 1995. Relative susceptibilities of rotifers and cladocerans to Microcystis aeruginosa. Archiv für Hydrobiologie 132: 309–336.
Sommer, T. R. & others, 2007. The collapse of pelagic fishes in the upper San Francisco Estuary. Fisheries 32: 270–277.
United States Environmental Protection Agency (US EPA), 1983. Methods for chemical analysis of water and wastes. Washington, DC. Technical Report EPA-600/4-79-020.
United States Geological Survey (USGS), 1985. Methods for determination of inorganic substances in water and fluvial sediments. United States Geological Survey. Open file report 85-495.
Utermöhl, H., 1958. Zur Vervollkommung der quantitativen Phytoplankton-methodik. Mitteilumgen Internationale Verejunigung fur Theoretische und Angewandtet Limnologie 9: 1–38.
Utkilen, H. & N. Gjølme, 1992. Toxin production by Microcystis aeruginosa as a function of light in continuous cultures and its ecological significance. Applied and Environmental Microbiology, 58: 1321–1325.
Van der Westhuizen, A. J. & J. N. Eloff, 1985. Effect of temerpataure and light on the toxicity and growth of the blue-green alga Microcystis aeruginosa (UV-006)*. Planta 163: 55–59.
Vollenweider, R. A., 1974. A Manual on methods for measuring primary production in aquatic environments. International Biological program Handbook 12. Balckwell Scientific Publications, Oxford.
Watson, S. B., E. McCauley & J. A. Downing, 1997. Patterns in phytoplankton taxonomic composition across temperate lakes of differing nutrient status. Limnology and Oceanography 42: 487–495.
White, S. H., L. J. Duivenvoorden & L. D. Fabbro, 2005. A decision-making framework for ecological impacts associated with the accumulation of cyanotoxins (cylindrospermopsin and microcystin). Lakes and Reservoirs: Research and Management 10: 25–37.
Wiedner, C., P. M. Visser, J. Fastner, J. S. Metcalf, G. A. Codd & L. R. Mur, 2003. Effects of light on the microcystin content of Microcystis strain PCC 7806. Applied and Environmental Microbiology 69: 1475–1481.
Wood, S. A., L. R. Briggs, J. Sprosen, J. G. Ruck, R. G. Wear, P. T. Holland & M. Bloxham, 2006. Changes in concentrations of microcystins in rainbow trout, freshwater mussels and cyanobacteria in Lakes Rotoiti and Totoehu. Environmental Toxicology 21: 205–222.
Yunes, J. S., P. S. Salomon, A. Matthiensen, K. A. Beattie, S. L. Raggett & G. A. Codd, 1996. Toxic blooms of cyanobacteria in the Patos Lagoon Estuary, southern Brazil. Journal of Aquatic Ecosystem Health 5: 223–229.
Zurawell, R. W., H. Chen, J. M. Burke & E. E. Prepas, 2004. Hepatotoxic cyanobacteria: a review of the biological importance of microcystins in freshwater environments. Journal of Toxicology and Environmental Health 8: 1–37.
Acknowledgments
This research was funded by a special study grant from the Sacramento-San Joaquin Delta Interagency Ecological Program. Many people assisted with the field sampling, E. Santos, M. Dempsey, K. Gehrts, S. Philippart and K. Clark and phytoplankton analysis, M. Bentencourt.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: D. Hamilton
Rights and permissions
About this article
Cite this article
Lehman, P.W., Boyer, G., Satchwell, M. et al. The influence of environmental conditions on the seasonal variation of Microcystis cell density and microcystins concentration in San Francisco Estuary. Hydrobiologia 600, 187–204 (2008). https://doi.org/10.1007/s10750-007-9231-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-007-9231-x