Abstract
Many models of infectious disease ignore the underlying contact structure through which the disease spreads. However, in order to evaluate the efficacy of certain disease control interventions, it may be important to include this network structure. We present a network modeling framework of the spread of disease and a methodology for inferring important model parameters, such as those governing network structure and network dynamics, from readily available data sources. This is a general and flexible framework with wide applicability to modeling the spread of disease through sexual or close contact networks. To illustrate, we apply this modeling framework to evaluate HIV control programs in sub-Saharan Africa, including programs aimed at concurrent partnership reduction, reductions in risky sexual behavior, and scale up of HIV treatment.





Similar content being viewed by others
References
Amaral LAN, Scala A, Barthlmy M, Stanley HE (2000) Classes of small-world networks. PNAS 97(21):11,149–11,152
Anderson RM, May RM (1991) Infectious diseases of humans: dynamics and control. Oxford University Press, Oxford
Badri M, Lawn S, Wood R (2006) Short-term risk of AIDS or death in people infected with HIV-1 before antiretroviral therapy in South Africa: a longitudinal study. Lancet 368(9543):1254–1259
Banks J, Carson JS, Nelson BL, Nicol DM (2009) Discrete-event simulation, 5th edn. Prentice Hall
Bendavid E, Young SD, Katzenstein DA, Bayoumi AM, Sanders GD, Owens DK (2008) Cost-effectiveness of HIV monitoring strategies in resource-limited settings: a southern African analysis. Arch Intern Med 168(17):1910–1918
Carter MW, Kraft JM, Koppenhaver T, Galavotti C, Roels TH, Kilmarx PH, Fidzani B (2007) A bull cannot be contained in a single kraal: concurrent sexual partnerships in Botswana. AIDS Behav 11(6):822–830
Drummond M, McGuire A (2001) Economic evaluation in health care: merging theory with practice. Oxford University Press
Enns EA, Brandeau ML, Igeme TK, Bendavid E (2011) Assessing effectiveness and cost-effectiveness of concurrency reduction for HIV prevention. Working Paper, Stanford University
Fenton K, Johnson A, McManus S, Erens B (2001) Measuring sexual behaviour: methodological challenges in survey research. Sex Transm Infect 77(2):84–92
Ghani AC, Swinton J, Garnett G (1997) The role of sexual partnership networks in the epidemiology of gonorrhea. Sex Transm Dis 24(1):45–56
Goldie S, Yazdanpanah Y, Losina E, Weinstein MC, Anglaret XRW, Hsu H, Kimmel A, Holmes C, Kaplan J, Freedberg K (2006) Cost-effectiveness of HIV treatment in resource-poor settings–the case of Cote d’Ivoire. N Engl J Med 355(11):1141–1153
Grant M, Boyd S (2010) CVX: Matlab software for disciplined convex programming, version 1.21. cvxr.com/cvx
Gray RH, Wawer MJ, Brookmeyer R, Sewankambo NK, Serwadda D, Wabwire-Mangen F, Lutalo T, Li X, vanCott T, Quinn TC (2001) Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet 357(9263):1149–1153
Green EC, Halperin DT, Nantulya V, Hogle JA (2006) Uganda’s HIV prevention success: the role of sexual behavior change and the national response. AIDS Behav 10(4):335–346
Helleringer S, Kohler H (2007) Sexual network structure and the spread of HIV in Africa: evidence from Likoma Island, Malawi. AIDS 21(17):2323–2332
Jackson M (2008) Social and economic networks. Princeton University Press
Kiss IZ, Green DM, Kao RR(2006) Infectious disease control using contact tracing in random and scale-free networks. J R Soc Interface 3(6):55–62
Klovdahl AS (1985) Social networks and the spread of infectious diseases: the AIDS example. Soc Sci Med 21(11):1203–1216
Klusmann D (2002) Sexual motivation and the duration of partnership. Arch Sex Behav 31(3):275–287
Kretzschmar M, van Duynhoven YTHP, Severijnen AJ (1996) Modeling prevention strategies for gonorrhea and chlamydia using stochastic network simulations. Am J Epidemiol 144(3):306–317
Liljeros F, Edling CR, Amaral LAN, Stanley HE, Åberg Y (2001) The web of human sexual contacts. Nature 411:907–908
Mah T, Halperin D (2008) Concurrent sexual partnerships and the HIV epidemics in Africa: evidence to move forward. AIDS Behav 14(1):11–16
MEASURE DHS. Demographic and health surveys. www.measuredhs.com. Accessed January 2009
MEASURE DHS (2003) Tanzania demographic and health survey. www.measuredhs.com
Morris M, Kretzschmar M (2000) A microsimulation study of the effect of concurrent partnerships on the spread of HIV in Uganda. Math Popul Stud 8(2):109–133
National AIDS Coordinating Agency Botswana (2009) National campaign plan—multiple concurrent partnerships. www.comminit.com/en/node/304747/2781
Newman MEJ, Strogatz SH, Watts DJ (2001) Random graphs with arbitrary degree distributions and their applications. Phys Rev E 64(2):026,118
Parker M, Ward H, Day S (1998) Sexual networks and the transmission of HIV in London. J Biosoc Sci 30(1): 63–83
Serlemitsos E (2009) Mass media campaign on multiple concurrent sexual partnerships (MCP) in Zambia. www.harvardaidsprp.org
Strogatz SH (2001) Exploring complex networks. Nature 410(6825):268–276
The United Republic of Tanzania Prime Minister’s Office (2009) National policy on HIV/AIDS. www.tanzania.go.tz/pdf/hivaidspolicy.pdf
Troitzsch KG (2004) Validating simulation models. In: Proceedings of the 18th European simulation multiconference. Society for Modeling and Simulation, Germany
Tumbo D, Jana M, Nkambule M (2008) One love: Multiple and concurrent sexual partnerships in southern Africa. www.onelovesouthernafrica.org
UNAIDS (2008) Report on the global AIDS epidemic. www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008
US Census Bureau, P.D. (2008) International data base. www.census.gov/ipc/www/idb/tables.html
Vieira IT, Cheng RCH, Harper PR, Senna V (2010) Small world network models of the dynamics of HIV infection. Ann Oper Res 178(1):173–200
Wadsworth J, Johnson A, Wellings K, Field J (1996) What’s in a mean?—an examination of the inconsistency between men and women in reporting sexual partnerships. J R Stat Soc A 159(1):111–123
Watts DJ, Strogatz SH (1998) Collective dynamics of ‘small-world’ networks. Nature 393(6684):440–442
Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, Kiwanuka N, Kigozi G, Kiddugavu M, Lutalo T, Nalugoda F, Wabwire-Mangen F, Meehan MP, Quinn TC (2005) Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis 191(9):1403–1409
Weeks MR, Clair S, Borgatti SP, Radda K, Schensul JJ (2002) Social networks of drug users in high-risk sites: finding the connections. AIDS Behav 6(2):193–206
WHO (1999) Removing obstacles to healthy development. www.who.int/infectious-disease-report/
WHO (2006) Life tables for WHO member states. www.who.int/whosis/database/life_tables/life_tables.cfm
WHO (2008) Millennium development goals: Combat HIV/AIDS, malaria and other diseases. www.who.int/topics/millennium_development_goals/
Wylie JL, Jolly A (2001) Patterns of chlamydia and gonorrhea infection in sexual networks in Manitoba, Canada. Sex Transm Dis 28(1):14–24
Acknowledgement
This research was funded by the National Institute on Drug Abuse, Grant Number R01-DA15612. Eva Enns is supported by a National Defense Science and Engineering Graduate Fellowship, a National Science Foundation Graduate Fellowship, and a Rambus Inc. Stanford Graduate Fellowship.
Author information
Authors and Affiliations
Corresponding author
Appendix: Details of Tanzania example
Appendix: Details of Tanzania example
This Appendix describes input parameters and key assumptions for the example analysis of the effects of concurrency reduction and other HIV prevention programs in Tanzania. Values for all input parameters are shown in Table 1.
We initialized the model with approximately 8,000 sexually active and potentially sexually active individuals aged 15–49 years old. We matched the age distribution of this population to gender-specific age distributions of Tanzania. Individuals age into the population at age 15 with an annual maturation rate determined from Tanzania demographic data [35]. Individuals experience a baseline gender- and age-specific mortality risk determined from country-specific life tables [42]. We matched the gender-specific concurrent partnership behavior reported in the most recent Demographic Health Survey conducted in Tanzania [24].
The disease model is a simplified version of a previously developed HIV progression model [5] and is described in detail in [8].
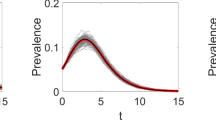
We modeled three discrete HIV disease states: acute, chronic, and on treatment. The HIV disease state determined the risk of transmission to sexual partners, with the acute state having the highest transmission risk and the treated state having the lowest. Per-act transmission probabilities were obtained from the literature [39] and then adjusted in model calibration to account for levels of sexual activity and condom use so that model-projected prevalence matched projected HIV prevalence trends in Tanzania.
We also modeled each individual’s CD4 counts. This provides a continuous measure of an individual’s immune function. CD4 counts determined HIV-related mortality risk and risks of opportunistic infections, which also increased mortality. In the chronic HIV state, CD4 counts decline slowly over time. Once CD4 counts fall below 200 cells/μL, an individual becomes HIV treatment eligible. HIV treatment causes a recovery in CD4 counts and thus a reduction in HIV-related mortality, though not all eligible individuals receive treatment in these resource-limited settings. The level of treatment coverage in Tanzania is estimated to be about 30% [34].
Rights and permissions
About this article
Cite this article
Enns, E.A., Brandeau, M.L. Inferring model parameters in network-based disease simulation. Health Care Manag Sci 14, 174–188 (2011). https://doi.org/10.1007/s10729-011-9150-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10729-011-9150-2