Abstract
SARS-CoV-2 infection is considered as a multi-organ disease, and several studies highlighted the relevance of the virus infection in the induction of vascular injury and tissue morphological alterations, including placenta. In this study, immunohistochemical analyses were carried out on placenta samples derived from women with COVID-19 infection at delivery (SARS-CoV-2 PCR+) or women healed from a COVID-19 infection (SARS-CoV-2 negative at delivery, SARS-CoV-2 PCR-) or women who gave birth before 2019 (Control). Angiotensin Converting Enzyme 2 (ACE2) receptor, Cluster of differentiation 147 (CD147), endothelial CD34 marker, Vascular Endothelial Growth Factor (VEGF) and total Microtubule-associated protein 1 Light Chain 3B marker (LC3B) were investigated in parallel with SPIKE protein by standard IHC. Multiplexed Immunohistochemical Consecutive Staining on Single Slide (MICSSS) was used to examine antigen co-expression in the same specimen. SPIKE protein was detected in villi and decidua from women with ongoing infection, with no significant differences in SPIKE staining between both biopsy sites. VEGF was significantly increased in SARS-CoV-2 PCR + biopsies compared to control and SARS-CoV-2 PCR- samples, and MICSSS method showed the co-localization of SPIKE with VEGF and CD34. The induction of autophagy, as suggested by the LC3B increase in SARS-CoV-2 PCR + biopsies and the co-expression of LC3B with SPIKE protein, may explain one of the different mechanisms by which placenta may react to infection. These data could provide important information on the impact that SARS-CoV-2 may have on the placenta and mother-to-fetus transmission.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The RNA virus SARS-CoV-2, not identified in humans before December 2019, represents a new strain of beta Coronavirus and is responsible for COrona VIrus Disease (COVID-19) (Chams et al. 2020).
SARS-CoV-2 infection is highly heterogeneous and of varying severity. The most common symptoms of SARS-CoV-2 infection started from a common cold and fever to more serious respiratory syndromes such as pneumonia or bronchopneumonia, up to death (COVIDSurg Collaborative and GlobalSurg Collaborative 2022; Mohamadian et al. 2021; Sauter et al. 2020). Giving further evidence that SARS-CoV-2 infection has been interpreted as a multi-organ disease, this coronavirus could infect various tissues and organs, including gut, heart, kidneys and liver, or the vascular structures and male and female organs, including the placenta (Adil et al. 2021; Rakheja et al. 2022; Rizzo et al. 2021; Robba et al. 2020; Wastnedge et al. 2021; Zamboni et al. 2022).
Specifically, human placenta can be infected with SARS-CoV-2 and the virus can proliferate in placental cells (Argueta et al. 2022).
Angiotensin Converting Enzyme 2 (ACE2) receptor is widespread in tissues, is crucial for the entrance of SARS-CoV-2 in cells (Harky et al. 2023; Zaim et al. 2020) and it is expressed in the placenta (Azinheira Nobrega Cruz et al. 2021). Therefore, further attention has been paid to the study of susceptibility from SARS-CoV-2 during pregnancy (Kyle et al. 2022).
Cluster of differentiation 147 (CD147) is a transmembrane protein involved in tissue remodeling and appears to mediate macrophage activation leading to the expression of matrix metallopeptidase-9 and pro-inflammatory cytokines and chemokines (Agostinis et al. 2022; Bortolotti et al. 2021; Fenizia et al. 2021). In the placenta, CD147 is involved in the regulation of implantation, invasion and differentiation of human trophoblasts, and is reported to be involved in virus entry and considered in some literature manuscripts as a putative alternative receptor (Behl et al. 2022; Bortolotti et al. 2021). In addition, it is involved in HIV-1 infection and other viral infections (Pushkarsky et al. 2001).
The normal vascular pattern of placenta includes vasculogenesis and angiogenesis with the development of syncytial knots at the level of the villi and has been associated with the physiological expression of Vascular Endothelial Growth Factor (VEGF) but also with pathological conditions (Ahmad and Nawaz 2022; Alfaidy et al. 2020; Carmeliet 2005; Huang et al. 2021; Melincovici et al. 2018; Shchegolev et al. 2021).
It has been reported that SARS-CoV-2 infection significantly alters the vascular pattern of the placental tissue, inducing on the one hand impaired maternal vascular perfusion, and thus insufficient blood flow to the fetus (Di Girolamo et al. 2021), and on the other hand ‘intervillous thrombi’ and coagulation alterations, with increased thrombin production and increased intravascular inflammation (Schwartz et al. 2022; Wastnedge et al. 2021).
In the placenta, the autophagic process plays a key role in the early embryonic stages and promotes the development and remodeling of the endometrium (Mizushima and Levine 2010; Tsukamoto et al. 2008). Although the entire mechanism is not yet well understood, autophagy contributes to maintain the correct balance of the maternal-fetal components during normal placental development (Gong and Kim 2014). LC3, or microtubule-associated protein 1 Light Chain 3, is ubiquitously distributed in the body (Runwal et al. 2019), is expressed in the autophagosome membrane during the autophagic process and could elicit rapid degradation of mRNAs (Hwang et al. 2022), especially if it contains the sequence AAUAAA. The protein autophagosomal splice variant LC3B is involved in the autophagic response to SARS-CoV-2 infection, and it has also attracted considerable interest in in vitro studies with antiviral drug treatments (Chen and Zhang 2022; Gorshkov et al. 2020; Ivanova et al. 2023). Autophagy may have a dual role in the interaction between SARS-CoV-2 and host cells (Shan et al. 2023): on one hand, it could act as a defense mechanism, targeting and degrading viral components to limit viral replication, on the other hand, the virus may drive the autophagic process to its advantage, promoting its own survival and replication (Mao et al. 2019). During SARS-CoV-2 infection, autophagy is induced by both the innate and adaptive immune response and by Toll-like receptors (TLRs) (Carmona-Gutierrez et al. 2020). Recently, SARS-CoV-2, but not SARS-CoV, has been reported to induce autophagy and accumulation of autophagosomes. Autophagosomes appear to be central to both viral replication and virion release (Ivanova et al. 2023).
We characterized 15 tissue samples derived from placenta of women included in one of the following different conditions: (1) Pregnancy in the pre-pandemic period (Control); (2) SARS-CoV-2 positive subjects at the time of delivery (SARS-CoV-2 PCR+); (3) Subjects with infection during pregnancy but subsequent negativization at the time of delivery (SARS-CoV-2 PCR-).
Our results showed a clear influence of the viral infection on the molecules and processes studied.
Materials and methods
Study population
The project was designed as a case-control study with 15 pregnant women admitted at the time of delivery to the Azienda Ospedaliero-Universitaria Sant’Anna of Ferrara (Italy): 10 during 2020, 3 control pregnant women during 2019 and 2 control pregnant women during 2018.
The women were divided into 3 groups: 5 control pregnant women gave birth in the pre-pandemic period, 5 women were SARS-CoV-2 positive at the time of delivery (SARS-CoV-2 PCR+), 5 women contracted the infection during pregnancy but were negative at the time of delivery (SARS-CoV-2 PCR-). The infection was tested by molecular RT-PCR after nasal swab for the presence of SARS-CoV-2 RNA. Of note, none of the pregnant women were vaccinated against SARS-CoV-2. Clinical data were collected for each of the three cohorts.
Ethical statement
The study was conducted in accordance with the ethical principles for medical research involving human subjects as required by the 2013 revision of the Helsinki Declaration—WMA Declaration of Helsinki—The Ethical Principles for Medical Research Involving Human Subjects. The present study was approved by the Local Ethics Committee (Comitato Etico di Area Vasta Emilia Centro della Regione Emilia-Romagna, CE-AVEC) with the reference number 122/2021/Oss/AOUFe. All participants have signed informed consent to be included in the study.
Placenta sample collection and immunohistochemical analysis
The placenta samples were collected and sent to the Pathology Lab for optimal formalin fixation and histological analysis. One formalin-fixed paraffin-embedded block of placental parenchyma was selected from each case to perform immunohistochemical analyses. After macroscopic examination of the cut surfaces, multiple representative sections of the parenchyma were sampled for histology.
Placenta slides were deparaffined with xylene and rehydrated at decreasing ethanol concentrations. Heat antigen retrieval in Citrate Buffer Ph6 and Ph9, depending on the marker datasheet information, was performed. Slides were stained with the following antibodies, purchased from Abcam: SARS-CoV-2 SPIKE glycoprotein (Cat# ab272504, polyclonal, dilution 1:100), ACE2 (Cat# ab108252, clone EPR4435-2, dilution 1:250), CD147 (Cat# ab666, clone MEM-M6/1, dilution 1:100), CD34 (Cat# ab110643, clone EPR2999, dilution 1:250), and VEGF-A (Cat# ab1316, clone VG-1, 5 µg/ml concentration). LC3B antibody was purchased from Novus Biologicals (Cat# NB100-2220, polyclonal, dilution 1:250). Slides were counterstained with hematoxylin-eosin (H-E) and were imaged with Nikon Eclipse E100 microscope at different magnifications (10 or 20x). The tissues were scored based on number of positively stained cells/mm2 and the staining on selected areas was detected using ImageJ (64-bit Java 8) software.
Multiplexed immunohistochemical consecutive staining on single slide (MICSSS)
MICSSS method represents a multiplex immunohistochemistry (IHC) platform based on multiple cycles of staining and scanning a single slide with an antibody panel that can include up to 10 markers (Remark et al. 2016). Each MICSSS staining cycle is identical to single IHC staining. To remove the previous antibody and staining, coverslips were removed from the slides at 56 degrees and were immersed at 50% of ethanol, subsequently in 70% ethanol + 1% hydrochloric acid (12 N) for 2 min and in 100% ethanol for 5 min. Slides were then bathed at higher percentages of ethanol. Heat-induced Epitope Retrieval for re-marking was then performed following the standard protocol. After each staining cycle, slides were scanned by the slide scanner ScanScope (Leica Biosystems), and the images were observed and saved with ScanScope Console (v9.0.0.1516) program. Consequently, the staining was reproduced in a single image, initially with QuPath v0.2.3 software and in a subsequent step with ImageJ (64-bit Java 8) software for the superposition of the individual staining. With MICSSS, in addition to the SPIKE protein, CD34, VEGF and LC3B staining was acquired.
Staining quantification
After staining, tissue slides were observed under a light microscope in 20x, 1600 × 1200 pixel resolution at high contrast. For each slide, 4 representative areas of the villi and 4 of the decidua section were acquired using the NIS-Elements program by digitizing the images in TIFF format.
Imaging analysis was done by the ImageJ program (version bundled with 64-bit Java 8) using the IHC Profiler Plugin that allows to obtain the percentage of stained area at different intensities: very intense, moderate, mild, unmarked staining. The different percentages were used to calculate the H-score of each individual image.
Statistical analysis
The Shapiro-Wilk test was used to identify variables with normal distribution. Variables such as demographic, clinical and hematochemical data with normal distribution were expressed as mean ± standard deviation and compared with ANOVA for independent samples. As for H-score data of markers with non-normal distribution were compared by the Mann-Whitney or Kruskal-Wallis tests to assess the statistically significant differences of the examined markers among two or three groups, respectively.
The Rho Spearman correlation test was used to analyze the correlations between the different markers by considering all biopsies together, independent of group or biopsy site (N = 30). Statistical analysis was performed using GraphPad software (version GraphPad.Prism.8.0.1.244.2b), and statistical significance was set to a p-value < 0.05.
Results
Clinical data
Study participants were divided into three groups: women negative for SARS-CoV-2 since their samples derived from pregnancy occurred before the virus spread (Controls); pregnant women declared positive at the molecular swab (RT-PCR) for SARS-CoV-2 at the time of delivery (SARS-CoV-2 PCR+); pregnant women who contracted the infection during pregnancy but were RT-PCR negative at the time of delivery (SARS-CoV-2 PCR-). The period of negativity ranged from 199 to 41 days before delivery with a mean of 88.4 ± 71.0 days.
No significant differences were reported between subjects for age, body mass index (BMI) and gestational age at delivery, as shown in the clinical data table (Table 1). The group of SARS-CoV-2 PCR- parturients included one woman with pre-pregnancy obesity. Two women, the first in SARS-CoV-2 PCR- group and the second in the control group, had type 2 diabetes.
Besides the aforementioned conditions, the enrolled women had no comorbidities or preexisting diseases such as hypertension or heart problems, nor did they have pregnancy-related diseases such as preeclampsia.
The hospitalization period for mothers with previous infection was 2.6 ± 0.9 days, shorter than that of the positive mothers at delivery who were hospitalized for 6.4 ± 1.5 days. Regarding COVID-19 complications, one woman of SARS-CoV-2 PCR- group reported fever (2 months before delivery) and respiratory symptoms; in the SARS-CoV-2 PCR + group, a patient with diabetes developed fever and dyspnea that necessitated the use of oxygen therapy.
Biochemical data showed no significant differences in D-Dimer (indicator of clotting risk) and aPTT (prothrombin partial time indicating coagulation rate) values between the groups and these parameters were within the normal range. In contrast, regarding hemoglobin and fibrinogen parameters, the two groups with previous or ongoing SARS-CoV-2 infection showed statistically significant higher levels when compared with the control group.
Expression of SPIKE, ACE2 and CD147
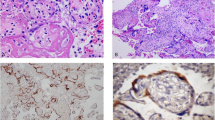
SPIKE protein expression was detected in decidua and chorionic villi of all samples of the SARS-CoV-2 PCR + women group, whereas it was absent in the placenta of control and previously infected group (Fig. 1).
The SPIKE staining was well outlined between the villi, especially at the level of the syncytiotrophoblast (Fig. 1a). Furthermore, different areas with a more relevant or, at variance, a weaker staining were observable, therefore suggesting a staining that follows viral protein quantity. The decidua was characterized by SPIKE islets of staining, mainly located toward its external surface (Fig. 1b).
Analysis of H-score calculated from IHC images did not report differences in SPIKE staining intensity between villi and decidua in corresponding samples (Fig. 1c).
ACE2 protein was expressed in the chorionic villi, along the surface of syncytiotrophoblast for all the samples, but the expression was increased in the SARS-CoV-2 PCR + as shown in Fig. 1d.
On the contrary in the decidua, ACE2 expression was slightly higher in SARS-CoV-2 PCR + versus control and SARS-CoV-2 PCR- while no differences were detected between control and SARS-CoV-2 PCR- groups (Fig. 1b and d; Table 2).
The transmembrane protein CD147 was present at basal levels in controls and was increased in SARS-CoV-2 PCR + samples, while its expression was lower in SARS-CoV-2 PCR- placentas (Fig. 1a, b and e). In the villi, the labeling is shown tagging along the outer edge of the syncytiotrophoblast laye; while in the decidua, the staining appeared distributed inside the tissue (Fig. 1a, b).
Rho Spearman correlation coefficient was assessed by considering all the three clinical groups and the scores were obtained for each protein (SPIKE and ACE2 or CD147) at the level of the villi and decidua for each woman (Supplementary Fig. 1a, b).
The expression of SPIKE was positively associated to the presence of ACE2 in the placenta tissue (Supplementary Fig. 1a). In contrast, there was a less significant correlation between the expression of SPIKE and CD147 as shown by the Rho- and p-value (Supplementary Fig. 1b).
Detection of SPIKE, ACE2, and CD147 in control, SARS-CoV-2 PCR + and SARS-CoV-2 PCR- placenta. a: IHC images of the chorionic villi; b: IHC staining of the decidua. Magnifications are 20x. c, d, e: H-score values of SPIKE, ACE2, and CD147, respectively. p-values were obtained by Mann-Whitney test. *0.05 < p-value < 0.01; ** 0.01 < p-value < 0.001
SARS-CoV-2 and the expression of CD34 and VEGF in the placenta
IHC analysis of placenta samples with anti-VEGF and anti-CD34 antibodies was also performed. The purpose was to analyze whether SARS-CoV-2 could influence the vascular organization of the tissue, using CD34 and VEGF as readouts. IHC analysis showed that CD34 staining was localized at the level of the endothelium, nearby the syncytiotrophoblast (Fig. 2a). In the decidua, CD34 was also appreciable on the surface of vascular endothelium, near the vessels of maternal circulation (Fig. 2b). Moreover, the expression of this marker appeared more intense during SARS-CoV-2 infection (SARS-CoV-2 PCR+), even if the increase did not reach the significatively comparing with the other two groups (p = 0.056 versus control and p = 0.095 versus SARS-CoV-2 PCR- in villi and p = 0.095 versus control and p = 0.095 versus SARS-CoV-2 PCR- in decidua) (Fig. 2a, b; Table 2).
A statistically significant difference was observed for the distribution of this marker between decidua and villi with high levels in the last one (Supplementary Table 1).
Regarding VEGF, its expression was lower in control samples and SARS-CoV-2 PCR- groups when compared with SARS-CoV-2 PCR + samples, both in the villi and decidua (Fig. 2d; Table 2). In the villi, VEGF was expressed at the level of syncytiotrophoblast and in the vascular endothelium (Fig. 2a) while in the decidua the expression was most detectable in the vascular endothelium lining the blood vessels (Fig. 2b).
Rho Spearman test revealed a significant positive correlation between CD34 and VEGF (Supplementary Fig. 2a) and between SPIKE and VEGF (Supplementary Fig. 2b).
Analysis of H-score calculated from IHC images confirmed that CD34, detectable also in control samples, was more appreciable during infection, while its expression was lower in SARS-CoV-2 PCR- samples. Similarly, VEGF expression was higher during infection, while it was less appreciable in control samples and past infection (Fig. 2c, d).
Expression of CD34 and VEGF in control, SARS-CoV-2 PCR + and SARS-CoV-2 PCR- placenta. a: IHC images of the chorionic villi; b: IHC staining of the decidua. Magnifications are 20x. c, d: H-score values of CD34 and VEGF respectively. p-values were obtained by Mann-Whitney test. ** 0.01 < p-value < 0.001. The arrows indicate the staining localization of CD34 and VEGF nearby the syncytiotrophoblast in the villi and on the surface of vascular endothelium in the decidua
SARS-CoV-2 induced autophagy
With the aim of analyzing the possible modulation of autophagy in our samples, the expression of LC3B protein was assessed. IHC analysis showed that in the normal placenta after delivery, LC3B was expressed either in the villi and in the decidua (Fig. 3a, b). In SARS-CoV-2 PCR + villi and decidua, an increase in both the spread and staining intensity was observed when compared with control and SARS-CoV-2 PCR- samples (Fig. 3; Table 2). Indeed, the H-score analysis showed a relevant increase of the PCR + samples (H-score 17–18) in comparison with the levels of control and PCR- ones. Surprisingly, LC3B expression was higher in the control than in the SARS-CoV-2 PCR- group in villi (Fig. 3; Table 2). The activation of autophagy during ongoing infection was also confirmed by the positive correlation between SPIKE and LC3B expression with the Rho Spearman correlation test (Supplementary Fig. 3).
Expression of LC3B in control, SARS-CoV-2 PCR + and SARS-CoV-2 PCR- placenta. a: IHC images of chorionic villi; b: IHC staining of decidua. Magnifications are 20x. c: H-score values of LC3B. p-values were obtained by Mann-Whitney test. The arrows indicate the staining localization of LC3B in the villi, more widespread in the decidua
Analysis of tissue vascular and autophagy readouts
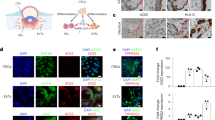
To first analyze the co-distribution of SPIKE, CD34 and VEGF, MICSSS was performed in placenta samples at the different conditions (Fig. 4).
Similar to previous results, CD34 characterized the areas of vascular endothelium in all the three conditions, with greater expression in villi than in decidua. In particular, CD34 demarcated the capillaries in the inner part, while the more intense and thicker marking precisely indicated the surface of the vascular endothelial cells that constitute the chorionic villi. Notably, in control placenta, CD34 represented essentially the only visible staining, as there was no detection of SARS-CoV-2 and only a slight expression of VEGF. Likewise, SPIKE was not detected in the SARS-CoV-2 PCR- condition, thus indicating the absence of the virus.
The appreciable aspect of multiple staining is the co-expression, and in this case, it was most strongly expressed in the positive condition. Indeed, the strong presence of SPIKE was distributed in villi and decidua areas characterized by a high expression of VEGF and CD34 (Fig. 4).
To further investigate the correlation of SARS-CoV-2 with the autophagic process, MICSSS with SPIKE protein and LC3B staining was also performed to detect whether the presence of the SPIKE protein was associated with increased autophagy (Fig. 5).
In both villi and decidua areas of SARS-CoV-2 PCR + placenta samples, the infection appeared very extensive as SPIKE staining was distributed throughout the tissue and LC3B is distributed in the proximity of the viral protein staining, both at the level of the endothelial cells that characterize the villi and in the extravillous trophoblast in the decidua (Fig. 5).
Analysis of co-expression of SPIKE, CD34 and VEGF in control, SARS-CoV-2 PCR + and SARS-CoV-2 PCR- groups in villi and decidua. The expression of the three markers SPIKE, CD34 and VEGF was co-localized. In detail, SPIKE protein was assigned the color cyan, VEGF the color red, and CD34 the color magenta. H-E: Hematoxilin Eosin. Magnifications are 20x. The arrows indicate points of co-localization of the three markers in the villi
Analysis of co-expression of SPIKE and LC3B. Comparison of MICSSS of control, SARS-CoV-2 PCR + and SARS-CoV-2 PCR- conditions showing the co-localization of SPIKE and LC3B in the villi and decidua sections. With overlapping images, SPIKE protein was assigned the color cyan and LC3B the color magenta. Magnifications are 20x. The arrows indicate points of co-localization of the two markers in the villi and in the decidua
Discussion
Since the onset of the COVID-19 pandemic, pregnant women have been particularly under observation in case of contact with SARS-CoV-2 virus to monitor the impact that its infection might have on gestation and fetus growth.
Indeed, the placenta was also considered as a possible target organ for the virus, and the scientific community has drawn attention to the possibility of vertical transmission of SARS-CoV-2 infection from mother to fetus (Moza et al. 2023; Simbar et al. 2023).
In this study, we analyzed the impact of SARS-CoV-2 infection in the modulation of markers involved in the vascular damage and the autophagic process.
The presence or the absence of the virus was assessed by the expression of the viral protein SPIKE, its main receptor ACE2 and the transmembrane protein CD147. We also analyzed the expression of VEGF, CD34 for vascular impairment and LC3B, considered as a discriminating factor in the presence or absence of autophagy.
IHC analysis confirmed the presence of SPIKE only in SARS-CoV-2 PCR + samples with staining concentrated at the level of syncytiotrophoblast and areas with staining islets in the decidua area.
ACE2 is expressed in placenta (Donoghue et al. 2000; Li et al. 2020; Valdes et al. 2006) and its expression could be increased by SARS-CoV-2 (Schiuma et al. 2023). Our data documented indeed a positive association between ACE2 and SPIKE detection.
CD147 is also present at the placental level and is involved in the placental development (Lee et al. 2013, 2022). In our samples, CD147 levels are higher in the two groups infected by SARS-CoV-2 (PCR- and PCR+) than in the control samples, suggesting that, at least in the villi, the expression is maintained even when infection is over.
SARS-CoV-2 infection can cause significant hematological and immunological alterations in maternal blood circulation that can be transmitted to the placenta. In this line, González-Mesa et al. have detected IgG anti-SARS-CoV-2 antibodies and the proinflammatory cytokines IL-1, IL-6 and IFN-γ in the umbilical cord blood of 79 pregnant women infected by SARS-CoV-2 during pregnancy (Gonzalez-Mesa et al. 2022). Inflammation is a powerful inducer of the proangiogenic marker VEGF. In fact, our results show a positive association between virus SPIKE protein and VEGF detection. These data are corroborated by the results obtained by Shchegolev et al., which showed an increase in placenta VEGF with the severity of the infection (Shchegolev et al. 2021) and increased levels of circulating VEGF has been measured in patients with worse COVID-19 prognosis (Josuttis et al. 2023; Smadja et al. 2021). Therefore, these data suggested that VEGF may represent a good predictor of adverse outcomes (Madureira and Soares 2023).
Our data showed that during SARS-CoV-2 infection, VEGF expression was increased, suggesting that this increase might be correlated to the presence of consistent alterations, disarrangements or remodeling of normal vasculature, associated with vascular endothelial injury and inflammation, presumably endothelitis. Moreover, the high expression of VEGF in SARS-CoV-2 PCR + samples was associated to the endothelial cell marker CD34, involved in various pathological conditions such as angiogenesis, or in pre-eclamptic placenta (Escudero et al. 2014). However, this aspect, which is of considerable importance, requires further investigation.
Another intriguing aspect that has been investigated was autophagy, that is a cytoplasmic process of degradation and is necessary to maintain the cellular homeostasis (Klionsky et al. 2021; Nakashima et al. 2017; Park et al. 2021; Zhao et al. 2020). Little has been studied on the role of autophagy during SARS-CoV-2 infection, especially at the placental level. Moreover, the modulation of this process in the post-infection phase is still being explored. Autophagy is a crucial process in the placenta to regulate trophoblast differentiation, endometrial development and remodeling following blastocyst implantation. Our results reported that the autophagosomal marker LC3B was expressed in healthy gestations and increased in SARS-CoV-2 PCR + placenta at the trophoblasts level but was reduced when SARS-CoV-2 infection was over (SARS-CoV-2 PCR- condition). The accumulation of LC3 in infected tissues or cells may be a mechanism exploited by SARS-CoV-2 through many cellular mechanisms to avoid the harmful effect of autophagy, as reported elsewhere (Zhou et al. 2023) and several publications also report how SARS-CoV-2 could even utilize the autophagy machinery to promote the virion production (Shan et al. 2023; Zhou et al. 2023). In our samples, an autophagic decrease in the post-infection phase could be related to the fact that the infection is overcome, and the tissue returned to a condition close to physiological. Consequently, the absence of the virus in SARS-CoV-2 PCR- placenta may prevent its own autophagosome-mediated replication with a significant decrease in LC3B marker.
It is therefore possible that autophagy could be involved in the response of placental cells to SARS-CoV-2. However, the specific implication of this process remains largely unclear and still require an in-depth analysis for different tissues, and most notably for the placenta.
It should be noted that in this study SARS-CoV-2 infection was tested, as previously detailed, at the time of delivery by a molecular swab test (RT-PCR) for the presence of SARS-CoV-2 RNA, and the placenta samples were immediately collected, fixed and subsequently analysed by means of immunohistochemistry.
The detection of the SPIKE viral glycoprotein was assumed as presence of active virus. As reported elsewhere, SPIKE was assumed as representative of SARS-CoV-2 presence in several different tissues, including lung (Bosmuller et al. 2021; Jeican et al. 2023), brain vessels (Zinserling et al. 2022), olfactory epithelium (Power Guerra et al. 2024) or liver (Swain et al. 2023).
Overall, these results resume and highlight some of the relevant modifications induced by SARS-CoV-2 infection at the placental level, that may be related with tissue and vascular damage and have effects on the mother, on the fetus and also on pregnancy.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- ACE2:
-
Angiotensin Converting Enzyme 2
- COVID-19:
-
Corona VIrus Disease 2019
- CD147:
-
Cluster of differentiation 147
- CD34:
-
Cluster of differentiation 34
- VEGF:
-
Vascular Endothelial Growth Factor
- LC3B:
-
Microtubule-associated protein 1 Light Chain 3B
- MICSSS:
-
Multiplexed Immunohistochemical Consecutive Staining on Single Slide
- H-E:
-
Hematoxylin-Eosin
- IHC:
-
Immunohistochemistry
- IL-1:
-
Interleukin 1
- IL-6:
-
Interleukin 6
- IFN-γ:
-
Interferon-gamma
References
Adil MT et al (2021) SARS-CoV-2 and the pandemic of COVID-19. Postgrad Med J 97:110–116. https://doi.org/10.1136/postgradmedj-2020-138386
Agostinis C et al (2022) SARS-CoV-2 modulates virus receptor expression in placenta and can induce trophoblast fusion, inflammation and endothelial permeability. Front Immunol 13:957224. https://doi.org/10.3389/fimmu.2022.957224
Ahmad A, Nawaz MI (2022) Molecular mechanism of VEGF and its role in pathological angiogenesis. J Cell Biochem 123:1938–1965. https://doi.org/10.1002/jcb.30344
Alfaidy N et al (2020) The emerging role of the prokineticins and Homeobox Genes in the vascularization of the placenta: physiological and pathological aspects. Front Physiol 11:591850. https://doi.org/10.3389/fphys.2020.591850
Argueta LB et al (2022) Inflammatory responses in the placenta upon SARS-CoV-2 infection late in pregnancy. iScience 25:104223. https://doi.org/10.1016/j.isci.2022.104223
Azinheira Nobrega Cruz N, Stoll D, Casarini DE, Bertagnolli M (2021) Role of ACE2 in pregnancy and potential implications for COVID-19 susceptibility. Clin Sci (Lond) 135:1805–1824. https://doi.org/10.1042/CS20210284
Behl T et al (2022) CD147-spike protein interaction in COVID-19: get the ball rolling with a novel receptor and therapeutic target. Sci Total Environ 808:152072. https://doi.org/10.1016/j.scitotenv.2021.152072
Bortolotti D et al (2021) Relevance of VEGF and CD147 in different SARS-CoV-2 positive digestive tracts characterized by thrombotic damage. FASEB J 35:e21969. https://doi.org/10.1096/fj.202100821RRR
Bosmuller H, Matter M, Fend F, Tzankov A (2021) The pulmonary pathology of COVID-19. Virchows Arch 478:137–150. https://doi.org/10.1007/s00428-021-03053-1
Carmeliet P (2005) VEGF as a key mediator of angiogenesis in cancer. Oncol 69 Suppl 3:4–10. https://doi.org/10.1159/000088478
Carmona-Gutierrez D, Bauer MA, Zimmermann A, Kainz K, Hofer SJ, Kroemer G, Madeo F (2020) Digesting the crisis: autophagy and coronaviruses. Microb Cell 7:119–128. https://doi.org/10.15698/mic2020.05.715
Chams N et al (2020) COVID-19: a multidisciplinary review. Front Public Health 8:383. https://doi.org/10.3389/fpubh.2020.00383
Chen D, Zhang H (2022) Autophagy in severe acute respiratory syndrome coronavirus 2 infection. Curr Opin Physiol 29:100596. https://doi.org/10.1016/j.cophys.2022.100596
COVIDSurg Collaborative, GlobalSurg Collaborative (2022) SARS-CoV-2 infection and venous thromboembolism after surgery: an international prospective cohort study. Anaesthesia 77:28–39. https://doi.org/10.1111/anae.15563
Di Girolamo R et al (2021) Placental histopathology after SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM 3:100468. https://doi.org/10.1016/j.ajogmf.2021.100468
Donoghue M et al (2000) A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res 87:E1–9. https://doi.org/10.1161/01.res.87.5.e1
Escudero C et al (2014) Increased placental angiogenesis in late and early onset pre-eclampsia is associated with differential activation of vascular endothelial growth factor receptor 2. Placenta 35:207–215. https://doi.org/10.1016/j.placenta.2014.01.007
Fenizia C, Galbiati S, Vanetti C, Vago R, Clerici M, Tacchetti C, Daniele T (2021) SARS-CoV-2 entry: at the crossroads of CD147 and ACE2. Cells 10. https://doi.org/10.3390/cells10061434
Gong JS, Kim GJ (2014) The role of autophagy in the placenta as a regulator of cell death. Clin Exp Reprod Med 41:97–107. https://doi.org/10.5653/cerm.2014.41.3.97
Gonzalez-Mesa E et al (2022) Transmitted fetal Immune response in cases of SARS-CoV-2 infections during pregnancy. Diagnostics (Basel) 12. https://doi.org/10.3390/diagnostics12020245
Gorshkov K et al (2020) The SARS-CoV-2 cytopathic effect is blocked with autophagy modulators. bioRxiv. https://doi.org/10.1101/2020.05.16.091520
Harky A, Ala’Aldeen A, Butt S, Duric B, Roy S, Zeinah M (2023) COVID-19 and Multiorgan Response: the long-term impact. Curr Probl Cardiol 48:101756. https://doi.org/10.1016/j.cpcardiol.2023.101756
Huang Z, Huang S, Song T, Yin Y, Tan C (2021) Placental angiogenesis in mammals: a review of the Regulatory effects of Signaling pathways and functional nutrients. Adv Nutr 12:2415–2434. https://doi.org/10.1093/advances/nmab070
Hwang HJ, Ha H, Lee BS, Kim BH, Song HK, Kim YK (2022) LC3B is an RNA-binding protein to trigger rapid mRNA degradation during autophagy. Nat Commun 13:1436. https://doi.org/10.1038/s41467-022-29139-1
Ivanova T et al (2023) Autophagy and SARS-CoV-2-Old players in New games. Int J Mol Sci 24. https://doi.org/10.3390/ijms24097734
Jeican II et al (2023) Histopathological lung findings in COVID-19 B.1.617.2 SARS-CoV-2 Delta variant. J Pers Med 13. https://doi.org/10.3390/jpm13020279
Josuttis D, Schwedler C, Heymann G, Gumbel D, Schmittner MD, Kruse M, Hoppe B (2023) Vascular endothelial growth factor as potential biomarker for COVID-19 severity. J Intensive Care Med 38:1165–1173. https://doi.org/10.1177/08850666231186787
Klionsky DJ et al (2021) Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)(1). Autophagy 17:1–382. https://doi.org/10.1080/15548627.2020.1797280
Kyle MH, Hussain M, Saltz V, Mollicone I, Bence M, Dumitriu D (2022) Vertical Transmission and neonatal outcomes following maternal SARS-CoV-2 infection during pregnancy. Clin Obstet Gynecol 65:195–202. https://doi.org/10.1097/GRF.0000000000000667
Lee CL et al (2013) Identification of CD147 (basigin) as a mediator of trophoblast functions. Hum Reprod 28:2920–2929. https://doi.org/10.1093/humrep/det355
Lee CL et al (2022) Dysregulation of the CD147 complex confers defective placental development: a pathogenesis of early-onset preeclampsia. Clin Transl Med 12:e826. https://doi.org/10.1002/ctm2.826
Li M, Chen L, Zhang J, Xiong C, Li X (2020) The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS ONE 15:e0230295. https://doi.org/10.1371/journal.pone.0230295
Madureira G, Soares R (2023) The misunderstood link between SARS-CoV-2 and angiogenesis. A narrative review. Pulmonology 29:323–331. https://doi.org/10.1016/j.pulmoe.2021.08.004
Mao J, Lin E, He L, Yu J, Tan P, Zhou Y (2019) Autophagy and viral infection. Adv Exp Med Biol 1209:55–78. https://doi.org/10.1007/978-981-15-0606-2_5
Melincovici CS et al (2018) Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Rom J Morphol Embryol 59:455–467
Mizushima N, Levine B (2010) Autophagy in mammalian development and differentiation. Nat Cell Biol 12:823–830. https://doi.org/10.1038/ncb0910-823
Mohamadian M, Chiti H, Shoghli A, Biglari S, Parsamanesh N, Esmaeilzadeh A (2021) COVID-19: Virology, biology and novel laboratory diagnosis. J Gene Med 23:e3303. https://doi.org/10.1002/jgm.3303
Moza A et al (2023) Outcome of newborns with confirmed or possible SARS-CoV-2 Vertical Infection-A scoping review. Diagnostics (Basel) 13. https://doi.org/10.3390/diagnostics13020245
Nakashima A et al (2017) Role of autophagy in oocytogenesis, embryogenesis, implantation, and pathophysiology of pre-eclampsia. J Obstet Gynaecol Res 43:633–643. https://doi.org/10.1111/jog.13292
Park H, Cho M, Do Y, Park JK, Bae SJ, Joo J, Ha KT (2021) Autophagy as a therapeutic target of Natural products enhancing embryo implantation. Pharmaceuticals (Basel) 15. https://doi.org/10.3390/ph15010053
Power Guerra N, Bierkamper M, Pablik J, Hummel T, Witt M (2024) Histochemical Evidence for Reduced Immune Response in nasal mucosa of patients with COVID-19. Int J Mol Sci 25. https://doi.org/10.3390/ijms25084427
Pushkarsky T et al (2001) CD147 facilitates HIV-1 infection by interacting with virus-associated cyclophilin A. Proc Natl Acad Sci U S A 98:6360–6365. https://doi.org/10.1073/pnas.111583198
Rakheja D et al (2022) SARS-CoV-2 immunohistochemistry in Placenta. Int J Surg Pathol 30:393–396. https://doi.org/10.1177/10668969211067754
Remark R et al (2016) In-depth tissue profiling using multiplexed immunohistochemical consecutive staining on single slide. Sci Immunol 1:aaf6925. https://doi.org/10.1126/sciimmunol.aaf6925
Rizzo R et al (2021) SARS-CoV-2 nucleocapsid protein and ultrastructural modifications in small bowel of a 4-week-negative COVID-19 patient. Clin Microbiol Infect 27:936–937. https://doi.org/10.1016/j.cmi.2021.01.012
Robba C, Battaglini D, Pelosi P, Rocco PRM (2020) Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expert Rev Respir Med 14:865–868. https://doi.org/10.1080/17476348.2020.1778470
Runwal G, Stamatakou E, Siddiqi FH, Puri C, Zhu Y, Rubinsztein DC (2019) LC3-positive structures are prominent in autophagy-deficient cells. Sci Rep 9:10147. https://doi.org/10.1038/s41598-019-46657-z
Sauter JL et al (2020) Insights into pathogenesis of fatal COVID-19 pneumonia from histopathology with immunohistochemical and viral RNA studies. Histopathology 77:915–925. https://doi.org/10.1111/his.14201
Schiuma G et al (2023) Effect of SARS-CoV-2 infection in pregnancy on CD147, ACE2 and HLA-G expression. Placenta 132:38–43. https://doi.org/10.1016/j.placenta.2023.01.004
Schwartz DA et al (2022) Placental tissue Destruction and Insufficiency from COVID-19 causes stillbirth and neonatal death from hypoxic-ischemic Injury. Arch Pathol Lab Med 146:660–676. https://doi.org/10.5858/arpa.2022-0029-SA
Shan T, Li LY, Yang JM, Cheng Y (2023) Role and clinical implication of autophagy in COVID-19. Virol J 20:125. https://doi.org/10.1186/s12985-023-02069-0
Shchegolev AI, Kulikova GV, Lyapin VM, Shmakov RG, Sukhikh GT (2021) The number of Syncytial knots and VEGF expression in Placental Villi in Parturient Woman with COVID-19 depends on the Disease Severity. Bull Exp Biol Med 171:399–403. https://doi.org/10.1007/s10517-021-05236-x
Simbar M, Nazarpour S, Sheidaei A (2023) Evaluation of pregnancy outcomes in mothers with COVID-19 infection: a systematic review and meta-analysis. J Obstet Gynaecol 43:2162867. https://doi.org/10.1080/01443615.2022.2162867
Smadja DM et al (2021) Placental growth factor level in plasma predicts COVID-19 severity and in-hospital mortality. J Thromb Haemost 19:1823–1830. https://doi.org/10.1111/jth.15339
Swain LA, Ambasta A, Munhoz EP, Omodon O, Urbanski SJ, Nguyen HH (2023) Acute severe hepatitis as a presenting symptom in clinically stable patients admitted with SARS-CoV-2 Omicron infection. Hepatol Commun 7. https://doi.org/10.1097/HC9.0000000000000115
Tsukamoto S, Kuma A, Mizushima N (2008) The role of autophagy during the oocyte-to-embryo transition. Autophagy 4:1076–1078. https://doi.org/10.4161/auto.7065
Valdes G et al (2006) Distribution of angiotensin-(1–7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta 27:200–207. https://doi.org/10.1016/j.placenta.2005.02.015
Wastnedge EAN, Reynolds RM, van Boeckel SR, Stock SJ, Denison FC, Maybin JA, Critchley HOD (2021) Pregnancy and COVID-19. Physiol Rev 101:303–318. https://doi.org/10.1152/physrev.00024.2020
Zaim S, Chong JH, Sankaranarayanan V, Harky A (2020) COVID-19 and Multiorgan Response. Curr Probl Cardiol 45:100618. https://doi.org/10.1016/j.cpcardiol.2020.100618
Zamboni P et al (2022) Bowel ischemia as onset of COVID-19 in otherwise asymptomatic patients with persistently negative swab. J Intern Med 291:224–231. https://doi.org/10.1111/joim.13385
Zhao X, Jiang Y, Jiang T, Han X, Wang Y, Chen L, Feng X (2020) Physiological and pathological regulation of autophagy in pregnancy. Arch Gynecol Obstet 302:293–303. https://doi.org/10.1007/s00404-020-05607-1
Zhou H, Hu Z, Castro-Gonzalez S (2023) Bidirectional interplay between SARS-CoV-2 and autophagy. mBio 14:e0102023. https://doi.org/10.1128/mbio.01020-23
Zinserling VA, Semenova NY, Bikmurzina AE, Kruglova NM, Rybalchenko OV, Markov AG (2022) SARS-CoV-2-Induced Pathology-Relevance to COVID-19 pathophysiology. Pathophysiology 29:281–297. https://doi.org/10.3390/pathophysiology29020021
Acknowledgements
The authors are grateful to Dr. Chiara Marina Semprini (Medical Department, University Hospital of Ferrara, Italy) for collecting samples, Dr. Cristina Bosi (Department of Translational Medicine, University of Ferrara, Italy) for histological sample preparation, and Aurora Zanetti (Department of Translational Medicine, University of Ferrara, Italy) for help in developing markers staining in IHC.
Funding
This study was supported by current University research funds “Fondo di Ateneo per la Ricerca Scientifica” (FAR) to C.S. (project code 2023-FAR.L_SVEB_SCA) and to L.M.N. (2023-FAR.L_medtras_NL)
Open access funding provided by Università degli Studi di Ferrara within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
C. Simioni, J.M. Sanz, L.M. Neri and A. Passaro conceptualized the work; C. Simioni, J.M. Sanz and R. Gafà performed the methodology; C. Simioni, J.M. Sanz., R. Gafà, L.M. Neri and A. Passaro validated the results; C. Simioni and J.M. Sanz focused on software analysis; C. Simioni and L.M. Neri found resources; C. Simioni and J.M. Sanz dealt with data curation; C. Simioni wrote and prepared the original draft; C. Simioni, J.M. Sanz, R. Gafà, L.M. Neri and A. Passaro reviewed and edited the manuscript; L.M. Neri and A. Passaro supervised the work; A. Passaro, C. Simioni and L.M. Neri administrated the project.All the authors were involved in drafting and revising the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Simioni, C., Sanz, J.M., Gafà, R. et al. Effects of SARS-COV-2 on molecules involved in vascularization and autophagy in placenta tissues. J Mol Histol (2024). https://doi.org/10.1007/s10735-024-10228-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10735-024-10228-y