Abstract
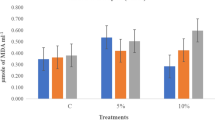
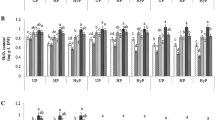
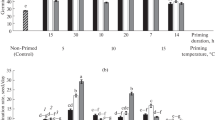
Chilling stress is one of primary constraints to tobacco production in many parts of the world. The present study was conducted to induce chilling tolerance in tobacco by seed priming with putrescine (Put) in relation to physiological changes, using seeds from two tobacco varieties, MSk326 (chilling sensitive variety) and Honghuadajinyuan (HHDJY, chilling tolerant variety). Seed germination, seedling antioxidant enzyme activities and malondialdehyde (MDA) concentration, as well as polyamine concentration were determined under low temperature. During chilling stress at 11°C, seed priming with 0.01 mM Put for 48 h (Put0.01mM48 h) and seed priming with 0.1 mM Put for 48 h (Put0.1mM48 h) significantly increased germination percentage, germination index, seedling length and dry weight of both varieties compared to the controls without Put treatment. When seedlings of 4-leaf stage suffered a short chilling stress (5°C), Put 0.1 mM 48 h improved the activities of antioxidant enzymes including superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) and ascorbate peroxidase (APX), increased endogenous Put, Spd and Spm concentration and decreased the MDA concentration. The results showed that Put priming treatments were available to enhance the chilling tolerance of tobacco seedlings. The optimal treatment of Put was Put0.1 mM48 h.








Similar content being viewed by others
Abbreviations
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- EDTA:
-
Ethylenediamine tetra-acetic acid
- MDA:
-
Malondialdehyde
- NBT:
-
Nitroblue tetrazolium
- PA:
-
Polyamine
- POD:
-
Peroxidase
- Put:
-
Putrescine
- SOD:
-
Superoxide dismutase
- Spd:
-
Spermidine
- Spm:
-
Spermine
- TBA:
-
2-Thiobarbituric acid
- TCA:
-
Trichloroacetic acid
References
Afzal I, Munir F, Ayub CM, Basra SMA, Hameed A, Nawaz A (2009) Changes in antioxidant enzymes, germination capacity and vigour of tomato seeds in response of priming with polyamines. Seed Sci Technol 37(3):765–770
Alcazar R, Altabella T, Marco F, Bortolotti C, Reymond M, Koncz C, Carrasco P, Tiburcio A (2010) Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231:1237–1249
Altman A (2006) Polyamines and wounded storage tissues–inhibition of RNase activity and solute leakage. Physiol Plant 54(2):194–198
An LZ, Liu GX, Zhang MX, Chen T, Liu YH, Feng HY, Xu SJ, Qiang WY, Wang XL (2004) Effect of enhanced UV-B radiation on polyamine content and membrane permeability in cucumber leaves. Russ J Plant Physiol 51:658–662
Besford RT, Richardson CM, Campos JL, Tiburcio AF (1993) Effect of polyamines on stabilization of molecular complexes in thylakoid membranes of osmotically stressed oat leaves. Planta 189:201–206
Bolkhina O, Virolainen E, Fagerstedt K (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Bowler C, Van Montagu M, Inze D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 43:83–116
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol 98:1222–1227
Cao DD, Hu J, Gao CH, Guan YJ, Zhang S, Xiao JF (2008) Chilling tolerance of maize (Zea mays L.) can be improved by seed soaking in putrescine. Seed Sci Technol 36:191–197
Chattopadhayay MK, Tiwari BS, Chattopadhayay G, Bose A, Sengupta DN, Ghosh B (2002) Protective role of exogenous polyamines on salinity-stressed rice (Oriza sativa) plants. Physiol Plantarum 116:192–199
Cuevas JC, Lopez-Cobollo R, Alcazar R, Zarza X, Koncz C, Altabella T, Salinas J, Tiburcio AF, Ferrando A (2008) Putrescine is involved in Arabidopsis freezing tolerance and cold acclimation by regulating abscisic acid levels in response to low temperature. Plant Physiol 148:1094–1105
Dadlani M (1992) Seed coating to improve stand establishment in rice. Seed Sci Technol 20:307–313
Farooq M, Wahid A, Lee DJ (2009) Exogenously applied polyamines increase drought tolerance of rice by improving leaf water status, photosynthesis and membrane properties. Acta Physiol Plant 31:937–945
Flores HE, Galston WA (1982) Analysis of polyamine in higher plants by high performance liquid chromatography. Plant Physiol 69:701–706
Gao CH, Hu J, Zhang S (2009) Association of polyamines in governing the chilling sensitivity of maize genotypes. Plant Growth Regul 57:31–38
Gechev T, Willekens H, Van Montagu M, Inzé D, Van Camp W, Toneva V, Minkov I (2003) Different responses of tobacco antioxidant enzymes to light and chilling stress. Plant Physiol 160:509–515
Guo Z, Ou W, Lu S, Zhong Q (2006) Differential responses of antioxidative system to chilling and drought in four rice cultivars differing in sensitivity. Plant Physiol Biochem 44:828–836
Ha HC, Sirisoma NS, Kuppusamy P, Zweier JL, Woster PM, Casero RA (1998) The natural polamine spermie functions directly as a free radical scavenger. Biochemistry 95:11140–11145
Han JF, Zhu DH, Lin XW, Han W (1992) Effects of polyamines on tobacco root growth and development. Plant Physiol Commun 28(2):124–126
Kasinathan V, Wingler A (2004) Effect of reduced arginine decarboxylase activity on salt tolerance and on polyamine formation during salt stress in Arabidopsis thaliana. Physiol Plantarum 121:101–107
Kim TE, Kim SK, Han TJ, Lee JS, Chang SC (2002) ABA and polyamines act independently in primary leaves of cold-stressed tomato (Lycopersicon esculentum). Physiol Plantarum 115:370–376
Kovacs Z, Simon-Sarkadi L, Szucs A, Kocsy G (2010) Differential effects of cold, osmotic stress and abscisic acid on polyamine accumulation in wheat. Amino Acids 38:623–631
Kubiś J (2006) Exogenous spermidine alters in different way membrane permeability and lipid peroxidation in water stressed barley leaves. Acta Physiologiae Plantarum 28:27–33
Li YP, Xu SC, Ma WG, Zheng YY, Hu J (2009) Identification of chilling-tolerance in tobacco cultivars at germination and seedling growth stages. Bull Sci Technol 25(6):807–811
Lin JM, Sung JM (2001) Pre-sowing treatments for improving emergence of bitter gourd seedlings under optimal and sub-optimal temperatures. Seed Sci Technol 29(1):39–50
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplast. Plant Cell Physiol 22:867–880
Nayyar H (2005) Putrescine increases floral retention, pod set and seed yield in cold stressed chickpea. J Agron Crop Sci 191(5):340–345
Nayyar H, Chander S (2004) Protective effects of polyamines against oxidative stress induced by water and cold stress in chickpea. J Agron Crop Sci 190:355–365
Ndayiragije A, Lutts S (2006) Do exogenous polyamines have an impact on the response of a salt-sensitive rice cultivar to NaCl? J Plant Physiol 163:506–516
Qiu J, Wang R, Yan J, Hu J (2005) Seed film coating with uniconazole improves rape seedling growth in relation to physiological changes under waterlogging stress. Plant Growth Regul 47:75–81
Rao KVM, Sresty TVS (2000) Antioxidant parameters in the seedlings of pigeon pea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci 157:113–128
Rodríguez-Kessler M, Alpuche-Solís AG, Ruiz OA, Jiménez-Bremont JF (2006) Effect of salt stress on the regulation of maize (Zea mays L.) genes involved in polyamine biosynthesis. Plant Growth Regul 48:175–185
Sanchez DH, Cuevas JC, Chiesa MA, Oscar RA (2005) Free spermidine and spermine content in Lotus glaber under long-term salt stress. Plant Sci 168:541–546
Simon EW, Minchin A, McMenamin MM, Smith JM (1976) The low temperature limit for seed germination. New Phytol 77:301–311
Urano K, Yoshiba Y, Nanjo T, Igarashi Y, Seki M, Sekiguchi F, Yamaguchi-Shinozaki K, Shinozaki K (2003) Characterization of Arabidopsis genes involved in biosynthesis of polyamines in abiotic stress responses and developmental stages. Plant Cell Environ 26:1917–1926
Wallace MH, Fraser VA, Hughes A (2003) A perspective of polyamine metabolism. Biochem J 376:1–14
Wu QS, Zou YN, He XH (2010) Exogenous putrescine, not spermine or spermidine, enhances root mycorrhizal development and plant growth of trifoliate orange (Poncirus trifoliata) seedlings. Int J Agri Biol 12:576–580
Zhang XZ (1992) The measurement and mechanism of lipid peroxidation and SOD, POD and CAT activities in biological system. In: Zhang XZ (ed) Research methodology of crop physiology. Agriculture Press, Beijing, pp 208–211
Zhang S, Hu J, Zhang Y, Xie XJ, Knapp A (2007) Seed priming with brassinolide improves lucerne (Medicago sativa L.) seed germination and seedling growth in relation to physiological changes under salinity stress. Aust J Agr Res 58:811–815
Zhao ZF, Guo AH, Feng ZW (2003) Amelioration of chilling stress by triadimefon in cucumber seedlings. Plant Growth Regul 39(3):277–283
Acknowledgments
The research was supported by the Yunnan Province Tobacco Company (No. 07A02, 09YN008), Major Science and Technology Special Project (priority subject) of Zhejiang Province (No. 2008C12005-1) and Major special project of Ministry of Agriculture (No. 2008ZX08005-005), China. The authors are grateful to the editor and anonymous reviewers for comments that improved the presentation of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, S., Hu, J., Li, Y. et al. Chilling tolerance in Nicotiana tabacum induced by seed priming with putrescine. Plant Growth Regul 63, 279–290 (2011). https://doi.org/10.1007/s10725-010-9528-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-010-9528-z