Abstract
Neuropilin-1 (NRP-1) is an essential regulator of maternal immune tolerance, placentation, and angiogenesis. Its dysregulation in preeclampsia (PE) and human immunodeficiency virus (HIV) infection implicates NRP-1 in disease susceptibility and progression. Therefore, this study investigates placental NRP-1 immunoexpression in HIV-complicated preeclamptic pregnancies in South African women of African ancestry receiving antiretroviral therapy. Immunohistochemistry of recombinant anti-neuropilin-1 antibody was performed on placental tissue from 30 normotensive and 60 early onset (EOPE) and late-onset (LOPE) preeclamptic women stratified by HIV status. Qualitative analysis of NRP-1 immunostaining within the chorionic villi revealed a predominant localization in trophoblasts and syncytial knots as well as endothelial, fibroblast-like, and Hofbauer cells. Following morphometric evaluation, we report that PE and HIV infection and/or antiretroviral usage independently downregulate placental NRP-1 immunoexpression; however, as a comorbidity, this decline is further augmented within the conducting and exchange villi. Furthermore, reduced immunoexpression of NRP-1 in EOPE compared with LOPE villi may be due to maternal–fetal maladaptation. It is plausible that the decreased NRP-1 immunoexpression in PE placentae facilitates syncytiotrophoblast apoptosis and subsequent deportation of NRP-1 into the maternal circulation, contributing to the anti-angiogenic milieu of PE. We hypothesize that the intense NRP-1 immunoreactivity observed in Hofbauer cells at the maternal–fetal interface may contribute to the natural prevention mechanism of HIV vertical transmission.
Similar content being viewed by others
Introduction
Maternal mortality is a major global concern in sub-Saharan Africa, accounting for 66% of the deaths. Despite a significant decline in institutional maternal mortality rate within South Africa (SA), hypertensive disorders of pregnancy (HDP) and nonpregnancy-related infections, particularly human immunodeficiency virus (HIV) infection, still remain a major burden to national healthcare (Woldesenbet et al. 2021). In SA, HIV prevalence in pregnant women is 30%, with the highest occurring within the KwaZulu-Natal province (40.9%) (Woldesenbet et al. 2021). Approximately 18% of all maternal deaths in SA result from HDP, predominantly due to preeclampsia (PE) and eclampsia (National Committee for Confidential Enquiry into Maternal Deaths 2018).
The decidualised endometrium plays an essential role in embryonic development by protecting the embryo from maternal immunological rejection and providing nourishment prior to placentation (Mori et al. 2016; Vinketova et al. 2016). Furthermore, the decidua secretes proteins that induce trophoblast growth and invasion and promotes angiogenesis (formation of new capillaries from preexisting vasculature) during spiral artery remodeling (Wang et al. 2018). The uteroplacental vasculature undergoes a significant morphological and physiological transformation to sustain a healthy pregnancy (Singh et al. 2011). Ideally, cytotrophoblast (CT) cells derived from anchoring fetal chorionic villi form a multinucleated syncytiotrophoblast (ST) layer, which encompasses the placental villi, thus establishing the maternal–fetal interface for efficient nutrient and gaseous exchange. The extravillous trophoblast (EVT) infiltration of the decidua and the inner myometrium occurs in a set time sequence (Valenzuela et al. 2012; Pijnenborg 1990; Cartwright et al. 2010). These EVTs also invade the fibrinoid-type material that replaces the musculo-elastic media within the spiral artery wall, transforming it into low-resistance large sinusoidal-like vessels (dilated five to tenfold) that facilitates an adequate blood supply, which meets the oxygen and nutrient supply required by the growing fetus. (Brosens et al. 1967; Cartwright et al. 2010). As placentation advances, EVT migration reduces the distance between the fetal vasculature in the villous stroma and the maternal blood in the intervillous space, thereby enhancing the exchange at the maternal–fetal interface. (Huppertz 2008). These changes are typically achieved by 20 weeks of gestation; however, complications may predispose to PE development (Whitley and Cartwright 2009).
Preeclampsia is categorized by time of onset (> 20 weeks) i.e., early onset preeclampsia (EOPE < 34 weeks), and late-onset preeclampsia (LOPE ≥ 34 weeks) (Brown et al. 2018). Although the pathogenesis of PE remains multifactorial, it is widely accepted that PE emanates from maternal risk factors (nulliparity, chronic prepregnancy disease, genetic predisposition, etc.) inducing placental/trophoblast stress (Staff 2019). Staff (2019) hypothesizes that EOPE follows an extrinsic pathway implicating dysfunctional uterine tolerization of the allogeneic trophoblast, deficient trophoblast invasion, and a lack of the physiological conversion of spiral arteries within the myometrium (Staff 2019). In comparison, LOPE follows an intrinsic pathway without placental maladaptation but involves placental stress/hypoxia exerted directly by maternal risk factors or exceeding placental capacity (Staff 2019). Nonetheless, both extrinsic and intrinsic pathways lead to the second stage of PE, i.e., pervasive multiorgan endothelial dysfunction as a result of reduced endothelial nitric oxidase synthase bioavailability, vasoconstriction, hypoxia, oxidative stress, and an anti-angiogenic microenvironment leading to the clinical manifestation of PE (presence of hypertension, proteinuria, liver dysfunction, cerebral edema, eclampsia, etc.) (Kvietys and Granger 2012; Brown et al. 2018; Wagner 2004; Redman and Sargent 2005; Rana et al. 2019).
Globally, SA has the highest antiretroviral therapy (ART) rollout program for the treatment of HIV, including the use of ARTs for the prevention of mother-to-child transmission (PMTCT) (Pattinson 2014). While the immunosuppressive state of HIV infection may reduce the risk of PE development in pregnant women, ART usage induces immune reconstitution, impairs decidualization, and is associated with endothelial injury, eventuating in PE development (Naidoo et al. 2021; Sebitloane and Moodley 2017b, 2017a; Frank et al. 2004; Pattinson 2014).
Neuropilin-1 (NRP-1) is a versatile transmembrane protein involved in cell migration and invasion, angiogenesis, tumorigenesis, axonal guidance, immune response regulation, and entry of several viruses (i.e., severe acute respiratory syndrome coronavirus 2, human T-cell lymphotropic virus type 1, and Epstein–Barr virus) into the host cell (Naidoo et al. 2022). It serves as a co-receptor for the binding of angiogenic ligands such as vascular endothelial growth factor (VEGF), transforming growth factor beta (TGF-β), human growth factor (HGF), and platelet-derived growth factor (PDGF) (Elpek 2015). The expression of NRP-1 in normal female reproductive tissue is essential for decidualization and the maintenance of maternal immune tolerance and placental angiogenesis (Arad et al. 2017). Of note, previous studies observed a downregulation of placental NRP-1 expression in PE (Maulik et al. 2016; Xu et al. 2016; Arad et al. 2017). Moreover, upregulated NRP-1 expression occurs within macrophages and dendritic cells demonstrate HIV resistance and reduced infectivity (Wang et al. 2022). Therefore, the expression of NRP-1 is implicated in the susceptibility to and progression of PE and HIV infection.
Given the paucity of knowledge on the role of NRP-1 in PE comorbid with HIV infection in an African population, our study is the first to investigate placental NRP-1 immunoexpression in HIV-complicated preeclamptic pregnancies in South African women of African ancestry.
Materials and methods
Study population
Ethical approval was obtained from the Biomedical Research Ethics Committee (BREC), University of KwaZulu-Natal, for this prospective study (BREC/00003307/2021). Ninety archived placental samples were obtained from pregnant women attending a regional hospital in the KwaZulu-Natal province of South Africa. The study population was grouped into normotensive (blood pressure ± 120/80 mmHg) and preeclamptic pregnancies, i.e., new-onset hypertension (> 20 weeks) with a blood pressure of ≥ 140/90 mmHg and proteinuria of ≥ 300 mg/24 h (Brown et al. 2018). The preeclamptic group was subdivided into EOPE and LOPE and all pregnancy types were further stratified by HIV status (n = 15 per subgroup), i.e., normotensive HIV negative (N), normotensive HIV positive (N+), early onset preeclamptic HIV negative (EOPE−), early onset preeclamptic HIV positive (EOPE+), late-onset preeclamptic HIV negative (LOPE−), and late-onset preeclamptic HIV positive (LOPE+).
Inclusion criteria
Normotensive pregnant women and women diagnosed with PE, known HIV status (CD4+ cell count ≥ 200 cells/μL, if HIV+ and in receipt of ARVs), and singleton pregnancy were included in this study. All participants were ≥ 18 years old.
Exclusion criteria
Women with eclampsia, chronic hypertension, intrauterine death, abruption placentae, polycystic ovarian syndrome, chorioamnionitis, preexisting seizure disorders, gestational diabetes, chronic diabetes, systemic lupus erythematosus, chronic renal disease, sickle cell disease, thyroid disorder, antiphospholipid antibody syndrome, connective tissue disorder, cardiac disease, asthma, unknown HIV status, patients who did not consent to participation, patients who were unable to provide informed consent, and women without antenatal care were excluded from this study.
Placenta collection and tissue preparation
In the primary study, a segment from the central region of each placenta was cautiously excised, avoiding areas with macroscopic infarction and immediately fixed in 10% buffered formaldehyde solution. The placental tissue was dehydrated using an ascending ethanol series, cleared with xylene, and infiltrated with paraffin wax using an automated tissue processor (Sakura 5, Torrance, California, USA). The tissue was then embedded in an embedding station (Leica EG 1160 embedding station, Germany) and stored until required. Prior to the immunostaining procedure, the paraffin wax-embedded blocks were trimmed and cut into 3 μm sections using a rotatory microtome (Leica RM2135, UK). Sections were then floated on a 50 °C water bath (Leica HI1210, Leica Biosystems, UK), collected onto X-tra adhesive coated slides (Leica Biosystems, UK), and heat fixed on a 60 °C hot plate (Sakura, USA) overnight.
Immunohistochemistry
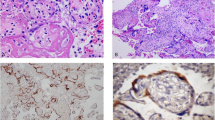
To expose the target antigens, antigen retrieval was performed using the Rabbit-specific HRP/DAB Detection Micro-polymer Kit (ab236469; Abcam, UK). The sections were de-paraffined, hydrated, and rinsed in deionized water. Thereafter, antigen retrieval was achieved by incubating the sections in preheated Tris–EDTA (pH 9.0) target retrieval solution for 20 min at 95 °C (ab93684; Abcam, UK). The slides were cooled at room temperature (20 min), rinsed, and placed in wash buffer solution (5 min). Following this, the sections were circled with a delimiting hydrophobic pap pen (ab2601, Abcam, UK). To quench endogenous peroxidase, tissue was placed in hydrogen peroxidase-blocking reagent in a humidity chamber (45 min). Following washing, the sections were incubated with a protein block (45 min). After washing, the slides were incubated at room temperature with the anti-NRP-1 primary antibody (Recombinant Anti-Neuropilin 1 Antibody, EPR3113, ab81321, Abcam, USA) in a 1:1000 dilution for 1 h to label the target antigen. Post-washing, the slides were incubated with a secondary antibody, goat anti-rabbit IgG horseradish peroxidase conjugate (20 min), followed by rinsing in wash buffer. Immunoreactivity was detected by 3,3′diamino-benzidine (DAB) chromogen. All sections were rinsed, counterstained with Mayer’s hematoxylin (30 s), and rinsed again in water (2 min). Thereafter, the samples were dehydrated, cleared in xylene, and cover slipped. Human placental tissue served as the positive control [Fig. 1a]. Replacement of the primary antibody with a buffer and nonimmune serum of the same IgG class as the primary served as the negative control [Fig. 1b].
Morphometric image analysis
An ApoTome 2 microscope (Carl Zeiss, Germany) was used to examine immunostaining in four fields of view per slide for each villus type (i.e., conducting and exchange) at an initial objective magnification of 20× and captured at 40× . Following this, Zen Blue 2.5 Pro software (Carl Zeiss, Germany) was used to optimize, capture and archive images. Fiji/ImageJ image analysis software (Wisconsin, USA) was used to segment and measure NRP-1 immunoexpression (Schneider et al. 2012). Due to histological variation between villus types, different morphometric frame strategies were applied. Each conducting villi was first framed/segmented. Thereafter, the amount of label within both villus types was determined by a two-phase threshold and expressed as a total percentage of NRP-1 immunostaining within the villus area.
Statistical analysis
Data were analyzed using GraphPad Prism software version 8.4.3 (GraphPad Software, San Diego, California, USA). Normality tests (D’Agostino and Pearson, Shapiro–Wilk, and Kolmogorov–Smirnov) revealed nonparametrically distributed patient data; therefore, a Kruskal–Wallis test and Dunn’s multiple comparison post hoc test was conducted for pregnancy types. These results were represented as the median and interquartile range (IQR). For the immunoexpression analysis, normality tests revealed parametric data; therefore, a one-way ANOVA was used to compare pregnancy types and subgroups followed by a Holm Sidak’s multiple comparison test. An unpaired t-test was used to compare HIV status irrespective of pregnancy types. These results were represented as a percentage (%) mean ± SD. A p-value of < 0.05 was considered statistically significant for all tests. Asterisks (*) denotes the degree of significance, i.e., *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Results
Patient characteristics
Table 1 summarizes the demographics and clinical data of the study population, which includes maternal age (years), systolic and diastolic blood pressure (mmHg), gestational age at delivery (weeks), maternal weight (kg), CD4+ T Cell Count (cells/µl), and neonatal birth weight (kg) across pregnancy types stratified by HIV status. Significantly higher systolic and diastolic blood pressures were noted in EOPE and LOPE compared with N pregnancies (p < 0.0001****). Gestational age was significantly higher in EOPE compared with N (p < 0.0001****) and LOPE (p < 0.0001****) pregnancies. Birth weight of neonates was significantly lower in EOPE compared with N and LOPE groups, i.e., p < 0.0001**** and p = 0.0002***, respectively.
Qualitative immunolocalization of NRP-1 in the placenta
Distinctive NRP-1 immunoexpression within the fetal chorionic villi was predominantly localized to the endothelial (Fig. 3a–f) and medial cells (Fig. 3e) of fetal arterial supply and venous drainage of conducting villi. Moreover, NRP-1 immunostaining was observed in the capillaries and mesenchymal stroma of exchange villi (Fig. 4a–f). High immunoreactivity was noted within the trophoblast cell populations [i.e., EVT (Fig. 2), ST, and CT cells (Figs. 3a and 4c)], and mesenchymal fibroblast-like cells (Fig. 3c, b, d) within both conducting and exchange villi. Syncytial knots (Fig. 3a, d, e] and shed syncytial knots/sprouts (Figs. 3d and 4d, f) were also immunostained for NRP-1. Hofbauer cells (HCs, placental tissue macrophages) were intensely stained for NRP-1 terminal exchange villi in HIV-infected exchange villi, regardless of pregnancy type (Fig. 4b, d, f). However, red bloods cells were negative for NRP-1 in fetal circulation and the intervillous space (maternal circulation) across villus types.
NRP-1 immunostaining within conducting villi (20×) in a N−, b N+ , c EOPE−, d EOPE+ , e LOPE−, and f LOPE+ groups. Scale bar, 200 μm. CT cytotrophoblast cell, EC endothelial cells, FB fibroblast cells, M medial cells, S syncytium, SS shed syncytial knot/sprout, ST syncytiotrophoblast cell, VSM vascular smooth muscle fiber
NRP-1 immunostaining within exchange villi (40×) in a N−, b N+ , c EOPE−, d EOPE+ , e LOPE−, and f LOPE+ groups. Scale bar, 100 μm. CT cytotrophoblast cell, EC endothelial cells, FB fibroblast cells, HC Hofbauer cell, S syncytium, SB syncytial bridge, SK syncytial knot, SS shed syncytial knot/sprout, ST syncytiotrophoblast cell
Morphometric analysis of NRP-1 immunostaining
Conducting Villi
Pregnancy types irrespective of HIV status: The NRP-1 immunoexpression was significantly lower in PE (11.21 ± 3.874) compared with N pregnancies (13.55 ± 4.156), p = 0.0099** (Fig. 5a). NRP-1 immunoexpression was significantly decreased in EOPE (9.686 ± 2.799) compared with N (13.55 ± 4.156) and LOPE pregnancies (12.74 ± 4.230); p = 0.0005*** and p = 0.0049**, respectively. NRP-1 immunoexpression were similar between LOPE and N groups (p = 0.4075) (Fig. 5b).
Graphical representation of NRP-1 immunoexpression comparisons between pregnancy types, HIV status, and across all groups in conducting and exchange villi. N normotensive, PE preeclampsia, EOPE early onset preeclampsia, LOPE late-onset preeclampsia, HIV− Human immunodeficiency virus negative, HIV+ Human immunodeficiency virus positive, N− normotensive HIV negative, N+ normotensive HIV positive, EOPE− early-onset preeclampsia HIV negative, EOPE+ early onset preeclampsia HIV positive, LOPE− late-onset preeclampsia HIV negative; LOPE+ late-onset preeclampsia HIV positive. Asterisks (*) denote significance: *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001
HIV status irrespective of pregnancy type: No significant difference in NRP-1 immunoexpression was detected between HIV+ (11.33 ± 4.276) and HIV− pregnancies (12.65 ± 3.850); p = 0.1278 (Fig. 5c).
Across all groups: NRP-1 immunoexpression was significantly lower in EOPE+ (9.085 ± 3.132) compared with N− (14.31 ± 4.170) and LOPE− (13.35 ± 3.755); p = 0.0045** and p = 0.0381*, respectively. No other subgroup comparisons were significantly different (Table 2, Fig. 5d).
Exchange Villi
Pregnancy type irrespective of HIV status: The NRP-1 immunoexpression was significantly lower in PE (24.63 ± 4.335) compared with N pregnancies (30.42 ± 4.708), p = < 0.0001**** (Fig. 5e). NRP-1 immunoexpression showed a significant reduction in both EOPE (22.62 ± 3.767) and LOPE (26.63 ± 3.962) compared with N pregnancies (30.42 ± 4.708); p = < 0.0001**** and p = 0.0007***, respectively. NRP-1 immunostaining was also significantly lower in EOPE compared with LOPE pregnancies (p = 0.0007***) (Fig. 5f).
HIV status irrespective of pregnancy type: NRP-1 immunoexpression was significantly lower in HIV + (25.39 ± 4.956) compared with HIV− pregnancies (27.73 ± 5.263), p = 0.0327* (Fig. 5g).
Across all groups: NRP-1 immunoexpression was significantly reduced in EOPE− (23.20 ± 3.553), EOPE+ (22.04 ± 4.005), and LOPE+ (25.13 ± 4.578) compared with N− pregnancies (31.84 ± 5.245); p = < 0.0001****, p = < 0.0001****, and p = 0.0002***, respectively. Both EOPE− and EOPE+ pregnancies showed decreased NRP-1 immunostaining compared with N+ pregnancies (29.00 ± 3.755); p = 0.0017** and p = 0.0001***, respectively. Furthermore, EOPE− and EOPE+ pregnancies also showed reduced NRP-1 immunoexpression compared with LOPE− pregnancies (p = 0.0109* and p = 0.0009*** respectively) (Table 2, Fig. 5h).
Discussion
The vital role of NRP-1 expression in ensuring pregnancy success has been highlighted by various studies (Naidoo et al. 2022). Dysregulation exacerbates the development of obstetric complications such as fetal growth restriction (FGR) and PE (Naidoo et al. 2022). To our knowledge, the current study is the first to (1) evaluate NRP-1 immunoexpression within EOPE and LOPE placentae independently, (2) investigate the effect of HIV infection on placental NRP-1 immunoexpression in pregnant women, and (3) to assess the influence of PE comorbid with HIV infection on NRP-1 placental immunoreactivity.
Irrespective of HIV status, placental NRP-1 immunoexpression within both conducting and exchange villi was downregulated in PE compared with normotensive pregnancies. Dependent on timing of onset, NRP-1 immunoexpression was reduced in EOPE compared with normotensive and LOPE pregnancies. NRP-1 immunoreactivity within exchange villi was also significantly reduced in LOPE compared with normotensive pregnancies. A similar trend was observed in conducting villi, albeit nonsignificantly. Our results in HIV-uninfected pregnant women are corroborated by a few studies that report decreased NRP-1 immunoexpression in PE compared with normotensive placentae (Yang et al. 2021; Xu et al. 2016; Arad et al. 2017). In addition, our findings of immunostaining within the trophoblast cell populations (i.e., ST, CT, and EVT cells) of both conducting and exchange villi are supported by Arad et al., who found reduced immunoexpression of NRP-1 in the ST layer of PE compared with normotensive villi (Arad et al. 2017). Furthermore, a decline in NRP-1 and the RNA-binding protein [quaking I-5 (QKI-5)] expression was observed in trophoblasts upon exposure to a hypoxic microenvironment, as seen in PE (Yang et al. 2021). However, they demonstrated that by upregulating QKI-5 within trophoblast cells, NRP-1 expression also increased and significantly improved their proliferation in vitro and in vivo since QKI-5 directly interacts with the 3′-untranslated region (UTR) of NRP-1 to promote cell proliferation and migration via matrix metalloprotease-9 (Yang et al. 2021).
In another in vivo study, immunolocalization analysis of normotensive second trimester placental bed tissue showed that NRP-1 expression within CT cells was upregulated as the these cells infiltrated the uterine wall, and strong immunostaining was also observed within endovascular trophoblast cells as well as within endothelial cells of the uterine vessels (Zhou et al. 2013). Additionally, FGR pregnancies complicated with absent end-diastolic flow in the umbilical artery have reduced placental NRP-1 expression, correlating with PE development (Maulik et al. 2016). NRP-1 downregulation also occurs in pregnancies following assisted reproductive technologies, a known risk factor for PE development (Omani-Samani et al. 2020). Since NRP-1 promotes sprouting angiogenesis, its downregulation may be implicated in deficient vascular branching observed in FGR pregnancies and preeclamptic placentae, thereby contributing to the anti-angiogenic milieu of PE (Maulik et al. 2016). Our findings add to the existing pool of evidence implicating a decline in NRP-1 immunoexpression in deficient trophoblast invasion and the subsequent inadequate spiral artery remodeling, and placental maladaptation predisposing PE development.
Notably, the trend in placental NRP-1 immunoexpression across pregnancy types (i.e., normotensive > LOPE > EOPE) observed in our study supports the revised two-stage placental model of PE (Staff 2019). The expression of NRP-1 in EOPE is considerably reduced compared with LOPE placentae due to the probable causal agents involved in the aforementioned extrinsic pathway (Staff 2019). While both extrinsic and intrinsic pathways lead to the second stage of PE (maternal endothelial dysfunction, hypertension, and proteinuria), FGR pregnancies are more common in EOPE compared with LOPE, implicating NRP-1 dysregulation in this phenomenon (Maulik et al. 2016; Staff 2019).
The syncytium remains viable for several weeks due to the presence of apoptosis inhibitors [B-cell lymphoma-2 (Bcl-2), myeloid cell leukemia 1 (Mcl-1), etc.]; thereafter, aged nuclei migrate toward the villous tips to form syncytial knots (Huppertz et al. 1998). Similar to apoptotic nuclei, the syncytial knots contain condense packed nuclei with no distinct nucleoli and the expression of apoptosis inhibitors are greatly reduced (Crocker 2007). Therefore, syncytial knot formation is considered the result of the apoptotic cascade within the syncytiotrophoblast layer and are eventually shed as membrane-sealed vesicles into maternal circulation (intervillous space) (Heazell and Crocker 2008). Notably, trophoblast apoptosis is believed to be exacerbated in PE (Naicker et al. 2013; Tomas et al. 2011). Similar to Arad et al., we report NRP-1 immunostaining within syncytial knots and shed syncytial sprouts/knots. Interestingly, NRP-1 regulates apoptosis in cancer and rheumatoid arthritis due to its regulation of Bcl-2 expression, Bcl-2 associated X protein (Bax) translocation, and Mcl-1 expression (Kim et al. 2006; Ochiumi et al. 2006; Bachelder et al. 2001; Riese et al. 2012; Wey et al. 2005). Therefore, reduced expression of NRP-1 in PE placentae may facilitate this apoptotic process (Arad et al. 2017). Furthermore, Awoyemi et al. reports NRP-1 immunoexpression within the shed ST microparticles (STBMs), particularly in smaller STMBs, in the intervillous space (maternal circulation) (Awoyemi et al. 2022). Shedding of STBM from the placenta into maternal blood occurs in normal pregnancies and is exacerbated during PE also due to elevated apoptosis or aponecrosis (Huppertz et al. 1998). Subsequently, the microparticles evoke systemic inflammation (Naidoo et al. 2021). It is plausible that the expulsion of essential NRP-1 into the maternal circulation via shed syncytial knots/sprouts (Burton and Jones 2009) and STBM upregulates the anti-angiogenic soluble form of NRP-1 (sNRP-1) (Klagsbrun et al. 2002). Consequently, sNRP-1 may bind and sequester angiogenic ligands (VEGF, TGF-β, HGF), hindering angiogenic signaling, thereby inducing endothelial dysfunction, and ultimately predisposing to PE development (Mamluk et al. 2002; Smárason et al. 1993). This implication is supported by Naidoo (2020), who reported an increase in circulating sNRP-1 in PE (Naidoo 2020).
Irrespective of pregnancy type, the immunoexpression of NRP-1 within exchange villi was influenced by HIV status. A similar trend was observed in conducting villi, albeit nonsignificantly. This result is corroborated by Jia et al., who showed that the trans-activator of transcription (tat), an HIV accessory protein, mimics VEGF through structural homology and inhibits VEGF165 binding to tyrosine kinase receptors and their coreceptor NRP-1 in endothelial cells (Jia et al. 2001). Consequently, dysregulated basic fibroblast growth factor (bFGF) and VEGF-induced extracellular signal-related kinase (ERK) activation and mitogenesis in endothelial cells inhibited angiogenesis in vitro at concentrations similar to those that impeded VEGF receptor (VEGFR) binding (Jia et al. 2001). Furthermore, the tat protein induces apoptosis of endothelial cells, independent of VEGF and bFGF (Jia et al. 2001).
NRP-1 immunoexpression in the conducting villi was only significantly different between EOPE+ compared with normotensive− and LOPE− groups, whereas exchange villi showed great variability across all groups. In exchange villi, NRP-1 immunoexpression was significantly reduced in EOPE−, EOPE+, and LOPE+ compared with normotensive placentae. Both EOPE− and EOPE+ placentae showed decreased NRP-1 immunostaining compared with normotensive+ placentae. Furthermore, EOPE− and EOPE+ pregnancies also showed reduced NRP-1 immunoexpression compared with LOPE− pregnancies. Of note, all HIV-infected pregnant women in our study received combinations of ART, including nucleoside/nucleotide reverse transcriptase inhibitors (tenofovir disoproxil fumarate, efavirenz, emtricitabine, lamivudine), protease inhibitors (lopinavir/ritonavir), and integrase inhibitors (dolutegravir) in accordance with national guidelines for improving immunological responses and for PMTCT (World Health Organization 2010; National Department of Health South Africa 2019). The immune reconstitution induced by ART in HIV-infected pregnant women may increase their risk of severe comorbidity with PE (Tooke et al. 2016; French et al. 2000). Several studies show that ARTs, such as nucleoside/nucleotide reverse transcriptase inhibitors and protease inhibitors, dysregulates decidualization and placentation, inducing maternal endothelial dysfunction and the hypertensive manifestation denoting PE (Naidoo et al. 2021; Hernández et al. 2017; Song et al. 2018; Autran et al. 1999; Powis and Shapiro 2014).
Given the antiinflammatory and proangiogenic nature of NRP-1, the trend observed in NRP-1 immunostaining across all groups (i.e., normotensive− > normotensive+ ≈ LOPE− > LOPE+ > EOPE− > EOPE+) implicates HIV infection and/or ART usage in NRP-1 downregulation in HIV-infected placentae. Furthermore, the decrease in NRP-1 placental immunoexpression in EOPE+ and LOPE+ compared with their HIV-negative counterparts suggest that HIV infection and/or ART usage further exacerbates the risk of PE development. However, one study revealed no significant differences between the proportion of peripheral blood CD4+ and CD8+ T cells expressing NRP-1 as a result of ART-treated or untreated HIV infection when compared with HIV-seronegative controls (Lim et al. 2006).
Placental NRP-1 also shows dysregulation in other infections such as SARS-CoV-2 (Argueta et al. 2022). During SARS-CoV-2 infection, NRP-1 serves as a receptor for viral internalization and infectivity (Cantuti-Castelvetri et al. 2020). Interestingly, NRP-1 expression is upregulated in SARS-CoV-2-infected placental cells but not in uninfected cells; therefore the utilizing of NRP-1 receptors for SARS-CoV-2 entry is believed to potentially deter the physiological signaling action of NRP-1 in pregnancy, thereby dysregulating angiogenesis (Argueta et al. 2022).
Our qualitative observations of a predominance of HCs in HIV-infected placentae irrespective of pregnancy type was unexpected. Surprisingly, these cells were all intensely immunopositive for NRP-1. Hofbauer cells are placental villous macrophages of fetal origin present throughout pregnancy (Reyes and Golos 2018). Functionally, HCs resemble alternatively activated macrophages or M2-like macrophages that play a vital role in placental vasculogenesis and angiogenesis (Reyes and Golos 2018). Hofbauer cells comprise an array of M2a, M2b, and M2c macrophages that vary in surface marker expression, cytokine secretion, and functions, supporting the idea of a regulatory rather than inflammatory role of these cells (Loegl et al. 2016). While the number of HCs is reportedly reduced in PE, downregulation of CD74 and a human leukocyte antigen class II histocompatibility antigen-y chain on HCs is believed to alter macrophage polarization from an immunoregulatory M2 phenotype toward a proinflammatory signature impairing vital macrophage–trophoblast signaling, promoting maternal immune intolerance in PE (Yang et al. 2017; Koi et al. 2001) However, we cannot conclusively extrapolate our data to the regulation of HCs in HIV-infected PE placentae as CD68 or DC-SIGN immunostaining was not concurrently performed.
Despite the presence of the HIV receptors and coreceptors (i.e., CD4, CCR5, CXCR4, and DC-SIGN) on HCs, the rate of HIV infection in utero in the absence of interventions is only about 15–20% of exposed infants (Johnson and Chakraborty 2012; Teasdale et al. 2011). These cells have a decreased ability to replicate HIV in vitro and may contribute to the prevention of vertical transmission of HIV infection, and are believed to induce immunoregulatory cytokines such as IL-10, thereby protecting the maternal–fetal interface during continued HIV exposure (Johnson and Chakraborty 2012). NRP-1 has been recently identified as an antiviral protein for HIV infection (Wang et al. 2022). The high expression of transmembrane NRP-1 in macrophages and dendritic cells (DCs) compared with CD4+ T cells results in HIV resistance (Wang et al. 2022). Furthermore, NRP1 gene silencing significantly promotes the transmission of HIV in macrophages and DCs, thereby amplifying HIV infectivity (Wang et al. 2022). Therefore, it is plausible to hypothesize that the intense NRP-1 immunoreactivity observed in HCs at the maternal–fetal interface emanates from HIV resistance, thus contributing to the natural mechanism of PMTCT.
In summary, our study provides a novel insight into the downregulation of NRP-1 expression in HIV-infected preeclamptic placentae. This study shows that, independently, PE and HIV infection downregulate placental NRP-1 immunoexpression; however, as a comorbidity, this decline is further augmented within ST, CT, and endothelial cells of exchange and conducting (to a lesser extent) villi. Moreover, the decline in NRP-1 immunoexpression in EOPE compared with LOPE villi may be due to maternal immune intolerance, deficient trophoblast invasion, and incomplete spiral artery remodeling. Furthermore, it is plausible that the reduced immunoexpression of NRP-1 in PE placentae facilitates ST apoptosis and subsequent deportation of NRP-1 into the maternal circulation via shed syncytial knots/sprouts, which may bind and sequester angiogenic ligands contributing to the anti-angiogenic milieu of PE. Finally, considering the recent study showing HIV resistance in macrophages due to NRP-1 expression, we hypothesize that the intense NRP-1 immunoreactivity observed in HCs at the maternal–fetal interface emanates from HIV resistance thus contributing to the natural mechanism of PMTCT.
Strengths, limitations, and future recommendations
The morphometric evaluation of placental NRP-1 immunoexpression was performed and inspected by two independent researchers, ensuring the reliability of the results. All HIV-infected pregnant women in our study received ART; therefore, it is unclear whether the observed placental NRP-1 immunoexpression resulted from HIV infection, ART usage, or both. This study did not control for EOPE gestational age using patients with other preterm delivery complications, such as preterm premature rupture of membranes or FGR, since they were uncommon at the site in which the study was conducted. It may also prove beneficial to establish a correlation between NRP-1 and other angiogenic and inflammatory factors across pregnancy types (including mild, moderate, and severe PE) and across all trimesters during gestation. Moreover, further large-scale studies are warranted to validate the role of NRP-1 in pregnancy, PE, HIV infection, and ART usage. The regulation of NRP-1 within HCs in HIV-infected PE placentae is inconclusive with our data as CD68 or DC-SIGN immunostaining was not concurrently performed; therefore, it necessitates clarification. Future studies are also invited to confirm our hypothesis associating NRP-1 immunoexpression in HCs with the natural PMTCT of HIV infection.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ART:
-
Antiretroviral therapy
- ARV:
-
Antiretrovirals
- Bcl-2:
-
B-cell lymphoma-2
- bFGF:
-
Basic fibroblast growth factor
- CT:
-
Cytotrophoblast
- DG-SIGN:
-
Dendritic cell-specific ICAM-3-grabbing nonintegrin
- EC:
-
Endothelial cell
- EVT:
-
Extravillous trophoblast cell
- EOPE:
-
Early onset preeclampsia/preeclamptic
- FGR:
-
Fetal growth restriction
- HC:
-
Hofbauer cell
- HDP:
-
Hypertensive disorders of pregnancy
- HGF:
-
Human growth factor
- HIV:
-
Human immunodeficiency virus
- IL-10:
-
Interleukin-10
- KZN:
-
KwaZulu-Natal
- LOPE:
-
Late-onset preeclampsia/preeclamptic
- Mcl-1:
-
Myeloid cell leukemia 1
- PDGF:
-
Platelet-derived growth factor
- PE:
-
Preeclampsia
- PMTCT:
-
Prevention of mother-to-child transmission
- QKI-5:
-
Quaking I-5
- SA:
-
South Africa
- sNRP-1 or NRP-1:
-
Soluble and/or neuropilin-1
- ST:
-
Syncytiotrophoblast
- STBMs:
-
Syncytiotrophoblast microparticles
- Tat:
-
Trans-activator of transcription
- TGF-β :
-
Transforming growth factor beta
- VEGF:
-
Vascular endothelial growth factor
- VEGFR:
-
Vascular endothelial growth factor receptor
References
Arad A, Nammouz S, Nov Y, Ohel G, Bejar J, Vadasz Z (2017) The expression of Neuropilin-1 in human placentas from normal and preeclamptic pregnancies. Int J Gynecol Pathol 36(1):42–49. https://doi.org/10.1097/pgp.0000000000000283
Argueta LB, Lacko LA, Bram Y, Tada T, Carrau L, Rendeiro AF, Zhang T, Uhl S, Lubor BC, Chandar V, Gil C, Zhang W, Dodson BJ, Bastiaans J, Prabhu M, Houghton S, Redmond D, Salvatore CM, Yang YJ, Elemento O, Baergen RN, tenOever BR, Landau NR, Chen S, Schwartz RE, Stuhlmann H (2022) Inflammatory responses in the placenta upon SARS-CoV-2 infection late in pregnancy. Iscience 25(5):104223. https://doi.org/10.1016/j.isci.2022.104223
Autran B, Carcelaint G, Li TS, Gorochov G, Blanc C, Renaud M, Durali M, Mathez D, Calvez V, Leibowitch J, Katlama C, Debre P (1999) Restoration of the immune system with anti-retroviral therapy. Immunol Lett 66(1–3):207–211. https://doi.org/10.1016/s0165-2478(98)00159-x
Awoyemi T, Iaccarino DA, Motta-Mejia C, Raiss S, Kandzija N, Zhang W, Vatish M (2022) Neuropilin-1 is uniquely expressed on small syncytiotrophoblast extracellular vesicles but not on medium/large vesicles from preeclampsia and normal placentae. Biochem Biophys Res Commun 619:151–158. https://doi.org/10.1016/j.bbrc.2022.06.041
Bachelder RE, Crago A, Chung J, Wendt MA, Shaw LM, Robinson G, Mercurio AM (2001) Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res 61(15):5736–5740
Brosens I, Robertson WB, Dixon HG (1967) The physiological response of the vessels of the placental bed to normal pregnancy. J Pathol Bacteriol 93(2):569–579. https://doi.org/10.1002/path.1700930218
Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, Hall DR, Warren CE, Adoyi G, Ishaku S (2018) Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension 72(1):24–43. https://doi.org/10.1161/hypertensionaha.117.10803
Burton GJ, Jones CJ (2009) Syncytial knots, sprouts, apoptosis, and trophoblast deportation from the human placenta. Taiwan J Obstet Gynecol 48(1):28–37. https://doi.org/10.1016/s1028-4559(09)60032-2
Cantuti-Castelvetri L, Ojha R, Pedro LD, Djannatian M, Franz J, Kuivanen S, van der Meer F, Kallio K, Kaya T, Anastasina M, Smura T, Levanov L, Szirovicza L, Tobi A, Kallio-Kokko H, Österlund P, Joensuu M, Meunier FA, Butcher SJ, Winkler MS, Mollenhauer B, Helenius A, Gokce O, Teesalu T, Hepojoki J, Vapalahti O, Stadelmann C, Balistreri G, Simons M (2020) Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 370(6518):856–860. https://doi.org/10.1126/science.abd2985
Cartwright JE, Fraser R, Leslie K, Wallace AE, James JL (2010) Remodelling at the maternal-fetal interface: relevance to human pregnancy disorders. Reprod 140(6):803–813. https://doi.org/10.1530/rep-10-0294
Crocker I (2007) Gabor than award lecture 2006: pre-eclampsia and villous trophoblast turnover: perspectives and possibilities. Placenta. https://doi.org/10.1016/j.placenta.2007.01.016
D’Costa GF, Khadke K, Patil YV (2007) Pathology of placenta in HIV infection. Indian J Pathol Microbiol 50(3):515–519
Elpek G (2015) Neuropilins and liver. World J Gastroenterol 21(23):7065–7073. https://doi.org/10.3748/wjg.v21.i23.7065
Frank KA, Buchmann EJ, Schackis RC (2004) Does human immunodeficiency virus infection protect against preeclampsia-eclampsia? Obstet Gynecol 104(2):238–242. https://doi.org/10.1097/01.aog.0000130066.75671.b2
French MA, Lenzo N, John M, Mallal SA, McKinnon EJ, James IR, Price P, Flexman JP, Tay-Kearney ML (2000) Immune restoration disease after the treatment of immunodeficient HIV-infected patients with highly active antiretroviral therapy. HIV Med 1(2):107–115. https://doi.org/10.1046/j.1468-1293.2000.00012.x
Heazell AE, Crocker IP (2008) Live and let die - regulation of villous trophoblast apoptosis in normal and abnormal pregnancies. Placenta 29(9):772–783. https://doi.org/10.1016/j.placenta.2008.07.003
Hernández S, Catalán-García M, Morén C, García-Otero L, López M, Guitart-Mampel M, Milisenda J, Coll O, Cardellach F, Gratacós E (2017) Placental mitochondrial toxicity, oxidative stress, apoptosis, and adverse perinatal outcomes in HIV pregnancies under antiretroviral treatment containing zidovudine. J Acquir Immune Defic Syndr 75(4):e113–e119. https://doi.org/10.1097/qai.0000000000001334
Huppertz B (2008) The anatomy of the normal placenta. J Clin Pathol 61(12):1296–1302. https://doi.org/10.1136/jcp.2008.055277
Huppertz B, Frank HG, Kingdom JC, Reister F, Kaufmann P (1998) Villous cytotrophoblast regulation of the syncytial apoptotic cascade in the human placenta. Histochem Cell Biol 110(5):495–508. https://doi.org/10.1007/s004180050311
Jia H, Lohr M, Jezequel S, Davis D, Shaikh S, Selwood D, Zachary I (2001) Cysteine-rich and basic domain HIV-1 Tat peptides inhibit angiogenesis and induce endothelial cell apoptosis. Biochem Biophys Res Commun 283(2):469–479. https://doi.org/10.1006/bbrc.2001.4790
Johnson EL, Chakraborty R (2012) Placental Hofbauer cells limit HIV-1 replication and potentially offset mother to child transmission (MTCT) by induction of immunoregulatory cytokines. Retrovirology 9:101. https://doi.org/10.1186/1742-4690-9-101
Kim WU, Kang SS, Yoo SA, Hong KH, Bae DG, Lee MS, Hong SW, Chae CB, Cho CS (2006) Interaction of vascular endothelial growth factor 165 with neuropilin-1 protects rheumatoid synoviocytes from apoptotic death by regulating Bcl-2 expression and Bax translocation. J Immunol 177(8):5727–5735. https://doi.org/10.4049/jimmunol.177.8.5727
Klagsbrun M, Takashima S, Mamluk R (2002) The role of neuropilin in vascular and tumor biology. Adv Exp Med Biol 515:33–48. https://doi.org/10.1007/978-1-4615-0119-0_3
Koi H, Zhang J, Parry S (2001) The mechanisms of placental viral infection. Ann NY Acad Sci 943:148–156. https://doi.org/10.1111/j.1749-6632.2001.tb03798.x
Kvietys PR, Granger DN (2012) Role of reactive oxygen and nitrogen species in the vascular responses to inflammation. Free Radic Biol Med 52(3):556–592. https://doi.org/10.1016/j.freeradbiomed.2011.11.002
Lim AY, Price P, Beilharz MW, French MA (2006) Cell surface markers of regulatory T cells are not associated with increased forkhead box p3 expression in blood CD4+ T cells from HIV-infected patients responding to antiretroviral therapy. Immunol Cell Biol 84(6):530–536. https://doi.org/10.1111/j.1440-1711.2006.01467.x
Loegl J, Hiden U, Nussbaumer E, Schliefsteiner C, Cvitic S, Lang I, Wadsack C, Huppertz B, Desoye G (2016) Hofbauer cells of M2a, M2b and M2c polarization may regulate feto-placental angiogenesis. Reprod 152(5):447–455. https://doi.org/10.1530/rep-16-0159
Mamluk R, Gechtman Z, Kutcher ME, Gasiunas N, Gallagher J, Klagsbrun M (2002) Neuropilin-1 binds vascular endothelial growth factor 165, placenta growth factor-2, and heparin via its b1b2 domain. J Biol Chem 277(27):24818–24825. https://doi.org/10.1074/jbc.m200730200
Maulik D, De A, Ragolia L, Evans J, Grigoryev D, Lankachandra K, Mundy D, Muscat J, Gerkovich MM, Ye SQ (2016) Down-regulation of placental neuropilin-1 in fetal growth restriction. Am J Obstet Gynecol 214(2):279.e271-279.e279. https://doi.org/10.1016/j.ajog.2015.09.068
Mori M, Bogdan A, Balassa T, Csabai T, Szekeres-Bartho J (2016) The decidua-the maternal bed embracing the embryo-maintains the pregnancy. Semin Immunopathol 38(6):635–649. https://doi.org/10.1007/s00281-016-0574-0
Naicker T, Dorsamy E, Ramsuran D, Burton GJ, Moodley J (2013) The role of apoptosis on trophoblast cell invasion in the placental bed of normotensive and preeclamptic pregnancies. Hypertens Pregnancy 32(3):245–256. https://doi.org/10.3109/10641955.2013.796969
Naidoo N (2020) The regulation of Osteopontin and soluble Neuropilin-1 in HIV-associated preeclampsia. Dissertation, University of KwaZulu-Natal.
Naidoo N, Moodley J, Naicker T (2021) Maternal endothelial dysfunction in HIV-associated preeclampsia comorbid with COVID-19: a review. Hypertens Res 44(4):386–398. https://doi.org/10.1038/s41440-020-00604-y
Naidoo N, Moodley J, Khaliq OP, Naicker T (2022) Neuropilin-1 in the pathogenesis of preeclampsia, HIV-1 and SARS-CoV-2 infection: a review. Virus Res 319:198880. https://doi.org/10.1016/j.virusres.2022.198880
National Committee for Confidential Enquiry into Maternal Deaths (2018) Saving Mothers Report 2017. Annual Report on Confidential inquiries into maternal death in South Africa. Pretoria: South African Department of Health
National Department of Health South Africa (2019) ART clinical guidelines for the management of HIV in adults, pregnancy, adolescents, children, infants and neonates. South African Department of Health, Pretoria
Ochiumi T, Kitadai Y, Tanaka S, Akagi M, Yoshihara M, Chayama K (2006) Neuropilin-1 is involved in regulation of apoptosis and migration of human colon cancer. Int J Oncol 29(1):105–116. https://doi.org/10.3892/ijo.29.1.105
Omani-Samani R, Alizadeh A, Almasi-Hashiani A, Mohammadi M, Maroufizadeh S, Navid B, Khedmati Morasae E, Amini P (2020) Risk of preeclampsia following assisted reproductive technology: systematic review and meta-analysis of 72 cohort studies. J Matern Fetal Neonatal Med 33(16):2826–2840. https://doi.org/10.1080/14767058.2018.1560406
Pattinson R (2014) Saving Mothers 2011–2013: The Sixth Report of the National Committee for Confidential Enquiries into Maternal Deaths in South Africa. South African Department of Health, Pretoria
Pijnenborg R (1990) Trophoblast Invasion and Placentation in the Human: Morphological Aspects. In: Denker H-W, Aplin JD (eds) Trophoblast invasion and endometrial receptivity: novel aspects of the cell biology of embryo implantation. Springer, US, Boston, MA, pp 33–47
Powis KM, Shapiro RL (2014) Protease inhibitors and adverse birth outcomes: is progesterone the missing piece to the puzzle? J Infect Dis 211(1):4–7. https://doi.org/10.1093/infdis/jiu397
Rana S, Lemoine E, Granger JP, Karumanchi SA (2019) Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res 124(7):1094–1112. https://doi.org/10.1161/circresaha.118.313276
Redman CW, Sargent IL (2005) Latest advances in understanding preeclampsia. Science 308(5728):1592–1594. https://doi.org/10.1126/science.1111726
Reyes L, Golos TG (2018) Hofbauer cells: their role in healthy and complicated pregnancy. Front Immunol 9:2628. https://doi.org/10.3389/fimmu.2018.02628
Riese A, Eilert Y, Meyer Y, Arin M, Baron JM, Eming S, Krieg T, Kurschat P (2012) Epidermal expression of neuropilin 1 protects murine keratinocytes from UVB-induced apoptosis. PLoS ONE 7(12):e50944. https://doi.org/10.1371/journal.pone.0050944
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675. https://doi.org/10.1038/nmeth.2089
Sebitloane HM, Moodley D (2017a) The impact of highly active antiretroviral therapy on obstetric conditions: a review. Eur J Obstet Gynecol Reprod Biol 210:126–131. https://doi.org/10.1016/j.ejogrb.2016.12.008
Sebitloane HM, Moodley J (2017b) Maternal and obstetric complications among HIV-infected women treated with highly active antiretroviral treatment at a Regional Hospital in Durban. South Africa Niger J Clin Pract 20(11):1360–1367. https://doi.org/10.4103/njcp.njcp_328_16
Singh M, Chaudhry P, Asselin E (2011) Bridging endometrial receptivity and implantation: network of hormones, cytokines, and growth factors. J Endocrinol 210(1):5–14. https://doi.org/10.1530/joe-10-0461
Smárason AK, Sargent IL, Starkey PM, Redman CW (1993) The effect of placental syncytiotrophoblast microvillous membranes from normal and pre-eclamptic women on the growth of endothelial cells in vitro. Br J Obstet Gynaecol 100(10):943–949. https://doi.org/10.1111/j.1471-0528.1993.tb15114.x
Song L, Ding S, Ge Z, Zhu X, Qiu C, Wang Y, Lai E, Yang W, Sun Y, Chow SA, Yu L (2018) Nucleoside/nucleotide reverse transcriptase inhibitors attenuate angiogenesis and lymphangiogenesis by impairing receptor tyrosine kinases signalling in endothelial cells. Br J Pharmacol 175(8):1241–1259. https://doi.org/10.1111/bph.14036
Staff AC (2019) The two-stage placental model of preeclampsia: an update. J Reprod Immunol 134–135:1–10. https://doi.org/10.1016/j.jri.2019.07.004
Teasdale CA, Marais BJ, Abrams EJ (2011) HIV: prevention of mother-to-child transmission. BMJ Clin Evid 2011:0909
Tomas SZ, Prusac IK, Roje D, Tadin I (2011) Trophoblast apoptosis in placentas from pregnancies complicated by preeclampsia. Gynecol Obstet Invest 71(4):250–255. https://doi.org/10.1159/000320289
Tooke L, Riemer L, Matjila M, Harrison M (2016) Antiretrovirals causing severe pre-Eclampsia. Pregnancy Hypertens 6(4):266–268. https://doi.org/10.1016/j.preghy.2016.04.006
Valenzuela FJ, Perez-Sepulveda A, Torres MJ, Correa P, Repetto GM, Illanes SE (2012) Pathogenesis of preeclampsia: the genetic component. J Pregnancy. https://doi.org/10.1155/2012/632732
Vinketova K, Mourdjeva M, Oreshkova T (2016) Human decidual stromal cells as a component of the implantation niche and a modulator of maternal immunity. J Pregnancy 2016:8689436. https://doi.org/10.1155/2016/8689436
Wagner LK (2004) Diagnosis and management of preeclampsia. Am Fam Physician 70(12):2317–2324
Wang XB, Qi QR, Wu KL, Xie QZ (2018) Role of osteopontin in decidualization and pregnancy success. Reprod 155(5):423–432. https://doi.org/10.1530/rep-17-0782
Wang S, Zhao L, Zhang X, Zhang J, Shang H, Liang G (2022) Neuropilin-1, a myeloid cell-specific protein, is an inhibitor of HIV-1 infectivity. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.2114884119
Wey JS, Gray MJ, Fan F, Belcheva A, McCarty MF, Stoeltzing O, Somcio R, Liu W, Evans DB, Klagsbrun M, Gallick GE, Ellis LM (2005) Overexpression of neuropilin-1 promotes constitutive MAPK signalling and chemoresistance in pancreatic cancer cells. Br J Cancer 93(2):233–241. https://doi.org/10.1038/sj.bjc.6602663
Whitley GSJ, Cartwright JE (2009) Trophoblast-mediated spiral artery remodelling: a role for apoptosis. J Anat 215(1):21–26. https://doi.org/10.1111/j.1469-7580.2008.01039.x
Woldesenbet SA, Lombard C, Manda S, Kufa T, Ayalew K, Cheyip M, Puren A (2021) The 2019 National Antenatal Sentinel HIV Survey. South Africa
World Health Organisation (2010) WHO Guidelines Approved by the Guidelines Review Committee. In: Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants: Recommendations for a Public Health Approach: 2010 Version. World Health Organization.,Geneva.
Xu X, Yang XY, He BW, Yang WJ, Cheng WW (2016) Placental NRP1 and VEGF expression in pre-eclamptic women and in a homocysteine-treated mouse model of pre-eclampsia. Eur J Obstet Gynecol Reprod Biol 196:69–75. https://doi.org/10.1016/j.ejogrb.2015.11.017
Yang SW, Cho EH, Choi SY, Lee YK, Park JH, Kim MK, Park JY, Choi HJ, Lee JI, Ko HM, Park SH, Hwang HS, Kang YS (2017) DC-SIGN expression in Hofbauer cells may play an important role in immune tolerance in fetal chorionic villi during the development of preeclampsia. J Reprod Immunol 124:30–37. https://doi.org/10.1016/j.jri.2017.09.012
Yang X, Chen D, He B, Cheng W (2021) NRP1 and MMP9 are dual targets of RNA-binding protein QKI5 to alter VEGF-R/ NRP1 signalling in trophoblasts in preeclampsia. J Cell Mol Med 25(12):5655–5670. https://doi.org/10.1111/jcmm.16580
Zhou Y, Gormley MJ, Hunkapiller NM, Kapidzic M, Stolyarov Y, Feng V, Nishida M, Drake PM, Bianco K, Wang F, McMaster MT, Fisher SJ (2013) Reversal of gene dysregulation in cultured cytotrophoblasts reveals possible causes of preeclampsia. J Clin Invest 123(7):2862–2872. https://doi.org/10.1172/jci66966
Acknowledgements
Dr N Naidoo and Dr T Abel were financially supported by the National Research Foundation (grant numbers: 131660 and 130992, respectively). The running expenses of this experiment were supported by the publication funds of Professors T Naicker and J Moodley. The authors would like to thank Dr Shoohana Singh for her laboratory assistance.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation was performed by NN and TA. Data and statistical analysis were conducted by NN. The first draft of the manuscript was written by NN. All authors contributed to the review and editing process and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Naidoo, N., Abel, T., Moodley, J. et al. Immunoexpression of neuropilin-1 in the chorionic villi of HIV-infected preeclamptic South African women of African ancestry. Histochem Cell Biol 160, 307–319 (2023). https://doi.org/10.1007/s00418-023-02213-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-023-02213-5