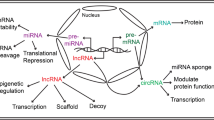
Abstract
Hypertrophic cardiomyopathy (HCM) is the most common heritable cardiomyopathy and is characterized by increased left ventricular wall thickness, but existing diagnostic and treatment approaches face limitations. MicroRNAs (miRNAs) are type of noncoding RNA molecule that plays crucial roles in the pathological process of cardiac remodelling. Accordingly, miRNAs related to HCM may represent potential novel therapeutic targets. In this review, we first discuss the different roles of miRNAs in the development of HCM. We then summarize the roles of common miRNAs as diagnostic and clinical biomarkers in HCM. Finally, we outline current and future challenges and potential new directions for miRNA-based therapeutics for HCM.


Similar content being viewed by others
Availability of data and material
The datasets from the current study are available from the corresponding author upon reasonable request.
References
Maron BJ, Ommen SR, Semsarian C, Spirito P, Olivotto I, Maron MS (2014) Hypertrophic cardiomyopathy: present and future, with translation into contemporary cardiovascular medicine. J Am Coll Cardiol 64:83–99
Angelopoulos A, Oikonomou E, Vogiatzi G, Antonopoulos A, Tsalamandris S, Georgakopoulos C, Papanikolaou P, Lazaros G, Charalambous G, Siasos G, Vlachopoulos C, Tousoulis D (2021) MicroRNAs as biomarkers in hypertrophic cardiomyopathy: current state of the art. Curr Med Chem 28:7400–7412
Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, Evanovich LL, Hung J, Joglar JA, Kantor P, Kimmelstiel C, Kittleson M, Link MS, Maron MS, Martinez MW, Miyake CY, Schaff HV, Semsarian C, Sorajja P (2020) 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 142:e558–e631
Maron BJ (2018) Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med 379:1977
Semsarian C, Ingles J, Maron MS, Maron BJ (2015) New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol 65:1249–1254
Geske JB, McKie PM, Ommen SR, Sorajja P (2013) B-type natriuretic peptide and survival in hypertrophic cardiomyopathy. J Am Coll Cardiol 61:2456–2460
Kawahara C, Tsutamoto T, Sakai H, Nishiyama K, Yamaji M, Fujii M, Yamamoto T, Horie M (2011) Prognostic value of serial measurements of highly sensitive cardiac troponin I in stable outpatients with nonischemic chronic heart failure. Am Heart J 162:639–645
Small EM, Olson EN (2011) Pervasive roles of microRNAs in cardiovascular biology. Nature 469:336–342
Zhou H, Tang W, Yang J, Peng J, Guo J, Fan C (2021) MicroRNA-related strategies to improve cardiac function in heart failure. Front Cardiovasc Med 8:773083
Roma-Rodrigues C, Raposo LR, Fernandes AR (2015) MicroRNAs based therapy of hypertrophic cardiomyopathy: the road traveled so far. Biomed Res Int 2015:983290
Liu N, Olson EN (2010) MicroRNA regulatory networks in cardiovascular development. Dev Cell 18:510–525
Lewis BP, Burge CB, Bartel DP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120:15–20
Kumarswamy R, Anker SD, Thum T (2010) MicroRNAs as circulating biomarkers for heart failure: questions about MiR-423–5p. Circ Res 106:e8; author reply e9
Wang GK, Zhu JQ, Zhang JT, Li Q, Li Y, He J, Qin YW, Jing Q (2010) Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J 31:659–666
Roncarati R, Viviani Anselmi C, Losi MA, Papa L, Cavarretta E, Da Costa MP, Contaldi C, Saccani Jotti G, Franzone A, Galastri L, Latronico MV, Imbriaco M, Esposito G, De Windt L, Betocchi S, Condorelli G (2014) Circulating miR-29a, among other up-regulated microRNAs, is the only biomarker for both hypertrophy and fibrosis in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 63:920–927
Palacin M, Reguero JR, Martin M, Diaz Molina B, Moris C, Alvarez V, Coto E (2011) Profile of microRNAs differentially produced in hearts from patients with hypertrophic cardiomyopathy and sarcomeric mutations. Clin Chem 57:1614–1616
Weidemann F, Niemann M, Warnock DG, Ertl G, Wanner C (2011) The Fabry cardiomyopathy: models for the cardiologist. Annu Rev Med 62:59–67
Care A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Hoydal M, Autore C, Russo MA, Dorn GW 2nd, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G (2007) MicroRNA-133 controls cardiac hypertrophy. Nat Med 13:613–618
Greaves SC, Roche AH, Neutze JM, Whitlock RM, Veale AM (1987) Inheritance of hypertrophic cardiomyopathy: a cross sectional and M mode echocardiographic study of 50 families. Br Heart J 58:259–266
Deng J, Zhong Q (2016) Advanced research on the microRNA mechanism in heart failure. Int J Cardiol 220:61–64
Wang J, Liew OW, Richards AM, Chen YT (2016) Overview of MicroRNAs in cardiac hypertrophy, fibrosis, and apoptosis. Int J Mol Sci 17
Xu X, Su YL, Shi JY, Lu Q, Chen C (2021) MicroRNA-17-5p promotes cardiac hypertrophy by targeting Mfn2 to inhibit autophagy. Cardiovasc Toxicol 21:759–771
Zhang S, Yin Z, Dai FF, Wang H, Zhou MJ, Yang MH, Zhang SF, Fu ZF, Mei YW, Zang MX, Xue L (2019) miR-29a attenuates cardiac hypertrophy through inhibition of PPARdelta expression. J Cell Physiol 234:13252–13262
Wang W, Wu C, Ren L, Bao Y, Han Y, Li C, Li Y (2020) MiR-30e-5p is sponged by Kcnq1ot1 and represses angiotensin II-induced hypertrophic phenotypes in cardiomyocytes by targeting ADAM9. Exp Cell Res 394:112140
Mo B, Wu X, Wang X, Xie J, Ye Z, Li L (2019) miR-30e-5p mitigates hypoxia-induced apoptosis in human stem cell-derived cardiomyocytes by suppressing Bim. Int J Biol Sci 15:1042–1051
Yu XJ, Huang YQ, Shan ZX, Zhu JN, Hu ZQ, Huang L, Feng YQ, Geng QS (2019) MicroRNA-92b-3p suppresses angiotensin II-induced cardiomyocyte hypertrophy via targeting HAND2. Life Sci 232:116635
Hinkel R, Batkai S, Bahr A, Bozoglu T, Straub S, Borchert T, Viereck J, Howe A, Hornaschewitz N, Oberberger L, Jurisch V, Kozlik-Feldmann R, Freudenthal F, Ziegler T, Weber C, Sperandio M, Engelhardt S, Laugwitz KL, Moretti A, Klymiuk N, Thum T, Kupatt C (2021) AntimiR-132 attenuates myocardial hypertrophy in an animal model of percutaneous aortic constriction. J Am Coll Cardiol 77:2923–2935
Yan H, Li Y, Wang C, Zhang Y, Liu C, Zhou K, Hua Y (2017) Contrary microRNA expression pattern between fetal and adult cardiac remodeling: therapeutic value for heart failure. Cardiovasc Toxicol 17:267–276
Ding YQ, Zhang YH, Lu J, Li B, Yu WJ, Yue ZB, Hu YH, Wang PX, Li JY, Cai SD, Ye JT, Liu PQ (2021) MicroRNA-214 contributes to Ang II-induced cardiac hypertrophy by targeting SIRT3 to provoke mitochondrial malfunction. Acta Pharmacol Sin 42:1422–1436
Jin L, Zhou Y, Han L, Piao J (2020) MicroRNA302-367-PI3K-PTEN-AKT-mTORC1 pathway promotes the development of cardiac hypertrophy through controlling autophagy. In Vitro Cell Dev Biol Anim 56:112–119
Zeng N, Wen YH, Pan R, Yang J, Yan YM, Zhao AZ, Zhu JN, Fang XH, Shan ZX (2021) Dickkopf 3: a novel target gene of miR-25-3p in promoting fibrosis-related gene expression in myocardial fibrosis. J Cardiovasc Transl Res 14:1051–1062
Zhang W, Wang Q, Feng Y, Chen X, Yang L, Xu M, Wang X, Li W, Niu X, Gao D (2020) MicroRNA-26a protects the heart against hypertension-induced myocardial fibrosis. J Am Heart Assoc 9:e017970
Liu ZY, Lu M, Liu J, Wang ZN, Wang WW, Li Y, Song ZJ, Xu L, Liu Q, Li FH (2020) MicroRNA-144 regulates angiotensin II-induced cardiac fibroblast activation by targeting CREB. Exp Ther Med 20:2113–2121
Zhou Y, Ng DYE, Richards AM, Wang P (2020) microRNA-221 inhibits latent TGF-beta1 activation through targeting thrombospondin-1 to attenuate kidney failure-induced cardiac fibrosis. Mol Ther Nucleic Acids 22:803–814
Shi Y, Zhang Z, Yin Q, Fu C, Barszczyk A, Zhang X, Wang J, Yang D (2021) Cardiac-specific overexpression of miR-122 induces mitochondria-dependent cardiomyocyte apoptosis and promotes heart failure by inhibiting Hand2. J Cell Mol Med 25:5326–5334
Zhou F, Fu WD, Chen L (2019) MiRNA-182 regulates the cardiomyocyte apoptosis in heart failure. Eur Rev Med Pharmacol Sci 23:4917–4923
Zakowicz H, Yang HS, Stark C, Wlodawer A, Laronde-Leblanc N, Colburn NH (2005) Mutational analysis of the DEAD-box RNA helicase eIF4AII characterizes its interaction with transformation suppressor Pdcd4 and eIF4GI. RNA 11:261–274
Simmen T, Aslan JE, Blagoveshchenskaya AD, Thomas L, Wan L, Xiang Y, Feliciangeli SF, Hung CH, Crump CM, Thomas G (2005) PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. EMBO J 24:717–729
Li H, Zhu H, Xu CJ, Yuan J (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491–501
Chen K, Zhang B, Sun Z (2021) MicroRNA 379 regulates Klotho deficiency-induced cardiomyocyte apoptosis via repression of Smurf1. Hypertension 78:342–352
Zhu X, Lu X (2019) MiR-423-5p inhibition alleviates cardiomyocyte apoptosis and mitochondrial dysfunction caused by hypoxia/reoxygenation through activation of the wnt/beta-catenin signaling pathway via targeting MYBL2. J Cell Physiol 234:22034–22043
Seok H, Lee H, Jang ES, Chi SW (2018) Evaluation and control of miRNA-like off-target repression for RNA interference. Cell Mol Life Sci 75:797–814
Hinkel R, Ramanujam D, Kaczmarek V, Howe A, Klett K, Beck C, Dueck A, Thum T, Laugwitz KL, Maegdefessel L, Weber C, Kupatt C, Engelhardt S (2020) AntimiR-21 prevents myocardial dysfunction in a pig model of ischemia/reperfusion injury. J Am Coll Cardiol 75:1788–1800
Piegari E, Cozzolino A, Ciuffreda LP, Cappetta D, De Angelis A, Urbanek K, Rossi F, Berrino L (2020) Cardioprotective effects of miR-34a silencing in a rat model of doxorubicin toxicity. Sci Rep 10:12250
Zhu JN, Fu YH, Hu ZQ, Li WY, Tang CM, Fei HW, Yang H, Lin QX, Gou DM, Wu SL, Shan ZX (2017) Activation of miR-34a-5p/Sirt1/p66shc pathway contributes to doxorubicin-induced cardiotoxicity. Sci Rep 7:11879
Stegmayer G, Di Persia LE, Rubiolo M, Gerard M, Pividori M, Yones C, Bugnon LA, Rodriguez T, Raad J, Milone DH (2019) Predicting novel microRNA: a comprehensive comparison of machine learning approaches. Brief Bioinform 20:1607–1620
Shieh JT, Huang Y, Gilmore J, Srivastava D (2011) Elevated miR-499 levels blunt the cardiac stress response. PLoS One 6:e19481
Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Roxe T, Muller-Ardogan M, Bonauer A, Zeiher AM, Dimmeler S (2010) Circulating microRNAs in patients with coronary artery disease. Circ Res 107:677–684
Economou EK, Oikonomou E, Siasos G, Papageorgiou N, Tsalamandris S, Mourouzis K, Papaioanou S, Tousoulis D (2015) The role of microRNAs in coronary artery disease: from pathophysiology to diagnosis and treatment. Atherosclerosis 241:624–633
Briasoulis A, Sharma S, Telila T, Mallikethi-Reddy S, Papageorgiou N, Oikonomou E, Tousoulis D (2019) MicroRNAs in atrial fibrillation. Curr Med Chem 26:855–863
Moushi A, Michailidou K, Soteriou M, Cariolou M, Bashiardes E (2018) MicroRNAs as possible biomarkers for screening of aortic aneurysms: a systematic review and validation study. Biomarkers 23:253–264
Moushi A, Pillar N, Keravnou A, Soteriou M, Shomron N, Cariolou MA, Bashiardes E (2020) MicroRNAs in ascending thoracic aortic aneurysms. Biosci Rep 40
Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E, Shah A, Willeit J, Mayr M (2010) Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res 107:810–817
Briasoulis A, Tousoulis D, Vogiatzi G, Siasos G, Papageorgiou N, Oikonomou E, Genimata V, Konsola T, Stefanadis C (2013) MicroRNAs: biomarkers for cardiovascular disease in patients with diabetes mellitus. Curr Top Med Chem 13:1533–1539
Callis TE, Wang DZ (2008) Taking microRNAs to heart. Trends Mol Med 14:254–260
Chen JF, Murchison EP, Tang R, Callis TE, Tatsuguchi M, Deng Z, Rojas M, Hammond SM, Schneider MD, Selzman CH, Meissner G, Patterson C, Hannon GJ, Wang DZ (2008) Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc Natl Acad Sci USA 105:2111–2116
Albinsson S, Suarez Y, Skoura A, Offermanns S, Miano JM, Sessa WC (2010) MicroRNAs are necessary for vascular smooth muscle growth, differentiation, and function. Arterioscler Thromb Vasc Biol 30:1118–1126
Zhao Y, Ransom JF, Li A, Vedantham V, von Drehle M, Muth AN, Tsuchihashi T, McManus MT, Schwartz RJ, Srivastava D (2007) Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell 129:303–317
Hoelscher SC, Doppler SA, Dressen M, Lahm H, Lange R, Krane M (2017) MicroRNAs: pleiotropic players in congenital heart disease and regeneration. J Thorac Dis 9:S64–S81
Torrini C, Cubero RJ, Dirkx E, Braga L, Ali H, Prosdocimo G, Gutierrez MI, Collesi C, Licastro D, Zentilin L, Mano M, Zacchigna S, Vendruscolo M, Marsili M, Samal A, Giacca M (2019) Common regulatory pathways mediate activity of MicroRNAs inducing cardiomyocyte proliferation. Cell Rep 27:2759–2771 e5
Porrello ER, Johnson BA, Aurora AB, Simpson E, Nam YJ, Matkovich SJ, Dorn GW 2nd, van Rooij E, Olson EN (2011) MiR-15 family regulates postnatal mitotic arrest of cardiomyocytes. Circ Res 109:670–679
Ohtani K, Dimmeler S (2011) Control of cardiovascular differentiation by microRNAs. Basic Res Cardiol 106:5–11
Bagnall RD, Tsoutsman T, Shephard RE, Ritchie W, Semsarian C (2012) Global microRNA profiling of the mouse ventricles during development of severe hypertrophic cardiomyopathy and heart failure. PLoS One 7:e44744
Kuster DW, Mulders J, Ten Cate FJ, Michels M, Dos Remedios CG, da Costa Martins PA, van der Velden J, Oudejans CB (2013) MicroRNA transcriptome profiling in cardiac tissue of hypertrophic cardiomyopathy patients with MYBPC3 mutations. J Mol Cell Cardiol 65:59–66
Song L, Su M, Wang S, Zou Y, Wang X, Wang Y, Cui H, Zhao P, Hui R, Wang J (2014) MiR-451 is decreased in hypertrophic cardiomyopathy and regulates autophagy by targeting TSC1. J Cell Mol Med 18:2266–2274
Maron BJ, Maron MS (2013) Hypertrophic cardiomyopathy. Lancet 381:242–255
Schwartz RA, Fernandez G, Kotulska K, Jozwiak S (2007) Tuberous sclerosis complex: advances in diagnosis, genetics, and management. J Am Acad Dermatol 57:189–202
Bhaskar PT, Hay N (2007) The two TORCs and Akt. Dev Cell 12:487–502
Ming S, Shui-Yun W, Wei Q, Jian-Hui L, Ru-Tai H, Lei S, Mei J, Hui W, Ji-Zheng W (2018) miR-139–5p inhibits isoproterenol-induced cardiac hypertrophy by targetting c-Jun. Biosci Rep 38
Sun D, Li C, Liu J, Wang Z, Liu Y, Luo C, Chen Y, Wen S (2019) Expression profile of microRNAs in hypertrophic cardiomyopathy and effects of microRNA-20 in inducing cardiomyocyte hypertrophy through regulating gene MFN2. DNA Cell Biol 38:796–807
Chen KH, Guo X, Ma D, Guo Y, Li Q, Yang D, Li P, Qiu X, Wen S, Xiao RP, Tang J (2004) Dysregulation of HSG triggers vascular proliferative disorders. Nat Cell Biol 6:872–883
Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, Hewett TE, Jones SP, Lefer DJ, Peng CF, Kitsis RN, Molkentin JD (2000) The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J 19:6341–6350
Zhou J, Zhou Y, Wang CX (2018) LncRNA-MIAT regulates fibrosis in hypertrophic cardiomyopathy (HCM) by mediating the expression of miR-29a-3p. J Cell Biochem
Derda AA, Thum S, Lorenzen JM, Bavendiek U, Heineke J, Keyser B, Stuhrmann M, Givens RC, Kennel PJ, Schulze PC, Widder JD, Bauersachs J, Thum T (2015) Blood-based microRNA signatures differentiate various forms of cardiac hypertrophy. Int J Cardiol 196:115–122
Fang L, Ellims AH, Moore XL, White DA, Taylor AJ, Chin-Dusting J, Dart AM (2015) Circulating microRNAs as biomarkers for diffuse myocardial fibrosis in patients with hypertrophic cardiomyopathy. J Transl Med 13:314
Thottakara T, Lund N, Kramer E, Kirchhof P, Carrier L, Patten M (2021) A novel miRNA screen identifies miRNA-4454 as a candidate biomarker for ventricular fibrosis in patients with hypertrophic cardiomyopathy. Biomolecules 11
Ebrahimkhani S, Vafaee F, Young PE, Hur SSJ, Hawke S, Devenney E, Beadnall H, Barnett MH, Suter CM, Buckland ME (2017) Exosomal microRNA signatures in multiple sclerosis reflect disease status. Sci Rep 7:14293
Sanz-Rubio D, Martin-Burriel I, Gil A, Cubero P, Forner M, Khalyfa A, Marin JM (2018) Stability of circulating exosomal miRNAs in healthy subjects. Sci Rep 8:10306
De Toro J, Herschlik L, Waldner C, Mongini C (2015) Emerging roles of exosomes in normal and pathological conditions: new insights for diagnosis and therapeutic applications. Front Immunol 6:203
Liu W, Bu H (2021) ECG-based parameters combined exosomes in atrial fibrillation: Diagnosis potential and role as clinical biomarkers. Int J Cardiol
Xiang K, Akram M, Elbossaty WF, Yang J, Fan C (2021) Exosomes in atrial fibrillation: therapeutic potential and role as clinical biomarkers. Heart Fail Rev
van Rooij E, Olson EN (2012) MicroRNA therapeutics for cardiovascular disease: opportunities and obstacles. Nat Rev Drug Discov 11:860–872
Sartorio CL, Lazzeroni D, Bertoli G, Camici PG (2017) Theranostic biomarkers in hypertrophic cardiomyopathy: insights in a long road ahead. Front Biosci (Landmark Ed) 22:1724–1749
Ucar A, Gupta SK, Fiedler J, Erikci E, Kardasinski M, Batkai S, Dangwal S, Kumarswamy R, Bang C, Holzmann A, Remke J, Caprio M, Jentzsch C, Engelhardt S, Geisendorf S, Glas C, Hofmann TG, Nessling M, Richter K, Schiffer M, Carrier L, Napp LC, Bauersachs J, Chowdhury K, Thum T (2012) The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat Commun 3:1078
Batkai S, Genschel C, Viereck J, Rump S, Bar C, Borchert T, Traxler D, Riesenhuber M, Spannbauer A, Lukovic D, Zlabinger K, Hasimbegovic E, Winkler J, Garamvolgyi R, Neitzel S, Gyongyosi M, Thum T (2021) CDR132L improves systolic and diastolic function in a large animal model of chronic heart failure. Eur Heart J 42:192–201
Taubel J, Hauke W, Rump S, Viereck J, Batkai S, Poetzsch J, Rode L, Weigt H, Genschel C, Lorch U, Theek C, Levin AA, Bauersachs J, Solomon SD, Thum T (2021) Novel antisense therapy targeting microRNA-132 in patients with heart failure: results of a first-in-human Phase 1b randomized, double-blind, placebo-controlled study. Eur Heart J 42:178–188
Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S (2008) MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 456:980–984
van Rooij E, Liu N, Olson EN (2008) MicroRNAs flex their muscles. Trends Genet 24:159–166
Montgomery RL, Hullinger TG, Semus HM, Dickinson BA, Seto AG, Lynch JM, Stack C, Latimer PA, Olson EN, van Rooij E (2011) Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation 124:1537–1547
Karakikes I, Chaanine AH, Kang S, Mukete BN, Jeong D, Zhang S, Hajjar RJ, Lebeche D (2013) Therapeutic cardiac-targeted delivery of miR-1 reverses pressure overload-induced cardiac hypertrophy and attenuates pathological remodeling. J Am Heart Assoc 2:e000078
Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, Ponimaskin E, Schmiedl A, Yin X, Mayr M, Halder R, Fischer A, Engelhardt S, Wei Y, Schober A, Fiedler J, Thum T (2014) Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest 124:2136–2146
Ganesan J, Ramanujam D, Sassi Y, Ahles A, Jentzsch C, Werfel S, Leierseder S, Loyer X, Giacca M, Zentilin L, Thum T, Laggerbauer B, Engelhardt S (2013) MiR-378 controls cardiac hypertrophy by combined repression of mitogen-activated protein kinase pathway factors. Circulation 127:2097–2106
Nagalingam RS, Sundaresan NR, Gupta MP, Geenen DL, Solaro RJ, Gupta M (2013) A cardiac-enriched microRNA, miR-378, blocks cardiac hypertrophy by targeting Ras signaling. J Biol Chem 288:11216–11232
Carrer M, Liu N, Grueter CE, Williams AH, Frisard MI, Hulver MW, Bassel-Duby R, Olson EN (2012) Control of mitochondrial metabolism and systemic energy homeostasis by microRNAs 378 and 378*. Proc Natl Acad Sci USA 109:15330–15335
Nagalingam RS, Sundaresan NR, Noor M, Gupta MP, Solaro RJ, Gupta M (2014) Deficiency of cardiomyocyte-specific microRNA-378 contributes to the development of cardiac fibrosis involving a transforming growth factor beta (TGFbeta1)-dependent paracrine mechanism. J Biol Chem 289:27199–27215
Bernardo BC, Nguyen SS, Winbanks CE, Gao XM, Boey EJ, Tham YK, Kiriazis H, Ooi JY, Porrello ER, Igoor S, Thomas CJ, Gregorevic P, Lin RC, Du XJ, McMullen JR (2014) Therapeutic silencing of miR-652 restores heart function and attenuates adverse remodeling in a setting of established pathological hypertrophy. FASEB J 28:5097–5110
Baptista PV (2014) Gold nanobeacons: a potential nanotheranostics platform. Nanomedicine (Lond) 9:2247–2250
van Rooij E, Kauppinen S (2014) Development of microRNA therapeutics is coming of age. EMBO Mol Med 6:851–864
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was prepared in accordance with the Committee on Publication Ethics (COPE) guidelines to respect third party rights, such as copyright and/or moral rights. Ethical approval was not required to conduct this project, as the data are not individualized and primary data were not collected.
Consent for publication
All authors have read and approved the content and agree to submit the final manuscript for consideration and publication in your journal.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Luo, F., Liu, W. & Bu, H. MicroRNAs in hypertrophic cardiomyopathy: pathogenesis, diagnosis, treatment potential and roles as clinical biomarkers. Heart Fail Rev 27, 2211–2221 (2022). https://doi.org/10.1007/s10741-022-10231-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-022-10231-z