Abstract
In this study, 36 cultivars and genotypes of quince fruits were harvested as materials from the collection garden of Yalova Atatürk Horticultural Central Research Institute. This study aims to determine the nutritional contents of quince cultivars and genotypes, including vitamin C (L-ascorbic acid mg/100 g), reducing sugar content (glucose mg/100 g), mineral substances (N, Fe, Mn, Zn, Cu, K, Ca, Mg, P; mg/100 g, Ash %), total antioxidant activity (DPPH, FRAP, ABTS, CUPRAC; µmol/100 g TE), total phenolic content (mg/100 g GAE), SSC (%), titratable total acidity (%), and pH and color values (L*, a*, b*). Based on these evaluations, quince cultivars and types with the highest nutritional content beneficial to human health were determined. This study can contribute to recommending high-nutrient cultivars and types to producers for cultivation in broder areas and promoting their widespread adoption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Türkiye holds the position of being the largest producer of many fruit types worldwide. One of these fruits is the quince (Cydonia vulgaris Pers.), which is classified among temperate climate fruit species and has its origins in Northwestern Iran, the Northern Caucasus, the region around the Caspian Sea, and Northern Anatolia. Quince (Cydonia oblonga Miller), belonging to the Rosaceae family in the Pomoidea subfamily, is a soft-seeded fruit with a unique, pleasant fragrance, characterized by its golden yellow color (Stojanović et al. 2017). According to the data for 2022, the global quince production is approximately 692,263 tons, with Türkiye ranking first with a production of 197,503 tons (Turkish Statistical Institute-TUIK 2022). Approximately 73% of these quince orchards are located in six provinces: Sakarya, Denizli, Bursa, Bilecik, İzmir, and Manisa in Türkiye (Fig. 1).
Provinces with the largest share in Turkey’s quince product (2021, %), (Özüdoğru 2022)
Quince has a low fat content and is a significant source of organic acids, dietary fiber, sugars, and minerals, such as potassium, phosphorus, and calcium. In many investigations, quinces have been recognized as a good and cost-effective natural source of phenolic acids and flavonoids. The fruit is well-known primarily for its high pectin content, which allows it to be used in fruit jam production and the canning industry (Rop et al. 2011; Pawlak-Lemańska et al. 2018; Antoniewska et al. 2019; Byczkiewicz et al. 2021). In addition to being consumed fresh, quinces can be utilized in various forms, such as jams, marmalades, and canned products. Moreover, they are used as dwarf rootstocks for pear trees in moist soils (Okatan et al. 2022; Ünal and Okatan 2023). Quince is an important fruit for human health due to its valuable nutritional content, making fresh consumption highly beneficial.
There has been a recent growing interest in foods of natural origin and with valuable nutritional values. Due to the high therapeutic and health potential and properties of quince, these fruits are gaining increasing popularity among food producers and consumers. Considering not only their undesirable sensory properties but also the seasonal availability of fresh quince fruits, the food market is dominated mainly by processed products derived from these fruits (Magalhćes et al. 2009). Therefore, this study aims to determine the effects of physicochemical and bioactive properties of fresh quince fruit on cultivars grown in Türkiye for producers who want to convert quince fruit into table and high-value-added products.
Depending on the cultivars, quince has a bitter and tart taste with a firm texture. Besides being consumed fresh, quince is well known for being a good source of pectin, giving it a technological advantage over other dietary fiber sources as it exhibits gelling, thickening, and stabilizing properties. Ascorbic acid content in quince fruit was reported to vary between 18 and 233 mg per 100 g−1, the variation was influenced by environmental factors and fruit genotype (Rubinskienė et al. 2014; Curi et al. 2018). Quince fruit, as a natural source of antioxidants, may be used in foods or nutraceuticals for disease prevention (Magalhćes et al. 2009; Rop et al. 2011). Pleasant flavour, high content of organic acids and fibre makes quince fruit valuable and interesting raw material for developing various, healthy food products (Byczkiewicz et al. 2021). Therefore, quince fruit can be used more widely to produce similar foods. This study can guide us to evaluate the biochemical composition and antioxidant activity of quince fruit cultivars and types as a potential source of biologically active compounds. Understanding the physicochemical properties of fruit raw materials is crucial for determining their suitability for various uses, including direct consumption and technological processing, as well as the quality of the derived products (Curi et al. 2018). Due to the high nutritional value of quince, identifying and popularizing the cultivars or genotypes with the highest content in Türkiye through this study will also attract attention from the industry. Thus, this research holds original value as, to our knowledge, it is the first time 36 cultivars and types have been evaluated in Türkiye. The richness of quince cultivars and the differences among them necessitate the use of appropriate cultivars in the industry. For this study, 36 quince cultivars and types from the Atatürk Central Horticultural Research Institute collection garden were used as materials. Through statistical analysis of the tests conducted, quince cultivars and types with high nutritional content were identified. This study aims to establish a profile for 36 genotypes and cultivars of quince fruits through physicochemical analyses.
Material and methods
Material
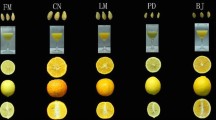
This study was conducted at the Atatürk Fruit Cultures Central Research Institute, located in Yalova, Türkiye, in 2022. The cultivars and types studied in this project were previously evaluated in the project titled “Quince Variety Selection” under the TAGEM/IY/96/06/01/003 project number in 1999 (Buyukyilmaz 1999). Quince fruits from the Quince-A clone tree, grafted at the full fruiting stage (15 years old), were harvested from the variety collection garden, which consists of 36 genotypes and cultivars at the institute. Routine cultural practices, such as irrigation and fertilization, were performed in the garden according to the prescribed schedule. The 36 quince cultivars and genotypes used as materials were as follows: Eşme-1, Eşme-2, Eşme-3, Eşme-4, Eşme-5, Eşme-6, Eşme-7, Eşme-8, Eşme-9, Eşme-10, Eşme-11, Eşme-12, Eşme-13, Eşme-14, Limon-1, Limon-2, 1/1, 10/3, 2/19, 22/1, 25/2, 27/1, 6/3, 7/2, Sapanca Eşme, Altin (Yalova), Ekmek (Keles), Viranyadevi, Eşme (Arifiye), Tekkeş, Bencikli, Gördes, Şekergevrek, Limon (Yalova), Demir-1, and Bardak (Fig. 2). During this research, fruits from three randomly selected trees of each cultivar were harvested, and those showing significantly deviant characteristics from the average were excluded, leaving 20 remaining fruits for the measurements and analyses described below. Quince fruits used as plant material are Eşme-1, Eşme-2, Eşme-3, Eşme-4, Eşme-5, Eşme-6, Eşme-7, Eşme-8, Eşme-9, Eşme-10 Eşme-11, Eşme -12, Eşme-13, Eşme-14, Limon-1, Limon-2, Sapanca Eşme, Altin (Yalova), Ekmek (Keles), Viranyadevi, Eşme (Arifiye), Tekkeş, Bencikli, Gördes, Şekergevrek, Limon (Yalova), Demir-1, Bardak as registered cultivars; 27/1, 22/1, 19/2, 10/3, 25/2, 6/3, 1/1, 7/2 were used as genotypes. The pomological characteristics of accessions were published elsewhere (Buyukyilmaz 1999). Some pomological measurements, observations and the origin of these cultivars and genotypes are given in Tables 6, 7 and 8 and at the end of this study. Quince fruits were harvested when they reached maturity, indicated by the green fruit skin starting to turn yellow and the presence of air inside the fruit that could be easily removed. Harvesting was performed before the onset of late autumn frosts.
Method
For these analyses, the optimum time for quince fruits was determined, and samples were taken. Fruits harvested at their maturity stage were brought to the Food Technology Laboratory. In the present study, various types and cultivars of quince were subjected to analyses, including Vitamin C, Minerals, Reducing sugars, Total Antioxidants (DPPH, FRAP, CUPRAC, ABTS), Total Phenols, Titratable Acidity, pH, and L* a* b* color analysis (Fig. 3). All experiments were carried out in triplicate, and results were determined by two parallel measurements.
The color analysis of the harvested fruits was determined using L* a* b* values with the Konica-Minolta CR 400 device. The CIE Minolta Chromameter (Minolta, CR-300, Japan) was used to measure L* (brightness), a* (+ red–green), and b* (yellow–blue) values at three different points on the product. Before the measurements, the Minolta Chromameter was calibrated with a standard white plate. L* represents the lightness or darkness of the color, where a higher value approaches 100, indicating maximum reflectance of 100% of the incident light on the surface. The a* value indicates the shift from green to red, while the b* value indicates the shift from yellow to blue. Increasingly negative or positive values imply a darkening of the color.
pH measurement
Fresh quince samples, 10 g each, were taken and homogenized in distilled water to make a 250 ml solution. The homogenized mixture was blended for 2 min and then measured using a glass electrode and a digital pH meter (CONSORT pH 514 model) (Anonim 2001).
Titratable acidity
From the harvested quince samples, 10 g were taken and homogenized with distilled water to make a 250 ml solution. After filtering the mixture, 25 ml of the filtered liquid was titrated with 0.1 N NaOH until it reached pH 8.1. The amount of NaOH used in the titration was determined to calculate the titratable acidity in terms of citric acid. The total acidity was calculated using the following equation (Anonim 2002):
Total Acidity (malic acid) % = Amount of NaOH used (ml) × N (0.1) × 0.067 × 100/sample quantity.
Determination of Total Soluble Solid Content (TSS): A digital refractometer (PAL-BX/ACID2, Atago Co. Ltd., Tokyo, Japan) was used to determine the soluble solid content of the samples (i.e., Brix values (%)) in fresh quince. The brix values of Quince were calculated using the equation.
Extraction of bioactive compounds
The extraction process for the analysis was performed according to (Thaipong et al. 2006). 3 g of the blended samples were taken and homogenized with 25 ml of pure methanol using a Silverson brand homogenizer for 2 min. The samples were then kept overnight at + 4 °C. The next day, the samples were centrifuged at 15,000 rpm for 20 min using an Eppendorf centrifuge. The upper phase was collected into amber vials using a Pasteur pipette and stored at − 20 °C until analysis. This extract was used in the analyses of total phenolic contents and antioxidant activity assays, which were performed using four different methods (2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric reducing antioxidant potential assay (FRAP) and Cupric reducing antioxidant capacity assay (CUPRAC), and ABTS (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)) to determine the bioactive properties of fresh quince fruit.
Antioxidant activity assays
For the determination of antioxidant activity, antioxidant analysis was performed using four different methods. The DPPH (2,2-diphenyl-1-picrylhydrazyl) assay procedure described by Thaipong et al. (2006) was used with slight modifications. DPPH stock solution was prepared as 24 mg/100 mL methanol (chromatographic grade) and stored at − 20 °C before use. The working solution was prepared by diluting the stock solution with methanol to the final absorbance of 1.20 ± 0.02 at 515 nm. For the calibration curve, Trolox® solution with a final concentration of less than 50 µM in a spectrophotometer cuvette was used. In the experiments, extract or standard (150 µL) and DPPH working solution (2850 µL) were mixed in a test tube, and the reaction was continued for 24 h in a dark environment. At the end of this period, the absorbance of coloured solutions was read at 515 nm, and results were expressed as µmol TE per 100 g. Extracts were diluted if their absorbance reading was over the linear range of the calibration curve.
Ferric Reducing Antioxidant Power (FRAP) method is employed to determine antioxidant activities od fresh quince fruit. The FRAP experiment relies on the ability of phenolic compounds to reduce Fe(+ 3) and Fe(+ 2) ions (Thaipong et al. 2006). Stock solution included acetate buffer is prepared by mixing 300 mM sodium acetate (3.1 g C2H3NaO2·3H2O) and 16 ml glacial acetic acid (C2H4O2), pH 3.6 and 10mMTPTZ (2,4,6-tripyridyls- triazine) solution in 40 mM HCl and 20 mM FeCl3_6H2O solution. Fresh working solution was prepared by mixing 25 mL of acetate buffer, 2.5 mL of TPTZ solution and 2.5 mL of FeCl3_6H2O solution and then kept at 37 °C before use. To analyze the samples, 150 µl of the extract is mixed with 2850 ml of the FRAP working solution and left in the dark for 30 min. The absorbance is then measured at 593 nm using a Hitachi spectrophotometer. Evaluation was performed using a standard curve prepared with Trolox® in the concentration range of 25–800 µM, and the results were expressed as µM TE per 100 g of sample. Extracts were diluted if their absorbance reading was over the linear range of the calibration curve.
ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) solution: 7.4 mM ABTS and 2.6 mM potassium persulfate are mixed. The working solution was prepared by mixing equal volumes of these two stock solutions. The mixture was kept in the dark for 12 h until the absorbance at 734 nm reaches 1.1 ± 0.02. Then, 1 ml of the ABTS working solution was diluted with 60 ml of methanol. A fresh working solution was prepared for each analysis. 150 ml of the sample extract was mixed with 2850 ml of ABTS solution and left in the dark for two hours. Afterward, the absorbance was measured at 734 nm using a spectrophotometer against the blank reagent. Results were expressed as µM Trolox equivalent (TE) per 100 g fresh weight.
In CUPRAC (Copper Reducing Antioxidant Capacity) method, an antioxidant solution is mixed with a copper (II) chloride solution, a neocuproine alcoholic solution and an ammonium aqueous buffer at pH 7. Subsequently, the absorbances of mixtures are determined at 450 nm after 60 min (Apak et al. 2004). The antioxidant activities of quince fruit solution (10 mM), neocuproine solution (Nc) of 7.5 mM and ammonium acetate (NH4 Ac) buffer (pH 7) solutions were mixed in a test tube, and extract (or standard) solution (× mL) and H2O ((1.1 − x) mL) were added to the test tube to make the final volume 4.1 mL. Test tubes were screw-capped and left for an hour at room temperature, and then the absorbance at 450 nm was recorded against a reagent blank. The calibration curve was constructed with trolox as absorbance against each concentration. The molar absorptivity of the CUPRAC method for each antioxidant was found from the slope of the calibration line concerned, and the antioxidant activity values of fresh quince fruits were expressed as mg Trolox equivalents (TE) per 100 g.
Total phenolic content (TP)
The total phenolic contents of samples were determined by the Folin-Ciocalteu (FC) method (Singleton and Rossi 1965). Analysis was conducted using the Folin Ciocalteu method. 150 µL of the extract was mixed with 2400 µL of water and 150 µL of Folin, then vortexed. Afterward, 300 µL of Na2CO3 was added, and the mixture was kept in the dark for two hours. The same procedure was run to prepare the blank using 150 µL deionized water instead of extract. Absorbance values were read at 750 nm using a spectrophotometer against the blank reagent. Various concentrations of gallic acid solutions (50, 100, 200, 300, 400 and 500 mg/L) were used to plot a calibration curve. Results were expressed as mg gallic acid equivalent (GAE) per 100 g fresh weight.
L-ascorbic acid (vitamin C) determination
L-ascorbic acid analysis was performed using Reflectoquant® Ascorbic Acid Test analysis kits (Orikasa et al. 2014). Approximately 5 g of fruit flesh was weighed and homogenized in 50 ml of 1% oxalic acid solution (14 g/l). Homogenized samples were centrifuged (7500 rpm for 10 min). The supernatant solution from the sample was used for analysis. The results were recorded as mg/l.
Fresh fruit mineral analyses
The collected fruit samples were first washed in tap water, then sequentially washed with 0.1 N HCl and deionized water to remove impurities and contaminants. After that, they were allowed to air-dry on filter papers. Subsequently, the dried samples were transferred to paper bags and further dried in a vacuum drying cabinet at 70 °C until a constant weight was achieved. The dried samples were then ground to a fine powder using a mill with a 0.5 mm mesh diameter, preparing them for analysis (Kacar and Inal 2008).
The total nitrogen content of the samples was determined using the Kjeldahl method (Kacar and Inal 2008). For the analysis of phosphorus, potassium, calcium, magnesium, iron, manganese, zinc, and copper, 0.5 g of sample was taken and mixed with 10 ml of HNO3. The mixture was subjected to microwave digestion at high temperature. The resulting solutions were then filtered through blue ribbon filter paper into 50 ml volumetric flasks and made up to volume with deionized water. The elemental concentrations in the filtered solutions were measured using an ICP-OES device (Kacar and Inal 2008). To ensure the accuracy of the analyses, NIST brand reference apple leaves (1515) and peach leaves (1547) were used together.
Experimental design and statistical analysis
The experiment was conducted according to a randomized complete block design. All experiments were performed with three replications and evaluated using analysis of variance (ANOVA). LSD test was performed to determine whether there were significant differences among factors. Correlations between data were calculated using Pearson correlation coefficient with a significance level of 0.05 (p = 0.05).
Result and discussion
The measurement and analysis results for various quince cultivars and types examined in this study are presented in Table 1, 2, 3, 4 and 5.
Color values
The L*, a* and b* values were used for measuring fruit skin color. It has been reported that skin color is an important quality criterion for quinces (Çalhan and Koyuncu 2018; Karaçali 2012; Maddi et al. 1996). In quinces, as in other pome fruits, the loss of green color in the skin occurs due to the degradation of chlorophyll during ripening. As ripening begins, the yellow color pigment in the skin becomes more pronounced. This study found statistically significant effects (P < 0.005) of all considered cultivars and types on fruit skin color values (Table 1). When individually examining the L*, a*, and b* color values of the fruits, it was determined that the highest L* brightness value was observed in the Eşme-7 (82.41), Eşme-13 (81.81), and 6/3 (81.93) samples. In contrast, the lowest L* brightness was recorded in the Sapanca Eşme (73.76). The highest a* value was found in the Sapanca Eşme cultivar (0.30) and the lowest value was detected in the Altin Yalova (− 5.02) cultivar. Regarding the a* color value, it was observed that as the negative values increased, the colors tended towards green, while as the values approached positive, they were closer to yellow. As for the b* color value, the highest value was observed in Tekkeş (59.77) and the lowest in Eşme-13 (25.43) and 6/3 (25.65). The study of Nanos et al. (2015) reported that in quinces, with the progress of ripening, the yellow color becomes more dominant, resulting in an increase in b* values, while a* values decrease (Gunes and Dumanoglu 2005). According to Najman et al. (2023), who looked at fresh quinces, the greatest L* color value was 82.11 and the highest b* color value was 58.76, which is similar with the results of our investigation. L* values were between 76.0 and 82.2, a* values between − 1.9 and 5.5, and b* values between 21.4 and 30.0 in several quince cultivars and fruits gathered at various times (Cascales and García 2021). This study demonstrates the consistency of the L*a*b* color values found in the 36 different cultivars of quince fruits. It should be mentioned that the hues of fruits differ depending on the time of year they are harvested, the conditions under which they were grown, the local environment, and the cultivars used.
Total phenolic substances, total antioxidants
Thirty-six (36) quince genotypes and cultivars were analyzed, and the differences between the results of total antioxidant and total phenolic compounds were significant (P < 0.005). Quince fruit is rich in phenolic compounds that contribute positively to health, such as antioxidant, anti-inflammatory, anti-allergic, anti-bacterial and anti-carcinogenic capacities. Total phenolic content (Table 2) values of quince cultivars and types examined in this study were analyzed in the range of highest 167,40 mg/100 g GAE (Eşme-9) to lowest 65,31 mg/100 g GAE (27/1). Phenolic compounds play a protective role against heart diseases and are very important for health because they show high antioxidant activity. In the research, the total amount of phenolic substances detected in quince cultivars was between 65.73 mg GAE/100 g and the highest 68.13 mg GAE/100 g were found, and the amounts we analyzed were in this range (Karadeni̇z et al. 2005; Fattouch et al. 2007; Rasheed et al. 2018). The results of the phenolic contents of recent research are almost identical to the findings reported by us.
The total antioxidant activity of quince was analyzed by four different methods: CUPRAC, DPPH, ABTS and FRAP. The results are given in Table 2 with μmol /100 g Trolox equivalent fw value. Antioxidant activity values: highest 474,88 µmol/100gTE (Eşme-4)-lowest 33,12 µmol/100gTE (27/1) by CUPRAC method; highest 15,861,82 µmol/100gTE (Eşme-4)-lowest 6016,365 µmol/100gTE (Limon-1) by DPPH method; The highest 7296,67 µmol/100gTE (Eşme-4)- the lowest 1393,33 µmol/100gTE (Lemon-1) by ABTS method; the highest 2072,80 µmol/100gTE (Eşme7)- the lowest 1132,80 µmol/100gTE (22/1) by FRAP method. Thus, the effect of cultivars and types on antioxidant activity was determined. It was determined that genotype 25/2 showed the highest antioxidant activity according to DPPH, ABTS and FRAP treatments. It was determined that the 25/2 genotype contained the highest total phenolic matter with high antioxidant activity according to these three methods. Again, while the highest amount of phenolic matter was analyzed in the Limon-2 cultivar, DPPH and ABTS antioxidant activities were the highest. Given that phenolic substances have a considerable effect on antioxidant activity, it is usual to determine that the cultivar with the highest antioxidant activity contains the highest phenol content (Bandonienė and Murkovic 2002).
Wojdyło et al. (2013) reported that the antioxidant activity measured in 22 quince cultivars and genotypes ranged from 2.4 to 0.9 for ABTS- + , 2.5 to 0.9 for DPPH and 1.5–0.4 Trolox equivalent µmol/g dw TEAC for FRAP. It was reported that the differences in antioxidant activity results varied according to both cultivar differences and phenolic matter content. In the analysis of four different quince cultivars grown in Spain and fruits harvested in different periods, total phenolic matter content was 120–229 mg/100 g; antioxidant activity FRAP was 734–1258 µmol/100 g TEAC. The results of our study are consistent with these results and it was determined that there were differences according to the cultivars (Cascales and García 2021). Considering the evaluation of these preliminary results and the raw material needs of the quince industry, future research will be appropriate to address traits, such as the relationship between cultivars, origin, harvest time, ripeness index, climatic conditions and soil (Leonel et al. 2016; İzli and Yildiz 2021). Quince fruit and its products have been identified as a potential source of biologically active compounds in many investigations (Rubinskienė et al. 2014; İzli and Yildiz 2021; Cascales and García 2021; Najman et al. 2023).
Quince fruit is a rich source of polyphenols with an average total phenolic content (TPC) of 96.82 mg AGE/g FM (mg Gallic Acid Equivalent/g Fresh Matter) ranging from 0.4 to 1.8 mg AGE/g recorded for apple fruit. FM) reported by (Colin-Henrion 2008). In our study, the amount of total phenolic matter was different among the cultivars, but as determined in previous research, many close values and higher amounts were determined. One of the important points to be revealed is that each bioactive compound contributes to the amount of antioxidant activity. When the contents of polyphenolic compounds were correlated separately with CUPRAC, ABTS, DPPH, FRAP and even L-ascorbic acid, we could see that the phenolic content is highly correlated with all these assays.
It was determined that the total amount of phenolic substances detected in quince fruit was higher than in apple, grape, or pear. Fruits with high polyphenol content are very important for human health and for this reason, the World Health Organization recommends fruit consumption. It is recommended to consume fruit once a day, or 400 g, to reduce the risk of chronic diseases. Therefore, the consumption of quince fruit is beneficial for health and nutrition.
The study results have reported significant differences in phenolic content and antioxidant activity among various types and cultivars of fruits, which could be attributed to variations in plant genotype, soil conditions, and environmental factors, as previously reported. The quince fruit is one of the potential sources of natural phenolic antioxidants and is known for its health benefits due to its richness in organic acids, amino acids, and polyphenols (Silva et al. 2002, 2005; Al-Zughbi and Krayem 2022). Quince stands out as a good and cost-effective natural source of powerful antioxidants, such as phenolic acids and flavonoids (Silva et al. 2005, 2008; Oliveira et al. 2007). Particularly noteworthy is its high level of phytochemicals and low amount of amygdalin (a molecule that can lead to cyanide poisoning); therefore, including this fruit in food technology and daily dietary recipes as a functional food could be highly beneficial (Byczkiewicz et al. 2021; Ionică and Corbu 2022).
Vitamin C, reducing sugar content
After conducting analyses on 36 cultivars of quince types, significant differences were found among the cultivars (P < 0.005), according to the statistical evaluation. Vitamin C is highly important in terms of providing valuable contributions to antioxidant capacity and the health benefits of fruits. As a result of the analyses conducted on fruits, Table 3 presents the Vitamin C values ranging from the highest 25.56 mg/100 g (Eşme 14) to the lowest 8.67 mg/100 g (Eşme-12; 22/1). In their study, Rasheed et al. (2018) determined the average C vitamin content of different quince cultivars obtained from various sources to be 15.46 mg/100 g. Our obtained C vitamin results were within this range and were consistent with theirs. In another study that examined 13 different quince cultivars and 5 genotypes, L-ascorbic acid activity was reported to vary between 5.86 and 26.42 mg/100 g (Wojdyło et al. 2013). These findings align with our study, as they also fall within the same range. Additionally, Sharma et al. (2011) conducted analyses on quince samples obtained from edible parts and determined the average C vitamin content to be 16.8 mg/100 g.
When compared with the results of our study, values close to this range were analyzed. It is thought that the different values are influenced by the characteristics of the cultivar and the characteristics of the place of cultivation. Ascorbic acid concentration can be affected by many factors, such as climatic conditions, cultivar, storage conditions, processing technologies and harvesting practices (Kader 1988; Cemeroğlu 2009). Therefore, the variation in ascorbic acid among the quince cultivars and types used in this study may be affected by the above factors. Rop et al. 2011, analyzed the L-ascorbic acid content in 22 different genotypes and cultivars; the L-ascorbic acid content in these 22 quince fruits ranged from 41.1 to 79.3 mg/100 g fw. According to Szeto et al. (2002), the maximum value of L-ascorbic acid in quince fruit is comparable to apple, while its content in pear does not exceed half of this value. 55.56 mg/100 g. The range of ascorbic acid content (mg/100 g) in quince fruit compared to other fruits was higher than nectarine (4.8–13.2), peach (3.6–12.6), litchi (13.8) and plum (2.5–10.2) but lower than mango (60.5) and papaya (92.9). The USDA reference value for vitamin C is 15 mg/100 g. This value varies according to the quince cultivar but ranges from 50 to 80 mg/100 g in the Czech cultivar and increases to about 80 mg/dl in the 'Muškatová' cultivar (Rop et al. 2011). In our study, it was observed that vitamin C values varied between cultivars and types as in the literature.
TSS, PH, TEA
The TEA, pH and TSS values of quince types and cultivars were measured. The differences between cultivars and types in TEA, pH and TSS values were statistically significant (P < 0.005) (Table 4). The amount of TSS is an important component in the definition of juiciness and flavor balance and is often used to aid in the technological identification of fruits (Cemeroğlu 2009; Guneş and Dumanoglu 2005). The nutrient composition of fruits, especially the TSS, provides information to consumers in defining the nutritional properties of fruits. In the present study, it was analyzed that the Brix value of TSS in quince cultivars and types was between the highest 19.20% (Altn-Yalova) and the lowest 9.0% (Limon-1). Rasheed et. al. 2018 determined between 14.17% and 13.94% and Mir et al. (2016) determined between 13.9% and 14.26%. It was determined that the results of this study were compatible with previous research. The Total Organic Acid (TEA) value of the quince fruit has been calculated regarding malic acid content, as it is the most abundant organic acid in the Rosaceae family fruit species. In this study, the TEA value was measured between the highest 1.23% (6/3) and the lowest 0.55% (Eşme-6) in quince cultivars and types. The pH value of quince cultivars ranged from the highest 3.86 (Altin Yalova) to the lowest 3.17 (10/3). Rasheed et al. (2018) determined pH (3.43), total soluble solids (14.22°Brix), acidity (1.25%) as average in their analysis of quinces collected from different places in their study. The results found in our study were consistent with this study. Again, Ali et al. (2015) and Sharma et al. (2011) determined that the pH value varied between 3.3 and 3.36, which is almost consistent with the findings reported by Ali. The findings reported by Sharma and Ali regarding acidity indicated that acidity levels ranged from 1.05 to 1.31% and from 1.2 to 2.0%. In our study, the acidity values we found fell within this range and were consistent among different cultivars. These variations in acidity among the quinces in our study could be attributed to the influence of fruit cultivars and environmental conditions. In a study evaluating the characteristics of fresh quinces harvested from different cultivars and at different times (Cascales and García 2021), it was determined that quinces harvested at different times and from different origins exhibited variations in their contents. This aspect should be considered as quinces serve as a raw material for the quince industry. In their study, Cascales and García (2021) found that quinces from four different cultivars, harvested at two different periods, had pH values ranging from 3.6 to 3.8; TSS values between 13.4 and 19.5%; and titratable acidity between 0.5 and 0.9 g/100 g. These results were within the same range as the values obtained in our study. Therefore, our study's results were consistent with the literature.
Amounts of mineral matter
The evaluation of many products in our diet as useful foods depends on the concentration of mineral substances, so it is very important to determine the amount of mineral substances since it will affect the nutritional properties of the food. In this study, the differences between the mineral content values of quince types and cultivars were significant (P < 0.005). In Table 5, the highest N value is 434,81 mg/100 g (7/2) and the lowest is 263,06 mg/100 g (Gördes); Fe value is the highest 3,63 (1/1)—the lowest 2,03 mg/100 g (Eşme (arifiye)); Mn value is the highest 0,18 (1/1)- lowest 0,09 (Ekmek (Keles)) mg/100 g; Zn value is the highest 1,80 (1/1)- lowest 0,93 mg/100 g (Eşme -14); Cu value: highest 0,64 (1/1)- lowest 0,37 mg/100 g (Eşme-8); K values were highest at 975,12 (25/2)- lowest 566,19 mg/100 g (Eşme-14); Ca values were highest at 90,90 (1/1)- lowest at 46,15 mg/100 g (25/2); Mg values were highest at 56,47 (22/1)- lowest at 35,64 mg/100 g (Şekergevrek, Limon Yalova); and p-values were highest at 110,88 (27/1)- lowest at 62,58 mg/100 g (Eşme-14). The highest value of ash content was 3.08% (27/1), and the lowest value was 1.24% (Eşme-5). Rasheed et al. (2018) determined the value of ash content between 0.62% and 0.42% in their study. It has been reported that differences in ash content may be due to differences in fruit cultivars as well as plant genotype, soil and environmental factors (Leonel et al. 2016). Silva et al. (2005), Fattouch et al. (2007), Najman et al. (2023) stated that fruits, such as quince, are a source of valuable minerals, such as potassium, calcium, magnesium and are a valuable fruit for health. Quince, rich in minerals, such as sodium, calcium, potassium and phosphorus, has been reported to be a richer source of mineral elements than apples (almost twice as much as quince) (Rop et al. 2011). In addition, the properties of quince pulp (pH, moisture, ascorbic acid, total phenols) were reported by Rasheed et al. (2018).
The taste of the quince fruit depends on many factors, such as the cultivar and degree of ripeness or the harvest date of the fruit; but the fruit is usually hard, sour and astringent (Silva et al. 2002; Szychowski et al. 2014). However, this affects the sensory properties of fruits and the content of organic acids, sugars, phenolic component, aromatic compounds and pectins (Silva et al. 2006; Szychowski et al. 2014). The physicochemical properties of fruit raw materials determine their intended use, that is, their suitability for direct consumption, their technological suitability and their processing potential, which affects the quality of the resulting products (Mir et al. 2016; Najman et al. 2023). The physicochemical properties of fruit raw materials determine their intended use, that is, their suitability for direct consumption, their technological suitability and their processing potential, which affects the quality of the resulting products (Najman et al. 2023) reported that the content in phytochemical compounds, beside the cultivar, may be affected by many factors, also climatic conditions, the agrochemical composition of the soil and the maturity index (Blanda et al. 2020; Bystrická et al. 2017). Therefore, in our study, it is crucial to determine the physicochemical properties (total phenolic component, reducing sugar, titratable acidity, total soluble solids, macro and micro element contents) of quince fruits.
Topcu et al. (2015) evaluated the genetic similarity and relationships in 40 quince cultivars for quince fruit species with great morphological variability. It was observed that 36 genotypes and cultivars considered in our study were examined regarding genetic similarity in this study. According to the study conducted by Topcu et al., different genotypes grown under the name of Eşme show different rates of similarity at the genetic level, indicating that they may show differences in the analyzed traits as in our study. In this study, where genetic traits were determined, 'Sapanca-Eşme' and 'Eşme-3' were the most closely related cultivars with 95% similarity. In cluster I, the closest similarity in this cluster was surrounded by 'Esme-7' and 'Esme-9' accessions, while 'Esme-12', 'Esme-1,' 'Esme Arifiye' and 'Ekmek Yalova' were in the same group, respectively. Cluster II included only the genotype '10/3'. Cluster III also formed two sub-clusters. In the first subculture, '6/3', '7–2,' 'Viranyadevi,' 'Limon-2,' 'Esme-6,' 'Esme-11,' 'Esme-5,' 'Sekergevrek' and 'Bencikli' cultivars and genotypes were included. In the second subculture, Esme-10,' 'Gordes, 27/1,' 'Tekkes,' 'Esme-14,' 'Esme-8,' 'Limon Yalova,' 'Esme-13,' '19/2,' 'Limon-1’, ‘Esme-4,’ 'Esme-3,' 'Sapanca Esme,' 'Esme-2,' 'Ekmek Keles,' 'Demir-1,' 'Bardak' and 'Altin Yalova' were found. For example, in Cluster I, the most closely related cultivars 'Esme-7' and 'Esme-9' have different tree and fruit characteristics. 'Esme-7' has a light yellow fruit skin color when ripe and 'Esme-9' has a greenish yellow skin color. 'Esme-7' has an oval and necked fruit shape while 'Esme-9' has oval fruits with less neck. Büyükyilmaz, 1999 determined in his study that fruit productivity is different even though they are genetically similar.
According to this study, although genotypes and cultivars showing genetic similarity were included in the clusters mentioned above and showed genetic similarity, regarding the components examined in our study, it was observed that although the amounts of some mineral substances and physicochemical components gave close values, they were not in the same group statistically. The high level of genetic diversity among genotypes and cultivars indicates that they may not synthesize similar products because they are molecularly similar to each other.
Conclusion
Despite significant differences in the biochemical composition among the 36 quince types and cultivars obtained from the collection garden of Atatürk Horticultural Research Institute, they all contain sufficient nutrients, including phenolic compounds and ascorbic acid, which contribute to antioxidant activity. Quince fruit's high content of health-promoting values makes it important for a wide range of applications in both the food and pharmaceutical industries. These variations among genotypes and cultivars are believed to be due to the inherent characteristics of each fruit. The results we have obtained suggest that quince can be used in various products, such as jams, jellies, and puddings, and can also be utilized as a garnish or breakfast item, similar to pears. However, the actual benefits of quince lie in its consumption with the peel and flesh, as it is rich in beneficial nutrients like vitamins, minerals, phenolic compounds and antioxidants, making it a valuable addition to daily dietary intake.
Due to Türkiye's significant position regarding quince production and foreign trade at the international level, there is a lack of literature, especially concerning its economic aspects. Based on this, the present study aims to determine the essential nutritional content of 36 cultivars and types of quince produced in Türkiye, which are particularly important for human health. Additionally, a profile will be created for the quince cultivars and types with high nutritional content, identifying their cultivation areas and promoting their widespread cultivation.
This study reveals the properties of quince that have a positive impact on human health. Therefore, we should emphasize the importance of the consumption of the fruit to benefit from the total bioactive compounds. The results obtained draw attention to the need to take into account the synergistic effects of quince polyphenols and clearly show that quince is characterized by high biological activity, especially regarding phenolic substances.
These compounds are present in significant quantities in quince, making it a very valuable fruit from a chemical point of view. Since quince is an important raw material in the processing of various foods and a source of biologically active compounds, it should be grown in larger areas and used both fresh and processed. Among the cultivars examined in this study, Eşme-9 was the cultivar with the highest total phenolic content and antioxidant activity. In the same way, it was determined that genotype 25/2 stood out in terms of total phenol and antioxidant activity. Quince has an important amount of potassium. Thus, in this study, it was determined that genotypes 25/2 and 6/3 contained the highest amount of potassium. In the same way, the amount of phosphorus was determined to be high in these genotypes (25/2 and 6/3). It was also observed that genotype 1/1 contained higher amounts of Fe, Mn, Zn and Cu mineral substances. When all these are evaluated, it will benefit human health to grow and consume Eşme -9 cultivars and 25/2, 6/3 and 1/1 genotypes in larger areas.
The identification of constituents for quince fruit can provide a starting point for the development of functional products that are health-promoting, high in value and have a wide range of applications in both the food and pharmaceutical industries by enabling producers to obtain high quality fruit and by processing quince fruit in various ways.
References
Ali N, Abbas Y, Ali A et al (2015) Physio-chemical nutritional and sensory evaluation of local quince fruit of Nomal Village, Gilgit-Baltistan, Pakistan. Int J Nutr Food Sci 4:600–608. https://doi.org/10.11648/j.ijnfs.20150406.11
Al-Zughbi I, Krayem M (2022) Quince fruit cydonia oblonga mill nutritional composition, antioxidative properties, health benefits and consumers preferences towards some industrial quince products: a review. Food Chem 393:133362. https://doi.org/10.1016/j.foodchem.2022.133362
Anonim (2001) TS 1728/ ISO 1842/Subat 2001, Meyve ve Sebze Ürünleri pH Tayini Standardi, Türk Standartlari Enstitüsü Yayini, Ankara.
Anonim (2002) TSE 1125 ISO 750/Mart 2002, Meyve ve Sebze Ürünleri Titre Edilebilir Asitlik Tayini Standardi. Türk Standartlari Enstitüsü Yayini, Ankara
Antoniewska A, Rutkowska J, Pineda MM (2019) Antioxidative, sensory and volatile profiles of cookies enriched with freeze-dried Japanese quince (Chaenomeles japonica) fruits. Food Chem 286:376–387. https://doi.org/10.1016/j.foodchem.2019.02.029
Apak R, Güçlü K, Özyürek M, Karademir SE (2004) Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J Agric Food Chem 52:7970–7981. https://doi.org/10.1021/jf048741x
Bandonienė D, Murkovic M (2002) On-Line HPLC-DPPH screening method for evaluation of radical scavenging phenols extracted from apples (Malus domestica L.). J Agric Food Chem 50:2482–2487. https://doi.org/10.1021/jf011475s
Blanda G, Rodríguez-Roque MJ, Comandini P, Flores- Córdoba MA, Salas-Salazar NA, Cruz-Álvarez O, Soto-Caballero MC (2020) Phenolic profile and physicochemical characterization of quince (Cydonia oblonga Mill) fruits at different maturity index. Notul Bot Hortic Agrobot 48:2306–2315
Buyukyilmaz M (1999) Quince cultivar selection. Ataturk Horticultural Central Research Institute, Türkiye. Publication No:125
Bystrická J, Musilová J, Lichtnerová H, Lenková M, Kovarovič J, Chalas M (2017) The content of total polyphenols, ascorbic acid and antioxidant activity in selected varieties of quince (Cydonia oblonga Mill.). Potravinarstvo: Slovak Journal of Food Sciences, 11(1): 77–81.
Byczkiewicz S, Szwajgier D, Cisowska JK et al (2021) Comparative examination of bioactive phytochemicals in quince (Chaenomeles) fruits and their in vitro antioxidant activity. Emir J Food Agric 33:293–302. https://doi.org/10.9755/ejfa.2021.v33.i4.2667
Çalhan Ö, Koyuncu MA (2018) Eşme Ayva (Cydonia oblonga Mill.) Çeşidinde Optimum Derim Tarihini Belirlemek İçin Uygun Kriterlerin Seçimi. Yuzuncu Il Univ J Agric Sci 28:215–225. https://doi.org/10.29133/yyutbd.397084
Cascales EV, García JMR (2021) Assessment of the characteristics of the fresh quince (Cydonia oblonga Miller) from different geographical origin and year of harvesting. Emir J Food Agric. https://doi.org/10.9755/ejfa.2021.v33.i6.2715
Cemeroğlu B (2009), Meyve ve Sebze işleme Teknolojisi, Gida Teknolojisi Yayinlari No: 39, 2. Cilt Syf. p 479–504
Colin-Henrion M (2008) De la pomme à la pomme transformée : impact du procédé sur deux composés d?intérêt nutritionnel Caractérisation physique et sensorielle des produits transformés. Phd thesis, Université d’Angers
Curi PN, Coutinho G, Matos M et al (2018) Characterization and marmelade processing potential of quince cultivars cultivated in tropical regions. Rev Bras Frutic 40:1–7. https://doi.org/10.1590/0100-29452018986
Fattouch S, Caboni P, Coroneo V et al (2007) Antimicrobial activity of Tunisian quince (Cydonia oblonga Miller) pulp and peel polyphenolic extracts. J Agric Food Chem 55:963–969. https://doi.org/10.1021/jf062614e
Gunes NT, Dumanoglu H (2005) Some fruit attributes of quince (Cydonia oblonga) based on genotypes during the pre-harvest period. N Z J Crop Hortic Sci 33:211–217. https://doi.org/10.1080/01140671.2005.9514352
Ionica ME, Corbu AR (2022) The evolution of some chemical compounds of quince (Cydonia oblonga MILL.) during growth and ripening. South West J Hortic, Biol Environ 13(1):59–66
İzli G, Yildiz G (2021) Evaluation of high intensity ultrasound pre-treatment effects on the physical properties and bioactive compounds of convective dried quince samples. Int J Fruit Sci 21:645–656. https://doi.org/10.1080/15538362.2021.1918604
Kacar B, Inal A (2008) Plant analysis. Nobel Pres 1241:891
Kader AA (1988) Influence of preharvest and postharvest environment on nutritional composition of fruits and vegetables. J Hortic Hum Health 4:18–32
Karaçali İ (2012) Bahçe Ürünlerinin Muhafaza ve Pazarlanmasi, Ege Üniversitesi Ziraat Fakültesi Yayinlari, No: 494
Karadeniz F, Burdurlu HS, Koca N, Soyer Y (2005) Antioxidant activity of selected fruits and vegetables grown in Turkey. Turk J Agric for 29:297–303
Leonel M, Leonel S, Tecchio MA et al (2016) Characteristics of quince fruits cultivars’ (Cydonia oblonga Mill.) grown in Brazil. Aust J Crop Sci 10:711–716. https://doi.org/10.3316/informit.203321426595075
Maddi SR (1996) Personality theories: a comparative analysis. Thomson Brooks/Cole Publishing Co, California
Magalhćes A, Silva B, Pereira J, Paula B, Andrade P, Valentćo P, Carvalho M (2009) Protective effect of quince (Cydonia oblonga Miller) fruit against oxidative hemolysis of human erythrocytes. Food Chem Toxicol 47:1372–1377
Mir SA, Wani SM, Wani TA et al (2016) Comparative evaluation of the proximate composition and antioxidant properties of processed products of quince (Cydonia oblonga Miller). Int Food Res J 23:816–821
Najman K, Adrian S, Sadowska A et al (2023) Changes in physicochemical and bioactive properties of quince (Cydonia oblonga Mill.) and its products. Molecules 28:3066. https://doi.org/10.3390/molecules28073066
Nanos GD, Mpezou A, Georgoudaki T (2015) Effects of 1-MCP and storage temperature on quince fruit quality. Acta Hortic. https://doi.org/10.17660/ActaHortic.2015.1079.59
Okatan V, Aşkin MA, Polat M, Bulduk İ, Çolak AM, Güçlü SF, Caruso G (2022) Farkli kültür sistemlerinde yetiştirilen çilek çeşitlerinde melatonin dozunun meyve verimi, kalitesi ve antioksidanlar üzerine etkileri. Tarim 13(1):71
Oliveira AP, Pereira JA, Andrade PB et al (2007) Phenolic profile of Cydonia oblonga Miller leaves. J Agric Food Chem 55:7926–7930. https://doi.org/10.1021/jf0711237
Orikasa T, Koide S, Okamoto S et al (2014) Impacts of hot air and vacuum drying on the quality attributes of kiwifruit slices. J Food Eng 125:51–58. https://doi.org/10.1016/j.jfoodeng.2013.10.027
Özüdoğru T (2022) Agricultural products market-Quince. Agricultural Economics and Policy Development Institute
Pawlak-Lemańska K, Włodarska K, Przybylska M, Tyrakowska B (2018) Wpływ dodatku pigwy i pigwowca na właściwości prozdrowotne i sensoryczne soku jabłkowego. Nauka Przyr Technol 12:3. https://doi.org/10.17306/J.NPT.2018.1.3
Rasheed M, Hussain I, Rafiq S et al (2018) Chemical composition and antioxidant activity of quince fruit pulp collected from different locations. Int J Food Prop 21:2320–2327. https://doi.org/10.1080/10942912.2018.1514631
Rop O, Balík J, Řezníček V et al (2011) Chemical characteristics of fruits of some selected quince (Cydonia oblonga Mill.) cultivars. Czech J Food Sci 29:65–73. https://doi.org/10.17221/212/2009-CJFS
Rubinskienė M, Viškelis P, Viškelis J et al (2014) Biochemical composition and antioxidant activity of Japanese quince (Chaenomeles japonica) fruit, their syrup and candied fruit slices. Lith Res Cent Agricu Aleksandras Stulginskis Univ 33:45–52
Sharma R, Joshi VK, Rana JC (2011) Nutritional composition and processed products of quince (Cydonia oblonga Mill.). IJNPR 2:354–357
Silva BM, Andrade PB, Ferreres F et al (2002) Phenolic profile of quince fruit (Cydonia oblonga Miller) (pulp and peel). J Agric Food Chem 50:4615–4618. https://doi.org/10.1021/jf0203139
Silva BM, Andrade PB, Martins RC et al (2005) Quince (Cydonia oblonga Miller) fruit characterization using principal component analysis. J Agric Food Chem 53:111–122. https://doi.org/10.1021/jf040321k
Silva BM, Andrade PB, Martins RC, Seabra RM, Ferreira MA (2006) Principal component analysis as tool of characterization of quince (Cydonia oblonga Miller) jam. Food Chem 94(4): 504–512.
Silva BM, Valentão P, Seabra RM, Andrade PB (2008) Quince (Cydonia Oblonga Miller): an interesting source of bioactive compounds. Food chemistry research developments, 1st edn. Nova Science Publishers, Porto/Portugal, pp 243–266
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158. https://doi.org/10.5344/ajev.1965.16.3.144
Stojanović BT, Mitić SS, Stojanović GS et al (2017) Phenolic profiles and metal ions analyses of pulp and peel of fruits and seeds of quince (Cydonia oblonga Mill.). Food Chem 232:466–475. https://doi.org/10.1016/j.foodchem.2017.04.041
Szeto YT, Tomlinson B, Benzie IFF (2002) Total antioxidant and ascorbic acid content of fresh fruits and vegetables: implications for dietary planning and food preservation. Br J Nutr 87:55–59. https://doi.org/10.1079/BJN2001483
Szychowski PJ, Munera-Picazo S, Szumny A, Ángel A, Carbonell-Barrachina ÁA, Hernández F (2014) Quality parameters, bio-compounds, antioxidant activity and sensory attributes of Spanish quinces (Cydonia oblonga Miller). Sci Hortic 165:163–170
Thaipong K, Boonprakob U, Crosby K et al (2006) Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compos Anal 19:669–675. https://doi.org/10.1016/j.jfca.2006.01.003
Topcu H, Kafkas S, Dogan A, Akcay ME, Ercisli S (2015) Genetic relatedness among quince (Cydonia Oblonga Miller) accessions from turkey using amplified fragment length polymorphisms. J Appl Bot Food Qual 88:197–201
Turkish Statistical Institute (2022) TUİK newsletter, 31 March, number: 45505
Ünal N, Okatan ve V (2023) Kuraklik stresi uygulamasinin çilek çeşitlerinin fitokimyasal içerikleri üzerine etkileri. Sci Hortic 316:112013
Wojdyło A, Oszmiański J, Bielicki P (2013) Polyphenolic Composition, antioxidant activity, and polyphenol oxidase (PPO) activity of quince (Cydonia oblonga Miller) varieties. J Agric Food Chem 61:2762–2772. https://doi.org/10.1021/jf304969b
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This study was supported by the project numbered TAGEM/HSGYAD/E/18/A3/P3/378.
Author information
Authors and Affiliations
Contributions
ZG, She took part in the construction of this study, analysis and writing of the results.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Pomological measurements, observations and origin of quince fruits are given Tables 6, 7 and 8 (Buyukyilmaz 1999).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Göksel, Z. Comparison of physicochemical and bioactive contents of 36 different quince cultivars and genotypes. Genet Resour Crop Evol (2024). https://doi.org/10.1007/s10722-024-01866-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10722-024-01866-y