Abstract
The carbonate (CO −23 ) produced by Sporosarcina pasteurii was injected electrokinetically to enhance the mechanical properties of soft clay soils. In this method the Ca2+ was injected into the anode chamber and moved towards the cathode by electromigration and electroosmotic flow. Then the released CO −23 from a blend of bacteria and urea was injected into the cathode chamber. The CO −23 ions were moved from the cathode to the anode under electromigration mechanism. The CaCO3 was precipitated in the presence of calcium in porous medium of the soil, and consequently increased the shear strength of the soil. The polarity reversal was applied to have a homogeneous distribution of CaCO3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Electrokinetic (EK) stabilization is a ground improvement technique for treating subsurface soils without excavation, unlike conventional methods. Several researchers have studied the applications of EKs principles on engineering properties of soils and soil improvement (Esrig and Gemeinhardt 1967; Johnston and Butterfield 1977; Shang and Dunlap 1996; Ozkan et al. 1999; Barker et al. 2004; Ou et al. 2009; Chien et al. 2012). The injection of agents under electric fields could be used to overcome problems in heterogeneous and/or low permeability soils (Alshawabkeh 2001). The movement of stabilizing agents into the soil mass is governed by the principles of EKs, whereas the mechanisms of stabilization can be explained by the principles of chemical stabilization. When ions are used as stabilizing agents, the ions migrate into soils through processes of electro migration and osmotic advection. These ions improve the soil strength by three mechanisms, ion replacement, mineralisation and precipitation of species in the pore fluid. It is precipitation that provides to the greatest contribution to increase of the strength (Mohamedelhassan and Shang 2002; Alshawabkeh and Sheahan 2003; Asavadorndeja and Glawe 2005; Barker et al. 2004).
Biogrouting is a biological ground improvement method, focuses on microbially induced carbonate precipitation (MICP), in which microorganisms are used to induce carbonate precipitation in the subsurface in order to increase the strength and stiffness of granular soils (Whiffin 2004; De Jong et al. 2006, 2010; Whiffin et al. 2007; Ivanov and Chu 2008; Van Paassen et al. 2010).
Most studies on biogrouting use microorganisms containing the enzyme urease, in particular, the bacterium of Sporosarcina pasteurii. The microbial urease catalysis the hydrolysis of urea into ammonium and carbonate (CO3 2−) (Eq. 1).
The produced carbonate ions precipitate in the presence of calcium ions as calcium carbonate crystals (CaCO3), which form cementing bonds between the grains of the soil (Eq. 2).
There are some limitations related to the bacterial injection in fine grain soils when it is pumped. As bacteria have a typical size of 0.5–3 μm, it is hard to be transported through silty or clayey soils. Bioclogging could occur when bacteria are adsorbed or strained by the solid grains, resulting a heterogeneous CaCO3 precipitation in the soil (Mitchell and Santamarina 2005).
Previous research of biogrouting investigated the improvement of coarse grain soils (Whiffin 2004; De Jong et al. 2006; Whiffin et al. 2007; Ivanov and Chu 2008; Harkes et al. 2010; Van Paassen et al. 2009, 2010). As the permeability of coarse soil is high, passing the bacteria through the soil is possible. However, in fine grain soil, because the permeability of the soil is low, the transport of bacteria is difficult. Subsequently, the precipitation of CaCO3 in porous media in the biomineralisation process could be heterogeneous. Furthermore, the control of grout pressure and pumping rate is difficult in various types of soils (i.e., fine and coarse soils) and depth. When high-pressure pumps are used to inject fluids into the formation, as pressure builds, soil structure fails and the fluid causes soil to separate or “fracture”. As the injection progresses, this separation propagates through the formation in a very unpredictable manner. Moreover, the flow velocity of grout can effect CaCO3 distribution. The lack of CaCO3 close to the injection point in the experiments could be the result of higher flow velocity, causing more bacterial flush out and hence, lower activity and less CaCO3 precipitation. Another explanation for the lack of CaCO3 around the injection points, considers the kinetics of CaCO3 precipitation and the transport of crystals (Van Paassen et al. 2010).
The aim of this study is EK stabilization of soft clay soil using the product of bacteria (CO −23 ) and calcium (Ca2+).
2 Materials and Methods
2.1 Bacterial Growth Condition
The strain S. pasteurii (ATCC 11859) was used in this study. The medium used for harvesting S. pasteurii was NH4-YE prepared as follows. Yeast extract (20.0 g) was dissolved in 100 ml of distilled water (ingredient A), (NH4)2SO4 (10.0 g) in 100 ml of distilled water (ingredient B), and Tris buffer (15.75 g) in 800 ml distilled water (ingredient C). Ingredients A, B and C were then autoclaved separately (121 °C for 15 min). After sterilization, each ingredient was allowed to cool and then the ingredients were mixed in a 1,000 ml Erlenmeyer flask. The NH4-YE medium has a pH value of 9.0, which is appropriate for S. pasteurii growth. After, the bacteria were moved to NH4-YE broth in an incubator (30 °C) and shaken at 200 rpm while mouth of the Erlenmeyer flask was plugged with a silicon cork to allow the cultivation under aerobic conditions. The color of the culture broth turned muddy within 2–3 days. An overnight culture was harvested by centrifugation (Sartorius AG, Sigma 3–18 K, Germany) at 8,000g for 10 min with an optical density of 0.5 at 600 nm (Labomed UVD 2950) (Mortensen et al. 2011).
2.2 Experimental Setup
Figure 1 shows the EK setup which was made of Plexiglas with 30-cm long, 10-cm wide and 15-cm high The setup was designed with two chambers (6 cm length) in the left and right of the soil specimen holder (18 cm length). Two graphite electrodes (Graphite Laminate SLS) were placed into the chambers. Two Mariote bottles and two pH controllers were connected to each compartment. They maintained a constant water level across the specimen and prevented any hydraulic gradient. The pH controllers with two acid and base tanks were designed to adjust the pH in the chamber solution. 0.5 M NaOH and 0.5 M HCl solutions were used for pH adjustment.
Any excess fluid transported owing to electroosmosis flow was collected in cylinders placed at the end of the chambers. Normally, the electroosmotic flow will be from the anode to the cathode. The DC current was applied by a power supply in this study. A voltmeter and a thermometer respectively measured the voltage potential between two points across the specimen and the temperature because of the electrical current in the compartments.
2.3 Soil Properties
Table 1 shows the engineering properties of the soil used in this study. This soil had a low hydraulic permeability about 2.7 × 10−7 cm/s. Figures 2 and 3 illustrate scanning electron microscopy image and energy-dispersive X-ray analysis of kaolinite minerals respectively.
3 Experimental Procedures
3.1 Sample Preparation
The soil specimens were prepared at 85 % maximum dry density (1.3 g/cm3) and 21 % of moisture to make the soil more permeable. The soil was placed in the test container as the basis of sample preparation (ASTM D698-07). Filter papers were used at both ends of the specimen to avoid material loss and to prevent contamination of the electrode fluids by soil particles. The soil sample was allowed to saturate by adding distilled water for 48 h before EK treatment.
3.2 Electrokinetic Experiments
Table 2 lists the detailed test procedure of the EK treatment. In the experiment, a calcium chloride solution (2 M) was injected into the anode chamber over a period of 3 days (T1). A blend of bacterial suspension and urea (1 M) about 150 ml was prepared and then bacteria were allowed to be activated aerobically. When the bacteria were exposed to the urea, they released the urease enzyme and made carbonate ions (CO −23 ). Then the solution was passed through a filter paper (0.2 μm) allowing the bacteria and particles retained and the carbonate solution remained. The product of bacterial activity (i.e. CO −23 solution) was injected into the cathode chamber in treatment time of 4 days for normal polarity and 4 days for reversing polarity in the process (T2 and T3).
The voltage gradient of 60 mV was held constant in this EK experiment between the anode and cathode (18 cm). During the experiments, electrical potentials over time and across the specimen were monitored (T4). After 7 days curing time, the shear strengths were measured using a 12.7 mm vane across the soil specimen at the horizontal distance of 4, 8, 12 and 16 cm from the cathode to the anode (BS1377-7-3: 1990). For each horizontal distance, the vane was positioned at two points at the same level in order to take the average value of the vane shear strength. The center-to-center distance between the two points of measurements was 4 cm (T5). Water content and percentage of CaCO3 were also measured on each section (T6). The water content of each sample was measured by weight as the ratio of the mass of water present to the oven dry weight of the soil sample. The CaCO3 percentage was determined using the acid washing technique (Mortensen et al. 2011). In this technique, the oven dried mass of the soil samples across the specimen were measured before and after an acid wash in 5 M solution of HCl. The dissolved calcium chloride after treatment was filtered. The difference in the two measured masses before and after treatment was taken as the mass of CaCO3.
4 Results and Discussion
4.1 Undrained Shear Strength
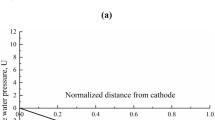
Undrained shear strength tests demonstrated the improvement of strength and stiffness of the soft clay after EK treatment. The undrained shear strength of the untreated soil was 6 kPa with moisture content 60 % from the cathode to the anode. To examine the effect of EK treatments, the shear resistance of the soil at the horizontal distance of 4, 8, 12 and 16 cm from the cathode was obtained. Figure 4 shows the undrained shear strength and the corresponding moisture content of the soil which had an increase in strength 10 times after a 7-day curing. The highest improvement in strength was found near the cathode because of the high concentration of carbonate ions (CO −23 ). The water content increased from the anode to the cathode (73–77 %), indicating that the increase in strength is as a result of some chemical reactions.
4.2 Detection of Calcium Carbonate
Figure 5 shows the percentage of precipitation CaCO3 across the specimen, which is an evidence for soil improvement during EK treatment. The CaCO3 content was from 10 to 16 % across the soil sample (T6). The increase in shear strength was due to the increase of CaCO3 across the sample. Scanning electron microscopy shows microstructure of calcium carbonate crystals between the soil particles (Fig. 6).
4.3 Variation of pH in EK Treatment
Figure 7 shows the pH variation in the compartments during the tests T1, T2 and T3 (i.e. 11 days). The electrolysis of water at the anode and the cathode due to the application of direct electric current through electrodes produces oxygen and hydrogen, respectively (Eqs. 3, 4). Oxidation of water at the anode generates an acid medium, whereas reduction at the cathode produces a basic medium that causes a pH gradient between the two electrodes (Acar et al. 1990).
As the CaCO3 precipitation needs an alkaline condition, two-pH controllers adjusted the pH in both the anode and cathode compartments. The pH value in the pump controller was set at 8.5 for both compartments. There was no need for pH adjustment during T1 experiment (1–3 days), because during this period, Ca2+ were injected into the anode chamber. A significant change in pH was also observed in T1, reaching a maximum of 12.5 and a minimum of 1.5 in the cathode and the anode chambers respectively. However, the pH was adjusted during T2 experiment (4–5 days) and in the polarity reversal T3 experiment (6–7 days).
4.4 Variation of Voltage and Temperature
Figure 8 shows the variation of voltage distribution with time during the EK treatment. At first day, the voltage distribution was linear between the electrodes in the soil because of the uniform electric conductivity. After 2 days, the voltage increased near the anode due to the presence of calcium ions. The maximum voltage distribution continued due to the electrolysis reactions and the electromigration of Ca2+ from the anode to the cathode until 4 days (T1 experiment). During the injection of production of bacteria (T2 experiment), as CO3 2− were induced from the cathode to the anode, an increase of electrical potential was observed initially in the soil (7 days). Then, a zone of high electrical resistance developed near to the cathode. This is probably due to the precipitation of CaCO3 into the soil. During polarity reversal (T3 experiment), the decrease of electrical potential near the cathode demonstrated a higher electrical resistance. After 11 days, a sharp drop in the electrical potential occurred due to the precipitation of CaCO3 across the soil specimen.
4.5 EK Treatment Mechanism
The applied electrical potential into the soil was initiated by both electroosmosis, and electromigration. The calcium ions (Ca2+) were moved from the anode chamber through the soil specimen towards the cathode chamber by both electromigration and electroosmosis during 3 days. Figure 9 illustrates the cumulative volume of the discharge fluid and its cation compositions against elapsed time by the electroosmotic flow in the cathode chamber during 3 days. The carbonate ions that have a negative charge were moved through the soil specimen towards the anode chamber. The carbonate quickly connected with the calcium ions (Ca2+). Ion migration can be used for injection of bacterial product (i.e. CO −23 ) and Ca2+ to enhance the stabilization of the soils.
Previous studies showed that the EKs has delivered significant benefits in terms of both efficiency and cost when applied to difficult ground engineering (Alshawabkeh and Sheahan 2003; Barker et al. 2004; Ahmad et al. 2010; Chien et al. 2012). It can be designed to target a specific area. The energy demands are really relatively small. Without having to excavate the entire area, money and time are saved. Therefore, EK stabilization of soft clay using the production of bacteria is considered a clean method. This method has two advantages. Firstly, the microbial carbonate precipitation is safe and environmental friendly compared to the chemical materials used in EK stabilization such as sodium silicate, aluminum hydrates, phosphoric acid (Asavadorndeja and Glawe 2005; Kamarudin et al. 2006; Ou et al. 2009; Abdullah and Al-Abadi 2010; Chien et al. 2012). Secondly, using bacterial production (i.e. CO −23 ) for deposition of CaCO3 homogeneously in fine soils is more effective compared to biocementation techniques (soil mixing and biogrouting).
5 Conclusions
Electrokinetic treatments were conducted on soft clay (kaolinite) using produced carbonate of bacterial activity and calcium. The calcium (Ca2+) electrokinetically was transported by electrooomotic flow and electromigration across the soil specimen from the anode to the cathode. A blend of bacteria and urea solution that released high concentrations of carbonate were filtered and injected into the cathode chamber. As the carbonate ions (CO −23 ) were moved from the cathode to the anode, the CaCO3 was precipitated in the presence of calcium (Ca2+) in the soil. The injection of carbonate solution and calcium could be effectively flowed in soft clay during EK treatment. The water content increased from the anode to the cathode by the electroosmotic flow from 73 to 77 %. Therefore, a significant strength increase up to 60 kPa of untreated soil (10 times) was observed in the soft clay across the specimen, with increasing CaCO3 precipitation. The CaCO3 content was from 10 to 16 % across the soil sample. It can be concluded that the EK stabilization technique is a sustainable and environmental friendly method to improve the mechanical properties of the soft soils due to the microbial carbonate precipitation.
References
Abdullah WS, Al-Abadi AM (2010) Cationic–electrokinetic improvement of an expansive soil. Appl Clay Sci 47(3–4):343–350
Acar YB, Gale RJ, Hamed J, Putnam G (1990) Electrochemical processing of soils; theory of pH gradient development by diffusion and linear convection. J Environ Sci Health A 25(6):687–714
Ahmad KB, Taha MR, Kassim KA (2010) Electrokinetic treatment on a tropical residual soil. Proc ICE-Ground Improv 164(1):3–13
Alshawabkeh AN (2001) Basics and applications of electrokinetic remediation. Handouts prepared for short course. Federal University of Rio de Janeiro, Rio de Janeiro, Brazil, 19–20 Nov 2001
Alshawabkeh AN, Sheahan TC (2003) Soft soil stabilization by ionic injection under electric fields. Ground Improv 7:177–185
Asavadorndeja P, Glawe U (2005) Electrokinetic strengthening of soft clay using the anode depolarization method. Bull Eng Geol Environ 64:237–245
ASTM (2007) Moisture–density relations for soils and soil–aggregate mixtures. D698-07e1, American Society for Testing and Materials 04.08, USA
Barker JE, Rogers CDF, Boardman DI, Peterson J (2004) Electrokinetic stabilization: an overview and case study. Ground Improv 8(2):47–58
British Standard Institution (1990) Methods of test for soils for civil engineering purposes. BSI1377, part 1–9, HMSO, London
Chien SC, Ou CY, Lo WW (2012) Electro-osmotic chemical treatment of clay with interbedded sand. J Geotech Eng ASCE 167(1):1–10
De Jong JT, Fritzges MB, Nusslein K (2006) Microbially induced cementation to control sand response to undrained shear. J Geotech Geoenviron Eng 132(11):1381–1392
De Jong JT, Mortensen BM, Martinez BC, Nelson DC (2010) Bio-mediated soil improvement. Ecol Eng 36(2):197–210
Esrig MI, Gemeinhardt JP Jr (1967) Electrokinetic stabilization of an illitic clay. J Soil Mech Found Div ASCE 93(SM3):109–128
Harkes MP, Van Paassen LA, Booster JL, Whiffin VS, Van Loosdrecht MCM (2010) Fixation and distribution of bacterial activity in sand to induce carbonate precipitation for ground reinforcement. Ecol Eng 36(2):112–117
Head KH (1982) Manual of soil laboratory testing. Pentech Press, London
Ivanov V, Chu J (2008) Applications of microorganisms to geotechnical engineering for bioclogging and biocementation of soil in situ. Rev Environ Sci Biotechnol 7(2):139–153
Johnston IW, Butterfield R (1977) A laboratory investigation of soil consolidation by electro-osmosis. Aust Geomech J G7:21–32
Kamarudin A, Anuar KK, Raihan TM (2006) Electroosmotic flows and electromigrations during electrokinetic processing of tropical residual soil. Malays J Civil Eng 18(2):74–88
Mitchell JK, Santamarina JC (2005) Biological considerations in geotechnical engineering. J Geotech Geoenviron Eng 131(10):1222–1233
Mohamedelhassan E, Shang JQ (2002) Effect of electrode materials and current intermittence in electro-osmosis. Ground Improv 5:3–11
Mortensen BM, Haber MJ, DeJong JT, Caslake LF, Nelson DC (2011) Effects of environmental factors on microbial induced calcium carbonate precipitation. J Appl Microbiol 111(2):338–349
Ou C-Y, Chien S-C, Chang H-H (2009) Soil improvement using electroosmosis with the injection of chemical solutions: field tests. Can Geotech J 46(6):727–733
Ozkan S, Gale RJ, Seals RK (1999) Electrokinetic stabilization of kaolinite by injection of Al and PO -34 ions. Ground Improv 3(4):135–144
Shang JQ, Dunlap WA (1996) Improvement of soft clays by high voltage electrokinetics. J Geotech Eng ASCE 122(4):274–280
Van Paassen LA, Harkes M P, Van Zwieten GA, Van der Zon WH, Van der Star WRL, Van Loosdrecht MCM (2009) Scale up of BioGrout: a biological ground reinforcement method. In: Hamza M, Shahien M, Mossallamy YE (eds) Proceedings of 17th international conference on soil mechanics and geotechnical engineering (ICSMGE), pp 2328–2333
Van Paassen LA, Daza CM, Staal M, Sorokin DY, Van der Zon W, Van Loosdrecht MCM (2010) Potential soil reinforcement by microbial denitrification. Ecol Eng 36(2):168–175
Whiffin VS (2004) Microbial CaCO3 precipitation for the production of biocement. Ph.D. thesis, School of Biological Sciences and Biotechnology, Murdoch University, Perth Australia
Whiffin VS, Van Paassen LA, Harkes MP (2007) Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol J 24(5):417–423
Acknowledgments
Financial supports from the Research Management Center (RMC) of the Universiti Putra Malaysia under Grant No. 5527094 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Keykha, H.A., Huat, B.B.K. & Asadi, A. Electrokinetic Stabilization of Soft Soil Using Carbonate-Producing Bacteria. Geotech Geol Eng 32, 739–747 (2014). https://doi.org/10.1007/s10706-014-9753-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10706-014-9753-8