Abstract
Malnutrition is common in heart failure (HF), and it is associated with higher hospital readmission and mortality rates. This review aims to answer the question whether nutritional interventions aiming to increase protein and energy intake are effective at improving outcomes for patients with HF who are malnourished or at risk of malnutrition or cachexia. Systematic searches of four databases (Medline, Embase, CINAHL and Cochrane Central Register of Controlled Trials (CENTRAL)) were conducted on 21 June 2019. Randomized controlled trials (RCTs) or other interventional studies using protein or energy supplementation for adult HF patients who are malnourished or at risk of malnutrition or cachexia were included. Two independent reviewers assessed study eligibility and risk of bias. Five studies (four RCTs and one pilot RCT) met the inclusion criteria. The majority of studies were small and of limited quality. The pooled weighted mean difference (WMD) for body weight showed a benefit from the nutritional intervention by 3.83 kg (95% confidence interval (CI) 0.17 to 7.50, P = 0.04) from three trials with no significant benefit for triceps skinfold thickness (WMD = − 2.14 mm, 95% CI − 9.07 to 4.79, P = 0.55) from two trials. The combination of personalized nutrition intervention with conventional treatment led to a decrease in all-cause mortality and hospital readmission in one study. Findings of this review suggest that nutritional interventions could potentially improve outcomes in HF patients who are malnourished or at risk of malnutrition. However, the strength of the evidence is poor, and more robust studies with a larger number of participants are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
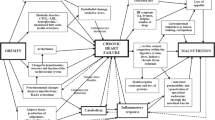
Heart failure (HF) is considered as a present-day epidemic, with 26 million cases worldwide [1] causing a burden on health care systems; for example, it has been valued that 2% of the National Health Service (NHS) budget is spent on HF alone [2]. The incidence of HF tends to increase with age due to age-associated changes in the heart’s function and structure [3], making HF one of the most common reasons for hospitalization in older adults [4]. The 1-year mortality rate among HF patients admitted to hospital has been estimated at 29.6% by the recent Annual National HF Audit in England [5]. Additionally, patients with HF tend to also have a high hospital readmission rate with almost 25% of patients being readmitted within 30 days [6]. Although maintaining a good quality of life is important for patients’ survival and outlook [7], it has been shown that the quality of life for patients with HF is lower than in any other chronic disease [8].
Malnutrition is common among patients with HF [9], and it predicts worse mortality and hospital readmission outcomes [10, 11]. The prevalence of malnutrition among such a group of patients has been reported to be as high as 69% depending on the screening tool being used [12], and it can be attributed to illness-related factors, such as reduced calorie intake due to medication induced anorexia (e.g. diuretics), anxiety and the lack of energy to prepare food [13, 14]. Moreover, around 5–15% of HF patients tend to suffer from cardiac cachexia [15], defined as ‘involuntary progressive weight loss due to the reduction in skeletal muscle mass with or without depletion of adipose tissue’ [16]. Cachexia is caused by immunological and hormonal abnormalities, switching the body from an anabolic to a catabolic state by a decrease in the activity and levels of anabolic mediators such as insulin and growth hormone and an increase in activity and levels of catabolic mediators such as pro-inflammatory cytokines and glucocorticoids [17]. The above changes lead to a hypermetabolic state [18] and an increase in protein degradation [19], and therefore, result in muscle wasting.
Considering the pathophysiology of malnutrition and cachexia in HF, it has been hypothesised that the supplementation of protein or the increase in energy intake could reduce catabolic effects and increase in lean body mass tissue in these patients [20, 21]. However, no nutritional guidelines for the management of HF currently exist. Although systematic reviews have investigated the effectiveness of restrictive diets (e.g. low sodium and fluid restriction) for HF patients, no systematic review so far has focused on nutritional interventions tackling malnutrition in HF patients. Therefore, this systematic review, being the first of its kind, will focus on answering the question whether nutritional interventions aiming to increase protein or energy intake for malnourished or at risk of malnutrition or cachexia HF patients are effective at improving clinical outcomes including nutritional status, mortality and hospital readmission. The aim is to present the evidence regarding the effectiveness of nutritional interventions, which can potentially help form guidelines for nutritional support in HF patients.
Methods
Searches
A search strategy was created and applied with the assistance of a professional librarian to combine the following key concepts: HF, malnutrition/cachexia and oral nutrition supplements (ONS). The search strategy was applied to four databases: Embase, Medline, CINAHL and Cochrane Controlled Register of Trials (CENTRAL) on 21 June 2019. Detailed search strategy for Medline and Embase can be found in Appendix 1. Reference list searching from selected included papers was also undertaken. No restrictions were applied on language or publication date when applying the searches. A filter was applied for the Embase search to exclude conference abstracts. Findings were reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [22]. A PRISMA checklist is provided in Appendix 2.
Study selection
Independent screening of title, abstract and full text was performed by two reviewers (DH, MBM) who selected studies that met the inclusion criteria. Discussions took place between the two reviewers, and disagreements were resolved by discussion with a third reviewer (CA) when required. Studies that were not possible to be obtained in a full-text English version were excluded.
Types of studies included
Design
Randomized controlled trials (RCTs) or other interventional studies in humans.
Participants
Inclusion criteria:
-
Adult patients with a diagnosis of HF (any age > 18)
-
Malnutrition, cachexia or risk of malnutrition
Exclusion criteria:
-
Children/adolescents
-
Participants without a diagnosis of HF
Interventions
Inclusion criteria: (1) ONS, (2) food enrichment, (3) any other form of protein supplementation, (4) nutrition education targeting the increase of protein or energy intake, (5) combination of any of the methods mentioned above (no time limit for the duration of intervention or time limit for follow-up was set).
Exclusion criteria: (1) interventions aiming to reduce salt and water intake, (2) other interventions not focussing on increasing protein or energy intake (e.g. interventions aiming to reduce cholesterol), (3) vitamin supplementation (e.g. vitamin D) unless this formed part of a wider intervention aiming to increase protein/energy.
Comparator
Inclusion criteria: studies that compared the intervention group with:
-
1.
Standard care
-
2.
Placebo
-
3.
Other non-nutritional interventions applied to both the control and the nutritional intervention group, e.g. physical activity/exercise
-
4.
Pre-intervention measurements
Exclusion criteria: no suitable comparator (when it was not possible to separate the effect attributed purely to a nutritional intervention).
Outcomes
Inclusion criteria:
Studies with at least one of the following primary outcomes:
-
1.
Nutritional status assessed by any of the following:
-
(a)
Anthropometry (BMI, weight, mid upper arm circumference, calf circumference, triceps skinfold thickness, lean body weight, etc.)
-
(b)
Nutritional risk (measured by Mini Nutritional Assessment (MNA), Malnutrition Universal Screening Tool (MUST) or other validated tools)
-
(c)
Nutritional-related outcomes that are subjectively assessed (self-reported protein and energy intake, dietary recall, changes in dietary behaviour or knowledge)
-
2.
Hospital admission/readmission
-
3.
Mortality
Secondary outcomes:
-
1.
Quality of life
-
2.
Depression
-
3.
Physical performance
-
4.
Functioning
-
5.
Outcomes related to HF (e.g. breathlessness, exercise tolerance)
-
6.
Outcomes related to malnutrition (e.g. infections, pressure sores, etc.)
-
7.
Any other relevant clinical outcome (excluding biochemical outcomes that are unrelated to malnutrition)
Exclusion criteria: studies that measured exercise capacity only without measuring any of the main primary outcomes (nutrition, hospital readmission and mortality) were excluded.
Data extraction
Data was extracted by the main author (DH), who tabulated data for each study, including author, year, country, setting, study population, sample size, number of participants in intervention and control, mean age (intervention/control), sex (female/male), type of intervention, duration of intervention, control (type of comparator), period of follow-up, number of participants completing the follow-up, outcomes assessed and main findings. The authors of the articles included in this review were contacted by e-mail when information was unclear/missing.
Study quality assessment
The Cochrane Risk of Bias Tool for randomized controlled trials [23] was used to assess the methodological quality of the included studies. Two reviewers (DH and CA) independently assessed risk of bias.
Data synthesis and presentation
Data from included studies was extracted and included in tables summarising the results. A random effects pooled analysis was performed for outcomes where the length of the intervention and type of intervention was comparable, and the combined post-intervention effect was calculated. Results were converted into mean ± standard deviation when possible, and the authors were contacted when unpublished data was required for the meta-analysis. Heterogeneity was calculated using I2 statistical test and data in the pooled analysis was presented as difference in mean and 95% confidence intervals (CIs). P < 0.05 was considered statistically significant. Review Manager 5 (RevMan 5) software was used. Other outcomes were presented in a narrative way.
Study registration
The protocol of this review has been registered in the PROSPERO database (International Prospective Register of Systematic Reviews, University of York) (CRD42019142323) [24].
Results
Selection process
A total of 824 titles and abstracts were identified in the searches and five additional articles were identified through reference list screening of the included articles. After removing duplicates (n = 192), 632 + 5 articles were screened in which 621 titles and abstracts were excluded as found to be irrelevant. The remainder 16 articles were selected to be screened for eligibility. Of those 16 articles screened, 11 were rejected, as one did not have a suitable comparator [25], six did not have a relevant outcome [26,27,28,29,30,31], one was a protocol paper [32], one was reporting secondary outcomes of studies already selected to be included in the review [33], and one was rejected as the full-text English version was not available [34]. One further paper was found to be relevant, but it was excluded because the study population was mixed including patients with heart failure, acute myocardial infarction, pneumonia and chronic obstructive pulmonary disease (COPD), and the results were presented for the whole patient population without subgroup analysis for HF only [35]. Finally, five studies were included in this review. The PRISMA flow chart in Fig. 1 shows a summary of the selection process.
Study characteristics
Among the five studies that were included in this review, four were RCTs and one was a pilot RCT. The total number of participants included in these studies was 275. The country of origin varied, including Poland [36], Sweden [37], Mexico [38], Italy [39] and Spain [40]. Table 1 shows the characteristics of the studies included in this review.
Participants
More male than female participants were recruited in the selected trials (total males count 152 vs. total females count 123). Most of the studies included participants who were older than 60 (n = 4), while only one study recruited participants who are 42 years and older [36]. The studies selected for this review recruited HF patients who were malnourished or at risk of malnutrition or cachexia; however, the classification of HF as well as the definition and the diagnosis of malnutrition varied among the studies. One study [40] recruited participants with acute heart failure (AHF) (either decompensated chronic heart failure (CHF) or a new onset of HF) who were malnourished identified by MNA (Mini Nutritional Assessment) [41]. In another study, patients had stable CHF New York Heart Association NYHA-II (71%) and NYHA-III (29%) with severe depletion of lean muscle mass identified through having an arm muscle circumference measurement < 10th percentile of normal values for age and sex [39]. Another study recruited stable CHF NYHA-II patients who were cachectic, identified as having a weight loss of 7.5% over the past 6 months [36]. Moreover, another study included participants with stable CHF NYHA-I (60.9%), NYHA-II (31.1%), NYHA-III (6%) and NYHA-IV (2%), in which 62.1% of the participants were diagnosed as cachectic, identified by Bioelectrical Impedance Analysis (BIA) [38]. Finally, in another study patients with severe CHF (NYHA-III-IV) were recruited, the majority of whom had markedly low exercise capacity and oxygen uptake, although only two participants were diagnosed as malnourished (identified as having two nutritional-related variables as subnormal, one of which was anthropometric (weight index, triceps skinfold thickness and arm muscle circumference) and one of which was biochemical (transthyretin and albumin)). Seven patients had low serum albumin, five had low serum transthyretin and four had anthropometric values below the range. In the same study, muscle biopsies of patients with HF compared to healthy controls showed significant decreases of adenosine triphosphate (ATP), creatine, total creatine and glycogen at baseline, considered to be markers of cardiac cachexia [37].
Interventions
There was a variety of nutritional interventions, which included ONS only in three of the studies [36, 37, 39], personalised dietary intervention with ONS prescriptions in cases where nutritional goals were not reached in one study [40] and a combination of an ONS with a resistance exercise (RE) programme in one study [38].
More specifically, the intervention consisted of:
-
A high-calorie, high-protein ONS containing 20 g of protein, 26 g of fat, 72 g of carbohydrates and a total energy of 600 kcal [36]
-
A 500 ml daily dietary supplement containing 30 g of protein, 30 g of fat, 87.5 g of carbohydrate and a total energy of 750 kcal [37]
-
Supplementation of 8 g/day of an oral essential amino acid [39]
-
Conventional treatment plus a nutritional intervention which included diet optimization, specific nutritional recommendations and nutritional supplement prescriptions when nutritional goals were not reached [40]
-
A resistance exercise programme plus 10 g/day of branched chain amino acid (BCAA) supplementation [38]
Intervention length, adherence to intervention and follow-up period
There was variation in the length of interventions for the studies included in this review with the longest duration of intervention being 6 months [40] and the shortest 6 weeks [36]. Other studies included had a duration of intervention of 8 weeks [37], 12 weeks [38] and finally 2 months [39]. The adherence to the intervention was not reported for most of the studies (n = 4); only one study reported adherence which was measured through counting the number of amino acid packs remaining after the end of the intervention period (2 months), and judgement of patient’s reliability was made through taking venous blood samples and measuring fasting plasma leucine concentration. Although the compliance rate was not reported, the authors assumed that the compliance was satisfactory [39]. The follow-up period for the selected studies ranged from 6 weeks [36] to 12 months [40].
Risk of bias
The risk of bias assessment for the studies included in this review is presented in Fig. 2. Random sequence generation had a high risk in one of the studies with other studies either being low (n = 2) or unclear (n = 2). Allocation concealment (selection bias) was either low risk (n = 2) or unclear (n = 3) in the studies included. Blinding of participants and personnel (performance bias) was high risk in two studies, unclear in one study and the remainder (n = 2) low risk. Blinding of outcome assessment (detection bias) was unclear in most of the studies (n = 4) with one of the studies having low risk. Most of the studies (n = 4) had low risk for incomplete outcome data (attrition bias) with only one of the studies being unclear. Selection reporting risk of bias was high in one of the studies, low for three studies and unclear for one study. Other risk of bias was high in one of the studies with the rest of the studies being unclear (n = 4).
Primary outcomes
Nutritional status
Anthropometry and body composition
Of the five studies included, four studies assessed anthropometry, and two studies assessed body composition, with mixed findings across the studies. In one study, the supplementation of 8 g of essential amino acids (EAA) per day for 2 months did not lead to significant changes in anthropometric measurements when comparing the intervention group with the control group receiving no supplementation. However, a significant increase in body weight for the EAA supplemented group was observed compared to their baseline body weight [39]. Another study showed that the supplementation of 500 ml daily dietary supplement for 8 weeks significantly increased triceps skinfold thickness during the 8-week follow-up period in the supplemented intervention group when compared to the control group receiving a placebo [37]. Significant benefits were also observed in another study by Rozentryt et at. [36], which found that the supplementation of a high-caloric, high-protein ONS for 6 weeks increased body weight and fat tissue mass measured by dual X-ray absorptiometry (DEXA) during the 6-week and 18-week follow-up period, and a significant increase in lean tissue mass measured by DEXA was observed during the 6-week follow-up period only. Nonetheless, this study was a pilot RCT and data was analysed in the form of pre-/post-intervention comparison at 6 weeks and 12 weeks for the intervention arm only, with no comparison data available between ONS and placebo.
In another study, the combined intervention of resistance exercise (RE) program with the daily nutritional supplementation of 10 g of BCAA for 12 weeks resulted in a significant reduction in hip circumference during the 12-week follow-up period when compared with the control group receiving resistance exercise program only. A reduction in waist circumference and an increase in muscle strength were observed in both groups (RE and RE + ONS) at the end of the programme, but changes were not significant between the groups. Moreover, body composition parameters assessed by BIA did not significantly improve with the addition of ONS to the exercise programme. The authors assumed that fat mass could have decreased, and muscle mass increased as a result of the anabolic effect of exercise. They also hypothesised that reduced hip and waist circumference might be related to reduced third space water retention in both groups, probably associated with increased albumin concentration [38].
Body weight
Three studies that assessed body weight were eligible to be included in the meta-analysis. The intervention length was 8 weeks [37], 12 weeks [38] and 2 months [39]. No significant heterogeneity was detected (I2 = 0%, P = 0.76), and pooled results have shown that the nutritional intervention significantly increased body weight in HF patients (112 participants; weighed mean difference = 3.83 kg, 95% CI 0.17 to 7.50, P = 0.04) (Fig. 3).
Triceps skinfold thickness
Two studies that assessed triceps skinfold thickness were eligible to be included in the meta-analysis. The intervention length was 8 weeks [37] and 2 months [39]. Significant heterogeneity was detected (I2 = 85%, P = 0.009), and pooled results have shown that the nutritional intervention had no effect on triceps skinfold thickness for HF patients (57 participants; weighted mean difference = − 2.14 mm, 95% CI − 9.07 to 4.79, P = 0.55) (Fig. 4).
Dietary assessment
Three studies assessed dietary intake. Two of these studies showed that the nutritional intervention did not result in any significant improvement in nutritional intake measured by 7-day food recall method [39] and 24-h dietary recall method [38]. However, significant improvement in nutritional intake measured by the modified dietary history method was observed in one of the studies, in which ONS supplementation resulted in an increase in fat and non-protein energy intake during the 8-week follow-up period when compared with the control group receiving a placebo [37].
Malnutrition-related biological parameters
None of the three included studies [36,37,38] that assessed malnutrition-related biological parameters showed any significant outcomes.
Hospital readmission and mortality
Only one of the studies reported hospital readmission and mortality. In this study, the intervention consisted of a 6-week conventional treatment with diet optimisation, specific nutritional recommendations and nutritional supplement prescriptions when nutritional goals were not reached. This resulted in a significant reduction in the composite end point (death from all causes or readmission for worsening of HF), as well as isolated all-cause mortality and hospital readmission rates during the 12-month follow-up period when compared to the control group receiving the conventional treatment alone [40].
Secondary outcomes
Exercise capacity
Four included studies assessed outcomes that related to exercise capacity, with only one study showing no significant effects on giving a nutritional intervention on exercise capacity-related outcomes [37]. However, positive benefits were observed in the other studies. For instance, in the study by Aquilani et al. [39], the supplementation of 8 g/day of EAA for 2 months led to a significant increase in power output, peak VO2 (maximum rate of oxygen consumption measured during incremental exercise) and 6-min walk functional test during the 2 month follow-up period when compared with the control group receiving no supplementation. Moreover, in a study by Rozentryt et al. [36], the nutritional intervention of supplementing a high-caloric, high-protein ONS for 6 weeks resulted in a significant increase in 6-min walk functional test during the 6-week follow-up period but not for the 18-week follow-up period when compared with pre-intervention values. Furthermore, another study showed that the combined intervention of resistance exercise program with the daily nutritional supplementation of 10 g of BCAA for 2 months resulted in a significant increase in exercise diastolic blood pressure during the 2-month follow-up period when compared with the control group receiving resistance exercise program only [38].
Quality of life
Quality of life was assessed in one study only, which has found that the nutritional intervention consisting of supplementing a high-caloric, high-protein ONS for 6 weeks resulted in a significant increase in quality of life during the 6-week and 18-week follow-up period when compared to pre-intervention values [36].
Other clinical heart failure related outcomes
Three included studies assessed clinical outcomes that relate to HF. The effect of the nutritional intervention and the outcomes being assessed among the studies varied; for example, in one study, the nutritional intervention of supplementing a high-caloric, high-protein ONS for 6 weeks resulted in a non-significant difference in left ventricular ejection fraction during the 6-week and 18-week follow-up period when compared to pre-intervention values [36]. However, in another study, the supplementation of a 500-ml daily dietary supplement for 8 weeks resulted in a significant increase in P-norepinephrine during the 8-week follow-up period when compared to the control group receiving a placebo [37]. Moreover, positive benefits were observed in another study in which the combined intervention of resistance exercise program with the daily nutritional supplementation of 10 g of BCAA for 2 months resulted in a significant decrease in dyspnoea when compared with the control group receiving resistance exercise program only [38].
Discussion
Summary of findings
Five studies were identified (four RCTs and one pilot RCT) reporting conflicting results on different outcomes being tested. Pooled analysis of data from three studies [37,38,39] showed a significant increase in body weight with ONS supplementation. This is an interesting finding, given that there was no significant weight change in the isolated studies included in the analysis, with the exception of one study which has found that body weight significantly increased in the EAA supplemented group when compared to their baseline body weight [39], and this could be potentially explained by a lack of power in the individual studies. However, pooled analysis of data from two studies [37, 39] showed no significant benefit on triceps skinfold thickness. Moreover, one study found that the combination of conventional treatment with personalized nutritional intervention for 6 months led to a significant decrease in all-cause mortality and hospital readmission rates during the 12-month follow-up period [40].
The results for other outcomes were mixed with some benefits from nutritional intervention on outcomes relating to body composition post-intervention compared to baseline, but without comparison with control group post-intervention [36], non-protein and fat energy intake [37], exercise capacity-related outcomes [36, 39], quality of life [36] and clinical HF-related outcomes such as dyspnoea [38], with no significant effect on malnutrition-related biological parameters [36,37,38]. Inconsistency in results across the studies could potentially be explained by the differences in the severity of HF, as well as the setting where the study was undertaken. The overall quality of the studies was low, with recruitment of a small number of participants and no formal sample size calculation in most cases.
Comparison with existing literature
Observational studies have shown that patients with HF tend to have insufficient energy and protein intake [9, 42], and it has been suggested that nutritional interventions aiming to increase protein or energy could lead to a better coping mechanism in the anabolic/catabolic imbalances caused by the inflammation process and neurohormone activation, which are common among HF patients [43]. Although some researchers have argued that nutritional interventions aiming to increase protein or calorie intake do not reverse the physiological consequences involved in cardiac cachexia [44, 45], this review has demonstrated that the 6-week supplementation of a high-caloric, high-protein ONS for cachectic HF patients led to a significant increase in body composition values measured by DEXA, such as the increase in fat tissue mass during the 6-week and 18-week follow-up period, as well as an increase in lean tissue mass during the 6-week follow-up period when comparing post-intervention with baseline measurements [36]. Such findings are important, as the increase in fat mass has been linked to better survival outcomes and the increase in lean body mass has been linked to a better quality of life among HF patients [46].
Furthermore, data from a recent systematic review has shown that malnutrition among HF patients is associated with higher hospital readmission and mortality rates [47] and this can consequently lead to a series of other negative long-term outcomes. For instance, frequent hospital readmissions among HF patients have been associated with a decrease in quality of life and an increase in healthcare costs related to HF [48, 49]. Given such information, the finding that personalised nutritional intervention for malnourished HF patients led to a reduction in hospital readmission and mortality [40] is important, as the integration of nutritional support in standard care for HF patients could potentially contribute to better outcomes for such patients. However, hospital readmission and mortality were measured as outcomes in only one of the included studies, and the effectiveness of nutritional interventions would have to be tested in a larger number of participants before safe conclusions can be made.
It is also important to report here key findings of a multicentre RCT where older, malnourished, hospitalised adults suffering from various conditions including heart failure, acute myocardial infarction, pneumonia or COPD were randomised to receive a specialised ONS consisting of a high protein content and beta-hydroxy-beta methylbutyrate (HMB) (HP-HMB) (n = 328) or a placebo supplement (n = 324). Among the recruited patients, 157 had HF (HP-HMB n = 79, placebo n = 78). No effects were observed for the primary composite endpoint (event of death or readmission within 90 days post-discharge). However, the 90-day mortality rate was lower with HP-HMB compared to placebo (4.8% vs. 9.7%; relative risk 0.49, 95% confidence interval [CI], 0.27 to 0.90). Compared with placebo, HP-HMB resulted in improved odds of better nutritional status at day 90, and an increase in body weight at day 30. No between-group differences were observed for 90-day readmission rate, length of stay in hospital or activities of daily living [35].
In terms of outcomes that relate to exercise capacity, results from this review have shown that nutritional intervention consisting of high protein or energy could induce possible positive effects on functional outcomes such as 6-min walk test and VO2 max (which is a predictor of exercise performance) [36, 39]. While this was a secondary outcome in this study, other studies in the literature have also shown that the supplementation of individualised amino acids such as taurine [50] or the combination of essential and semi-essential amino acids [51] could improve exercise capacity in HF patients who are not malnourished. Though this is beyond the scope of this review, it is important to mention that the benefits of supplementing amino acids go beyond just their anabolic effects, but rather they are important for the maintenance of normal physiological functions [52], which should also be considered when understanding the physiological benefit of protein or amino acid supplementation.
Strengths and limitations of this review
This review has several strengths, one being that it is, to this date, the first systematic review focusing on whether nutritional interventions aiming to increase protein or energy intake are effective at improving outcomes for patients with HF who are malnourished or at risk of malnutrition. Additionally, a rigorous methodology was followed, and more specifically, two independent reviewers were involved during screening, assessing the risk of bias and extracting data from the studies.
However, certain limitations of this review also exist, and these are related to methodological challenges. Importantly, the definition and the method of diagnosing malnutrition, risk of malnutrition or cachexia varied across the studies. Although there is a significant overlap between malnutrition and cachexia [53], these two conditions are not synonymous; hence, one could argue that the total population could potentially be heterogeneous, despite sharing common features. Additionally, adherence to the nutritional interventions was either insufficiently reported or not reported. Furthermore, only one study reported on hospital readmission and mortality, despite them being important outcomes when considering malnourished HF patients. Also, dietary intake measured by dietary recall methods could be subject to recall bias. Moreover, most of the studies included recruited a small number of participants with high attrition rates, and they did not have a formal sample size calculation, which means that they may have been underpowered. Finally, other limitations of this review are that no grey literature search was undertaken, and non-English articles were excluded; hence, relevant articles could have been missed.
Implications for practice and research
Findings of this review suggest that there is some evidence that protein or energy nutritional supplements could potentially improve body weight, hospital readmission, and mortality in patients with HF who are malnourished or at risk of malnutrition. However, it is important to interpret the results from this review with caution, given that most of the evidence is generated from low- to moderate-quality studies with a small sample size and a short follow-up period. Therefore, this review calls for better-quality studies to be conducted in the future with robust methodology, adequate power size calculation, careful selection of participants and assessment of important clinical outcomes as mortality and hospital readmission. Additionally, consistent measurements and definition of malnutrition should be considered with further exploration on the type of supplement and dosage required to reach optimum benefits along with the physiological mechanisms involved.
Conclusion
There is a potential benefit from oral nutritional supplements to increase body weight in patients with heart failure who are malnourished or at risk of malnutrition, and a potential benefit from individualized dietary intervention in reducing mortality and hospital readmission. However, the quality of the evidence is low, and no recommendations can be currently made to inform clinical practice. More robust, better-quality RCTs with a larger number of participants are needed to establish the effectiveness of nutritional interventions for malnutrition in heart failure.
References
Ambrosy A, Fonarow G, Butler J, Chioncel O, Greene S, Vaduganathan M, Nodari S, Lam C, Sato N, Shah A, Gheorghiade M (2014) The global health and economic burden of hospitalizations for heart failure. J Am Coll Cardiol 63(12):1123–1133. https://doi.org/10.1016/j.jacc.2013.11.053
Braunschweig F, Cowie M, Auricchio A (2011) What are the costs of heart failure? Europace 13(suppl 2):ii13–ii17. https://doi.org/10.1093/europace/eur081
Dharmarajan K, Rich M (2017) Epidemiology, pathophysiology, and prognosis of heart failure in older adults. Heart Fail Clin 13(3):417–426. https://doi.org/10.1016/j.hfc.2017.02.001
Health care cost and utilization project (HCUP) (2019) Statistical brief #66 [online]. Available from: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb66.jsp (Accessed 18 April 2019)
NHFA (2016-2017) [online]. Available from: https://www.nicor.org.uk/wp-content/uploads/2018/11/Heart-Failure-Summary-Report-2016-17.pdf (Accessed 18 April 2019)
McHugh M, Ma C (2013) Hospital nursing and 30-day readmissions among Medicare patients with heart failure, acute myocardial infarction, and pneumonia. Med Care 51(1):52–59. https://doi.org/10.1097/MLR.0b013e3182763284
Alla F, Briançon S, Guillemin F, Juillière Y, Mertès PM, Villemot JP, Zannad F (2002) Self-rating of quality of life provides additional prognostic information in heart failure. Insights into the EPICAL study. Eur J Heart Fail . [Online] 4 (3):337–343. https://doi.org/10.1016/s1388-9842(02)00006-5
Juenger, J, Schellberg, D, Kraemer, S, Haunstetter, A, Zugck, C, Herzog, W, and Haass, M (2002) Health related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables. Heart. [online] 87 (3):235–241. https://doi.org/10.1136/heart.87.3.235
Aquilani R, Opasich C, Verri M, Boschi F, Febo O, Pasini E, Pastoris O (2003) Is nutritional intake adequate in chronic heart failure patients? J Am Coll Cardiol 42(7):1218–1223. https://doi.org/10.1016/s0735-1097(03)00946-x
Yoshihisa A, Kanno Y, Watanabe S, Yokokawa T, Abe S, Miyata M, Sato T, Suzuki S, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Suzuki H, Ishida T, Takeishi Y (2018) Impact of nutritional indices on mortality in patients with heart failure. Open Heart 5(1):e000730. https://doi.org/10.1136/openhrt-2017-000730
Agra Bermejo R, González Ferreiro R, Varela Román A, Gómez Otero I, Kreidieh O, Conde Sabarís P, Rodríguez-Mañero M, Moure González M, Seoane Blanco A, Virgós Lamela A, García Castelo A, González Juanatey J (2017) Nutritional status is related to heart failure severity and hospital readmissions in acute heart failure. Int J Cardiol 230:108–114. https://doi.org/10.1016/j.ijcard.2016.12.067
Sze S, Pellicori P, Kazmi S, Rigby A, Cleland J, Wong K, Clark A (2018) Prevalence and prognostic significance of malnutrition using 3 scoring systems among outpatients with heart failure a comparison with body mass index. JACC Heart Fail 6(6):476–486. https://doi.org/10.1016/j.jchf.2018.02.018
Lennie T, Moser D, Heo S, Chung M, Zambroski C (2006) Factors influencing food intake in patients with heart failure. J Cardiovasc Nurs 21(2):123–129
Moser, D. (2002) Psychosocial factors and their association with clinical outcomes in patients with heart failure: why clinicians do not seem to care. European Journal of Cardiovascular Nursing. [Online] 1 (3):183-188. https://doi.org/10.1016/S1474-5151(02)00033-6
Anker S, Ponikowski P, Varney S, Chua T, Clark A, Webb-Peploe K, Harrington D, Kox W, Poole-Wilson P, Coats A (1997) Wasting as independent risk factor for mortality in chronic heart failure. Lancet 349(9058):1050–1053. https://doi.org/10.1016/S01406736(96)07015-8
Sente T, Van Berendoncks A, Hoymans V, Vrints C (2015) Adiponectin resistance in skeletal muscle: pathophysiological implications in chronic heart failure. J Cachexia Sarcopenia Muscle 7(3):261–274. https://doi.org/10.1002/jcsm.12086
Rossignol P, Masson S, Barlera S, Girerd N, Castelnovo A, Zannad F, Clemenza F, Tognoni G, Anand I, Cohn J, Anker S, Tavazzi L, Latini R (2015) Loss in body weight is an independent prognostic factor for mortality in chronic heart failure: insights from the GISSI-HF and Val-HeFT trials. Eur J Heart Fail 17(4):424–433. https://doi.org/10.1002/ejhf.240
Argilés J, Fontes-Oliveira C, Toledo M, López-Soriano F, Busquets S (2014) Cachexia: a problem of energetic inefficiency. J Cachexia Sarcopenia Muscle 5(4):279–286. https://doi.org/10.1007/s13539-014-0154-x
Von Haehling S, Morley J, Anker S (2012) From muscle wasting to sarcopenia and myopenia: update 2012. J Cachexia Sarcopenia Muscle 3(4):213–217. https://doi.org/10.1007/s13539-012-0089-z
Houston D, Nicklas B, Ding J, Harris T, Tylavsky F, Newman A, Lee J, Sahyoun N, Visser M, Kritchevsky S (2008) Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the health, aging, and body composition (health ABC) study. Am J Clin Nutr 87(1):150–155. https://doi.org/10.1093/ajcn/87.1.150
Kalantar-Zadeh K, Anker S, Horwich T, Fonarow G (2008) Nutritional and anti-inflammatory interventions in chronic heart failure. Am J Cardiol 101(11):S89–S103. https://doi.org/10.1016/j.amjcard.2008.03.007
Moher D, Liberati A, Tetzlaff J, Altman D (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. https://doi.org/10.1136/bmj.d5928
Habaybeh D, De Moraes MB, Slee A, Avgerinou C. A systematic review of nutritional interventions for patients with heart failure who are malnourished or at risk of malnutrition. PROSPERO (International Prospective Register of Systematic Reviews) https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=142323. Accessed 26 Feb 2020
George M, Azhar G, Pangle A, Peeler E, Dawson A, Coker R, Coleman K, Schrader A, Wei E (2017) Feasibility of conducting a 6-months long home-based exercise program with protein supplementation in elderly community-dwelling individuals with heart failure. J Physiother Phys Rehabil 2(2):137. https://doi.org/10.4172/2573-0312.1000137
Fumagalli S, Fattirolli F, Guarducci L, Cellai T, Baldasseroni S, Tarantini F, Di Bari M, Masotti G, Marchionni N (2011) Coenzyme Q10 terclatrate and creatine in chronic heart failure: a randomized, placebo-controlled, double-blind study. Clin Cardiol 34(4):211–217. https://doi.org/10.1002/clc.20846
Macchi A, Franzoni I, Buzzetti F, Pedrigi M, Rosa I, Gaudio G, Margonato A (2010) The role of nutritional supplementation with essential amino acids in patients with chronic heart failure. Mediterr J Nutr Metab 3(3):209–214. https://doi.org/10.3233/s12349-010-0010-2
Carvalho A, Rassi S, Fontana K, Correa K, Feitosa R (2012) Influence of creatine supplementation on the functional capacity of patients with heart failure. Arq Bras Cardiol 99(1):623–629. https://doi.org/10.1590/s0066-782x2012005000056
Aquilani R, Viglio S, Iadarola P, Opasich C, Testa A, Dioguardi F, Pasini E (2008) Oral amino acid supplements improve exercise capacities in elderly patients with chronic heart failure. Am J Cardiol 101(11):S104–S110. https://doi.org/10.1016/j.amjcard.2008.03.008
Gordon A, Hultman E, Kaijser L, Kristjansson S, Rolf C, Nyquist O, Sylven C (1995) Creatine supplementation in chronic heart failure increases skeletal muscle creatine phosphate and muscle performance. Cardiovasc Res 30(3):413–418. https://doi.org/10.1016/S0008-6363(95)00062-3
Kuethe F, Krack A, Richartz B, Figulla H (2006) Creatine supplementation improves muscle strength in patients with congestive heart failure. Pharmazie 61(3):218–222
Takata M, Amiya E, Watanabe M, Hosoya Y, Nakayama A, Fujiwara T, Taya M, Oguri G, Hyodo K, Takayama N, Takano N, Mashiko T, Uemura Y, Komuro I (2017) An exploratory study on the efficacy and safety of a BCAA preparation used in combination with cardiac rehabilitation for patients with chronic heart failure. BMC Cardiovasc Disord 17(1):205. https://doi.org/10.1186/s12872-017-0639-6
Ramiro-Ortega E, Bonilla-Palomas J, Gámez-López A, Moreno-Conde M, López-Ibáñez M, Alhambra-Expósito R, Anguita Sánchez M (2018) Nutritional intervention in acute heart failure patients with undernutrition and normalbuminemia: a subgroup analysis of PICNIC study. Clin Nutr 37(5):1762–1764. https://doi.org/10.1016/j.clnu.2017.07.009
Bonilla-Palomas J, Gámez-López A, Castillo-Domínguez J, Moreno-Conde M, López-Ibáñez M, Anguita-Sánchez M (2018) Does nutritional intervention maintain its prognostic benefit in the long term for malnourished patients hospitalised for heart failure? Rev Clin Esp 218(2):58–60. https://doi.org/10.1016/j.rce.2017.10.005
Deutz N, Matheson EM, Matarese LE, Luo M, Baggs GE, Nelson JL, Hegazi RA, Tappenden KA, Ziegler TR, on behalf of the NOURISH Study Group (2016) Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: a randomized clinical trial. Clin Nutr 35(1):18–26. https://doi.org/10.1016/j.clnu.2015.12.010
Rozentryt P, von Haehling S, Lainscak M, Nowak J, Kalantar-Zadeh K, Polonski L, Anker S (2010) The effects of a high-caloric protein-rich oral nutritional supplement in patients with chronic heart failure and cachexia on quality of life, body composition, and inflammation markers: a randomized, double-blind pilot study. J Cachexia Sarcopenia Muscle 1(1):35–42. https://doi.org/10.1007/s13539-010-0008-0
Broqvist M, Arnqvist H, Dahlstorm U, Larsson J, Nylander E, Permert J (1994) Nutritional assessment and muscle energy metabolism in severe chronic congestive heart failure—effects of long-term dietary supplementation. Eur Heart J 15(12):1641–1650. https://doi.org/10.1093/oxfordjournals.eurheartj.a060447
Pineda-Juárez J, Sánchez-Ortiz N, Castillo-Martínez L, Orea-Tejeda A, Cervantes-Gaytán R, Keirns-Davis C, Pérez-Ocampo C, Quiroz-Bautista K, Tenorio-Dupont M, Ronquillo-Martínez A (2016) Changes in body composition in heart failure patients after a resistance exercise program and branched chain amino acid supplementation. Clin Nutr 35(1):41–47. https://doi.org/10.1016/j.clnu.2015.02.004
Aquilani R, Opasich C, Gualco A, Verri M, Testa A, Pasini E, Viglio S, Iadarola P, Pastoris O, Dossena M, Boschi F (2008) Adequate energy-protein intake is not enough to improve nutritional and metabolic status in muscle-depleted patients with chronic heart failure. Eur J Heart Fail 10(11):1127–1135. https://doi.org/10.1016/j.ejheart.2008.09.002
Bonilla-Palomas J, Gámez-López A, Castillo-Domínguez J, Moreno-Conde M, López Ibáñez M, Alhambra Expósito R, Ramiro Ortega E, Anguita-Sánchez M, Villar-Ráez A (2016) Nutritional intervention in malnourished hospitalized patients with heart failure. Arch Med Res 47(7):535–540. https://doi.org/10.1016/j.arcmed.2016.11.005
Vellas B, Guigoz Y, Garry PJ, Nourhashemi F, Bennahum D, Lauque S, Albarede JL (1999) The mini nutritional assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 15(2):116–122
Grossniklaus D, O'Brien M, Clark P, Dunbar S (2008) Nutrient intake in heart failure patients. J Cardiovasc Nurs 23(4):357–363. https://doi.org/10.1097/01.JCN.0000317433.52210.e0
Brink M, Anwar A, Delafontaine P (2002) Neurohormonal factors in the development of catabolic/anabolic imbalance and cachexia. Int J Cardiol 85(1):111–121. https://doi.org/10.1016/s0167-5273(02)00239-5
Sole M, Jeejeebhoy K (2000) Conditioned nutritional requirements and the pathogenesis and treatment of myocardial failure. Curr Opin Clin Nutr Metab Care 3(6):417–424
Pittman J, Cohen P (1964) The pathogenesis of cardiac cachexia. N Engl J Med 271(8):403–409. https://doi.org/10.1056/NEJM196408202710807
Cicoira M, Doehner W, Jankowska W, Zanolla L, Zardini P, Ponikowski P (2004) 280 body composition and prognosis in 511 chronic heart failure patients: results from three European centers. Eur J Heart Fail Suppl 3(1):69. https://doi.org/10.1016/S1567-4215(04)902007
Wawrzeńczyk A, Anaszewicz M, Wawrzeńczyk A, Budzyński J (2019) Clinical significance of nutritional status in patients with chronic heart failure—a systematic review. Heart Fail Rev 24(8):39–44. https://doi.org/10.1007/s10741-019-09793-2
Nieminen M, Dickstein K, Fonseca C, Serrano J, Parissis J, Fedele F, Wikström G, Agostoni P, Atar S, Baholli L, Brito D, Colet J, Édes I, Gómez Mesa J, Gorjup V, Garza E, González Juanatey J, Karanovic N, Karavidas A, Katsytadze I, Kivikko M, Matskeplishvili S, Merkely B, Morandi F, Novoa A, Oliva F, Ostadal P, Pereira-Barretto A, Pollesello P, Rudiger A, Schwinger R, Wieser M, Yavelov I, Zymliński R (2015) The patient perspective: quality of life in advanced heart failure with frequent hospitalisations. Int J Cardiol 191:256–264. https://doi.org/10.1016/j.ijcard.2015.04.235
Soundarraj D, Singh V, Satija V, Thakur R (2017) Containing the cost of heart failure management. Heart Fail Clin 13(1):21–28. https://doi.org/10.1016/j.hfc.2016.07.002
Beyranvand M, Kadkhodai Khalafi M, Roshan V, Choobineh S, Parsa S, Piranfar M (2011) Effect of taurine supplementation on exercise capacity of patients with heart failure. J Cardiol 57(3):333–337. https://doi.org/10.1016/j.jjcc.2011.01.007
Lombardi C, Carubelli V, Lazzarini V, Vizzardi E, Quinzani F, Guidetti F, Rovetta R, Nodari S, Gheorghiade M, Metra M (2014) Effects of oral amino acid supplements on functional capacity in patients with chronic heart failure. Clin Med Insights Cardiol 21(8):39–49. https://doi.org/10.4137/CMC.S14016
Taegtmeyer H, Harinstein M, Gheorghiade M (2008) More than bricks and mortar: comments on protein and amino acid metabolism in the heart. Am J Cardiol 101(11):S3–S7. https://doi.org/10.1016/j.amjcard.2008.02.064
Cederholm T, Jensen GL, Correia MITD, GLIM Core Leadership Committee, GLIM Working Group et al (2019) GLIM criteria for the diagnosis of malnutrition - a consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle 10(1):207–217. https://doi.org/10.1002/jcsm.12383
Acknowledgements
We would like to thank Sophie Pattison, Clinical Support Librarian at the Royal Free Hospital Medical Library, for her time and support with the search strategy. We also thank authors of the original studies who shared additional data [38].
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Habaybeh, D., de Moraes, M.B., Slee, A. et al. Nutritional interventions for heart failure patients who are malnourished or at risk of malnutrition or cachexia: a systematic review and meta-analysis. Heart Fail Rev 26, 1103–1118 (2021). https://doi.org/10.1007/s10741-020-09937-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-020-09937-9