Abstract
The sweetpotato whitefly, Bemisia tabaci Genn., is a major pest of tomato (Solanum lycopersicum) and other crops throughout the tropics and subtropics. The objectives of this study were to characterize 255 accessions of S. galapagense, S. cheesmaniae and S. pimpinellifolium for trichome types, and to evaluate selected accessions with high densities of glandular trichomes for resistance to whitefly. Twenty-two accessions classified as either sparse or abundant for type IV trichomes were selected and evaluated for numbers of adults, eggs, nymphs, and puparium of whitefly in choice bioassays, for adult mortality and egg numbers in no-choice bioassays, and for densities of type I, IV, V, and VI trichomes. The highest whitefly resistance was detected in S. galapagense accessions VI063177 and VI037239 based on choice and no-choice bioassays. In addition, we found high levels of whitefly resistance in S. cheesmaniae accession VI037240 based on the choice bioassay and in S. pimpinellifolium accession VI030462 based on the no-choice bioassay. Whitefly resistance in VI037240 and VI030462 is noteworthy because these species are closely related to cultivated tomato and introgression of whitefly resistance should be relatively straightforward. High densities of type IV trichomes and low densities of type V trichomes were associated with reduced numbers of whitefly adults, nymphs, puparium, and eggs in the choice bioassay and with high adult whitefly mortality in the no-choice bioassay. Preliminary trichome analysis followed by choice and no-choice assays facilitated rapid identification of whitefly-resistant accessions from a large pool of candidates of different species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tomato (Solanum lycopersicum L.) is one of the most widely consumed vegetables worldwide, with a global production of 162 million tons with a net value of more than $59 billion in 2012 (United Nations Food and Agriculture Organization (FAO 2012) statistics; http://faostat.fao.org). The sweetpotato whitefly (Bemisia tabaci Genn.) (family Aleyrodidae), also known as the silverleaf whitefly (B. argentifolii Bellows et Perring), is a major pest of cultivated plants in tropical, subtropical and warm temperate regions. The most predominant and damaging biotypes are biotype B (also known as Middle East-Minor Asia 1 genetic group (MEAM1), hereafter referred to as B) and biotype Q (also known as the Mediterranean genetic group (MED), hereafter referred to as Q). These biotypes have spread from their native ranges to as many as 60 countries (Pan et al. 2013) causing annual losses of $1 to 2 billion (Jiang et al. 2012). Whitefly feed on foliar phloem and produce a sticky secretion called honeydew, a substrate for fungi that results in black sooty mold on the leaf surface and reduces leaf photosynthetic efficiency; whitefly saliva may also cause irregular fruit ripening, which reduces fruit quality and increases the number of unmarketable fruit (Schuster et al. 1996). Most importantly, 114 virus species belonging to five different genera (Begomovirus, Crinivirus, Closterovirus, Ipomovirus and Carlavirus) are transmitted by whitefly (Jones 2003). Begomoviruses that cause tomato yellow leaf curl disease are the most numerous of the B. tabaci-transmitted viruses and early tomato yellow leaf curl infection can cause 100 % yield loss (Saikia and Muniyappa 1989).
Current whitefly control strategies rely primarily on insecticides to which whitefly are notorious for developing resistance (Denholm et al. 1996; Palumbo et al. 2001). Pesticide control of whitefly is often costly and can be hazardous to farmers and the environment, including natural enemies. Therefore, availability of whitefly-resistant cultivars could contribute to cost-effective and environmentally sound pest management.
Seven trichomes types have been identified in cultivated tomato and its wild relatives. Trichomes are categorized into non-glandular types without droplets (Types II, III and V) and glandular types with droplets (Types I, IV, VI and VII). Trichomes in cultivated tomato are mostly non-glandular type V, sometimes with sparse densities of glandular types I and VI, whereas some wild relatives of tomato have an abundance of glandular trichomes, especially types I, IV and VI (Gurr and McGrath 2001; McDowell et al. 2011; Simmons and Gurr 2005). Glandular trichomes play an important role in whitefly resistance through release of defense compounds such as acyl sugars, methylketones, and sesquiterpenes; these compounds may cause antixenosis (insect behavior changes resulting in reduced or no host colonization) and/or antibiosis (reduced insect fitness resulting from reduced survival, oviposition, and development) (Antonious et al. 2005; Bleeker et al. 2009, 2011, 2012; Freitas et al. 2002; Muigai et al. 2003). Glandular trichomes also may act as a physical barrier, and interfere with whitefly landing, feeding and oviposition (Dimock and Kennedy 1983; Snyder and Carter 1984; Channaryappa et al. 1992).
Recent studies of whitefly resistance in the wild tomato species S. galapagense S.C. Darwin et Peralta (formerly Lycopersicon cheesmaniae f. minor (Hook. f.) C.H. Mull.) reported diverse resistance levels between and within accessions (Firdaus et al. 2012; Lucatti et al. 2013). Spider mite and whitefly resistance has been reported in S. pimpinellifolium L. (formerly L. pimpinellifolium (Juslen.) Mill.) accession TO937 (Alba et al. 2009; Rodríguez-López et al. 2011). Solanum galapagense, along with S. cheesmaniae (L. Riley) Fosberg (syn L. cheesmaniae L. Riley) and S. pimpinellifolium are closely related to cultivated tomato and introgression of resistance from these species may be easier and faster compared to the green-fruited species S. chilense (Dunal) Reiche (formerly L. chilense Dun.), S. peruvianum L. (formerly L. peruvianum (L.) Mill.), S. pennellii Correll (formerly L. pennellii (Corr.) D’Arcy), and S. habrochaites S. Knapp and D.M. Spooner (formerly L. hirsutum Humb. and Bonpl.) (Peralta et al. 2008; Rick 1971). To date only a limited number of accessions/populations of S. galapagense, S. cheesmaniae and S. pimpinellifolium have been characterized for trichome types and evaluated for insect resistance. Screening for insect resistance can be laborious, especially for large numbers of accessions and plant populations (Bas et al. 1992). Availability of high throughput methods to assess efficiently and accurately large numbers of tomato accessions/plants for insect resistance would facilitate identification of resistance sources and improve breeding. Information on insect resistance levels among a large number of accessions and their underlying resistance mechanisms would be very useful for tomato breeders worldwide. Based on the strong association between insect resistance and presence of glandular trichomes, the objectives of this study were to characterize all available S. galapagense, S. cheesmaniae and S. pimpinellifolium accessions in AVRDC – The World Vegetable Center’s genebank for trichome types, and to evaluate the resistance to whitefly of selected accessions with high densities of glandular trichomes in choice and no-choice bioassays.
Materials and methods
Plant materials and growth conditions
Seeds of 260 accessions of S. galapagense, S. cheesmaniae and S. pimpinellifolium were obtained from the AVRDC genebank (Supplementary Table 1). AVRDC tomato line CL5915-93D4-1-0-3 was included as a whitefly-susceptible check. Five accessions were discarded due to low germination. The accessions with enough viable seeds were divided into two groups of 130 accessions and groups 1 and 2 were sown on 11 and 28 November 2014, respectively. Seeds were sown in 72-plug seedling trays with 40 ml peat moss per cell. The accessions and check were grown in an AVRDC greenhouse (26 ± 4 °C, 6/18 h day/night) and fertilized weekly.
Analysis of trichome morphology and density
Four-week-old plants were assessed for trichome type and density using a stereo microscope (SZH-ILLB; Olympus, Tokyo, Japan) equipped with a light system (LG-PS2; Olympus, Tokyo, Japan). Densities were determined from the interior middle section of the abaxial surface of the second leaf from the apex of six randomly selected plants per accession using a 0–3 visual scale adapted from Simmons and Gurr (2005) where 3 = abundant (>5 per mm2); 2 = sparse (1–5 per mm2); 1 = very sparse (<1 per mm2), and 0 = absent. Trichome type classification was carried out according to Luckwill (1943) and determined by morphology and presence/absence of trichome glands on the entire abaxial leaf surface of six randomly selected plants per accession with a stereo microscope. Six weeks after sowing, selected accessions classified for type IV trichome density as abundant or sparse were re-evaluated for density in a one mm2 area on the right and left side of the main vein at the leaflet base during choice and no-choice bioassays.
Choice and no-choice bioassays
Thirteen accessions from group 1 and nine accessions from group 2 were classified as either abundant or sparse for type IV trichomes and selected for whitefly choice and no-choice bioassays to determine resistance. The 22 accessions included four S. cheesmaniae, 11 S. galapagense and seven S. pimpinellifolium. Five weeks after sowing, 10 seedlings per accession and 20 seedlings of the susceptible check were transplanted into 12 cm2 pots with potting soil and moved from the plastic house to growth rooms. The growth room temperature was increased slowly (one degree per day) from 23 to 27 °C to allow plants to adjust to the higher optimal temperatures (27 ± 2 °C) and conditions (70 % RH, 16/8 h day/night) for whitefly. Plants were fertilized weekly with NPK 15-15-15 and were watered daily. Whitefly (B. tabaci, biotype B) used in choice and no-choice assays were collected from colonies originating from an AVRDC field and reared and maintained on cabbage plants (Brassica oleracea L., a non-tomato yellow leaf curl-susceptible host) in muslin-covered cages in a growth chamber at 23–30 °C. Six weeks after sowing, each plant was infested with 10 and 5 whitefly pairs for choice and no-choice bioassays, respectively. The experiments were conducted from November 2014 to February 2015 in an AVRDC greenhouses.
Each accession and the susceptible check in the choice assay were represented by six plants. Plants were arranged according to a completely randomized design with one plant per experimental unit. The experiment was conducted on stainless steel benches in the AVRDC Insectary and each bench held 18 plants (three rows of six plants). Plant spacing was 20 and 15 cm between and within accessions, respectively. Ten pairs of adult non-viruliferous whitefly were collected with a hand-held aspirator and adults were placed in each pot in the growth room. Adult whiteflies on each plant were counted three days after introduction by gently turning the plants and noting the number of adults on the abaxial side of the leaves. Adult whiteflies were removed from the plants by a handmade vacuum aspirator after counting. Numbers of egg, nymph and puparium were counted under the stereo microscope (10×) 3, 10 and 15 days, respectively, after infestation. Numbers of adults were counted again 19 days after introduction. Log transformation was used to normalize adult-whitefly data before analysis; egg, nymph and puparium data were transformed by natural logarithm (1n) before analysis.
Four plants per accession and the susceptible check were included for the no-choice bioassay. Plants were arranged according to a completely randomized design with one plant per replication (four replications). Two clip-on cages (2.5 cm in diameter and 1.0 cm in high), one each on the second and third fully expanded leaf from the apex, were placed on each plant. Five pairs of non-viruliferous adult whiteflies were collected with a hand-held aspirator and inserted into each clip-on cage. Dead and alive adult whiteflies and eggs were counted four days after whitefly introduction according to the methods described by Momotaz et al. (2010). The leaflets were cut from the plant to facilitate egg counting under the stereo microscope (10×). An arc-sin (Sqrt) transformation was applied to normalize adult mortality data, whereas a Sqrt (x + 1) transformation was applied to egg number before data analysis.
Statistical procedures were performed using the statistical software SAS (version 9.1; SAS Institute, Cary, NC). Data of whitefly resistance parameters in both choice and no-choice bioassays and densities of type I, IV, V and VI trichomes were subjected to one-way analysis of variance (ANOVA) followed by a least significant difference (LSD) test (P = 0.05). Linear correlations were calculated between densities of type I, IV, V and VI trichomes and whitefly resistance parameters in both choice and no-choice bioassays.
Cryo-Scanning electron microscopy was performed to visualize and compare trichome types of selected resistant and susceptible accessions. Samples taken from young leaves were ≤7 mm in area and ≤2 mm thick. Samples were first frozen in slush, prepared in an Oxford Alto 2500 cryosystem (Catan), and then analyzed in a JEOL JSM-6330F field emission scanning electron microscope (JEOL, Tokyo, Japan). Samples were examined with a 15 kV accelerating voltage and the resulting images were captured digitally.
Results
Trichome diversity, density, and morphology
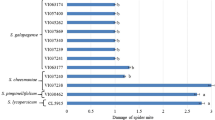
A total of 255 accessions of S. cheesmaniae, S. galapagense and S. pimpinellifolium were characterized (Supplementary Table 1) for presence and densities on the abaxial leaf surface of trichome types I, IV, V and VI associated with insect resistance in the genus Solanum. Types II and III were ignored because they are non-glandular trichomes and have not been associated with insect resistance. Most accessions had some type IV, V and/or VI trichomes but few produced type I. The susceptible control CL5915 lacked type IV trichomes entirely and produced some type I, V and VI trichomes. Type VI trichomes were almost all lobe shaped except for S. pimpinellifolium accession VI30462, which developed spherical-shaped type VI trichomes (Fig. 1). Trichome types I, V and VI were absent or sparse among the ten S. cheesmaniae accessions except VI037243, which had a high density of type V. Density of type IV trichomes among the S. cheesmaniae accessions ranged from 1 to 3, with the highest density observed in VI037240 (Fig. 1). All 13 S. galapagense, accessions produced abundant type IV trichomes except for VI063184, and almost no type V were found except on VI007099; type I trichomes were mostly absent and type VI trichomes were very sparse or absent. Of the 231 evaluated S. pimpinellifolium accessions, 131 (56.7 %) produced few type IV trichomes and only four (1.7 %), VI006086, VI007511, VI009549, and VI010049, were classified as abundant for type IV trichomes. Type I was mostly absent in the S. pimpinellifolium accessions while a wide range in densities of type VI trichomes were found; only accession VI009107 entirely lacked type VI trichomes.
Scanning electron micrographs of trichome morphology and density on abaxial leaf surfaces of four selected Solanum accessions/check evaluated in this study; a whitefly susceptible control S. lycopersicum (CL5915-93D4-1-0-3), b resistant S. galapagense VI037239, c resistant S. cheesmaniae VI37240, d susceptible S. cheesmaniae VI037238, e resistant S. pimpinellifolium VI030462, and f susceptible S. pimpinellifolium VI005575
After the initial assessment, a subset of 22 accessions (Tables 1, 2) characterized earlier as either abundant or sparse for type IV trichome densities were re-evaluated for trichome type and density in a one mm2 area six weeks after sowing. The analysis of variance (data not shown) revealed highly significant differences among accessions and the check for density of each trichome type (Tables 1, 2). Cultivated tomato check CL5915 lacked type IV and contained relatively high densities of type V trichomes. In contrast, high densities of type IV trichomes were detected in S. cheesmaniae accessions VI037240 and VI009635, S. pimpinellifolium VI030462, and all S. galapagense accessions. Types I and VI trichomes were sparse or absent in the evaluated accessions and high densities of type V were present in most S. pimpinellifolium, some S. cheesmaniae and two S. galapagense accessions.
Whitefly resistance in choice and no-choice bioassays
Selected accessions and the check were assessed for numbers of adults, eggs, nymphs and puparium between 3 and 19 days after whitefly infestation (DWF) (Table 1). Highly significant differences among accessions and the check for all whitefly resistance parameters were found (P < 0.001). Adult numbers between 3 and 19 DWF increased on check CL5915 (four-fold) and most S. pimpinellifolium accessions (1.3 to 21-fold increase) and they hosted high numbers of eggs, nymphs, and puparium. Mean numbers of adults, nymphs and puparium on accession VI030462 were generally lower but not statistically different from most of the other S. pimpinellifolium accessions. Except for VI007099, all S. galapagense accessions displayed high levels of whitefly resistance for all parameters, especially VI063174, VI063177 and VI037239. Most S. galapagense were inhospitable for whitefly adults and numbers dropped to zero at 19 DWF for eight of 10 accessions. Eggs were observed on all S. galapagense although counts on VI063177 and VI037239 were relatively fewer and none of those eggs hatched. Except for VI007099 and VI037241, all eggs that hatched on the S. galapagense accessions failed to develop into puparium. Three of four S. cheesmaniae accessions were good whitefly hosts; however, no whitefly adults were found on VI037240 at 19DFW and none of the eggs on this accession developed into puparium.
Whiteflies contained in clip-on cages were force infested on each accession and check, and numbers of whitefly adults and eggs were counted on the abaxial leaf sides four days after infestation. The ANOVA revealed highly significant differences among accessions and the check for adult mortality and egg number (P < 0.001). Whitefly adult mortality (Table 2) was generally greatest among the S. galapagense accessions, ranging from 34.7 to 100 % with complete adult mortality observed on VI037340 and very high mortality on VI057400 and VI063177; percent adult mortality on each S. galapagense accession was statistically different from check CL5915. Egg numbers on S. galapagense accessions ranged from 0–117 per leaf but only the means of VI057400 (15.8), VI037239 (12.02) and VI037869 (3.9) were significantly lower than the check (86.1). Compared to S. galapagense, adult mortalities were lower and egg numbers were higher on most of the S. pimpinellifolium and S. cheesmaniae accessions. Exceptions included S. pimpinellifolium accession VI030462 with high adult mortality and low egg numbers and VI005575 with low egg numbers. It is noteworthy that egg numbers on S. cheesmaniae accession VI009635 were relatively low (11.6) even though adult mortality was also low and not significantly different from CL5915.
Resistant tomato accessions showed type IV trichome densities of ≥9 mm2, whereas type IV densities on susceptible accessions were significantly lower and similar to susceptible check CL5915 (Tables 1, 2). However, some susceptible accessions had high densities of type IV trichomes such as S. galapagense VI007099 and S. cheesmaniae VI009635, but were susceptible based on resistance parameters in the choice bioassay (Table 1). Type V trichomes were abundant on leaves of most susceptible accessions, but were absent or sparse on the resistant accessions (Tables 1, 2).
Linear correlations were calculated between parameters in the choice test as well as the no-choice tests (Table 3). Correlations were subjectively categorized as low (±0.18–0.30), moderate (±0.31–0.50), high (±0.51–0.75) and very high (± ≥ 0.76). Adult whitefly numbers at 3 DWF and 19 DWF were highly correlated with numbers of eggs, nymphs, and puparium. Numbers of eggs, nymphs and puparium were very highly correlated among each other. Negative and highly significant correlations were obtained between adult mortality from the no-choice test and resistance variables from the choice test; the strength of these correlations ranged from moderate to high. Highly significant and positive but moderate correlations were found between number of eggs from the no-choice test and resistance variables from the choice test.
Correlations between trichome types and whitefly resistance parameters based on choice and no-choice bioassays
In the choice assay, significant negative correlations were found between type IV trichome density and numbers of adults, puparium, nymphs, and eggs (Table 4). In the no-choice assay, type IV trichome density was positively and negatively correlated with adult mortality and egg number, respectively. In contrast, significant positive correlations were detected between type V trichome density and numbers of adults, puparium, nymphs, and eggs. Type V trichome density was negatively correlated with adult mortality but the correlation between type V density and egg number was not statistically significant. In the choice assay, correlations between resistance parameters and type I trichomes and type VI trichomes were either weakly statistically significant or not significant; only the correlation between adult mortality in the no-choice assay showed a positive moderate correlation.
Discussion
The sweetpotato whitefly, B. tabaci, is a major global pest of tomato and other crops. Whitefly feeding can cause irregular ripening disorder, which reduces marketable fruit yields. Whiteflies vector several plant virus groups including begomoviruses, the cause of tomato yellow leaf curl disease, which is one of the most important tomato diseases worldwide. Farmers often resort to frequent applications of toxic pesticides for whitefly control, which increase production costs, contaminate themselves and the environment, and hasten the selection of pesticide resistant whitefly populations. For the above reasons, identification of whitefly resistance and development of resistant tomato cultivars is a high priority for tomato breeders.
The root knot nematode resistance gene Mi-1.2 was reported to confer whitefly resistance in older tomato plants (Nombela et al. 2003). However, the highest levels of insect/whitefly resistance were observed in some accessions of distant wild tomato species, particularly S. habrochaites accessions CGN1.1561, LA1777, PI127826, Pl134417 and PI134418 (Bleeker et al. 2009; Firdaus et al. 2012; Maliepaard et al. 1995; Momotaz et al. 2010; Oriani et al. 2011) and S. pennellii accessions LA716, LA1674, LA1735, LA1340 and LA2560 (Bleeker et al. 2009; Muigai et al. 2003; Nombela et al. 2000; Oriani and Vendramim 2010; Toscano et al. 2002). Transfer of resistance genes from these species into commercial tomato cultivars has been difficult because of the multigenic inheritance of insect resistance and the linkage of whitefly resistance genes with other genes from wild species conditioning poor horticultural traits (linkage drag). Problems with crossing barriers and linkage drag are expected to be much less with more closely related wild tomato species (Peralta et al. 2008; Rick 1971). Our approach was to assess and evaluate all available AVRDC genebank accessions of S. galapagense, S. cheesmaniae and S. pimpinellifolium for trichome type and density, identify those with glandular trichomes, and subject selected accessions for whitefly resistance testing in choice and no-choice trials. In this way we could quickly sort through several hundred accessions and select a manageable number for inclusion in the costly choice and no-choice assays. However, this approach would miss resistance that is independent of glandular trichomes such as Mi-1.2 (Nombela et al. 2003). It is expected that the choice bioassay would reveal accessions that confer both antixenosis and antibiosis resistance mechanisms whereas the no-choice bioassay assesses presence of antibiosis resistance mechanisms (Baldin and Beneduzzi 2010). The above process enabled us to identify/confirm whitefly resistant S. galapagense accessions VI063177 and VI037239 supporting earlier results by Firdaus et al. (2012) and Lucatti et al. (2013). In addition we discovered whitefly resistance in accessions S. cheesmaniae VI037240 and S. pimpinellifolium VI030462, which are closely related to cultivated tomato; this is the first report of whitefly resistance in these two accessions although we do not know the relationship between VI030462 and S. pimpinellifolium accession TO-937 reported by Rodríguez-López et al. 2011.
Differences in presence and densities of trichome types among and within species (and sometimes within accessions) were evident. The evaluated S. galapagense accessions developed few type I and VI, very few or no type V, but abundant type IV trichomes; the S. cheesmaniae accessions also produced few or no type I and VI trichomes and varying densities of type IV and V trichomes. Type V trichomes were abundant on most S. pimpinellifolium accessions but types I and IV were rare or absent while densities of type VI varied among accessions. These observations agree with previous studies (Firdaus et al. 2012; Simmons and Gurr 2005). The most resistant and least preferred accessions had high densities of glandular type IV trichomes and none or low densities of type V trichomes. Similar associations between trichome type and whitefly resistance were reported by others for S. galapagense (Firdaus et al. 2012; Lucatti et al. 2013), and S. habrochaites (Frelichowski and Juvik 2001; Momotaz et al. 2010). In this study, most S. galapagense exhibited high resistance in both choice and no-choice bioassays. In addition, we identified S. cheesmaniae VI037240 and S. pimpinellifolium VI030462 that possessed relatively high densities of type IV trichomes and demonstrated high levels of whitefly resistance. High densities of type IV trichomes were generally a strong indicator of whitefly-resistant plants. However, high densities of type IV trichomes did not necessarily result in whitefly resistance. S. galapagense VI007099, for example, was susceptible and produced many type IV trichomes, but also some type V trichomes that provide a favorable microclimate for whitefly oviposition and in nature help protect eggs and larvae from natural enemies (Butter and Vir 1989). Differences in chemical concentration in exudates from type IV trichomes are possible and may partly account for the susceptibility of S. galapagense VI007099. We did not find high variation among the evaluated accessions for densities of type VI trichomes, which may explain the very low correlation between type VI trichome density and whitefly resistance. Presence of type VI trichomes was associated with insect resistance in a cross derived from S. habrochaites (Chatzivasileiadis and Sabelis 1997; Lin et al. 1987). Furthermore, Ben-Israel et al. (2009) discerned three shapes of type VI trichomes (spherical, lobed, and intermediate) in F2 interspecific populations derived from S. habrochaites f. sp. glabratum and that the spherical trichomes were most closely associated with higher methylketone content. All accessions in this study developed lobed type VI trichomes except for whitefly resistant S. pimpinellifolium VI030462 with spherical type VI trichomes. It is possible that the high whitefly resistance of VI030462 is due to methylketone production but this needs to be determined. Although we did not study the chemical composition of glandular trichomes, their role in insect resistance and production of compounds such as methylketones, mono- and sesquiterpenes as well as acyl sugars has been discussed frequently (Bleeker et al. 2009, 2012; Maluf et al. 2001; Muigai et al. 2002; Schilmiller et al. 2008; Tissier 2012).
The choice and no-choice bioassays revealed overall differences among S. galapagense, S. cheesmaniae and S. pimpinellifolium species and accessions in whitefly resistance. In addition, significant variation for whitefly resistance was found within some accessions, an observation which agrees with Firdaus et al. (2012) and Lucatti et al. (2013). For instance, the six plants of S. cheesmaniae VI009635 in the choice bioassay included one highly susceptible, three moderately resistant, and two highly resistant plants carrying few non-viable eggs. Thus there is scope for single-plant selection within some accessions that are heterogeneous for trichome types and numbers.
Solanum galapagense VI063177 and VI037239 exhibited high resistance in the choice bioassay based on low numbers of whitefly adults that laid few eggs of which none hatched; these accessions were also highly resistant in the no-choice bioassay, suggesting presence of antixenosis and/or antibiosis. In some resistant S. galapagense accessions such as VI063174, VI037340, VI037869, VI045262 and VI057400 and S. cheesmaniae VI037240 only a few nymphs hatched from eggs and none of them developed into pupae, suggesting the possible presence of different resistance mechanisms in these accessions. All tested S. pimpinellifolium accessions were highly susceptible except VI030462, which whitefly tended to avoid in the choice bioassay and which exhibited high resistance in the no-choice bioassay. Variation for whitefly resistance was noted in this accession with two completely resistant plants (100 % adult mortality and no eggs) and two highly resistant (≥78 % adult mortality and ≤11 eggs) plants, indicating antibiosis to whitefly.
We performed choice and no-choice bioassays to evaluate resistance parameters based on the different stages of whitefly life history. High or very high positive correlations were detected between whitefly resistance parameters in the choice bioassay as expected. However, the correlations in choice bioassays between numbers of adult whiteflies 19 DWF with egg, nymph and puparium numbers were larger versus the comparable correlations for 3 DWF. Initial adult choice of a host plant for feeding and/or oviposition is influenced more by preference factors, but newly emerged adult females may be more affected by antibiosis factors. Whitefly choice of host plants to land on is determined by host color (van Lenteren and Noldus 1990), olfactory (Visser 1988) and semiochemicals (Bleeker et al. 2009, 2012). After landing, whiteflies apply labial dabbing, probing and feeding, and the results will determine whether they remain and oviposit or leave the plant (Bleeker et al. 2011). Unlike eggplant (Solanum melongena) and cucumber (Cucumis sativus) on which whitefly may remain several days, whitefly seldom stay on tomato plants for more than a few hours after probing and frequently change position between probes before leaving the plant (van Lenteren and Noldus 1990). The high correlations between numbers of eggs, nymphs and puparium in the choice assay suggest that resistance factor(s) do not affect egg hatching. Firdaus et al. (2012) also found high correlations between egg and nymphal densities including instar 1 to instar 4, so oviposition (egg density) was apparently affected by antibiosis and/or preference factor(s). These results also support the hypothesis that oviposition preference and host plant selection by female whiteflies has a profound effect on the fitness of offspring (van Lenteren and Noldus 1990; Nomikou et al. 2003).
Adult mortality in the no-choice bioassay was negatively correlated with the number of eggs determined in the same bioassay and also with all parameters in the choice bioassay. This strongly suggests that factor(s) affecting adult mortality are critical in tomato defense against whitefly. In addition, correlations of parameters within and between choice and no-choice bioassay show that antibiosis is the main cause for whitefly non-preference in choice situations. However, other factor(s) affecting whitefly non-preference of particular accessions also play a role in tomato defense against whitefly as we observed in accessions VI063177, VI063174 and VI037239. Metabolic compounds resulting in high mortality such as acyl sugars, sesquiterpenes and methylketones affect insect growth in some tomato wild relatives (Bleeker et al. 2009, 2011, 2012; Lin et al. 1987). Conversely, cultivated tomato does not produce these compounds, indicating a loss of expression or genomic information critical to their biosynthesis upon evolution and selection (Besser et al. 2009; Fridman et al. 2005; Sallaud et al. 2009).
Durable whitefly resistance, especially in the field, is more likely if tomato cultivars mount resistance based on a combination of antixenosis and antibiosis factors, thus forcing whiteflies to surmount a wide range of plant defenses (Anderson et al. 2010; Broekgaarden et al. 2011; Zangerl and Rutledge 1996). Capacity to repel and avoid whitefly landing, probing, and feeding is important to thwart whitefly as a vector of plant viruses, especially begomoviruses. Over 100 distinct begomovirus species infect tomato (Jones 2003) with continuous generation of new virus forms through recombination (Nawaz-ul-Rehman and Fauquet 2009). Begomovirus resistance/tolerance genes (Ty genes) have been introgressed from wild tomato accessions and some have been incorporated into resistant commercial tomato cultivars (Ji et al. 2007). However, Ty genes, alone or in combination, often act to reduce symptom severity and may allow infection by some viruses (Vidavski et al. 2008): plant co-infection by multiple begomoviruses offers opportunities to recombine and evolve virulent, resistance-breaking forms of the viruses. Combined vector and begomovirus resistance in tomato cultivars would be valuable in repelling whitefly, thus helping to preserve the durability of virus resistance genes and possibly making a contribution to slowing begomovirus evolution. Our results show that B. tabaci resistance was found in some accessions of S. galapagense, S. cheesmaniae and S. pimpinellifolium species, which may be exploited in breeding programs to develop whitefly-resistant cultivars.
References
Alba JM, Monserrat M, Fernández-Muñoz R (2009) Resistance to the two-spotted spider mite (Tetranychus urticae by acylsucroses of wild tomato (Solanum pimpinellifolium) trichomes studied in a recombinant inbred line population. Exp Appl Acarol 47:35–47
Anderson LK, Covey PA, Larsen LR, Bedinger P, Stack SM (2010) Structural differences in chromosomes distinguish species in the tomato clade. Cytogenet Genome Res 129:24–34
Antonious GF, Kochhar TS, Simmons AM (2005) Natural products: seasonal variation in trichome counts and contents in Lycopersicum hirsutum f. glabratum. J Environ Sci Health Part B 40:619–631
Baldin EL, Beneduzzi RA (2010) Characterization of antibiosis and antixenosis to the whitefly silverleaf Bemisia tabaci B biotype (Homoptera: Aleyrodidae) in several squash varieties. J Pest Sci 83:221–227
Bas N, Mollema C, Lindhout P (1992) Resistance in Lycopersicon hirsutum f. glabratum to the greenhouse whitefly (Trialeurodes vaporariorum) increases with plant age. Euphytica 64:189–195
Ben-Israel I, Yu G, Austin MB, Bhuiyan N, Auldridge M, Nguyen T, Schauvinhold I, Noel JP, Pichersky E, Fridman E (2009) Multiple biochemical and morphological factors underlie the production of methylketones in tomato trichomes. Plant Physiol 151:1952–1964
Besser K, Harper A, Welsby N, Schauvinhold I, Slocombe S, Li Y, Dixon RA, Broun P (2009) Divergent regulation of terpenoid metabolism in the trichomes of wild and cultivated tomato species. Plant Physiol 149:499–514
Bleeker PM, Diergaarde PJ, Ament K, Guerra J, Weidner M, Schütz S, de Both MTJ, Haring MA, Schuurink RC (2009) The role of specific tomato volatiles in tomato–whitefly interaction. Plant Physiol 151:925–935
Bleeker PM, Diergaarde PJ, Ament K, Schütz S, Johne B, Dijkink J (2011) Tomato-produced 7-epizingiberene and R-curcumene act as repellents to whiteflies. Phytochemistry 72(1):68–73
Bleeker PM, Mirabellaa R, Diergaardeb PJ, VanDoornb A, Tissierc A, Kantd MR, Prinsb M, de Vosb M, Haringa MA, Schuurinka RC (2012) Improved herbivore resistance in cultivated tomato with the sesquiterpene biosynthetic pathway from a wild relative. Proc Natl Acad Sci USA 109(49):20124–20129
Broekgaarden C, Snoeren TAL, Dicke M, Vosman B (2011) Exploiting natural variation to identify insect-resistance genes. Plant Biotech J 9(8):819–825
Butter NS, Vir BK (1989) Morphological basis of resistance in cotton to the whitefly Bemisia tabaci. Phytoparasitica 17(4):251–261
Channaryappa SG, Muniyappa V, Frist RH (1992) Resistance of Lycopersicon species to Bemisia tabaci, a tomato leaf curl virus vector. Can J Bot 70:2184–2192
Chatzivasileiadis EA, Sabelis MW (1997) Toxicity of methyl ketones from tomato trichomes to Tetranychus urticae Koch. Exp Appl Acarol 21:473–484
Denholm I, Cahill M, Byrne FJ, Devonshire AL (1996) Progress with documenting and combating insecticide resistance in Bemisia. In: Gerling D, Mayer RT (eds) Bemisia (1995) Taxonomy, biology, damage, control and management. Intercept, Andover, pp 577–603
Dimock MB, Kennedy YG (1983) The role of glandular trichomes in the resistance of Lycopersicon hirsutum f. glabratum to Heliothis zea. Entomol Exp Appl 33:263–268
FAO, FAOSTAT (2012) Agricultural statistics database. Food and Agriculture Organization, 2012 Rome. http://faostat.fao.org/site/567/DesktopDefault.aspx?PageID=567#ancor. Accessed 9 Mar 2015
Firdaus S, van Heusden A, Hidayati N, Supena ED, Visser RG, Vosman B (2012) Resistance to Bemisia tabaci in tomato wild relatives. Euphytica 187:31–45
Freitas JA, Maluf WR, Cardoso MD, Gomes LAA, Bearzotti E (2002) Inheritance of foliar zingiberene contents and their relationship to trichome densities and whitefly resistance in tomatoes. Euphytica 127:275–287
Frelichowski JE, Juvik JA (2001) Sesquiterpene carboxylic acids from a wild tomato species affect larval feeding behavior and survival of Helicoverpa zea and Spodoptera exigua (Lepidoptera: Noctuidae). J Econ Entomol 94:1249–1259
Fridman E, Wang J, Iijima Y, Froehlich JE, Gang DR, Ohlrogge J, Pichersky E (2005) Metabolic, genomic, and biochemical analyses of glandular trichomes from the wild tomato species Lycopersicon hirsutum identify a key enzyme in the biosynthesis of methylketones. Plant Cell 17:1252–1267
Gurr GM, McGrath D (2001) Effect of plant variety, plant age and photoperiod on glandular pubescence and host-plant resistance to potato moth (Phthorimaea operculella) in Lycopersicon spp. An Appl Biol 138:221–230
Ji Y, Scott JW, Hanson P, Graham E, Maxwell DP (2007) Sources of resistance, inheritance and location of genetic loci conferring to members of the tomato infecting begomoviruses. In: Czosnek H (ed) Tomato yellow leaf curl virus disease: management, molecular biology, and breeding for resistance. Springer, Dordrecht, pp 343–362
Jiang ZF, Xia F, Johnson KW, Bartom E, Tuteja JH, Stevens R, GrossmanRL Brumin M, White KP, Ghanim M (2012) Genome sequences of the primary endosymbiont “Candidatus portiera aleyrodidarum” in the whitefly Bemisia tabaci B and Q biotypes. J Bacteriol 194:6678–6679
Jones DR (2003) Plant viruses transmitted by whiteflies. Eur J Plant Pathol 109:195–219
Lin SYH, Trumble JT, Kunamoto J (1987) Activity of volatile compounds in glandular trichomes of Lycopersicon species against two insect herbivores. J Chem Ecol 13:837–850
Lucatti AF, van Heusden AW, de Vos RC, Visser RGF, Vosma B (2013) Differences in insect resistance between tomato species endemic to the Galapagos Islands. BMC Evol Biol 13:175
Luckwill LC (1943) The genus Lycopersicon: a historical, biological and taxonomic survey of the wild and cultivated tomatoes. Aberdeen University Press, Aberdeen, p 44
Maliepaard C, Bas N, van Heusden S, Kos K, Pet G, Verkerk R, Vrielink R, Zabel P, Lindhout P (1995) Mapping of QTLs for glandular trichome densities and Trialeurodes vaporariorum (greenhouse whitefly) resistance in an F2 from Lycopersicon esculentum × Lycopersicon hirsutum f. glabratum. Heredity 75:425–433
Maluf WR, Campos GA, Cardoso MG (2001) Relationships between trichome types and spider mite (Tetranychus evansi) repellence in tomatoes with respect to foliar zingiberene contents. Euphytica 121:73–80
McDowell ET, Kapteyn J, Schmidt A, Li C, Kang JH, Descour A, Shi F, Larson M, Schilmiller AL (2011) Comparative functional genomic analysis of Solanum glandular trichome types. Plant Physiol 155:524–539
Momotaz A, Scott JW, Schuster DJ (2010) Identification of quantitative trait Loci conferring resistance to Bemisia tabaci in an F2 population of Solanum lycopersicum × Solanum habrochaites Accession LA1777. J Am Soc Hort Sci 135:134–142
Muigai SG, Schuster DJ, Snyder JC, Scott JW, Bassett MJ, McAuslane HJ (2002) Mechanisms of resistance in Lycopersicon germplasm to Bemisia argentifolii (Homoptera: Aleyrodidae). Phytoparasitica 30:347–360
Muigai SG, Bassett MJ, Schuster DJ, Scott JW (2003) Greenhouse and field screening of wild Lycopersicon germplasm for resistance to the whitefly Bemisia argentifolii. Phytoparasitica 31:27–38
Nawaz-ul-Rehman MS, Fauquet CM (2009) Evolution of geminiviruses and their satellites. FEBS Lett 583:1825–1832
Nombela G, Beitia F, Muñiz M (2000) Variation in tomato host response to Bemisia tabaci (Hemiptera: Aleyrodidae) in relation to acyl sugar content and presence of the nematode and potato aphid resistance gene Mi. Bull Entomol Res 90:161–167
Nombela G, Williamson VM, Muñiz M (2003) The root-knot nematode resistance gene Mi-1.2 of tomato is responsible for resistance against the whitefly Bemisia tabaci. Mol Plant Microbe In 16:645–649
Nomikou M, Janssen A, Sabelis MW (2003) Herbivore host plant selection: whitefly learns to avoid host plants that harbour predators of her offspring. Oecologia 136:484–488
Oriani MAG, Vendramim JD (2010) Influence of trichomes on attractiveness and ovipositional preference of Bemisia tabaci (Genn.) B biotype (Hemiptera: Aleyrodidae) on tomato genotypes. Neotrop Entomol 39:1002–1007
Oriani MAD, Vendramim JD, Vasconcelos CJ (2011) No-choice ovipositional nonpreference of Bemisia tabaci (Gennadius) B biotype on tomato genotypes. Sci Agric 68:147–153
Palumbo JE, Horowitz AR, Prabhaker N (2001) Insecticidal control and resistance management for Bemisia tabaci. Crop Prot 20:739–765
Pan HP, Chu D, Liu BM, Xie W, Wang SL, Wu QJ (2013) Relative amount of symbionts in insect hosts changes with host-plant adaptation and insecticide resistance. Environ Entomol 42:74–78
Peralta IE, Spooner DM, Knapp S (2008) Taxonomy of wild tomatoes and their relatives (Solanum sect. Lycopersicoides, sect. Juglandifolia, sect. Lycopersicon; Solanaceae. Syst Bot Monogr 84:1–186
Rick CM (1971) Lycopersicon Mill. In: Wiggins IL, Porter DM (eds) Flora of the Galapagos Islands. Stanford University Press, Palo Alto, pp 468–471
Rodríguez-López MJ, Garzo E, Bonani JP, Fereres A, Fernández-Muñoz R (2011) Whitefly resistance traits derived from the wild tomato Solanum pimpinellifollium affect the preference and feeding behavior of Bemisia tabaci and reduce the spread of tomato yellow leaf curl virus. Phytopathology 101:1191–1201
Saikia AK, Muniyappa V (1989) Epidemiology and control of tomato leaf curl virus in southern India. Trop Agric 66:350–354
Sallaud C, Rontein D, Onillon S (2009) A novel pathway for sesquiterpene biosynthesis from Z, Z-farnesyl pyrophosphate in the wild tomato Solanum habrochaites. Plant Cell 21:301–317
Schilmiller AL, Last RL, Pichersky E (2008) Harnessing plant trichome biochemistry for the production of useful compounds. Plant J 54:702–711
Schuster DJ, Stansly PA, Polston JE (1996) Expressions of plant damage by Bemisia. In: Gerling D, Mayer RT (eds) Bemisia (1995) Taxonomy, biology, damage control and management. Intercept Andover, Hants, pp 153–165
Simmons AT, Gurr GM (2005) Trichomes of Lycopersicon species and their hybrids: effects on pests and natural enemies. Agric For Entomol 7:265–276
Snyder JC, Carter CD (1984) Leaf trichomes and resistance of Lycopersicon hirsutum and Lycopersicon esculentum to spider mites. J Am Soc Hort Sci 109:837–843
Tissier A (2012) Glandular trichomes: what comes after expressed sequence tags? Plant J 70:51–68
Toscano LC, Boiça JAL, Maruyama WI (2002) Nonpreference of whitefly for oviposition in tomato genotypes. Sci Agric 59:677–681
Van Lenteren JC, Noldus LLP (1990) Whitefly-plant relationships: behavioural and ecological aspects. In: Gerling D (ed) Whiteflies: their bionomics pest status and management. Intercept Andover, Hants, pp 47–89
Vidavski F, Czosnek H, Gazit S, Levy D, Lapidot M (2008) Pyramiding of genes conferring resistance to Tomato yellow leaf curl virus from different wild tomato species. Plant Breed 127:625–631
Visser JH (1988) Host-plant finding by insects: orientation, sensory input and search patterns. J Insect Physiol 34:259–268
Zangerl AR, Rutledge CE (1996) The probability of attack and patterns of constitutive and induced defense: a test of optimal defense theory. Am Nat 147:599–608
Acknowledgments
The authors thank Dr. Li Chi-Yi of the Taiwan Agricultural Chemicals and Toxic Substances Research Institute, Council of Agriculture for the scanning electron micrographs photos of trichomes and to Maureen Mecozzi for editing the manuscript. Financial support from the AVRDC Innovations Fund and Federal Ministry for Economic Cooperation and Development, Germany, Project Number 11.7860.7-001.00 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Rakha, M., Hanson, P. & Ramasamy, S. Identification of resistance to Bemisia tabaci Genn. in closely related wild relatives of cultivated tomato based on trichome type analysis and choice and no-choice assays. Genet Resour Crop Evol 64, 247–260 (2017). https://doi.org/10.1007/s10722-015-0347-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-015-0347-y