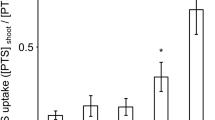
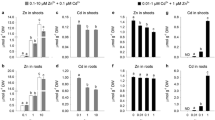
Abstract
It has long been recognized that the plasma membranes (PMs) play significant roles in the transport of ions across PMs, but kinetics of cadmium (Cd) transport between root and shoot via internal membrane system is not clear. Our experimental results showed that Cd2+ flux across the root PMs was well fitted by the Michaelis–Menten kinetics and the high-Cd-accumulation (HCA) variety displayed much higher maximum flux rate (Fmax) than the low-Cd-accumulation (LCA) variety. After Cd2+ was internalized, its flux into shoot PMs was linearly correlated with Cd2+ concentration in soluble fraction (FIII) of root cells. The efflux of Cd2+ across shoot PMs into cell wall was linearly correlated with Cd2+ concentration in shoot FIII. Only little amount of Cd2+ in cytosol was transported into organelles. The HCA variety displayed higher Cd2+ concentrations in FIII of root and shoot cells as well as higher Cd2+ flux rate by trans-cellular pathway than the LCA variety. However, efflux rate across shoot PMs to cell wall in the LCA variety was higher than that in the HCA variety. The low influx across PMs of root cells and high efflux across PMs of shoot cells probably are the special detoxification defense mechanisms of the LCA variety.







Similar content being viewed by others
References
Barberon M, Geldner N (2014) Radial transport of nutrients: the plant root as a polarized epithelium. Plant Physiol 166:528–537
Benitez-Alfonso Y (2014) Symplastic intercellular transport from a developmental perspective. J Exp Bot 65:1857–1863
Chen G, Liu Y, Wang R, Zhang J, Owens G (2013) Cadmium adsorption by willow root: the role of cell walls and their subfractions. Environ Sci Pollut Res Int 20(8):5665–5672
Chen D, Xu Y, Zheng W, Huang D, Wong W, Tai WC, Cho Y, Lau ATY (2015) Proteomic analysis of secreted proteins by human bronchial epithelial cells in response to cadmium toxicity. Proteomics 15:3075–3086
Degryse F, Shahbazi A, Verheyen L, Smolders E (2012) Diffusion limitations in root uptake of cadmium and zinc, but not nickel, and resulting bias in the Michaelis constant. Plant Physiol 160:1097–1109
Epstein E, Rains DW, Elzam OE (1963) Resolution of dual mechanisms of potassium absorption by barley roots. Proc Natl Acad Sci USA 49:684–692
Erfurt C, Roussa E, Thèvenod F (2003) Apoptosis by Cd2+ or CDMT in proximal tubule cells: different uptake routes and permissive role of endo/lysosomal CdMT uptake. Am J Physiol Cell Physiol 285:C1367–C1376
Etxeberria E, Pozueta-Romero J, Gonzalez P (2012) In and out of the plant storage vacuole. Plant Sci 190:52–61
Fu HH, Luan S (1998) AtKUP1: a dual-affinity K+ transporter from Arabidopsis. The Plant Cell 10:63–73
Fujimaki S, Suzui N, Ishioka NS, Kawachi N, Ito S, Chino M, Nakamura S (2010) Tracing cadmium from culture to spikelet: noninvasive imaging and quantitative characterization of absorption, transport, and accumulation of cadmium in an intact rice plant. Plant Physiol 152:1796–1806
Halimaa P, Lin Y-F, Ahonen VH, Blande D, Clemens S, Gyenesei A, Häikiö E, Kärenlampi SO, Laiho A, Aarts MGM, Pursiheimo J-P, Schat H, Schmidt H, Tuomainen MH, Tervahauta AI (2014) Gene expression differences between noccaea caerulescens ecotypes help to identify candidate genes for metal phytoremediation. Environ Sci Technol 48:3344–3353
Ishikawa S, Ishimaru Y, Igura M, Kuramata M, Abe T, Senoura T, Hase Y, Arao T, Nishizawa NK, Nakanishi H (2012) Ion-beam irradiation, gene identification, and marker-assisted breeding in the development of low-cadmium rice. Proc Natl Acad Sci USA 109:19166–19171
Ju X, Zhang C, Song Z, Han L, Lu Z, Wang J, Liu Z (2014) Changes in cadmium accumulation in rice organs during grain development and their relationship with genotype and cadmium levels in soil. Plant Physiol J 50:634–640
Kerkhove EV, Pennemans V, Swennen Q (2010) Cadmium and transport of ions and substances across cell membranes and epithelia. Biometals 23:823–855
Kitakura S, Vanneste S, Robert S, Löfke C, Teichmann T, Tanaka H, Friml J (2011) Clathrin mediates endocytosis and polar distribution of PIN auxin transporters in Arabidopsis. Plant Cell 23:1920–1931
Kobayashi NI, Tanoi K, Hirose A, Nakanishi TM (2013) Characterization of rapid intervascular transport of cadmium in rice stem by radioisotope imaging. J Exp Bot 64:507–517
Krzesłowska M (2011) The cell wall in plant cell response to trace metals: polysaccharide remodeling and its role in defense strategy. Acta Physiol Plant 33:35–51
Langelueddecke C, Lee WK, Thévenod F (2014) Differential transcytosis and toxicity of the hNGAL receptor ligandscadmium-metallothionein and cadmium-phytochelatin in colon-likeCaco-2 cells: implications for in vivo cadmium toxicity. Toxicol Lett 226:228–235
Miyadate H, Adachi S, Hiraizumi A, Tezuka K, Nakazawa N, Kawamoto T, Katou K, Kodama I, Sakurai K, Takahashi H, Satoh-Nagasawa N, Watanabe A, Fujimura T, Akagi H (2011) OsHMA3, a P1B-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytol 189:190–199
Nocito FF, Lancilli C, Dendena B, Lucchini G, Sacchi GA (2011) Cadmium retention in rice roots is influenced by cadmium availability, chelation and translocation. Plant Cell Environ 34:994–1008
Petraglia A, Benedictis MD, Degola F, Pastore G, Calcagno M, Ruotolo R, Mengoni A, Toppi LS (2014) The capability to synthesize phytochelatins and the presence of constitutive and functional phytochelatin synthases are ancestral (plesiomorphic) characters for basal land plants. J Exp Bot 65:1153–1163
Qiao X, Wang P, Shi G, Yang H (2015) Zinc conferred cadmium tolerance in Lemna minor L. via modulating polyamines and proline metabolism. Plant Growth Regul 77:1–9
Robbins NE II, Trontin C, Duan L, Dinneny JR (2014) Beyond the barrier: communication in the root through the endodermis. Plant Physiol 166:551–559
Sasaki A, Yamaji N, Yokosho K, Ma JF (2012) Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 24:2155–2167
Sasaki A, Yamaji N, Ma JF (2014) Overexpression of OsHMA3 enhances Cd tolerance and expression of Zn transporter genes in rice. J Exp Bot 65:6013–6021
Satoh-Nagasawa N, Mori M, Nakazawa N, Kawamoto T, Nagoto Y, Sakurai K, Takahashi H, Watanabe A, Akagi H (2012) Mutations in rice (Oryza sativa) heavy metal ATPase1 (OsHMA2) restrict the translocation of zinc and cadmium. Plant Cell Physiol 53:213–224
Siemianowski O, Barabasz A, Kendziorek M, Ruszczyńska A, Bulska E, Williams LE, Antosiewicz DM (2014) HMA4 expression in tobacco reduces Cd accumulation due to the induction of the apoplastic barrier. J Exp Bot 65:1125–1139
Singh S, Prasad SM (2015) IAA alleviates Cd toxicity on growth, photosynthesis and oxidative damages in eggplant seedlings. Plant Growth Regul 77:87–98
Sterckeman T, Redjala T, Morel JL (2011) Influence of exposure solution composition and of plant cadmium content on root cadmium short-term uptake. Environ Exp Bot 74:131–139
Takahashi R, Ishimaru Y, Senoura T, Shimo H, Ishikawa S, Arao T Nakanishi H, Nishizawa NK (2011) The OsNRAMP1 iron transporter is involved in Cd accumulation in rice. J Exp Bot 62:4843–4850
Ueno D, Yamaji N, Kono I, Huang CF, Ando T, Yano M, Ma JF (2010) Gene limiting cadmium accumulation in rice. Proc Natl Acad Sci USA 107:16500–16505
Ueno D, Koyama E, Yamaji N, Ma JF (2011) Physiological, genetic, and molecular characterization of a high-Cd-accumulating rice cultivar, Jarjan. J Exp Bot 62:2265–2272
Uraguchi S, Fujiwara T (2013) Rice breaks ground for cadmium-free cereals. Curr Opin Plant Biol 16:328–334
Uraguchi S, Kamiya T, Sakamoto T, Kasai K, Sato Y, Nagamura Y, Yoshida A, Kyozuka J, Ishikawa S, Fujiwara T (2011) Low-affinity cation transporter (OsLCT1) regulates cadmium transport into rice grains. Proc Natl Acad Sci USA 108:20959–20964
Wang P, Kinraide TB, Zhou D, Kopittke PM, Peijnenburg WJGM (2011) Plasma membrane surface potential: dual effects upon ion uptake and toxicity. Plant Physiol 155:808–820
Wang X, Gao Y, Feng Y, Li X, Wei Q et al (2014) Cadmium stress disrupts the endomembrane organelles and endocytosis during Picea wilsonii pollen germination and tube growth. PLoS ONE 9(4):e94721. doi:10.1371/journal.pone.0094721
Wu H, Chen C, Du J, Liu H, Cui Y, Zhang Y, He Y, Wang Y, Chu C, Feng Z, Li J, Ling H-Q (2012) Co-overexpression FIT with AtbHLH38 or AtbHLH39 in Arabidopsis-enhanced cadmium tolerance via increased cadmium sequestration in roots and improved iron homeostasis of shoots. Plant Physiol 158:790–800
Yu H, Xiang Z, Zhu Y, Wang J, Yang Z, Yang Z (2013) Subcellular and molecular distribution of cadmium in two rice genotypes with different levels of cadmium accumulation. J Plant Nutr 35:71–84
Zelazny E, Vert G (2014) Plant nutrition: root transporters on the move. Plant Physiol 166:500–508
Zelazny E, Vert G (2015) Regulation of iron uptake by IRT1: endocytosis pulls the trigger. Mol Plant 8:977–979
Zhang L, Chen Z, Zhu C (2012) Endogenous nitric oxide mediates alleviation of cadmium toxicity induced by calcium in rice seedlings. J Environ Sci 24:940–948
Zhu XF, Lei GJ, Jiang T, Liu Y, Li GX, Zheng SJ (2012) Cell wall polysaccharides are involved in P-deficiency-induced Cd exclusion in Arabidopsis thaliana. Planta 236:989–997
Zwiewka M, Nodzynski T, Robert S, Vanneste S, Friml J (2015) Osmotic stress modulates the balance between exocytosis and Clathrin-mediated endocytosis in Arabidopsis thaliana. Mol Plant 8:1175–1187
Acknowledgements
This work has been jointly financed by Funds for Science and Technology Innovation Project from the Chinese Academy of Agricultural Sciences and The Special Fund for Agro-scientific Research in the Public Interest (Grant No. 201403015). We thank Dr. Changrong Wang for manuscript editing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, Y., Zhang, S., Wen, N. et al. Modeling uptake of cadmium from solution outside of root to cell wall of shoot in rice seedling. Plant Growth Regul 82, 11–20 (2017). https://doi.org/10.1007/s10725-016-0233-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-016-0233-4