Abstract
Seeds of beech (Fagus sylvatica L.) that have been subjected to dormancy breaking consisting of 10 weeks of prechilling at 3 °C and 34 % water content (WC) and then desiccation to 10 % WC, are non-dormant (ND). ND seeds are characterised by greater sensitivity to storage conditions, than no prechilled, dormant (D) seeds. The aim of the present work was to investigate factors affecting the loss of seed viability during storage of D and ND beech seeds at different temperatures (4 and 20 °C) and humidity levels (45 and 75 % RH) for 3 weeks. In general, both D and ND seeds maintained a high germination capacity after storage at 4 °C. At 20 °C and 45 and 75 % RH the germination capacity of D seeds diminished to 80 and 28 %, respectively. Under the same conditions, ND seeds lost germination capacity to a greater degree, with only 62 and 7 % germinated seeds, respectively. At 20 °C, an increase in production of reactive oxygen species was observed, and the increase was significantly higher in ND seeds. The loss of germination capacity was coincident with an increase in electrolyte leakage and accumulation of free fatty acids, which suggests that membrane deterioration was the cause of the decline in germinability. ND seeds stored at 20 °C and 45 and 75 % RH showed a greater decrease than D seeds in contents of the primary phospholipids phosphatidylcholine (PC) and phosphatidylethanolamine (PE) as well as in polyunsaturated fatty acids (18:2 and 18:3). ND seeds possessed more unsaturated fatty acids, especially 18:3, than D seeds in the phospholipid fraction before storage. D seeds were characterised by a significantly higher level of α-tocopherol and UV-absorbing phenols. The level of ascorbate was similar in D and ND seeds. D seeds contained glutathione in both reduced (GSH) and oxidised (GSSG) forms, and GSSG dominated GSH. ND seeds contained more GSSG form than D seeds. We concluded that the membranes of ND seeds are exposed to greater oxidative stress during storage due to higher levels of unsaturation and lower levels of α-tocopherol, the main antioxidant that protects membranes against free radical attack.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The process of seed germination is strongly affected by environmental factors, which can significantly alter the rate and success of germination. Cold stratification is a method routinely utilised to maximise the germination potential of seeds (Yamauchi et al. 2004; Narsai et al. 2011). Seeds of woody plants are characterised by a deep dormancy, and it is often difficult to obtain a high percentage of seedlings from these seeds in nurseries. In temperate climates, spring often brings drought in combination with high temperatures, which can disturb the process of breaking dormancy. In view of the changing climate, this issue is particularly important. One method that leads to a high percentage of germination in nurseries, recommended for beech and seeds of other species by Suszka et al. (1996), is special preparation before sowing under conditions, particular to each type of seeds, that break dormancy, but hinders radicle protrusion. The prepared seeds, which had been released from dormancy but were still ungerminated are dried to approximately 10 % water content (WC) and stored at low temperatures (−3 to −10 °C). When the seeds are sowed in a nursery, a high percentage of germination is rapidly reached. However, after dormancy breaking and drying, seeds cannot be stored for as long as normal, dormant seeds. The causes of this phenomenon have not been investigated. However, Bentsink et al. (2006) showed that mutations within the DOG1 gene, which controls the dormancy of Arabidopsis seeds, were associated with seed longevity phenotype and indicated, that the absence of dormany may be a factor that reduces seed longevity. The molecular studies of Narsai et al. (2011), using 22 K Arabidopsis genome microarrays, showed that stratification is very important in the preparation of seeds for the germination process. The authors showed that the upregulation of transcripts encoding proteins involved protein synthesis and hormone metabolism occurred during the stratification period and that at the end of stratification, mitochondrial DNA synthesis and RNA processing began. In addition, the proteomic studies of Pawlowski (2007) on Fagus sylvatica seeds indicated that breaking dormancy involves proteins involved in many processes, beginning with hormone signal initiation, through signal transduction, transcription, protein synthesis, energy metabolism, and storage materials, and ending with the cell cycle. The arrest of many processes by desiccation of seeds after dormancy release but before germination can affect their sensitivity to storage conditions.
The common beech (F. sylvatica L.) is one of the most important broadleaved species in European forestry. Beech is propagated by seeds, but its seed set is irregular, with 5–10 years between good crops (Suszka et al. 1996). Consequently, it is necessary to store the seeds and to obtain a high germination percentage in nurseries. Seeds of beech belong to the suborthodox (intermediate) category of storage behaviour (Gosling 1991; León-Lobos and Ellis 2002; Pukacka et al. 2003). León-Lobos and Ellis (2002), on the basis of investigations of beech seed viability during storage under varied temperature and moisture conditions, established that the optimal temperature and moisture for storage of these seeds is 7.8–11.5 % water content and −10 to −20 °C. Pukacka et al. (2003) confirmed that these conditions are optimal through investigations of glass formation in embryonic axes of beech seeds and found that water sorption by these organs is greater than in cotyledons. The results suggested that in beech seeds, water content levels are the main cause of their sensitivity to storage conditions. It is thought that molecular mobility, which may be one of the main parameter that affects ageing (Buitink et al. 2000; Walters et al. 2005), is higher in embryonic axes because of the higher water content of these tissues. Thus, the processes accompanying seed ageing (mainly free-radical reactions) are more intensive in embryonic axes and, in effect, lead to the loss of germination capacity, which was confirmed by Pukacka and Ratajczak (2005) and by Ratajczak and Pukacka (2005). Seed ageing processes are controlled by temperature and moisture conditions and may be associated with various metabolic and biophysical alterations (Bailly 2004; Walters 1998; Walters et al. 2005, 2010, Ballesteros and Walters 2011). The accumulation of reactive oxygen species (ROS) is often indicated as the prime cause of seed deterioration (Bailly 2004; Pukacka and Ratajczak 2007). Cellular membranes are proposed to be one of the primary sites of injury during desiccation and storage of seeds (Pukacka 1991; Mc Donald 1999; Bailly 2004). This damage is mediated by oxidative attack, which promotes phospholipid degradation and the loss of membrane organisation (Pukacka and Ratajczak 2005, 2007). The antioxidative system plays a crucial role in protection of membranes and other cellular macromolecules in dry seeds. Low molecular weight antioxidants, which are directly able to detoxify ROS and free radicals, are particularly important. The very abundant membrane-associated antioxidant α-tocopherol plays an important role in membrane protection against ROS by limitation of non-enzymatic lipid oxidation during seeds storage (Sattler et al. 2004). Phenolic compounds, especially flavonoids, are also known to play a protective role for membranes (Hoekstra et al. 2001, Stevenson and Hurst 2007). These compounds appear in all seed organs and in embryos, where they protect cells against ROS and limit lipid peroxidation. Scavenging of ROS is also known to be undertaken by ascorbic acid (ASA) and glutathione, but these compounds may play a rather limited role in protecting dry seeds against ageing (Bailly 2004; Pukacka and Ratajczak 2007). However, Kranner et al. (2006) indicated that decrease of seed viability during ageing is tightly connected with the increase of half-cell reduction potential of glutathione (E GssG/2GSH).
Fagus sylvatica seeds are characterised by deep embryo dormancy which requires 10–12 weeks of moist chilling at +3 °C and 37–39 % WC for germination (Suszka et al. 1996). Restricted WC (32–34 %) allows dormancy to be released and delay readicle protrusion. After dormancy release, beech seeds can be dried to 10–12 % WC without loss of viability. In such a state, they can be stored at −3 °C for several months and will be ready for germination after imbibition. However, storage time is limited and lasts no longer than 1 year (Pukacka, unpublished). The aim of the present study was to establish the putative causes of the diminished storability of beech seeds after dormancy release. We focused on ROS production and membrane status during storage of dormant (D) seeds and in seeds after dormancy release (ND) under different temperature and moisture conditions and on the status of low molecular weight antioxidants in seeds before and after dormancy breaking and desiccation.
Materials and methods
The seeds of beech (F. sylvatica L.) were collected after shedding, from a single tree growing in the Arboretum of Kórnik (Poland) in 2009. They were dried at 18 °C and 60–70 % RH to 8–9 % WC and then stored at −10 °C in sealed aluminium foil bags until use.
Dormancy release and desiccation of non-dormant seeds
A portion of the seeds was remoistened to 34 % WC with distilled water according to Suszka et al. (1996) and then kept in boxes at +3 °C. Seed moisture content was checked every few days. After 10 weeks of prechilling, the seeds which were still ungerminated, were recognised as non dormant (ND), and in this point, they were desiccated at 15 °C and 40–60 % RH to ca. 10 % WC and kept at −3 °C in tightly sealed polyethylene bags until use. The second portion, dormant (D) seeds, were also transferred from −10 °C and kept at the same temperature at this point.
Ascorbic acid (ASA) assays
The level of ASA and its oxidised product DHA were assayed according to Kampfenkel et al. (1995). Four samples each consisting 15 embryonic axes and five cotyledons of D or ND seeds were taken for analyses. The extraction was performed in 6 % TCA (w/v) in an ice bath. The assay is based on the reduction of Fe3+ to Fe2+ by ASA in an acidic solution. Fe2+ forms complexes with bipyridyl, giving a pink colour with the maximum absorbance at 525 nm. Total ascorbate was determined after reduction of DHA to ASA by dithiothreitol (DTT) and DHA level was calculated from the difference between total ASA and ASA (without pretreatment with DTT).
Glutathione assays
Glutathione in the reduced (GSH) and oxidised (GSSG) forms was assayed according to Smith (1985). Four samples each consisting of 50 embryonic axes or 10 cotyledons of D or ND seeds were homogenized in 5 % (w/v) sulfosalicylic acid in an ice bath and centrifuged at 10,000g and 4 °C for 20 min. A 1 ml aliquot of the supernatant was removed and neutralised by adding 1.5 ml of 0.5 M K-phosphate buffer pH 7.5. This sample was used for the determination of total glutathione (GSH + GSSG). Another 1 ml of neutralised supernatant was pre-treated with 0.2 ml of 2-vinylpyridine for 1.5 h at 25 °C to mask GSH and to allow determination of GSSG alone. Both samples were extracted twice with 5 ml of diethylether. The incubation mixture contained: 0.5 ml of 0.1 M sodium phosphate buffer (pH 7.5) containing 5 mM EDTA, 0.2 ml of 6 mM 5,5′-dithiobis-(2-nitrobenzoic acid), 0.1 ml of 2 mM NADPH, 0.1 ml (1 unit) of glutathione reductase type III (Sigma, Poland), and 0.1 ml of extract. The change in absorbance at 412 nm was observed at 25 °C. A standard curve was prepared by using a GSH (Sigma-Aldrich) standard.
Determination of UV-absorbing phenols
Samples of 50 embryonic axes or 20 cotyledons of D or ND seeds were extracted in 10–20 ml of ethanol: water (80:20; v/v) solvent at 80 °C, according to Lois (1994). The extracts were clarified by centrifugation (16,000g) for 10 min at 4 °C. A spectral scan of UV-absorbing phenols was carried out with ethanolic extracts using a spectrophotometer (Shimadzu). Changes in UV-absorbing phenols were estimated by quantifying absorbance at 265 nm (characteristic for flavonoids) on 10 axes or one cotyledon.
The effect of storage at different temperatures and humidities on viability of D and ND seeds
To investigate the effect of temperature and humidity, aliquots from the batches of D and ND seeds were stored for 1 week at two humidity levels, 45 and 75 % RH (established above respective saturated salt solutions of K2CO3 and NaCl) (Sacandé et al. 2000), and at two temperatures 4 and 20 °C. Following the 1 week period, seeds were placed in laminated aluminum foil bags, hermetically sealed and stored for an additional 2 weeks at the respective temperatures. After this time, the samples of untreated (controls) and stored seeds, 2 × 100 seeds each from each variant, were allowed to germinate at 3 °C, after previous hydration at 100 % RH through 24 h, between moist rolled paper towels in separate boxes. Germination counts were made every week, and germinated seeds were removed upon counting (ISTA 1999). The germination of seeds was checked until the 26th week of stratification, as was described by León-Lobos and Ellis 2002. All seeds that had not germinated after this time were acknowledged as dead.
Electrolyte leakage
Three samples of 20 embryonic axes or 10 cotyledons each of D and ND seeds, before and after incubation at 4 and 20 °C and 45 and 75 % RH, were extracted from the pericarp and seed coats and then were placed in 10 ml of deionised water. The conductivity of the solutions was measured after 24 h of incubation at room temperature according to Pukacka and Ratajczak (2005). The results were expressed as a percentage of the untreated control.
ROS determination
ROS determination was conducted on D and ND seeds before (control) and after incubation at 4 and 20 °C and 45 and 75 % RH.
Superoxide production by seeds was assayed by their capacity to reduce nitroblue tetrazolium (NBT) in the dark at room temperature, as described by Doke (1983). Four samples, each containing five embryos were incubated in 3 ml of 0.05 M K-phosphate buffer, pH 7.8, containing 0.05 % NBT and 10 mM NaN3, at room temperature in the darkness, by 30 min. After that time, 2 ml of solution for each sample was warmed by 15 min on the water bath, and then cooled on the ice bath. The absorbance of the end product was measured at 530 nm. O2 − formation was expressed as ΔA530/g DW of the sample.
For determination of hydrogen peroxide, samples containing of five seeds each were ground to a fine powder in liquid nitrogen. They were then homogenised with 5 ml of 5 % (w/v) trichloroacetic acid (TCA) containing 10 mmol/l EDTA. The homogenate was centrifuged at 4 °C at 12,000g for 20 min. For hydrogen peroxide determination 0.5 ml of supernatant was analysed using the ferrithiocyanate method according to Sagisaka (1976).
Lipid extraction and analyses of phospholipids, fatty acids (FA), free fatty acids (FFA) and α-tocopherol
Phospholipids, FA and FFA were determined in D and ND seeds before (control) and after incubation at 4 and 20 °C and 45 and 75 % RH. α-Tocopherol, the other low molecular weight antioxidants was assayed only in non-incubated seeds. Samples of 50 embryonic axes or 20 cotyledons each were subjected to lipid extraction using chloroform: methanol (2:1, v/v) supplemented with 0.05 % butylated hydroxytoluene (BHT), according to Allen et al. (1966) and as described by Pukacka (1991). Aliquots of lipid extracts were separated on Sep-Pak silica cartridges (Waters Associates) according to Juaneda and Rocquelin (1985) and polar lipid (PL) containing mainly phospholipids and neutral lipid (NL) fractions were obtained. Phospholipids and their fatty acids were determined in aliquots of PL fractions. Particular phospholipids were determined in aliquots of PL fractions by separation on silica gel TLC plates (Merck) in a solvent containing chloroform:methanol:acetic acid:water (85:15:10:3.5, v/v/v/v) (Nichols et al. 1965), with the use of original phospholipid standards. Spots containing phospholipids were detected with iodine vapour and were scraped off for phosphorus analysis. The phosphorus content of spots was estimated according to Ames (1966). Samples of PL with heptadecanoic acid (17:0) as the internal standard were saponified and methylated according to Metcalfe et al. (1966). They were separated using a Hewlett Packard gas chromatograph equipped with a Supelco 2330 capillary column (30 m × 0.25 mm) at 220 °C (in isotherm) with He as the carrier gas.
The α-tocopherol and FFA contents were determined in aliquots of the total lipid fractions according to Kendal and McKersie (1989) after the silylation of samples with BSTFA (N–O-bis-trimethylsilyl-trifluoro-acetamide) and pyridine at room temperature in darkness. Silylated samples and internal standards (heptadecanoic acid 17:0 and α-cholestan) were separated on the gas chromatograph with a SPB 1 (Supelco) microcapillary column and identified by comparing retention times with those of authentic standards. Quantities of FFA were the sum of myristic + palmitic + stearic + oleic + linoleic + linolenic acids in the total lipid extract. The results were expressed as a percentage of non-treated control. The concentration of α-tocopherol was expressed in μg g−1 DW.
Statistical analysis
Data are presented as the mean ± standard deviation of four or three replicates. The significant differences between D and ND seeds, or between unstored and stored seeds, were tested using an analysis of variance (ANOVA). Levels of significance are indicated as *P < 0.05 and **P < 0.01.
Results
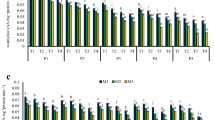
After release from dormancy and drying to 10 % WC, ND seeds contained a slightly greater amount of ASA and DHA in embryonic axes compared to D seeds. In cotyledons, the content of this antioxidant was similar for both types of seeds (Fig. 1a). Dormant and non-dormant seeds differed in glutathione contents. In embryonic axes and cotyledons of ND seeds, oxidized form of glutathione occurred in higher level than in D seeds (Fig. 1b). ND seeds were characterised by significantly lower levels of α-tocopherol than D seeds, 131 and 256 μg DW−1 in embryonic axes and 104 and 212 μg DW−1 in cotyledons, respectively (Fig. 1c). The level of UV-absorbing phenols at 265 nm was also significantly higher in embryonic axes and cotyledons of D seeds (Fig. 1d).
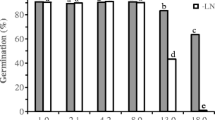
Storage of ND and D beech seeds for 3 weeks at 4 and 20 °C and 45 and 75 % RH showed that ND seeds are more sensitive to storage conditions than D seeds. Both D and ND seeds stored at 4 °C showed a high rate of germination (94–89 %), nearing that of the unstored control. After storage at 20 °C and 45 % RH, D seeds showed an 80 % germination rate, while ND seeds showed a 62 % germination rate. After storage at 20 °C and 75 % RH, germinability of D seeds decreased to 28 %, while that of ND seeds decreased to 7 % (Fig. 2a, b). The storage conditions also had an effect on the beginning of seed germination. Stored D seeds started to germinate earlier than non-stored control seeds, in contrast to stored ND seeds, which started to germinate later than non-stored control seeds. The decrease in germination capacity was accompanied by an increase in electrolyte leakage (Fig. 3) and an increased level of ROS (superoxide and hydrogen peroxide) (Table 1). In the case of D seeds, the increase in electrolyte leakage in embryonic axes and in cotyledons was visible only after storage at 20 °C and 75 % RH, while in the case of ND seeds the increase in electrolyte leakage was visible at 20 °C and both 45 and 75 % RH. ROS production was analysed in whole embryos. Compared to D seeds, the levels of superoxide anions and hydrogen peroxide in ND seeds were significantly higher after storage at 20 °C and 45 and 75 % RH. In addition, in control seeds (before temperature and humidity treatment), the level of ROS was slightly higher compared with ND seeds and decreased in seeds incubated at 4 °C. Strong negative correlations were noticed between germination capacity and superoxide and hydrogen peroxide contents in both D and ND seeds stored under various temperature and moisture conditions (Table 2). The FFA content was determined before and after storage of ND and D seeds under different temperature and humidity conditions. The level of FFA in D seeds was significantly higher (33 %) than in control seeds only under conditions of 20 °C/75 %, in both embryonic axes and cotyledons (Fig. 4). However, in embryonic axes of ND seeds, the FFA content increased 27, 77 and 85 % in comparison with the control under conditions of 4/75, 20/45 and 20 °C/75 %, respectively. In cotyledons, the FFA content increased 20 and 83 % under conditions of 20/45 and 20 °C/75 %, respectively. Table 3 shows the differences in membrane lipid components (phospholipids and fatty acids) in D and ND seeds before storage at different temperature and moisture conditions. Dormancy release caused a significant decrease in the level of phosphatidylcholine (PC) and an increase of phosphatidic acd (PA) in embryonic axes and cotyledons and a decrease in phosphatidylglycerol (PG) in embryonic axes only. In embryonic axes of ND seeds, the level of linolenic acid (18:3) was significantly higher than in D seeds, while the level of oleic acid (18:1), was significantly lower. The levels of the primary phospholipids PI (phosphatidylinositol), PC, PE (phosphatidyletanolamine) and PG in embryonic axes and cotyledons of D and ND seeds before and after storage under different temperature and humidity conditions are presented in Fig. 5a, b, c, and d and in Table 4. In ND seeds, a significant decrease in the phospholipids PC and PE was found under conditions of 20/45 and 20 °C/75 % (to 65.1 and 46.2 % for PC and to 66.9 and 47.5 % respectively for PE, compared to the control in embryonic axes, and to 76.2 and 63.8 % for PC and 75.5 and 58.0 %, respectively, for PE in cotyledons. In the case of D seeds, the decline in PC and PE contents was much smaller (to 87.8 and 81.0 % for PC and 72.2 and 63.55 %, respectively, for PE in embryonic axes and to 89.3 and 82.5 % for PC and 88.7 and 70.8 % for PE, respectively, compared to the control in cotyledons. The changes in fatty acids of the PL fraction are presented in Fig. 6a, b, c and d, and in Table 5. The largest decrease in the content of unsaturated fatty acids occurred in embryonic axes and cotyledons of ND seeds after storage under conditions of 20/45 and 20 °C/75 %, to 65.0 and 44.8 % for 18:2 and 58.4 and 41.6 for 18:3, respectively, in embryonic axes and to 76.8 and 57.8 % for 18:2 and 71.4 and 57.1 % for 18:3, respectively, compared to the control, in cotyledons. In D seeds, the decrease in polyunsaturated fatty acid contents was markedly smaller. The polar lipid fraction contained mainly phospholipids.
Changes in the primary phospholipid contents in embryonic axes and cotyledons of dormant (a, b) and non-dormant (c, d) F. sylvatica seeds, before (Control) and after 3 weeks of storage at 4 and 20 °C and 45 and 75 % RH. PI phosphatidylinositol, PC phosphatidylcholine, PE phosphatidylethanolamine, PG phosphatidylglycerol. Data are means of three replicates ±SD
Discussion
Beech seeds, similarly to other orthodox seeds, remain tolerant to desiccation after release from dormancy until the beginning of radicle protrusion (Buitink et al. 2003, 2006). Our results showing the germination capacity of beech seeds after 10 weeks of prechilling at 3 °C and 34 % WC, after desiccation to 10 % WC confirmed this previous finding (Fig. 2b) (control). The aim of the present study was to determine the causes of the greater decline in viability of beech seeds during storage after dormancy release and desiccation, in comparision with dormant seeds stored under similar conditions. Seed ageing is influenced by two environmental factors, humidity (RH) and temperature. On the basis of our previous studies (Pukacka and Ratajczak 2005; Ratajczak and Pukacka 2005) showing the effect of variations in storage temperatures and RHs on the viability of beech seeds, we chose temperatures of 4 and 20 °C and humidity levels of 45 and 75 % RH for 3 weeks of storage as the conditions for controlled deterioration (CD) of D and ND seeds. The germination capacity in these conditions clearly showed that ND seeds are more sensitive to storage than D seeds. The increase in electrolyte leakage accompanying the decrease in viability of seeds suggests that membrane destruction could be the main cause of the decline in viability of seeds during storage at higher temperature and humidity and that the underlying cause is oxidative stress. Similar cellular damage has been identified in seeds of other species of intermediate category, such as neem and coffee stored under similar temperature and humidity conditions (Sacande et al. 2001, Dussert et al. 2006). Analyses of ROS, the cause of oxidative stress in cells, superoxide anions and hydrogen peroxide contents, showed that in ND seeds, the production of these molecules was significantly higher than in D seeds under storage conditions of 20 °C and 45 and 75 % RH (Table 1). A strong negative correlation was evident between germination capacity and ROS production in D and ND seeds stored at different temperature and humidity levels (Table 2). Our previous research (Pukacka et al. 2003) showed that beech seeds stored at 45 and 75 % RH differ substantially in WC of embryonic axes. In the present study, the WC of embryonic axes of D seeds change for 0.07–0.08 g g−1 DW at 45 % RH to 0.13–0.14 g g−1 DW at 75 % RH. In cotyledons, the WC reached ca 0.05 and 0.08 g g−1 DW, respectively. The WC in ND seeds did not differ significantly. Nevertheless, storing seeds at higher WC and temperature evoked greater production of ROS in ND seeds compared to D seeds. The increase in ROS production in both D and ND seeds stored at 20 °C and 45 and 75 % RH could be evoked by the occurrence of free radical chain reactions initiated at higher WC levels and higher temperatures in embryonic axes. Such reactions were not visible at 4 °C. The increase in ROS production was accompanied by an increase in electrolyte leakage from embryonic axes and cotyledons and changes in membrane lipid components. In addition to the greater intensity of oxidative stress during the incubation of ND seeds, membranes of ND seeds may be more sensitive to it. Their greater sensitivity is visible in changes in the phospholipid composition, as well as in the content of unsaturated fatty acids in ND seeds before storage (Table 3) after dormancy breaking and desiccation. The increase in fatty acid unsaturation in the PL fraction may be the result of gibberellin activity during dormancy release (Grindstaff et al. 1996; Fernandez et al. 1997). Enzymatic modification of phospholipids is also possible during the cold stratification of seeds (Hallet and Bewley 2002). Along with peripheral membrane proteins, phospholipids play a part in signal transduction, leading to germination. Such changes could have an effect on the sensitivity of ND seed membranes to the increased levels of ROS attack. One of the consequences of increased ROS attack on the membranes is lipid peroxidation and phospholipid de-esterification (McKersie et al. 1988; Van Bilsen et al. 1994). The results of this study showed that the levels of FFA, the products of phospholipid de-esterification and neutral lipids hydrolysis due to free radical attack (McKersie et al. 1988; Dussert et al. 2006; Pukacka et al. 2011), were coincident with changes in seed viability. The FFA content increased more rapidly in embryonic axes of ND seeds stored at 20 °C and 45 and 75 % RH as well as at 4 °C and 75 % RH. In cotyledons, the increase in FFA content was very clearly visible after storage at 20 °C and 75 % RH. Membrane destruction was also evidenced by a decrease in levels of the primary phospholipids phosphatidylcholine (PC) and phosphatidylethanolamine (PE), as well as the contents of polyunsaturated fatty acids (18:2 and 18:3). This decrease was significantly greater in the case of ND seeds (Figs. 5, 6; Tables 4, 5). The degree of degradation of membrane lipid components was significantly higher in embryonic axes than in cotyledons, which was coincident with the higher water contents in these organs. The antioxidative system plays a crucial role in the protection of membranes and other macromolecules during the storage of seeds in a dry state. Low molecular weight antioxidants, which are directly able to detoxify ROS, are particularly important. The status of low molecular weight antioxidants, such as ascorbic acid (ASA), glutathione (GSH) in its reduced and oxidised forms, and α-tocopherol and UV-absorbing phenols, was determined in D and in ND seeds after desiccation but before storage under different temperature and humidity conditions. We expected that the levels of these compounds would be coincident with the possibility of protection against ROS during storage of seeds. ASA and GSH are important antioxidants that react directly with ROS and in the Halliwell-Asada pathway (Foyer and Noctor 2011). As our results show, D and ND beech seeds did not differ significantly in ASA content. In both D and ND seeds, the level of the reduced form, ASA, was higher than the level of the oxidised form, DHA. However, our results showed that ND seeds contained higher level the oxidised form of glutathione (GSSG) than D (Fig. 1b), which was most likely a result of prechilling and preparation for germination, as well as desiccation stress. During the above processes, enchanced production of ROS was observed (Oracz et al. 2009; Pukacka and Ratajczak 2006; Pukacka et al. 2011). High levels of GSSG can decrease the efficiency of ROS removal from cells of ND seeds (Bailly et al. 2008). It could also increase the half-cell reduction potential of glutathione in cells of ND seeds, which may be a part of the signalling cascade leading to programmed cell death (PCD) (Kranner et al. 2006). The low molecular weight antioxidant α-tocopherol protects membranes from the harmful activity of ROS. Sattler et al. (2004), working with mutants of Arabidopsis thaliana that were unable to synthesise tocopherols in seeds, showed that α-tocopherol is essential for seed longevity. Pukacka and Ratajczak (2007) reported a statistically significant relationship between α-tocopherol content and viability of beech seeds during natural ageing. The results presented in this study show that ND seeds contained significantly less α-tocopherol in embryonic axes and cotyledons than D seeds. Phenolic compounds, flavonoids and coumarins in particular, may also have antioxidant proprieties (Hernández et al. 2009). The presence of these compounds may indicate high absorption of UV at 265 nm by ethanolic extracts from seeds (Harborne 1998). These groups of phenolic compounds include many effective antioxidants, with antioxidative activity comparable to α-tocopherol (Oliver et al. 1998; Shirley 1998; Rice-Evans et al. 1997). In ND seeds, the level of UV-absorbing phenols was also significantly lower than in D seeds; however, the precise identification of these compounds requires further study.
In conclusion, our research showed that after prechilling by 10 weeks and desiccation, beech seeds are more sensitive to storage conditions of 20 °C and 45 and 75 % RH than dormant seeds. The cause of the rapid loss of viability under these conditions is the greater production of ROS (superoxide radicals and hydrogen peroxide) in cells, and the lower levels of low molecular weight antioxidants (glutathione, α-tocopherol and phenolic (flavonoid) antioxidants) in the embryonic axes and cotyledons of these seeds compared to dormant seeds. Oxidative stress induced greater destruction of membranes in ND seeds, which led to cell death. The membranes of ND seeds may be more sensitive to ROS because of the changes that occur during the dormancy release and desiccation processes.
References
Allen CF, Good P, Davis HF, Chisum P, Fowler SD (1966) Methodology for the separation of plant lipids and application to spinach leaf and chloroplast lamellae. J Am Oil Chem Soc 43:223–230
Ames DN (1966) Assay of inorganic phosphate, total phosphate and phosphatases. In: Colowick SP, Kaplan NO (eds) Methods in enzymology. Acad Press, New York, pp 115–118
Bailly C (2004) Active oxygen species and antioxidants in seed biology. Seed Sci Res 14:93–107
Bailly C, El-Maarouf-Bouteau H, Corbineau F (2008) From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. C R Biol 331:806–814
Ballesteros D, Walters C (2011) Detailed characterization of mechanical properties and molecular mobility within dry seed glasses: relevance to the physiology of dry biological systems. The Plant J 68:607–619
Bentsink L, Jowett J, Hanhart CJ, Koornneef M (2006) Cloning of DOG1, a quantitative trait locus controlling seed dormancy in Arabidopsis. Proc Natl Acad Sci USA 103:17042–17047
Buitink J, Leprince O, Hemminga MA, Hoekstra FA (2000) Molecular mobility in the cytoplasm: an approach to describe and predict lifespan of dry germplasm. Proc Natl Acad Sci USA 97:2386–2390
Buitink J, Ly VuB, Satour P, Leprince O (2003) The re-establishment of desiccation tolerancwe in germinated radicles of Medicago truncatula Gaertn. seeds. Seed Sci Res 13:273–286
Buitink J, Leger JJ, Guisle I, Ly VuB, Wuillème S, Lamirault G, Bars A, Le Meur N, Becker A, Küster H, Leprince O (2006) Transcriptome profiling uncovers metabolic and regulatory processes occurring during the transition from deciccation-sensitive to desiccation-tolerant states in Medicago truncatula seeds. Plant J 47:735–750
Doke N (1983) Generation of superoxide anion by potato tuber protoplasts during the hyper- sensitive response to hyphal wall components of Phytophthora infestans and specific inhibition of the reaction by suppressors of hypersensitivity. Physiol Plant Pathol 23:359–367
Dussert S, Davey MW, Laffargue A, Doulbeau S, Swennen R, Etienne H (2006) Oxidative stress, phospholipid loss and lipid hydrolysis during drying and storage of intermediate seeds. Physiol Plant 127:192–204
Fernandez H, Doumas P, Muller C, Bonnet-Masimbert M (1997) Endogenous gibbrellins and dormancy in beechnuts. In: Ellis RH, Black M, Hong TD (eds) Basic and applied aspects of seed biology. Kluwer Acad Publisch, Dordrecht, pp 311–321
Foyer CH, Noctor G (2011) Ascorbate and glutathione : the hearth of the redox hub. Plant Physiol 155:2–18
Gosling P (1991) Beechnuts storage: a review and practical interpretation of the scientific literature. Forestry 64:51–59
Grindstaff KK, Fielding LA, Brodl MR (1996) Effect of gibberellin and heath shock on the lipid composition of endoplasmic reticulum in barley aleurone layers. Plant Physiol 110:571–581
Hallett BP, Bewley JD (2002) Membranes and seed dormancy: beyond the anaesthetic hypothesis. Seed Sci Res 12:69–82
Harborne JB (1998) Phytochemical methods. Thompson Science London
Hernández I, Alegre L, Van Breusegem F, Munné-Bosch S (2009) How relevant are flavonoids as antioxidants in plants. Trends Plant Sci 14:125–132
Hoekstra FA, Golovina EA, Buitink J (2001) Mechanisms of plant desiccation tolerance. Trends Plant Sci 6:431–438
ISTA (1999) International rules for seed testing. Rules 1999. Seed Sci Technol 27:1–133. (Supplement)
Juaneda PG, Rocquelin G (1985) Rapid and convenient separation of phospholipids and non-phosphorus lipids from rat heart using silica cartridges. Lipids 20:40–41
Kampfenkel K, Van Montagu M, Inzé D (1995) Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal Biochem 225:165–167
Kendall EJ, McKersie BD (1989) Free radical and freezing injury to cell membranes of winter wheat. Physiol Plant 76:86–94
Kranner I, Birtic S, Anderson KM, Pritchard HW (2006) Glutathione haf-cell reduction potential: a universal stress marker and modulator of programmed cell death. Free Rad Biol Med 20:2155–2165
León-Lobos P, Ellis RH (2002) Seed storage behaviour of Fagus sylvatica and Fagus crenata. Seed Sci Res 12:31–37
Lois R (1994) Accumulation of UV-absorbing flavonoids induced by UV-B radiation in Arabidopsis thaliana L. Planta 194:498–503
Mc Donald MB (1999) Seed deterioration: physiology, repair and assessment. Seed Sci Technol 27:177–237
McKersie BD, Senaratna T, Walker MA, Kendall EJ, Hetherington PR (1988) Deterioration of membranes during aging in plants: evidence for free radical mediation. In: Nooden LD, Leopold AC (eds) Senescence and aging in plants. Acad Press, New York, pp 441–464
Metcalfe LD, Schmitz AA, Pelka JR (1966) Rapid preparation of fatty acids esters for gas chromatographic analysis. Anal Chem 38:514–515
Narsai R, Law SR, Carrie C, Xu L, Whelan J (2011) In-depth temporal transcriptome profiling reveals a crucial developmental switch with roles for RNA processing and organelle metabolism that are essential for germination in Arabidopsis. Plant Physiol 157:1342–1362
Nichols BW, Harris RV, James AT (1965) The lipid metabolism of blue-green algae. Biochim Biophys Res Commun 20:256–262
Oliver AE, Hincha DK, Crowe LM, Crowe JH (1998) Interaction of arbutin with dry and hydrated bilayers. Bioch Biophys Acta 1370:87–97
Oracz K, El-Maarouf-Bouteau H, Kranner I, Bogatek R, Corbineau F, Bailly C (2009) The mechanisms involved in seed dormancy alleviation by hydrogen cyanide unravel the role of reactive oxygen species as key factors of cellular signaling during germination. Plant Physiol 150:494–505
Pawlowski TA (2007) Proteomics of European beech (Fagus sylvatica L.) seed dormancy breaking: influence of abscisic and gibberellic acids. Proteomics 7:2246–2257
Pukacka S (1991) Changes in membrane lipid components and antioxidant levels during natural ageing of seeds of Acer platanoides. Physiol Plant 82:306–310
Pukacka S, Ratajczak E (2005) Production and scavenging of reactive oxygen species in Fagus sylvatica seeds during storage at varied temperature and humidity. J Plant Physiol 162:873–885
Pukacka S, Ratajczak E (2006) Antioxidative response of ascorbate-glutathione pathway and metabolites to desiccation of recalcitrant Acer saccharinum seeds. J Plant Physiol 163:1259–1266
Pukacka S, Ratajczak E (2007) Age related biochemical changes during storage of beech (Fagus sylvatica L.) seeds. Seed Sci Res 17:45–53
Pukacka S, Hoffmann SK, Goslar J, Pukacki PM, Wójkiewicz E (2003) Water and lipid reactions in beech (Fagus sylvatica L.) seeds and its effect on storage behaviour. Biochim Biophys Acta 1621:48–56
Pukacka S, Malec M, Ratajczak E (2011) ROS production and antioxidative system activity in embryonic axes of Quercus robur seeds under different desiccation rate conditions. Acta Physiol Plant 33:2219–2227
Ratajczak E, Pukacka S (2005) Decrease in beech (Fagus sylvatica) seed viability caused by temperature and humidity conditions as related to membrane damage and lipid composition. Acta Physiol Plant 27:3–11
Rice-Evans CA, Miller NJ, Paganga G (1997) Antioxidant properties of phenolic compounds. Trends Plant Sci 2:152–159
Sacandé M, Buitink J, Hoekstra FA (2000) A study of water relations in neem (Azadirachta indica) seed that is characterized by complex storage behaviour. J Exp Bot 51:635–643
Sacandé M, Golovina EA, van Aelst AC, Hoekstra FA (2001) Viability loss of neem (Azadirachta indica) seeds associated with membrane phase behaviour. J Exp Bot 52:919–931
Sagisaka S (1976) The occurrence of peroxide in a perennial plant Populus gelrica. Plant Physiol 57:309
Sattler SE, Gilliland LU, Magallanes-Lundback M, Pollard M, DellaPenna D (2004) Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell 16:1419–1432
Shirley BW (1998) Flavonoids in seeds and grains: physiological function, agronomic importance and genetics of biosynthesis. Seed Sci Res 8:415–422
Smith I (1985) Stimulation of glutathione synthesis in photorespiring plants by catalase inhibitors. Plant Physiol 79:1044–1047
Stevenson DE, Hurst RD (2007) Polyphenolic phytochemicals—just antioxidants or much more? Cel Mol Life Sci 64:2900–2916
Suszka B, Muller C, Bonnet-Masimbert M (1996) Seeds of forest broadleaves; from harvest to sowing. INRA, Paris
Van Bilsen DGJL, Hoekstra FA, Crowe LM, Crowe HJ (1994) Altered phase behavior in membranes of aging dry pollen may cause imbibitional leakage. Plant Physiol 104:1193–1199
Walters C (1998) Understanding the mechanisms and kinetics of seed aging. Seed Sci Res 8:223–244
Walters C, Wheeler LJ, Grotenhuis JM (2005) Longevity of seeds stored in a genebank: species characteristics. Seed Sci Res 15:1–20
Walters C, Ballesteros D, Vertucci VA (2010) Structural mechanics of seed deterioration: standing the test of time. Plant Sci 179:565–573
Yamauchi Y, Ogawa M, Kuwahara A, Hanada A, Kamiya Y, Yamaguchi S (2004) Activation of gibberellin biosynthesis and response pathways by low temperature during imbibition of Arabidopsis thaliana seeds. Plant Cell 16:367–378
Acknowledgments
This study was supported by research funds of the Polish Ministry of Science and Higher Education.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Pukacka, S., Ratajczak, E. Factors influencing the storability of Fagus sylvatica L. seeds after release from dormancy. Plant Growth Regul 72, 17–27 (2014). https://doi.org/10.1007/s10725-013-9832-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-013-9832-5