Abstract
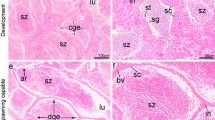
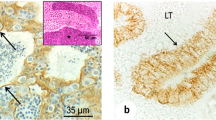
The present investigation was conducted to demonstrate laminin and α smooth muscle actin (αSMA) in the testis and epididymis of adult chickens, Sudani ducks, pigeons, and rabbits. This study may represent the first indication for the presence of laminin in the male reproductive organs of birds and rabbits and might therefore serve as a milestone for further reports. In the testis of chicken, Sudani duck, pigeon, and rabbit, the laminin was localized in the basal lamina of the seminiferous tubules and of the peritubular myoid cells, in the testicular capsule and to a small extent in the vicinity of Leydig cells. The testicular vasculature also exhibited intense laminin immunostaining. Weak laminin staining was additionally seen in the cytoplasm of the duck Sertoli cells. In the epididymis, the basal lamina of the epididymal epithelium showed a distinctly positive reaction in all birds and rabbit. The basal lamina of the periductal myoid cells also showed a positive reaction. In the interductal tissue, laminin immunostaining was particularly observed in chicken, duck and pigeon. Laminin positive reaction was also seen in the epididymal vasculatures of all birds and rabbit. Interestingly, weak to moderate laminin staining was observed in the apical surface of the ciliated cells of the proximal and distal efferent ductules in chicken, duck and pigeon. αSMA positive reaction was seen in the testicular capsule and in the peritubular myoid cells of all birds and rabbit. In the testicular capsule, αSMA staining was either observed in the inner portion (chicken) or throughout the tunica albuginea (Sudani duck and pigeon), or in the outer aspect (rabbit). Distinct αSMA reaction was additionally observed in the testicular vasculature. In the epididymis of all birds and rabbit, the αSMA was particularly seen in the periductal and interductal myoid cells as well as in the epididymal vasculatures. No αSMA specific staining was however detected in the epididymal epithelium, fibrous lamina propria, and luminal spermatozoa of all birds and rabbits. In conclusion, the distribution of laminin and αSMA in the testis and epididymis might point out to their roles in the male reproduction.



Similar content being viewed by others
References
Abd-Elmaksoud A (2005) Morphological, glycohistochemical, and immunohistochemical studies on the embryonic and adult bovine testis. Thesis, Institute of Veterinary Anatomy II, Faculty of Veterinary Medicine, LMU, Munich, Germany
Abd-Elmaksoud A, Sayed-Ahmed A, Mohamed SE et al (2009) Morphological and glycohistochemical studies on the epididymal region of the Sudani duck (Cairina moschata). Res Vet Sci 86:7–17
Aire TA (1997) The structure of the interstitial tissue of the active and resting avian testis. Onderstepoort J Vet Res 64:291–299
Aire TA, Ozegbe PC (2007) The testicular capsule and peritubular tissue of birds: morphometry, histology, ultrastructure and immunohistochemistry. J Anat 210:731–740
Aire TA, Ozegbe PC (2008) Immunohistochemistry of the cytoskeleton in the excurrent ducts of the testis in birds of the Galloanserae monophyly. Cell Tissue Res 333:311–321
Aire TA, Soley JT (2000) The surface features of the epithelial lining of the ducts of the epididymis of the ostrich (Struthio camelus). Anat Histol Embryol 29:119–126
Aire TA, Ozegbe PC, Soley JT et al (2008) Structural and immunohistochemical features of the epididymal duct unit of the ostrich (Struthio camelus). Anat Histol Embryol 37:296–302
Alkafafy M (2005) Glycohistochemical, immunohistochemical and ultrastructural studies of the bovine epididymis. Thesis, Institute of Veterinary Anatomy II, Faculty of Veterinary Medicine, LMU, Munich, Germany
Anthony CT, Rosselli M, Skinner MK (1991) Actions of the testicular paracrine factor (P-Mod-S) on Sertoli cell transferrin secretion throughout pubertal development. Endocrinology 129:353–360
Baker S, Hopkinson S, Fitchmun M et al (1996) Laminin-5 and hemidesmosomes: role of the alpha 3 chain subunit in hemidesmosome stability and assembly. J Cell Sci 109:2509–2520
Banks FC, Knight GE, Calvert RC et al (2006) Smooth muscle and purinergic contraction of the human, rabbit, rat, and mouse testicular capsule. Biol Reprod 74:473–480
Benazzi C, Sarli G, Preziosi R et al (1995) Laminin expression in testicular tumours of the dog. J Comp Pathol 112:141–150
Böck P, Breitenecker G, Lunglmayr G (1972) Contractile fibroblasts (myofibroblasts) in the lamina propria of human seminiferous tubules. Z Zellforsch Mikrosk Anat 133:519–527
Clulow J, Jones RC (1982) Production, transport, maturation, storage and survival of spermatozoa in the male Japanese quail, Coturnix coturnix japonica. J Reprod Fertil 64:259–266
Clulow J, Jones RC (1988) Studies of fluid and spermatozoal transport in the extratesticular genital ducts of the Japanese quail. J Anat 157:1–11
Colognato H, Yurchenco PD (2000) Form and function: the laminin family of heterotrimers. Dev Dyn 218:213–234
Davidoff MS, Breucker H, Holstein AF et al (1990) Cellular architecture of the lamina propria of human seminiferous tubules. Cell Tissue Res 262:253–261
Davis CM, Papadopoulos V, Sommers CL et al (1990) Differential expression of extracellular matrix components in rat Sertoli cells. Biol Reprod 43:860–869
Dym M (1994) Basement membrane regulation of Sertoli cells. Endocr Rev 15:102–115
Egger GF, Witter K (2009) Peritubular contractile cells in testis and epididymis of the dog, lupus familiaris. Acta Vet Brno 78:3–11
El Ouali H, Leheup BP, Gelly JL et al (1991) Laminin ultrastructural immunolocalization in rat testis during ontogenesis. Histochemistry 95:241–246
Erickson AC, Couchman JR (2000) Still more complexity in mammalian basement membranes. J Histochem Cytochem 48:1291–1306
Francavilla S, De Martino C, Scorza Barcellona P et al (1983) Ultrastructural and immunohistochemical studies of rat epididymis. Cell Tissue Res 233:523–537
Gelly JL, Richoux JP, Leheup BP et al (1989) Immunolocalization of type IV collagen and laminin during rat gonadal morphogenesis and postnatal development of the testis and epididymis. Histochemistry 93:31–37
Gülkesen KH, Erdogru T, Sargin CF et al (2002) Expression of extracellular matrix proteins and vimentin in testes of azoospermic man: an immunohistochemical and morphometric study. Asian J Androl 4:55–60
Hadley MA, Dym M (1987) Immunocytochemistry of extracellular matrix in the lamina propria of the rat testis: electron microscopic localization. Biol Reprod 37:1283–1289
Hadley MA, Byers SW, Suárez-Quian CA et al (1985) Extracellular matrix regulates Sertoli cell differentiation, testicular cord formation, and germ cell development in vitro. J Cell Biol 101:1511–1522
Holstein AF (1967) Smooth musculature of the testicular tunica albuginea and its influence on the transport of spermatozoa into the epididymis. Verh Anat Ges 62:103–108
Holstein AF, Maekawa M, Nagano T et al (1996) Myofibroblasts in the lamina propria of human seminiferous tubules are dynamic structures of heterogeneous phenotype. Arch Histol Cytol 59:109–125
Holt WV, Waller J, Moore A et al (2004) Smooth muscle actin and vimentin as markers of testis development in the harbour porpoise (Phocoena phocoena). J Anat 205:201–211
Hsu SM, Raine L, Fanger H (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29:577–580
Koch M, Olson PF, Albus A et al (1999) Characterization and expression of the laminin γ3 chain: a novel, non basement membrane-associated, laminin chain. J Cell Biol 145:605–618
Langhofer M, Hopkinson SB, Jones JC (1993) The matrix secreted by 804G cells contains laminin-related components that participate in hemidesmosomes assembly in vitro. J Cell Sci 105:753–764
Leeson CR, Forman DE (1981) Postnatal development and differentiation of contractile cells within the rabbit testis. J Anat 132:491–511
Lustig L, Denduchis B, Ponzio R et al (2000) Passive immunization with anti-laminin immunoglobulin G modifies the integrity of the seminiferous epithelium and induces arrest of spermatogenesis in the guinea pig. Biol Reprod 62:1505–1514
Maekawa M, Kamimura K, Nagano T (1996) Peritubular myoid cells in the testis: their structure and function. Arch Histol Cytol 59:1–13
Maretta M, Marettova E (2004) Immunohistochemical demonstration of myoid cells in the testis and its excurrent ducts in the domestic fowl. Br Poult Sci 45:585–589
Miner JH (2008) Laminins and their role in mammals. Microsc Res Tech 71:349–356
Noakes PG, Gautam M, Mudd J et al (1995) Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin beta 2. Nature 374:258–262
Ooba T, Ishikawa T, Yamaguchi K et al (2008) Expression and distribution of laminin chains in the testis for patients with azoospermia. J Androl 29:147–152
Palacios J, Regadera J, Paniagua R et al (1993) Immunohistochemistry of the human ductus epididymis. Anat Rec 235:560–566
Palombi F, Farini D, Salanova M et al (1992) Development and cytodifferentiation of peritubular myoid cells in the rat testis. Anat Rec 233:32–40
Pelliniemi LJ, Frojdman K (2001) Structural and regulatory macromolecules in sex differentiation of gonads. J Exp Zool 290:523–528
Pollanen PP, Kallajoki M, Risteli L et al (1985) Laminin and type IV collagen in the human testis. Int J Androl 8:337–347
Richardson LL, Kleinman HK, Dym M (1995) Basement membrane gene expression by Sertoli and peritubular myoid cells in vitro in the rat. Biol Reprod 52:320–330
Rodrίguez A, Rojas MA, Bustos-Obregόn E et al (1999) Distribution of keratins, vimentin, and actin in the testis of two South American camelids: vicuña (Vicugna) and llama (Lama glama). An immunohistochemical study. Anat Rec 254:330–333
Rothwell B, Tingari MD (1973) The ultrastructure of the boundary tissue of the seminiferous tubule in the testis of the domestic fowl (Gallus domesticus). J Anat 114:321–328
Ryan MC, Christiano AM, Engvall E et al (1996) The function of laminins: lessons from in vivo studies. Matrix Biol 15:369–381
Santamaria L, Martinez-Onsurbe P, Paniagua R et al (1990) Laminin, type IV collagen, and fibronectin in normal and cryptorchid human testes. An immunohistochemical study. Int J Androl 13:135–146
Schlatt S, Weinbauer GF, Arslan M et al (1993) Appearance of alpha-smooth muscle actin in peritubular cells of monkey testes is induced by androgens, modulated by follicle-stimulating hormone, and maintained after hormonal withdrawal. J Androl 14:340–350
Setchell BP, Maddocks S, Brooks DE (1994) Anatomy, vasculature, innervation, and fluids of the male reproductive tract. In: Knobil E, Neill JD (eds) The physiology of reproduction, 2nd edn. Raven Press, New York, pp 1063–1175
Skalli O, Ropraz P, Trzeciak A et al (1986) A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol 103:2787–2796
Skinner MK, Tung PS, Fritz IB (1985) Cooperativity between Sertoli cells and testicular peritubular cells in the production and deposition of extracellular matrix components. J Cell Biol 100:1941–1947
Smyth N, Vatansever HS, Murray P et al (1999) Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol 144:151–160
Stefanini MA, Orsi AM, Gregorio EA et al (1999) Morphologic study of the efferent ductules of the pigeon (Columba livia). J Morphol 242:247–255
Steger K, Wrobel KH (1994) Immunohistochemical demonstration of cytoskeletal proteins in the ovine testis during postnatal development. Anat Embryol 189:521–530
Steger K, Schimmel M, Wrobel KH (1994) Immunocytochemical demonstration of cytoskeletal proteins in seminiferous tubules of adult rams and bulls. Arch Histol Cytol 57:17–28
Timpl R (1996) Macromolecular assembly of basement membranes. Curr Opin Cell Biol 8:818–824
Tung PS, Fritz IB (1993) Interactions of Sertoli cells with laminin are essential to maintain integrity of the cytoskeleton and barrier functions of cells in culture in the two-chambered assembly. J Cell Physiol 156:1–11
van Nassauw L, Harrison F, Callebaut M (1993) Smooth muscle cells in the peritubular tissue of the quail testis. Eur J Morphol 31:60–64
Virtanen I, Lohi J, Tani T et al (1997) Distinct changes in the laminin composition of basement membranes in human seminiferous tubules during development and degeneration. Am J Pathol 150:1421–1431
Wrobel KH (1998) Male reproductive system. In: Dellmann HD, Eurell JA (eds) Textbook of veterinary histology, 5th edn. Williams and Wilkins, Pennsylvania, pp 226–235
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abd-Elmaksoud, A. Comparative expression of laminin and smooth muscle actin in the testis and epididymis of poultry and rabbit. J Mol Hist 40, 407–416 (2009). https://doi.org/10.1007/s10735-010-9254-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-010-9254-x